ABSTRACT
Many evaluations have been performed on the economic impact of pneumococcal vaccination in older adults (>64 y of age) in several countries, including Italy. However, these studies did not include the new data on the effectiveness of 13-valent conjugate pneumococcal vaccine (PCV13) in the elderly reported by the CAPiTA Study. The aim of the present study was to update our previous budget impact analysis of multi-cohort PCV13 vaccination in adults in Italy by including new scientific evidence. We also compared single-cohort vaccination strategies per year, in order to identify the cohort with the most favorable economic profile, in the event of the multi-cohort approach not being economically sustainable for the National Health System (NHS).
The new impact analysis highlights that the vaccination of one, two or three adult cohorts per year in Italy would lead to a considerable reduction in pneumococcal disease and its related costs over 5 y. The strategies proved cost-effective (ICERs ranging from €14,605 to €15,412/QALY), i.e. well below the threshold of €50,000/QALY. The ICERs were slightly lower than those calculated in the first published analysis and vaccination continued to be economically favorable. In the case of a mono-cohort strategy, the vaccination of 65-year-old subjects, albeit more expensive, proved to be more favorable than the vaccination of 70- or 75-year-old cohorts.
Finally, after the inclusion of the recent clinical evidence, the age-based PCV13 vaccination of the elderly in Italy continued to be economically justified from the NHS perspective in the short period. Vaccination of the elderly should therefore be strongly recommended nationwide in Italy.
Introduction
In the last decade, many economic evaluations have been performed in order to determine the financial sustainability and cost-effectiveness of the implementation of new vaccines and vaccination strategies against invasive diseases due to N. Meningitidis and, particularly, S. pneumoniae in national or regional immunisation programs in many countries,Citation1-10 including Italy.
The implementation of an age-based strategy of vaccination with the 13-valent conjugate pneumococcal vaccine (PCV13) in adult and elderly subjects (>64 y of age) is one of the issues most debated by scientists and decision-makers. While the clinical impact of pneumococcal vaccination is significantly evident,Citation11,12 its budget impact and cost-effectiveness profile are not entirely clear. This latter aspect is particularly relevant in Italy at the present time, as the current spending review has limited the resources available to regional and national health systems. Decision-makers need to know in advance what the budget impact of pneumococcal vaccination with PCV13 in the elderly will be in the short period (that of their mandate) and whether it is economically sustainable by the health system.
Some years ago, we made an economic evaluation of the potential clinical and economic impact of PCV13 vaccination in adults in Italy by developing an ad hoc population simulation model.Citation13 This mathematical model allowed us to evaluate the budget impact of multi-cohort PCV13 vaccination strategies targeting some specific adult age-groups each year on the entire elderly population over a 5-years period, according to the National Health System (NHS) perspective. These vaccination strategies were compared with a no-vaccination scenario, according to the general current situation of immunisation in Italy. The effect of herd immunity and the serotype replacement resulting from PCV13 immunisation were not included in the model. In that study, the age-based vaccination of one (65-year-old subjects), two (65- and 70-year-olds) or three cohorts (65-, 70- and 75-year-olds) each year over the 5-year period was assumed. Thus, after 5 y of the immunisation program, 5, 10 and 15 adult cohorts, respectively, were assumed to be vaccinated.
The results of that study indicated that age-based PCV13 vaccination in Italy in subjects >64 y of age would cost the NHS from €84 million to €222 million net in five years (€17 – €44 million per year), according to the vaccination strategy adopted. The average cost of vaccinating each adult cohort amounted to €17–15 million. At the same time, however, vaccination with PCV13 could prevent 5–15 million pneumococcal cases, including community-acquired pneumonia (CAP) and invasive pneumococcal diseases (IPD), with consequent economic savings of €7 – 19 million for the NHS due to the clinical cases avoided. Pneumococcal vaccination already proved economically justified for the NHS during the second year of the immunisation program, with ICERs ranging from €17,000 to €22,000/QALY, according to the number of adult cohorts immunised each year. However, in the study we emphasized that further additional health benefits could be expected on extending the period of study (to more than 5 years) and considering the number of pneumococcal cases prevented by 5-year PCV13 vaccination and the related clinical savings. Lastly, we suggested that continuing a mono-cohort pneumococcal vaccination strategy should be recommended after the first 5-year multi-cohort strategy (when the vaccinated cohorts begin to overlap) in order to maintain and increase the benefits already achieved during the first 5 y of implementation of pneumococcal immunisation.
However, our study had an important limitation, as was stated at the time; in the mathematical model, the efficacy of PCV13 against both IPD and CAP in adult subjects was assumed to be the same as in pediatric subjects immunised with 7-valent conjugate pneumococcal vaccine. This assumption was made because specific data on older subjects were not available at the time. Indeed, when the economic evaluation was performed, we were waiting for the effectiveness data on PCV13 vaccination in the elderly from the CAPiTA Study, which was ongoing in the Netherlands. Now, clinical evidence of PCV13 effectiveness in adults is available.Citation14 Meanwhile, other scientific evidence has become available, such as the distribution of pneumococcal CAP between bacteraemic and non-bacteraemic CAP, as reported by Said et al.Citation15 In the light of these considerations, our previous mathematical model and the related decisional tree were updated.
The main aim of the present study was therefore to update the previous budget impact analysis in order to obtained more accurate economic results. Moreover, given the more limited current economic resources of the National and Regional Health Services in Italy, single-cohort PCV13 vaccination strategies involving 65- or 70- or 75-year-old subjects per year were also comparatively analyzed in order to identify the elderly cohort with the most favorable profile, in the event of the multi-cohort approach not being economically sustainable for the National Health System owing to budgetary constraints.
Results
shows the number of clinical cases and deaths due to pneumococcal CAP (subdivided into bacteraemic and non-bacteraemic) and PM avoided in the adult Italian population during the 5 y of PCV3 immunization in Italy; these are compared with the estimated cases of disease and death in the same period in the non-vaccination scenario. The greatest impact of PCV13 vaccination on the absolute number of cases was due to the reduction in CAP (particularly outpatient non-bacteraemic pneumococcal CAP), while the greatest reduction rate concerned pneumococcal meningitides and bacteraemic CAP. By contrast, the greatest number of deaths avoided through PCV13 vaccination involved non-bacteraemic pneumococcal CAP, while the greatest reduction rate was seen in deaths due to pneumococcal meningitides, even though the number of deaths due to this disease was low.
Table 1. Avoided cases and deaths following PCV13 vaccination versus the no-vaccination scenario in the elderly population over the 5-year immunization period.
Table 2. Economic savings due to avoided cases, vaccination costs, net costs, net costs per immunised cohort, QALYs and ICERs related to PCV13 vaccination of the elderly population over the 5-year immunisation period in Italy (Euro).
The costs of the immunisation program for one, two or three Italian adult cohorts per year over the 5-year period ranged between €85 million and €234 million. The savings for the NHS due to pneumococcal cases avoided over the 5 y were between €6 million and €17 million. Thus, the net costs of PCV13 vaccination ranged between €79 million and €217 million, according to the number of cohorts immunised per year (). The net cost per vaccinated cohort proved to be about €15 million.
Table 3. Pneumococcal cases and deaths avoided, economic savings due to avoided cases, vaccination costs, net costs, QALY and ICERs related to the comparative single-cohort PCV13 vaccination strategies (Euro).
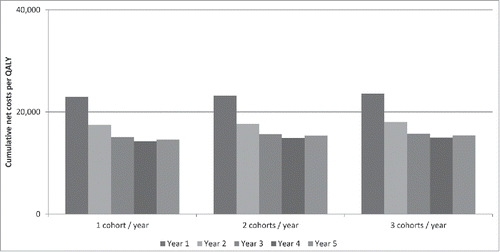
With regard to the quality-adjusted life-years gained by implementing PCV13 vaccination (ranging between 5,386 and 14,061 QALY), the cost/QALY due to PCV13 vaccination in the one, two or three adult cohorts simultaneously immunised each year was lower than €50,000/QALY, in accordance with WHO indications of the threshold value.Citation16 PCV13 vaccination in adults therefore proved highly cost-effective (). In addition, the PCV13 vaccination of elderly cohorts became economically justified the second year after the start of the immunisation program ().
The results of the comparative analysis of mono-cohort vaccination strategies are shown in . The vaccination of subjects aged 65 years, despite being the most expensive (probably owing to the greater number of subjects in this age group), would prevent the most cases of pneumococcal diseases, resulting in larger savings for the NHS, greater QALY gains, and therefore lower cost/QALY.
Table 4. Cost (Euro) per QALY in the sensitivity analysis.
In the sensitivity analysis, PCV13 vaccination of one, two or three cohorts proved economically cost-effective (<€50,000/QALY) on both univariate and multivariate analyses ().
Table 5. Input data in the population model (base case scenario).
Discussion and conclusions
The implementation of age-based PCV13 vaccination in adults is still one of the most debated issues among decision-makers and stakeholders in developed countries. The most critical point is the economic suitability of this preventive intervention in the elderly. Many pharmaco-economic studies have been published in the international and national literature. However, all these studies have had an important limitation, in that they did not consider the efficacy or effectiveness of PCV13 in the elderly, as those data were not available at the time. This issue is well highlighted in a recent review by Dirmesropian et al..Citation17 The authors of this review point out that the majority (9 out of 10) of economic evaluation studies identified concluded that PCV13 immunization was cost-effective in adults and/or the elderly, but that these results were based on assumptions that could not always be informed by robust evidence. Particularly, great uncertainties concerned the efficacy of PCV13 vaccine against non-invasive pneumonia.Citation17 Dirmesropian et al., therefore, concluded that publication of the CAPiTA Study results would oblige the economic evaluation of PCV13 in different countries to be reassessed in the light of this new clinical evidence. The present study meets the indication of these authors.
The main strength of our study is that it included the most recent clinical evidence of the efficacy of PCV13 in adults supplied by the CAPiTA Study,Citation14 thereby updating and confirming our previous results. Specifically, the results of our economic analysis highlighted the fact that age-based vaccination with PCV13 in one, two or three adult cohorts per year in Italy would lead to a considerable reduction in the burden of pneumococcal disease and its related costs over 5 y. The single-, double- and triple-cohort strategies proved highly cost-effective, with ICERs ranging from €14,605 to €15,412/QALY. Age-based adult PCV13 vaccination proved favorable from the economic standpoint two years after the start of the immunisation program, according to the cumulative net costs per QALY. The cost/QALY values obtained for adult PCV13 vaccination in this updated version of our population model were slightly lower than those calculated in the first version, in which PCV13 effectiveness data were not included. Therefore, PCV13 vaccination continued to be economically favorable. In addition, if indirect costs (as in the societal perspective) and non-medical direct costs were considered in the mathematical model, the results would be much more favorable.
In the case of a single-cohort immunisation strategy, the vaccination of 65-year old subjects, albeit more costly on account of the number of subjects in this age-group, was seen to be more suitable than the vaccination of 70- or 75-year-olds.
In conclusion, after incorporating the most recent epidemiological evidence and the results of the CAPiTA Study into our economic evaluation model, we confirmed that the age-based PCV13 vaccination of older subjects (≥65 y of age) in Italy was economically favorable and justified from the NHS perspective in the short period. A multi-cohort or single-cohort strategy can be chosen according to the economic resources of the NHS. In the case of a mono-cohort strategy, the cohort of 65-year-old subjects should be preferentially targeted on account of the greater health benefits obtained in spite of the higher costs. Like the data from previous economic evaluations, our results strongly suggest that age-based pneumococcal vaccination in the elderly should be recommended nationwide in Italy.
Materials and methods
The budget impact analysis was performed by updating the previous population model and its related decisional tree.Citation13 shows the new decisional tree and the health status (non-bacteraemic and bacteraemic CAP, and pneumococcal meningitis) included in the model.
Figure 2. Decisional tree in the population model (NB CAP: non-bacteraemic CAP; B CAP: bacteraemic CAP).
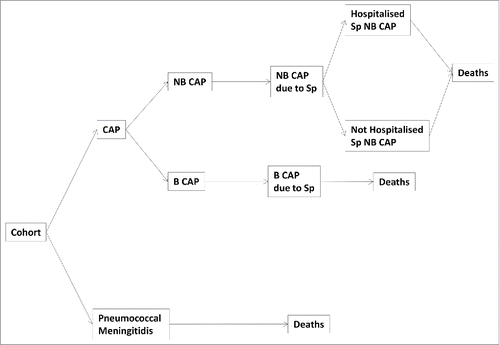
In the current model, the recent effectiveness data on PCV13 vaccination reported by the CAPiTA Study were used: 45% efficacy against CAP and 75% against IPD due to vaccine-type pneumococcal strains.Citation14 In addition, the rate of bacteraemic CAP due to S. pneumoniae infection among pneumococcal CAP (20%), derived from a recent meta-analysis,Citation15 were included in the model. Finally, the model was updated with the most recently available Italian population data: subjects included in the analysis were elderly (>64 y of age) residents in Italy on January 1, 2015.Citation18 All input data included in the model are shown in .Citation14,15,19-26
The effect of herd immunity and pneumococcal serotype replacement were not included in the model, owing to gaps in the available data on these issues in the elderly.
Univariate and multivariate sensitivity analyses were performed in order to evaluate the uncertainty of some input data (rate of CAP due to S. pneumonia, CAP and PM incidence, percentage of elderly vaccinated by general practitioners, vaccination coverage, cost of hospitalised CAP patients and vaccine delivery cost).
Disclosure of potential conflicts of interest
One of the authors (S.B.) had received a grant from Pfizer Italia to support a part-time researcher position.
References
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Lee BY, Zimmerman RK. Modeling of cost effectiveness of pneumococcal conjugate vaccination strategies in US older adults. Am J Prev Med 2013; 44:373-81; PMID:23498103; http://dx.doi.org/10.1016/j.amepre.2012.11.035
- Weycker D., Sato R, Strutton D, Edelsberg J, Atwood M, Jackson LA. Public health and economic impact of 13-valent pneumococcal conjugate vaccine in US adults aged ≥ 50 years. Vaccine 2012; 30:5437-44; PMID:22728289; http://dx.doi.org/10.1016/j.vaccine.2012.05.076
- Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine 2013; 31:3950-6; PMID:23806240; http://dx.doi.org/10.1016/j.vaccine.2013.06.037
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA 2012; 307:804-12; PMID:22357831
- Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine 2013; 31:6011-21; PMID:24148572; http://dx.doi.org/10.1016/j.vaccine.2013.10.024
- Boccalini S, Bechini A, Levi M, Tiscione E, Gasparini R, Bonanni P. Cost-effectiveness of new adult pneumococcal vaccination strategies in italy. Hum Vaccin Immunother 2013; 9:699-706; PMID:23295824; http://dx.doi.org/10.4161/hv.23268
- Liguori G, Parlato A, Zamparelli AS, Belfiore P, Gallé F, Di Onofrio V, Riganti C, Zamparelli B. Società Italiana di Health Horizon Scanning (SIHHS). Adult immunization with 13-valent pneumococcal vaccine in campania region, south italy: an economic evaluation. Hum Vaccin Immunother 2014; 10:492-7; PMID:24185467; http://dx.doi.org/10.4161/hv.26888
- Rozenbaum MH, van Hoek AJ, Fleming D, Trotter CL, Miller E, Edmunds WJ. Vaccination of risk groups in england using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ 2012; 345:e6879; PMID:23103369; http://dx.doi.org/10.1136/bmj.e6879
- Rozenbaum MH, Hak E, van der Werf TS, Postma MJ. Results of a cohort model analysis of the cost-effectiveness of routine immunization with 13-valent pneumococcal conjugate vaccine of those aged ≥ 65 years in the netherlands. Clin Ther 2010; 32:1517-32; PMID:20728764; http://dx.doi.org/10.1016/j.clinthera.2010.06.016
- Pradas R, Gil de Miguel A, Álvaro A, Gil-Prieto R, Lorente R, Méndez C, Guijarro P, Antoñanzas F. Budget impact analysis of a pneumococcal vaccination programme in the 65-year-old Spanish cohort using a dynamic model. BMC Infect Dis 2013; 13:175; PMID:23578307; http://dx.doi.org/10.1186/1471-2334-13-175
- Agenzia Italiana del Farmaco (AIFA). Prevenar 13. Available at: https://farmaci.agenziafarmaco.gov.it/bancadatifarmaci/farmaco?farmaco=039550
- European Medicines Agency (EMA). Prevenar 13. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001104/human_med_001220.jsp&mid=WC0b01ac058001d124
- Boccalini S, Bechini A, Levi M, Tiscione E, Gasparini R, Bonanni P. Cost-effectiveness of new adult pneumococcal vaccination strategies in Italy. Hum Vaccin Immunother 2013: 9(3):699-706; PMID:23295824; http://dx.doi.org/10.4161/hv.23268
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114-25; PMID:25785969; http://dx.doi.org/10.1056/NEJMoa1408544
- Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O'Brien KL, for the AGEDD Adult Pneumococcal Burden Study Team. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One 2013; 8(4):e60273; PMID:23565216; http://dx.doi.org/10.1371/journal.pone.0060273
- World Health Organization (WHO). Cost effectiveness and strategic planning (WHO-CHOICE). Cost-effectiveness thresholds. Available at: http://www.who.int/choice/costs/CER_thresholds/en/
- Dirmesropian S, Wood JG, MacIntyre CR, Newall AT. A review of economic evaluations of 13-valent pneumococcal conjugate vaccine (PCV13) in adults and the elderly. Hum Vaccin Immunother 2015; 11(4):818-25; PMID:25933180; http://dx.doi.org/10.1080/21645515.2015.1011954
- Demo.istat. Demografia in cifre. Istituto Nazionale di Statistica. Popolazione residente al 1 Gennaio 2015 per età, sesso e stato civile. Available at: http://demo.istat.it
- Viegi G, Pistelli R, Cazzola M, Falcone F, Cerveri I, Rossi A, Ugo Di Maria G. Epidemiological survey on incidence and treatment of community acquired pneumonia in Italy. Respir Med 2006; 100:46-55; PMID:16046113; http://dx.doi.org/10.1016/j.rmed.2005.04.013
- Bewick T, Sheppard C, Greenwood S, Slack M, Trotter C, George R, Lim WS. Serotype prevalence in adults hospitalized with pneumococcal non-invasive community-acquired pneumonia. Thorax 2012; 67:540-5; PMID:22374921; http://dx.doi.org/10.1136/thoraxjnl-2011-201092
- Merito M, Giorgi Rossi P, Mantovani J, Curtale F, Borgia P, Guasticchi G. Cost-effectiveness of vaccinating for invasive pneumococcal disease in the elderly in the Lazio region of Italy. Vaccine 2007; 25:458-65; PMID:17049685; http://dx.doi.org/10.1016/j.vaccine.2006.08.005
- Schito GC, Fadda G, Nicoletti G, Debbia EA. Streptococcus pneumoniae isolati da malattie invasive e infezioni respiratorie in soggetti adulti e anziani (> 50 anni) ospedalizzati in Italia: studio clinico-microbiologico retrospettivo. Giornale Italiano di Microbiologia Medica Odontoiatrica e Clinica 2011; 15:75-98
- Agenzia Nazionale per i Servizi Sanitari Regionali. (Age.na.s.). Ricoveri ospedalieri, i sistemi tariffari regionali vigenti nell'anno 2009. Available at: www.agenas.it/index.htm
- Potena A, Simoni M, Cellini M, Cartabellotta A, Ballerin L, Piattella M, Putinati S. Management of community-acquired pneumonia by trained family general practitioners. Int J Tuberc Lung Dis 2008; 12:19-25; PMID:18173872
- Sisk JE, Whang W, Butler JC, Sneller VP, Whitney CG. Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Ann Intern Med 2003; 138:960-8; PMID:12809452; http://dx.doi.org/10.7326/0003-4819-138-12-200306170-00007
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA 2012; 307:804-12; PMID:22357831