ABSTRACT
Pre-existing neutralizing antibodies (NAbs) directed against vaccine vectors have attracted considerable research attention. Therefore, our aim was to establish a high-throughput economical neutralization assay to investigate the epidemiology of adenovirus type 2 (Ad2)-specific immunity in China and developed countries, including in a Chinese Human immunodeficiency virus (HIV)-1-infected population, and to guide the application of Ad2-vectored vaccines. We established a FluoroSpot-based anti-Ad2-virus neutralization assay using a recombinant replication-deficient Ad2 that expresses enhanced green fluorescent protein and standardized the critical parameters, including the choice of cell line, cell concentration, viral infective dose, and incubation time. The sera of 561 healthy individuals from China and developed countries and from 230 HIV-1-infected Chinese individuals were screened with this assay for Nabs against Ad2. The prevalence of anti-Ad2 NAbs was high in both China (92.2%) and developed countries (86.9%). Of the Ad2-seropositive individuals, 64.6% in China and 77.4% in developed countries had high NAb titers (> 810). The frequency of anti-Ad2 NAbs was higher in Anhui (97.5%) than in Beijing (88.7%). Their prevalence differed significantly according to age in Beijing, but not in Anhui Province, but by sex in neither province. Ad2 seroprevalence was as high among HIV-1-infected individuals (88.7%) as among healthy individuals (92.2%) in China. In conclusion, a simple, intuitive, high-throughput, economical fluorescence-based neutralization assay was developed to determine anti-Ad2 NAbs titers. Ad2 exposure was high in both healthy and HIV-1-infected populations in China, so vectors based on Ad2 may be inappropriate for human vaccines.
Introduction
Several strategies have been developed to prevent the transmission of human immunodeficiency virus (HIV), including a preventive HIV vaccine. There are 3 main types of vaccines: recombinant viral vector vaccines,Citation1 subunit vaccines,Citation2,3 and DNA vaccines.Citation4 Some virus-based vectors derived from adenoviruses (Ads),Citation5 adeno-associated viruses (AAVs),Citation6 and pox virusesCitation7 have been used to construct vaccines against HIV. A recombinant adenovirus serotype 5 (rAd5) vector was considered the most promising of these vector, but the vaccines developed by Merck Research Laboratories and the NIH Vaccine Research Center have been disappointing because of the pre-existing neutralizing antibodies (Nabs) present in the recipients and other factors. ,8,9 Since the RV144 trial, pre-existing NAbs to vaccine vectors have attracted considerable research attention because of their potential to promote infection.Citation10-12 The development of vectors based on other rAd serotypes with lower seroprevalence than Ad5, such as Ad35 and Ad26, has shown promise in human trials.Citation13,14 The functions of most Ad2 and Ad5 proteins (from adenoviral species C) are similar and these are currently the most widely investigated adenoviruses. Several Ad2-vectored candidate vaccines have been developed in China, but the seroprevalence rates and titers of NAbs to Ad2 among healthy and HIV-positive individuals in China are unclear. Therefore, a novel high-throughput neutralization assay for Ad2 is urgently required.
Ad2-specific Nab titers are usually determined by the inhibition of replication and plaque scoring or by the inhibition of reporter gene expression.Citation15,16 A wide range of enzyme-activated chemiluminescence-based NAb detection tests have been used to detect Ad NAbs, including firefly luciferase,Citation17-19 the secreted alkaline phosphatase reporter gene (SEAP),Citation20,21 and β-galactosidase.Citation22 Enhanced green fluorescent protein (EGFP) has also been used in reporter gene expression inhibition assays, known as ‘fluorescence-based NAb detection tests'.Citation23 Here, we developed a novel neutralization assay for Ad2 using a replication-defective recombinant Ad2 containing the EGFP reporter gene (Ad2–EGFP) in a 96-well format. After infection, the number of fluorospots is counted with an ImmunoSpot reader (CTL, Shaker Heights, OH, USA), as in previous studies.Citation24 The use of FluoroSpot to screen for green fluorescent cells has several advantages over visual inspection with fluorescence microscopy or laborious detection methods involving flow cytometry, including its intuitiveness, objectivity, high throughput, and simplicity.Citation25 Previously, our laboratory has demonstrated that the NAb titers determined with FluoroSpot and flow cytometry show good consistency. Therefore, FluoroSpot is believed to be the ideal substitute for the classic EGFP–pseudovirion assay using flow cytometry.Citation26
In this study, we investigated the baseline levels of Nabs directed against Ad2 in representative Chinese subjects and foreigners from developed countries, and demonstrated their high seroprevalence and high titers. These results should guide the application of therapeutic and prophylactic Ad2-vectored HIV vaccines.
Results
Optimization of the neutralization assay
Two cell lines, HEK 293T and Vero cells, were infected with different doses of the Ad2–EGFP virus to determine the optimal cells for use in the neutralization assay ( and ). After incubation, the EGFP-positive cells were detected with an ImmunoSpot reader. Regardless of whether a high or low dose of virus was used for infection, low levels of EGFP-expressing Vero cells were detected, indicating that Vero cells are unsuitable for the assay. Therefore, the HEK 293T cell line was chosen for subsequent experiments. The numbers of fluorospots were observed after incubation for 48 h because the number of positive cells reached a plateau at that time. When we compared the use of 1 × 104 cells/well and 2 × 104 cells/well in a 96-well plate, the higher cell density gave better results because the 293T cells formed a monolayer after incubation for 48 h ( and ). Rather than preincubation, the mixed culture method was chosen for its simplicity and better reproducibility ( and ). The optimal concentration of the virus was also examined. shows the direct correlation between the Ad2–EGFP viral concentration and the number of EGFP-positive cells. HEK 293T cells were infected with a range of viral infective doses (0.1–1800 viral particles [vp]/cell), and the number of positive cells was maximum at 67 vp/cell. The 293T cell line showed a cytopathic effect after infection for 36 h with a dose of 100 vp/cell. For the easy observation of EGFP-expressing cells, counts of 600–1000 cells are required. Therefore, we selected an Ad2–EGFP viral concentration of 10 vp/cell. Next, to identify the optimal dose of virus for the assay, the neutralizing activities of serum samples from Ad2-vaccinated mice were determined and their effects on different Ad2–EGFP viral concentrations were compared (). Neutralization of the control virus was detected at 10 and 2 vp/cell with negative mouse serum. Similar dynamic curves for the Ad2-vaccinated mouse serum samples were also observed at viral concentrations of both 10 vp/cell and 2 vp/cell. In this assay, the mouse antiserum neutralized the Ad2–EGFP virus at 10 vp/cell and 2 vp/cell with titers of 8356 and 4656, respectively. Therefore, an infectious dose of 10 vp/cell was chosen as the optimal viral dose for this assay. The final format used to test human sera in the Ad2–EGFP-neutralizing assay is described in .
Figure 1. Optimization of the Ad2–EGFP neutralization assay. (A–B) HEK 293T and Vero cells were infected with the Ad2–EGFP virus at 10 vp/cell (A) or 100 vp/cell (B) in the neutralization assay. The x-axis indicates the hours after Ad2–EGFP infection and the y-axis indicates the counts of EGFP-positive cells. The dotted line represents the background level of cells. (C–D) Determination of 293T cell density. The x-axis indicates different doses of Ad2–EGFP that were added to 104 cells/well (C) or 2 × 104 cells/well (D) in a 96-well plate. The y-axis show the numbers of EGFP-positive cells after incubation for 48 h. (E–F) Influence of 293T cells seeded in a 96-well plate 1 day before the addition of the Ad2 virus. The x-axis indicates the different doses of Ad2–EGFP, and the y-axis show the numbers of EGFP-positive cells after incubation for 48 h with preinoculation (E) or mixing (F). (G) Dose–response between the Ad2–EGFP input and the numbers of EGFP-positive cells. The x-axis indicates the viral concentration (0.1–1800 vp/cell) added to 2 × 104 cells/well. The y-axis show the numbers of EGFP-positive cells after incubation for 48 h. (H) Two different concentrations of Ad2–EGFP (•, ▴, 10 vp/cell; ▪, ▾, 2 vp/cell) were used with various concentrations of mouse serum in the neutralization assay. The experiment was performed with mouse serum collected before (▴, ▾) and 1 week after (•, ▪) the second vaccination with Ad2. The x-axis indicates the different concentrations of mouse serum, and the y-axis indicates the percentage neutralization. Each data point represents the average of 3 experiments.
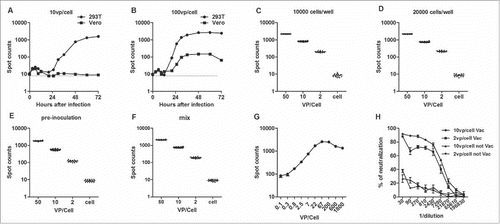
Table 1. Description of the final format of the anti-Ad2 neutralization assay for human sera.
Neutralization assay validated with sera from mice inoculated with different viruses
BALB/c mice were vaccinated twice with Ad2–EGFP, Ad5–EGFP, AAV1, or saline. Seven days after the second inoculation, the antisera were collected 3 times and the Ad2 Nabs were analyzed with the Ad2–EGFP neutralization assay. Neutralizing activity was only detected in the sera from Ad2-vaccinated mice, with a 50% neutralization titer of 4159 ± 2494. Only one sample vaccinated with Ad5 was positive for Ad2-specific Nabs, but at a low titer of 1:60. The AAV1-vaccinated and naïve mice showed no neutralizing activity (). When a 1:30 dilution of serum inhibited EFGP expression by ≥ 50%, the serum was deemed positive for Ad2 neutralizing capacity (). Taken together, these data demonstrate that Ad2-serotype-specific NAbs can be detected using the assay developed here, with good sensitivity and specificity.
Figure 2. NAbs against Ad2 after the administration of different viruses. NAbs directed against Ad2 were measured in sera collected 3 times from mice (n = 4 in each group) treated twice with Ad2, Ad5, or AAV1 (1 × 109 vp/animal) or saline by intramuscular injection. The titer is expressed as the serum dilution that inhibited 50% of the EGFP-positive cells. Statistically significant differences (P values) are shown above the figure.
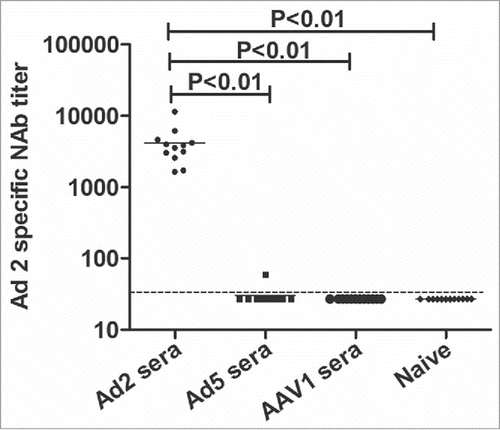
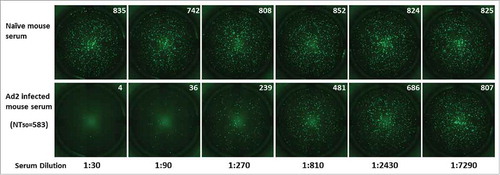
Figure 3. Fluorescence imaging of EGFP and determination of the 50% neutralization titers in mouse anti-Ad2 sera. The recombinant Ad2–EGFP virus was incubated with serial 3-fold dilutions (starting at 1:30) of mouse serum sampled before and after inoculation with Ad2 viral particles. FluoroSpot imaging of the negative control and Ad2-viru-infected mouse serum samples is shown. The numbers of fluorospots were counted after incubation, with an ImmunoSpot reader, and are shown in the upper right corner of each image. The 50% neutralization titer (NT50) for each serum sample was calculated with the Reed–Muench method.
Seroprevalence of anti-Ad2 NAbs in healthy human samples from China and developed countries
The percentage of normal individuals from the Anhui and Beijing Provinces with detectable NAbs against Ad2 was 92.2% (461/500). The prevalence of NAbs against Ad2 in Beijing Province (88.7%, 266/300) and Anhui Province (97.5%, 195/200; P < 0.01; ) were significantly different. The seroprevalence of NAbs against Ad2 was 79.3% in Beijing and 92.0% in Anhui Province at a serum dilution of > 1:80, and the difference was significant (P < 0.01; ). The numbers of donors with high titers (> 1:810) did not differ significantly between the 2 regions. However, a higher proportion of Ad2-seropositive individuals had low titers (1:30–1:90) in Beijing Province than in Anhui Province (P < 0.05; ).
Figure 4. Prevalence and magnitudes of anti-Ad2 NAbs in serum samples from China (n = 500) or developed countries (DC; n = 61). (A) Histogram showing the percentage of individuals in the Beijing and Anhui Provinces that were seropositive for anti-Ad2 NAbs. If a serum dilution > 1:30 (▪) or > 1:80 (γ) inhibited the EGFP-positive cells by ≥ 50%, the serum was considered positive. (B) Distribution of NAb titers against Ad2. To establish a qualitative reference for the potency of the positive sera, the NAb titer was divided into 3 groups: low (1:30–1:90), medium (1:90–1:810), and high (> 1:810). (C) Percentage of serum samples from China and DCs that neutralized infections by Ad2. Sera were considered positive if a serum dilution > 1:30 (▪) or > 1:80 (γ) inhibited EGFP-positive cells by ≥ 50%. (D) Arbitrary intervals were established as a qualitative reference for the potency of the sera from China or DCs: low (1:30–1:90), medium (1:90–1:810), and high (> 1:810). Statistically significant differences (P values) are shown above the figure.
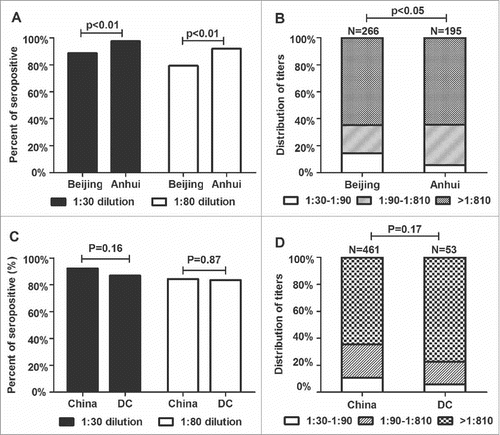
The NAb titers were divided into 4 age-related cohorts. In Beijing, the percentage of individuals positive for anti-Ad2 NAbs increased with increasing age, and age correlated positively with the percentage of positive individuals (P < 0.01). However, the percentage of individuals positive for anti-Ad2 NAbs did not increase significantly with age in Anhui Province (P = 0.80). The prevalence of NAbs against Ad2 did not differ statistically in the subgroups defined by sex (). The seroprevalence of anti-Ad2 NAbs in China and developed countries did not differ (P = 0.16; ). At a serum dilution of > 1:80, the prevalence of NAbs against Ad2 also did not differ between these populations (P = 0.87; ). The distribution patterns of anti-Ad2 NAb titers were similar in subjects from developed countries and those from China (P = 0.17; ). In total, no differences were observed in the prevalence of NAbs against Ad2 in China and developed countries.
Table 2. Epidemiological study of neutralizing antibodies in the general subjects in China.
Seroprevalence of anti-Ad2 NAbs in HIV-1-infected samples from China
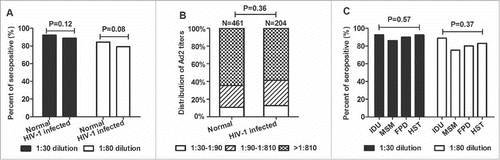
To determine whether Ad2 can be used as a therapeutic HIV vaccine vector, 230 HIV-1-infected sera were tested for the seroprevalence of anti-Ad2 NAbs. The seroprevalence of anti-Ad2 NAbs did not differ significantly between the healthy population and the subjects infected with HIV-1 at serum dilutions of 1:30 (92.2% and 88.7%, respectively; P = 0.12; ) and 1:80 (84.4% and 79.1%, respectively; P = 0.08; ). There was also no difference in the distributions of the Ad2 NAb titers in these 2 groups of individuals, and in both groups, most anti-Ad2 NAb titers were > 1:810 (P = 0.36; ). The HIV-1-infected individuals were divided into 4 subgroups: men who have sex with men (n = 130), intravenous drug users (n = 27), former commercial plasma donors (n = 20), and those infected by heterosexual transmission (n = 53). The differences between the subgroups were not statistically significant at dilutions of 1:30 and 1:80 (P = 0.57 and P = 0.37, respectively; ).
Figure 5. Comparison of the prevalence and magnitudes of anti-Ad2 NAbs in healthy (n = 500) and HIV-1-infected individuals (n = 230). (A) Percentages of serum samples from healthy and HIV-1-infected individuals in China that neutralized Ad2 infections. Sera were considered positive if a serum dilution > 1:30 (▪) or > 1:80 (γ) inhibited EGFP-positive cells by ≥ 50%. (B) Titers of anti-Ad2 NAbs. To establish a qualitative reference for the potency of the positive sera from healthy or HIV-1-infected individuals in China, the NAb titer was divided into 3 groups: low (1:30–1:90), medium (1:90–1:810), and high (> 1:810). (C) Histogram showing the percentages of HIV-1-infected individuals who were seropositive for anti-Ad2 NAbs among intravenous drug users (IDU, n = 27), men who have sex with men (MSM, n = 130), former commercial plasma donors (FPD, n = 20), and those infected by heterosexual transmission (HST, n = 53). Statistically significant differences (P values) are shown above the figure.
Discussion
With the failure of the rAd5-based-vector HIV vaccines developed by Merck Research Laboratories and the NIH Vaccine Research Center, other Ads, such as Ad2, Ad26, and Ad35, are being developed as vaccine vectors based on the assumption that they will outperform the Ad5 vector.Citation13,14 The seroprevalence rates and titers of NAbs directed against Ad2 have previously been reported in the Belgian population (n = 100) using traditional replication inhibition with a plaque assay, and in the Philadelphian population (n = 40) with neutralization assays, using rAd and the SEAP reporter gene.Citation15,20 Here, we developed a quantitative neutralization assay using rAd2 and the EGFP reporter gene. We screened healthy individuals and several HIV-1-positive populations in China for pre-existing humoral immunity to Ad2, for the first time.
With this EGFP-based method, the NAb titers are determined visually with FluoroSpot, which is used to analyze single cells secreting several cytokines in the ELISPOT assay.Citation27 Here, FluoroSpot was used to directly detect EGFP-positive cells infected with the rAd2–EGFP virus, rather than using fluorescence microscopy or flow cytometry. The results of EGFP-based neutralization assays have shown good consistency with those of flow cytometry and the FluoroSpot detection method. The use of EGFP as the reporter has several advantages over other reporter proteins, such as luciferase and SEAP, including its intuitiveness, objectivity, high throughput, simplicity, and economy, because no further substrates need be added. We also compared the sensitivity of this method with that of flow cytometry. The sensitivity of the FluoroSpot counts can be adjusted by changing the sensitivity parameter of the ImmunoSpot reader because too high sensitivity could yield high background noise and low specificity, whereas too low sensitivity might miss specific spots. Here, 215 was used as the optimal sensitivity parameter for this pseudovirion-based neutralization assay. At this sensitivity, about 300 fluorescent spots of EGFP were detected. If the rate of positive cells reaches 15%, which is the most suitable dose for flow cytometry, the 2 methods have similar sensitivity.Citation26
Standardizing the assay parameters, including the cell line, cell concentration, viral infective dose, and incubation method, is important for this assay, and can affect its sensitivity, specificity, and reproducibility. Cell lines that support viral replication, such as Hep2, A549, Vero, and HEK 293 cells, are required for infection with wild-type Ads.Citation17 In this study, we selected HEK 293T cells for the follow-up tests because the levels of infection and the expression of Ad2–EGFP were low in Vero cells. Most researchers have also selected HEK 293 cell to assay NAbs against Ads.Citation20,21 In our assay, a viral dose of 10 vp/cell was shown to produce results in the middle of the linear range of detection, with spot counts of 600–700, and was therefore appropriate for the assay, ensuring good sensitivity and reproducibility. Sprangers et al. also selected the viral dose for an Ad5 NAb assay to ensure that the corresponding luciferase expression was in the middle of the linear range.Citation17 Nie et al. selected 300–400 fluorescent spots per well as appropriate for an HPV NAb assay.Citation26 Because no anti-Ad2 human control serum was available, the assay established here was validated with serum from mice inoculated with the Ad2, Ad5, or AAV1 vector. The Ad2–EGFP neutralization assay detected the specific NAbs induced by the Ad2 vector, and no cross-reactivity between AAV1-inoculated serum or naïve serum and the NAbs against the Ad2–EGFP virus was observed. Only one Ad5-vector-inoculated serum showed a low titer of NAbs against the Ad2–EGFP virus, and may have been contaminated during the sampling or inactivation process because no cross-reaction was detected in the other 2 sera sampled from the same mouse at different time points.
In this study, the prevalence and potency of Ad2-specific NAbs were investigated in human populations from China and developed countries around the world. We demonstrated that the prevalence of anti-Ad2 NAbs was high in both China (92.2%) and developed countries (86.9%), and that the potency of the anti-Ad2 NAbs was also high, insofar as 64.6% of Chinese subjects and 77.4% of those from developed countries had high titers (> 810). A similar frequency of NAbs against Ad2 was described in the United States (n = 40, 24–69-year-old subjects), when about 82.5% and 65% of Ad2 titers were in the medium (201–1000) and high ranges (> 1000), respectively.Citation20 Data from locations in Europe, Asia, and the United States have also shown that Ad2 was neutralized efficiently by the majority of sera tested.Citation15 These data indicate that over 97% of humans have pre-existing antibodies against group C Ads, whereas about half have antibodies against the commonly used Ad serotype 2.Citation28 Surprisingly, the frequency of anti-Ad2 NAbs in the 19–40-year-old population was markedly higher in Anhui Province than in Beijing Province, which may be attributable to the different levels of health care available in the 2 provinces. Interestingly, the prevalence of anti-Ad2 NAbs differed significantly according to age in Beijing Province, but not in Anhui Province. The incidence of Ad2-neutralizing activity throughout childhood was relatively low, but with increasing age, the prevalence of Ad2-infected individuals increased. When people move outdoors after the age of 18 years, they are believed to be at greater risk of exposure to wild-type Ad2. We found no significant difference in the prevalence of anti-Ad2 NAbs according to sex in the Chinese subjects.
The co-occurrence of NAbs against Ad2 and Ad5 in China when the population was grouped by region, age, or sex is shown in Table S1. The Ad5-specific NAb titers for the same 206 healthy individuals from Beijing and Anhui Provinces have been reported in a previous study.Citation29 In total, 79.1% of the tested population carried NAbs against both Ad2 and Ad5. Only 4.9% of the sera were negative for both Ad2 and Ad5. The rate of co-occurrence of antibodies against Ad2 and Ad5 was higher in Anhui Province than in Beijing Province (P < 0.01), and was higher in adults than in children (P < 0.01). There was no significant difference in the co-occurrence rate by sex (P = 0.97). A previous study showed that human Ads are ubiquitous and that most people have been infected with one or more serotypes, conferring lifelong immunity.Citation30 In the present study, we have shown that Ad2 and Ad5 often co-occur in healthy individuals in China, but with no positive correlation between the Ad2 and Ad5 titers.
Pre-existing immunity to Ad is a major obstacle to the use of Ad-based vectors for vaccines. With the high prevalence of anti-Ad2 antibodies, several strategies are used to circumvent the effects of this immunity. The adenoviral coat has been physically shielded to protect the vector from Nabs.Citation31,32 Alternatively, several studies have demonstrated that the NAbs against one serotype do not cross-react with other serotypes, so researchers could develop HIV vaccines using nonhuman adenoviral serotypes or rare human serotypes.Citation15,33,34 Other inoculation strategies, such as oral administration or nasal inhalation, can also be used.
In summary, a simple, intuitive, high-throughput, economical EGFP-based neutralization assay was developed to determine the anti-Ad2 NAbs titers in both human and animal sera. This method of assaying anti-Ad2 NAbs is a useful tool for epidemiological surveys and for studying the Ad2 immune response during the design of Ad2-vector-based vaccines. The prevalence rates of NAbs against Ad2 are high in humans, not only in developing countries but also in developed countries. Overall, this epidemiological survey suggests that the use of the Ad2 vector may be limited by the pre-existing immune responses in human populations, as the use of the Ad5 vector has been.
Materials and methods
Cells and viruses
Vero (American Type Culture Collection [ATCC], CCL81) and human embryonic kidney (HEK) 293T cells (ATCC, CRL-3216) were used for the EGFP neutralizing assay. The cells were grown at 37 °C under 5% CO2 in high-glucose Dulbecco's modified Eagle's medium (HyClone, South Logan, UT) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), 2% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Gibco), 1% penicillin–streptomycin solution (Gibco), and 1% nonessential amino acids (HyClone). The Ad2–EGFP virus was a kind gift from Prof. Caijun Sun at the Institute of Respiratory Diseases, Guangzhou Medical College, Guangzhou, China.
Adenovirus and AAV antisera
Sixteen 5-week-old BALB/c mice were divided into 4 groups (4 per group). The mice in each group were inoculated intramuscularly twice (at an interval of 2 weeks) with a dose of 1 × 109 vp of one of 3 viruses (Ad2, Ad5, or AAV1) or with saline. Seven days after the second inoculation with Ad or AAV1, the antisera were collected 3 times (at intervals of 2 days) from the vena ophthalmica. Specific-pathogen-free BALB/c mice were purchased from the National Institutes for Food and Drug Control (Beijing, China). Before inoculation, all the animals were confirmed to be negative for NAbs to different Ads and AAV1. The National Guidelines for the Care and Use of Medical Laboratory Animals were followed.
Human serum samples
The study was approved by the Ethical Committee of the Beijing Red Cross Blood Center. Normal subjects were recruited from the Beijing Blood Center, the Anhui Blood Center, and the General Hospital of the Air Force. Sixty-one individuals (43 men, 18 women) from 10 developed countries were also enrolled in the study, as described previously.Citation35 A total of 230 subjects (209 men, 21 women) infected with HIV-1 were recruited from Beijing and Guangxi Provinces in China. The samples were heat inactivated for 1 h at 56 °C and then stored at 4 °C until assay.
EGFP-based Ad2 neutralization assay
The Ad2 NAb titers were quantified with a FluoroSpot-based assay that measured the reduction in the percentage of EGFP-positive cells caused by the Ad2 NAbs present in the samples. The diluted recombinant Ad2–EGFP virus (10 vp/cell, 50 μl) and serial 3-fold dilutions (starting at 1:30) of the serum to be tested (100 μl) were incubated together in a 96-well plate at 37 °C for 1 h. The virus was incubated without serum as the control. After incubation, 2 × 104 293T cells (50 μl) were added to the mixture. The plates were incubated for 48 h at 37 °C under 5% CO2. After incubation, the number of fluorospots was counted with an ImmunoSpot reader. An optimal sensitivity parameter of 215 was used for the neutralization assay. The 50% neutralization titer for the serum sample was calculated with the Reed–Muench method. Titers ≥ 30 were considered positive. The serum titers were further categorized as low (< 1:90), medium (1:90–1:810), and high (> 1:810).
Statistical analysis
The Ad2 seroprevalence in the different groups was compared with a χ2 test. Graphs were computed with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA) and statistical analyses were performed with SPSS ver. 18.0 (IBM, Armonk, NY). P values < 0.05 were considered to indicate statistically significant differences.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Q.Q.L., Q.L., W.J.H., and Y.C.W. designed the experiments. Q.Q.L., Q.L., A.J.S., C.Y.Z., and J.J.W. performed the experiments and analyzed the data. W.J.H. and Y.C.W. supervised the project. Q.Q.L., Q.L., and Y.C.W. wrote the manuscript, with input from all the authors. All the authors approved the manuscript.
1281487_Supplemental_Material.pdf
Download PDF (120.2 KB)Acknowledgments
We thank Dr. Caijun Sun and Dr. Xiaoyan Dong for providing the rAd2–EGFP and AAV vectors, respectively. We also thank Dr. Xuerong Jia of the Beijing Wantai Biological Pharmacy, Professor Jingyun Li of the Beijing Institute of Microbiology and Epidemiology, Dr. Dongying Gao of the Beijing Red Cross Blood Center, and Dr. Lisi Yao of the Chinese Academy of Inspection and Quarantine for providing the human sera. We greatly appreciate the cooperation of the human donors involved in the epidemiological study.
Funding
This study was supported by grants from the National Science and Technology Major Project (2012ZX10004701–001). The funding body had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
References
- D'Souza MP, Yang OO. Adenovirus vectors as HIV-1 vaccines: where are we? What next? AIDS 2015; 29:395-400; PMID:25630039; https://doi.org/10.1097/QAD.0000000000000548
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 2006; 194:1661-71; PMID:17109337; https://doi.org/10.1086/508748
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005; 191:654-65; PMID:15688278; https://doi.org/10.1086/428404
- Kong WP, Wu L, Wallstrom TC, Fischer W, Yang ZY, Ko SY, Letvin NL, Haynes BF, Hahn BH, Korber B, et al. Expanded breadth of the T-cell response to mosaic human immunodeficiency virus type 1 envelope DNA vaccination. J Virol 2009; 83:2201-15; PMID:19109395; https://doi.org/10.1128/JVI.02256-08
- Harro C, Sun X, Stek JE, Leavitt RY, Mehrotra DV, Wang F, Bett AJ, Casimiro DR, Shiver JW, DiNubile MJ, et al. Safety and immunogenicity of the Merck adenovirus serotype 5 (MRKAd5) and MRKAd6 human immunodeficiency virus type 1 trigene vaccines alone and in combination in healthy adults. Clin Vaccine Immunol 2009; 16:1285-92; PMID:19605598; https://doi.org/10.1128/CVI.00144-09
- Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther 2011; 11:321-30; PMID:21557723; https://doi.org/10.2174/156652311796150354
- Wan Y, Wu L, Liu L, Xu J, Liu Y, Shao Y. Comparison of immunogenicity between codon optimized HIV-1 Thailand subtype B gp140 and gp145 vaccines. Vaccine 2007; 25:4949-59; PMID:17350736; https://doi.org/10.1016/j.vaccine.2007.01.118
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 2008; 372:1894-905; PMID:19012957; https://doi.org/10.1016/S0140-6736(08)61592-5
- Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Eng J Med 2013; 369:2083-92; PMID:24099601; https://doi.org/10.1056/NEJMoa1310566
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372:1881-93; PMID:19012954; https://doi.org/10.1016/S0140-6736(08)61591-3
- Esparza J. A brief history of the global effort to develop a preventive HIV vaccine. Vaccine 2013; 31:3502-18; PMID:23707164; https://doi.org/10.1016/j.vaccine.2013.05.018
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Eng J Med 2012; 366:1275-86; PMID:22475592; https://doi.org/10.1056/NEJMoa1113425
- Keefer MC, Gilmour J, Hayes P, Gill D, Kopycinski J, Cheeseman H, Cashin-Cox M, Naarding M, Clark L, Fernandez N, et al. A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PloS One 2012; 7:e41936; PMID:22870265; https://doi.org/10.1371/journal.pone.0041936
- Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, Johnson JA, Kleinjan J, Yanosick KE, Perry J, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 2013; 207:240-7; PMID:23125444; https://doi.org/10.1093/infdis/jis670
- Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 2003; 77:8263-71; PMID:12857895; https://doi.org/10.1128/JVI.77.15.8263-8271.2003
- Appaiahgari MB, Pandey RM, Vrati S. Seroprevalence of neutralizing antibodies to adenovirus type 5 among children in India: implications for recombinant adenovirus-based vaccines. Clin Vaccine Immunol 2007; 14:1053-5; PMID:17596429; https://doi.org/10.1128/CVI.00173-07
- Sprangers MC, Lakhai W, Koudstaal W, Verhoeven M, Koel BF, Vogels R, Goudsmit J, Havenga MJ, Kostense S. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol 2003; 41:5046-52; PMID:14605137; https://doi.org/10.1128/JCM.41.11.5046-5052.2003
- Paris R, Kuschner RA, Binn L, Thomas SJ, Colloca S, Nicosia A, Cortese R, Bailer RT, Sullivan N, Koup RA. Adenovirus type 4 and 7 vaccination or adenovirus type 4 respiratory infection elicits minimal cross-reactive antibody responses to nonhuman adenovirus vaccine vectors. Clin Vaccine Immunol 2014; 21:783-6; PMID:24623627; https://doi.org/10.1128/CVI.00011-14
- Cheng C, Gall JG, Nason M, King CR, Koup RA, Roederer M, McElrath MJ, Morgan CA, Churchyard G, Baden LR, et al. Differential specificity and immunogenicity of adenovirus type 5 neutralizing antibodies elicited by natural infection or immunization. J Virol 2010; 84:630-8; PMID:19846512; https://doi.org/10.1128/JVI.00866-09
- Aste-Amezaga M, Bett AJ, Wang F, Casimiro DR, Antonello JM, Patel DK, Dell EC, Franlin LL, Dougherty NM, Bennett PS, et al. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum Gene Ther 2004; 15:293-304; PMID:15018738; https://doi.org/10.1089/104303404322886147
- Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 2010; 28:950-7; PMID:19925902; https://doi.org/10.1016/j.vaccine.2009.10.145
- Kuriyama S, Tominaga K, Kikukawa M, Nakatani T, Tsujinoue H, Yamazaki M, Nagao S, Toyokawa Y, Mitoro A, Fukui H. Inhibitory effects of human sera on adenovirus-mediated gene transfer into rat liver. Anticancer Res 1998; 18:2345-51; PMID:9703877
- Small JC, Haut LH, Bian A, Ertl HC. The effect of adenovirus-specific antibodies on adenoviral vector-induced, transgene product-specific T cell responses. J Leukoc Biol 2014; 96:821-31; PMID:25082150; https://doi.org/10.1189/jlb.1A0813-451RR
- Wu X, Zhang C, Feng S, Liu C, Li Y, Yang Y, Gao J, Li H, Meng S, Li L, et al. Detection of HPV types and neutralizing antibodies in Gansu province, China. J Med Virol 2009; 81:693-702; PMID:19235880; https://doi.org/10.1002/jmv.21435
- Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, Bian A, Zhou DM, Hutnick N, Yuan S, Gray C, et al. Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae. J Virol 2010; 84:10522-32; PMID:20686035; https://doi.org/10.1128/JVI.00450-10
- Nie J, Liu Y, Huang W, Wang Y. Development of a Triple-Color Pseudovirion-Based Assay to Detect Neutralizing Antibodies against Human Papillomavirus. Viruses 2016; 8:107; PMID:27120611; https://doi.org/10.3390/v8040107
- Ahlborg N, Axelsson B. Dual- and triple-color fluorospot. Methods Mol Biol 2012; 792:77-85. PMID: 21956502
- Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther 2010; 17:295-304; PMID:19907498; https://doi.org/10.1038/gt.2009.148
- Liu Q, Nie J, Huang W, Meng S, Yuan B, Gao D, Xu X, Wang Y. Comparison of two high-throughput assays for quantification of adenovirus type 5 neutralizing antibodies in a population of donors in China. PloS One 2012; 7:e37532; PMID:22655054; https://doi.org/10.1371/journal.pone.0037532
- Wold WS, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther 2013; 13:421-33; PMID:24279313; https://doi.org/10.2174/1566523213666131125095046
- Beer SJ, Matthews CB, Stein CS, Ross BD, Hilfinger JM, Davidson BL. Poly (lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther 1998; 5:740-6; PMID:9747453; https://doi.org/10.1038/sj.gt.3300647
- Chillon M, Lee JH, Fasbender A, Welsh MJ. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther 1998; 5:995-1002; PMID:9813671; https://doi.org/10.1038/sj.gt.3300665
- Kremer EJ, Boutin S, Chillon M, Danos O. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J Virol 2000; 74:505-12; PMID:10590140; https://doi.org/10.1128/JVI.74.1.505-512.2000
- Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, Wilson JM, Ertl HC. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol 2002; 76:2667-75; PMID:11861833; https://doi.org/10.1128/JVI.76.6.2667-2675.2002
- Liu Q, Huang W, Zhang H, Wang Y, Zhao J, Song A, Xie H, Zhao C, Gao D, Wang Y. Neutralizing antibodies against AAV2, AAV5 and AAV8 in healthy and HIV-1-infected subjects in China: implications for gene therapy using AAV vectors. Gene Ther 2014; 21:732-8; PMID:24849042; https://doi.org/10.1038/gt.2014.47
