ABSTRACT
The effective protective HIV vaccine should elicit either protective antibodies or effective T cell response, or both. To improve the efficacy of HIV-1 vaccines, HLA polymorphism and HIV-1 diversity are 2 key factors to be considered for vaccine development. In this study, we expressed a recombinant multi-epitope protein MEP1 which has the same amino acid sequence as a DNA vaccine for Chinese population in our previous report. We found that MEP1 alone could elicit moderate levels of humoral and cellular immune responses, but these responses could not provide protection from challenge with a recombinant virus rTTV-lucgag, which expresses Gag of HIV-1 CRF_07BC. Nevertheless, when MEP1 was immunized with aluminum adjuvant, both humoral and cellular immune responses were significantly increased, and they were protective against virus infection; meanwhile, MEP1 with aluminum not only elicited early (10 d post immunization) but also a long-term (at least 44 weeks post immunization) immune responses in BALB/c mice. These results suggested that MEP1 has the potential to be developed as an effective vaccine candidate, and that suitable adjuvant is necessary for this protein to generate protective immune responses.
Introduction
Breadth, magnitude and duration of immune responses are must-considered factors for vaccine development, especially for pathogens with highly diversity such as HIV, influenza virus and malaria. Since HIV-1 was identified as the pathogen of AIDS 3 decades ago, developing of an effective vaccine has been one of the major tasks to prevent and treat AIDS. Although several strategies have been attempted,Citation1-5 there are no licensed HIV vaccines to prevent or treat AIDS,Citation6 only one vaccine in clinical trial RV144 has been proved to provide 31% protection to the vaccinated population.Citation7 So far, it is believed that a successful protective HIV vaccine should elicit either protective antibodies (neutralizing or/and ADCC),Citation8,9 or effective T cell response,Citation10,11 or both.Citation12-14 To elicit a protective immune response with more breadth, either broadly neutralizing epitopes, or conserved ADCC epitopes, or conserved T cell epitopes (which are fully recognized by the target populations) are more widely used for vaccine rational design.Citation1,4,15,16
The target antigens are immunized in various forms, including plasmid DNA, viral vector (CMV, Ad5 and poxviruses),Citation2,17,18 and recombinant protein,Citation19,20 which has its own merits and limitations. Vaccines in recombinant protein form are proved to be more effective to elicit humoral immune responses with greater safety compared with DNA vaccines and viral vector based vaccines.Citation21,22 In our previous study, we designed several multi-epitope DNA constructs for Chinese populations, and evaluated their immunogenicity in BALB/c mice and obtained a potential DNA vaccine candidate MEG1, which consists of epitopes matching Chinese dominant MHC alleles and highly conserved among virus subtypes prevalent in China. Experimental data illustrated that MEG1 not only elicited strong cellular immune responses to each single epitope,Citation23 but also provided partial protection against HIV-1 infection using rTTV-lucgagCitation24 in mouse model, suggesting that such a multiple epitope construct might be an effective antigen candidate for vaccine design.
In this study, we selected the same amino acid sequence as MEG1 and successfully expressed the MEP1 protein in E.coli. We proved that aluminum (hereinafter, alum) adjuvant promoted MEP1 to generate increased humoral and cellular immune responses, which confer partial protection from challenge with rTTV-lucgag (CRF_07BC). Moreover, MEP1 with alum induced strong cellular immune responses at early stage and long-term humoral and cellular immune responses at least 44 weeks after immunization, suggesting that MEP1 is potential to be developed as an effective vaccine candidate against HIV-1 infection.
Results
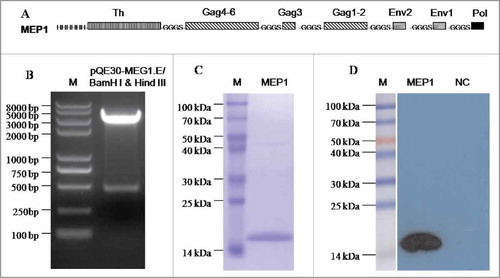
Expression and characterization of recombinant HIV multi-epitope MEP1 protein
Due to good immunogenicity of MEG1 DNA vaccineCitation23 in our previous study, we selected the same amino acid sequence of MEG1 to express the recombinant protein MEP1 in E. coli..The codon-optimized gene was cloned into the prokaryotic plasmid pQE30, which contains an N-terminal His-tag (). Hence, the recombinant plasmid pQE30-MEG1.E was identified correctly by restriction enzyme digestion () and DNA sequencing. After IPTG induction, the target MEP1 protein was mainly expressed induced inclusion bodies. The protein was purified by Ni-charged resin, and re-natured by gradient dialysis, with the recovery rate of 40%. The protein was shown with high purity and specificity in SDS-PAGE () and WB analyses ().
Figure 1. Expression and purification of a recombinant protein MEP1 for Chinese population. (A) Schematic diagram of the recombinant protein MEP1, it contains 7 fragments in the specific order linked with a flexible space sequence “GGGS.” In the diagram, H stands for histidine, Th represents gp120197–232 (NTSVITQACPKVSFEPIPIHYCAPAGFAILKCN- NKT); Gag4–6 represents p24128–151 (EIYKRWIILGLNKIVRMYSPTSIL); Gag3 represents p2461–73 (GHQAAMQMLKETI); Gag1–2: denotes p1718–36 (KIRLRPGGKKKYKLKHIVW); Env2 stands for gp120108–117 (IISLWDQSLK); Env1 represents gp12032–45 (KLWVTVYYGVPVWR); Pol is Integrase177–188 (QMAVFIHNFKRK). (B) Enzyme restriction analysis of the recombinant plasmid pQE30-MEG1.E by agarose gel electrophoresis. (C) SDS-PAGE analysis of the purified protein MEP1. (D) Western-blot analysis of the recombinant protein MEP1 using anti-His6 antibody as primary antibody.
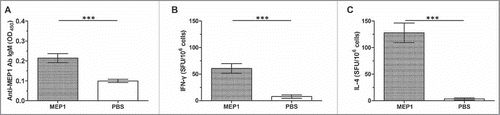
Recombinant MEP1 protein rapidly induced early immune responses after single-dose immunization
A vaccine with capability to rapidly promote early immune responses is beneficial for the host to prevent against HIV-1 infection.Citation25 To test the ability of MEP1 protein to induce early immune responses, we immunized BALB/c mice with single dose of this protein without adjuvant, and collected mouse sera and splenocytes on day 10 post-immunization to detect the generation of immune responses. ELISA results showed that MEP1 quickly stimulated mice to generate anti-MEP1 specific IgM antibody (p < 0.001, ), but low levels of IgG, IgG1 and IgG2a antibodies (data not shown). ELISPOT results further demonstrated that MEP1-immunized mice had significantly high spot forming unit (SFU) of IFN-γ () and IL-4 (), although the level of responses were not so high (61 SFU/106 cells for IFN-γ and 123 SFU/106 cells for IL-4). These results indicated that MEP1 had good immunogenicity, and the protein itself induced a Th2-biased Th1/Th2 immune responses at early stage of immunization.
Figure 2. Early immune responses in BALB/c mice immunized with MEP1 without any adjuvants on day 10 post immunization. BALB/c mice were intramuscularly immunized with MEP1 at a dose of 10 µg in 200 µl of PBS once, then, were killed on day 10 post immunization. Serum from each mouse was collected, and the specific anibody IgM, IgG and its subclass IgG1, IgG2a were detected by indirect ELISA, only anti-MEP1 IgM (A) was significantly higher than that in the immunized mice, the value was presented as OD450 at 1/50 dilution. Splenocytes were harvested and stimulated with peptide-pools for 48 hours to detect IFN-γ (B) and IL-4 (C)-secreting cells by ELISPOT assay to observe the cellular immune responses. *** denotes p < 0.001.
MEP1 without adjuvants elicited moderate levels of immune responses in capable of protecting against rTTV-lucgag challenge
To detect whether MEP1 without adjuvant could induce protective immune responses, we immunized BALB/c mice intramuscularly with MEP1 alone or PBS control, for 3 times at a 4-week interval. Results demonstrated that MEP1 induced high levels of MEP1-specific IgG (), IgG1 () and IgG2a () antibodies, reached about 104, significantly higher than those induced by the PBS control. In addition, the ratio of IgG1 to IgG2a was about 2, indicating that MEP1 alone was able to induce a relatively balanced Th1/Th2 immune responses. Particularly, compared with mice injected with PBS, the MEP1- immunized mice had significantly increased numbers of IFN-γ-and IL-4-secreting cells, which averaged at 63 and 21 SFU/106 cells respectively (). However, these numbers were not significantly higher than those induced at early stage of immunization, suggesting that immunization of MEP1 alone was not effective to enhance cellular immune responses.
Figure 3. Immune responses and protective efficacy in BALB/c mice induced by MEP1 without adjuvants 2 weeks after final immunization. Six to 8 week-old female BALB/c mice (n = 6) were intramuscularly immunized with MEP1 at a dose of 10 µg in 200 µl of PBS 3 times with 4-week interval. Two weeks after the final immunization, sera were collected and MEP1-specific IgG (A), IgG1 (B) and IgG2a (C) was measured by ELISA; and splenocytes were stimulated with pool-peptides for 48 hours to detect cellular immune response by ELISPOT assay (D&E). The MEP1-immunized mice and PBS control (n = 15) were intraperitoneally injected with the recombinant virus rTTV-lucgag (TCID50 value was 2 × 108), 3 d later, the protective efficacy was evaluated by measuring the luciferase activity of the ovary homogenate; the higher luciferase intensity stands for higher titer of virus infection (F), which means less protection. * denotes p < 0.05, *** denotes p < 0.001, and n.s. denotes no significance.
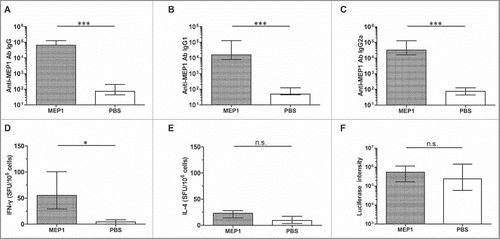
To evaluate whether MEP1 alone without adjuvant could induce protective immunity against HIV-1 infection, we used an rTTV-lucgag infection model, in which vaccine efficacy is reflected as the luciferase intensity of ovary tissue homogenate from each mouse with lower intensity for better protection.Citation24 As was shown in , there was no significant difference between 2 groups, indicating that MEP1 protein without adjuvant could not induce sufficient magnitude of immune responses to protect mice from rTTV-lucgag challenge. Therefore, an adjuvant might be necessary for MEP1 protein to elicit protective immune responses.
Alum adjuvant promoted MEP1 to generate protective immune responses against HIV-1 infection in BALB/c mice
To test protective immune responses of MEP1, alum adjuvant, the first and the most common use vaccine adjuvant for human useCitation26 was formulated with MEP1 protein. Mice were immunized with single dose of MEP1 with or without alum, and immune responses were detected on day 10 post-immunization. As shown in , alum significantly increased cellular immune responses (including IFN-γ and IL-4, ) elicited by MEP1, but humoral immune responses, including IgM (), IgG, IgG1 and IgG2a antibodies, were not significantly augmented, indicating that alum is capable of enhancing cellular immune responses at early stage of immunization. These results demonstrated that cellular immune responses were elicited earlier than antibody responses.
Figure 4. Comparison the early immune responses in BALB/c mice immunized with MEP1 in the presence or absence of alum on day 10 post immunization. BALB/c mice were intramuscularly immunized with MEP1 (with or without adjuvant alum) at a dose of 10 µg in 200 µl of PBS once, then, were killed on day 10 post immunization.Serum from each mouse was collected, the specific antibody IgM, IgG and its subclass IgG1, IgG2a were detected by indirect ELISA at 1/50 dilution, the value was presented as OD450, only anti-MEP1 IgM (A) was presented. Splenocytes were harvested and stimulated with peptide-pools for 48 hours to detect IFN-γ (B) and IL-4 (C) -secreting cells by ELISPOT assay to observe the cellular immune responses, and was expressed as SFU per million cells. * denotes p<0.05, n.s. denotes no significance.
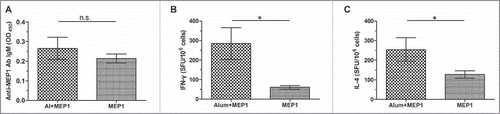
Next, we further compared immune responses on day 14 after 3 doses of immunization. As shown in , mice immunized with MEP1 plus alum had 10-fold higher titer of anti-MEP1 IgG antibodies () and Th2-associated subclass IgG1 antibodies (), while the titers of Th1-associated IgG2a antibodies (), was not significantly different between 2 groups. Thus, the IgG1/IgG2a ratio was about 15, which is much higher than that of the mice immunized with MEP1 alone suggesting that alum was more efficient to enhance humoral immune responses. ELISPOT assay further showed that mice immunized with MEP1 plus alum generated significantly high numbers of IFN-γ- and IL-4-secreting cells than those immunized with MEP1 alone (), which were 5- and 11-fold respectively, indicating that alum is capable of enhancing cellular immune responses. In addition, the ratio of IFN-γ to IL-4 in mice immunized with MEP1 plus alum (1.5) was lower than that in mice immunized with MEP1 alone (3.1), indicating that alum is superior to enhance humoral immune responses.
Figure 5. Comparison the immune responses and protective efficacy in BALB/c mice immunized with MEP1 in the presence or absence of alum 2 weeks after final immunization. Six to 8 week-old female BALB/c mice were intramuscularly immunized with MEP1 (with or without adjuvant alum) at a dose of 10 µg in 200 µl of PBS 3 times with 4-week interval. Two weeks after the final immunization, sera were collected and MEP1-specific IgG (A), IgG1 (B) and IgG2a (C) were measured by ELISA; and splenocytes were stimulated with pool-peptides for 48 hours to detect cellular immune response by ELISPOT assay (D&E). The immunized mice were intraperitoneally injected with the recombinant virus rTTV-lucgag (TCID50 value was 2 × 108), 3 d later, the protective efficacy was evaluated by measuring the luciferase activity of the ovary homogenate; the higher intensity stands for higher titer of virus infection (F). The value was expressed as median with interquartile range except ELISPOT. * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.001, and n.s. denotes no significance.
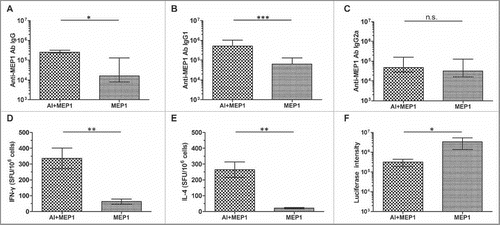
Evaluation of the efficacy of MEP1 with alum in protection against virus infection demonstrated that titers of rTTV-lucgag in the mice immunized with MEP1 plus alum (1.46 × 105−1.52 × 106) was significantly lower than those in mice inoculated with MEP1 alone (5.38 × 105−2.77 × 107) (), illustrating that the enhanced immune responses by alum adjuvant provided partial protection of mice against virus challenge.
Long-term immune responses were elicited by MEP1 protein with alum adjuvant
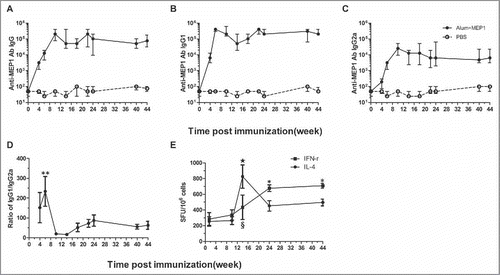
Analysis of the kinetics of MEP1-induced immune responses revealed that anti-MEP1 specific IgG (), IgG1 () and IgG2a () antibodies elevated gradually with the time of immunization, peaked at 2 weeks post-final immunization (i.e. week 10 post-first immunization), and sustained at the similar levels until week 44 post-first immunization. In addition, the ratio of IgG1 to IgG2a at different time points peaked at 150 at week 6 (i.e., 2 weeks post-secondary immunization), had a sharp decrease to 11 at week 10 (i.e., 2 weeks post-final immunization), and then sustained at the similar levels until week 44 (), suggesting that cellular immune responses increased with immunization time, and this conclusion was further proved by ELISPOT results (). Furthermore, the kinetics of IFN-γ- and IL-4-producing cells were different: the numbers of IFN-γ-producing cells generated at early phase, and gradually increased with time (week 24 and 44 v.s. day 10 and week 10), while IL-4 peaked at week 14 and decreased to moderate levels at week 24 and kept at similar level till week 44 (); the numbers of IFN-γ-producing cells at week 24 and 44 had tendency to be higher than that of IL-4 but with no statistic significance. These results indicated that MEP1 protein with alum adjuvant is capable of stimulating good memory immune responses (including B and T cell responses), which is beneficial for host to protect against virus infection.
Figure 6. Kinetics of the immune responses elicited by MEP1with alum in BALB/c mice. BALB/c mice immunized with MEP1 in combination with alum 3 times with 4-week interval, and sera were collected at different time point after immunization, specific anti-MEP1 antibody IgG (A), IgG1 (B) and IgG2a (C) were detected by ELISA, and the ratio of IgG1 to IgG2a was calculated for the immunized mice (** denotes p < 0.01, week 6 vs week10, 14, 18, 22, 24, 40 and 44) (D); Cellular immune responses were measured by ELISPOT assay (E) at day 10 (i.e.,week 1.5), week 10, 14, 24 and 44 post first immunization., * denotes p < 0.05 at week 24 and 44 v.s. week 1.5 and 10 for IFN-γ; ☆ stands for p < 0.01 at week14 v.s. week 1.5 and 10 for IL-4; § stands for p < 0.05 IFN-γ v.s. IL-4 at week 14.
Discussion
Broadly neutralizing antibodies (BNAbs) against HIV-1 are of great interest and encouraging for researchers to prevent and treat AIDS,Citation1,8,15 however, how to elicit BNAbs by vaccination is still an unraveled question.Citation27,28 Besides BNAbs, potential correlates of protection also include ADCC antibodiesCitation29 and effective T cell responses.Citation30,31 Efforts to elicit broadly-directed, co-dominant responses to conserved epitopes of protective CD4 and CD8 T cell responses have been sustained for many yearsCitation10,32,33 although such responses might lead to partial protection from HIV-1 infection, rather than sterile prevention or virus eradication.Citation34 So far, several multi-epitope-based HIV-1 vaccines in clinical trial phase have proven to be safe and effective, and more data will be obtained in the future, such as a therapeutic vaccine (Ad26.Mos.HIV and MVA-Mosaic vaccine) in clinical trial phase 1/2a,Citation35 a phase I human vaccine trial of a novel polypeptide containing HIV-T helper epitopes (EP-1043),Citation36 and a step MRKAd5/HIV-1 gag/pol/nef studyCitation37 . In our previous study, we designed a multi-epitope DNA vaccine MEG1 for Chinese populations (both Chinese HLA restriction and HIV-1 diversity in China). In this study, we expressed a multi-epiotope protein MEP1 in E.coli, which amino acid sequence is the same as MEG1, and evaluated its immunogenicity and efficacy in BALB/c mice.
We first expressed the multi-epiotope protein containing 12 conserved epitopes. Among of them, fragment Th, Gag4–6 and Gag1–2 are overlapping epitopes instead of separate epitopes, our previous study indicated that DNA vaccine using this design was more effective in eliciting immune responses than that using separate epitope design; results in this study of MEP1 again demonstrated its efficiency. These results are consistent with other's observation, the longer conserved overlapping epitopes may be more efficient to be processed in natural processing pathway, and possibly are targeted by diverse MHC haplotypes, which might lead to more effective immune responses.Citation38,39
We then evaluated immunogenicity and efficacy of MEP1 without any adjuvants. Out of our expectation, immunization with MEP1 alone once was capable to induce early immune responses including IgM and low levels of cellular immune responses at day 10 post immunization, predicting its good immunogenicity in mice. However, boosting immunization twice just led to significant elevation of humoral immune responses with Th1/Th2 balance, not enhanced cellular immune responses, which were not enough to provide protection against challenge with rTTV-lucgag. These results are much different from DNA vaccine MEG1, which elicited more robust cellular immune responses and provided partial protection from challenging with rTTV-lucgag in absence of any adjuvants,Citation23 suggesting that adjuvant is necessary for MEP1 to induce protective immune responses against challenge with rTTV-lucgag. Vaccine adjuvants are widely used in many forms and are expected to regulate the onset, magnitude, duration and quality of immune responses elicited by an antigen, leading to better protection from pathogen infection, especially important for novel vaccines, such as subunit vaccines and peptide vaccines.Citation40,41
To improve the immune responses elicited by MEP1, adjuvant aluminium hydroxide (referred to as alum) was formulated with MEP1, because alum is the first FDA-licensed and most commonly used adjuvant for human vaccine.Citation42 We found that alum significantly promoted both Th1-associted IFN-γ production and Th2-associsted IL-4 production in splenocytes from the immunized mice were stimulated with peptide pool at early stage, when mice were immunized with MEP1 plus alum once on day 10, indicating that MEP1 is capable to elicit early immune responses, which benefits for virus clearance after vaccination at early stage. What's more, alum also significantly promoted both Th2-biased Ab response and IFN-γ (which is similar to DNA vaccine MEG1) and IL-4 production on day 10 after mice were immunized thrice, which conferred partial protection from rTTV-lucgag infection. Although the rTTV-lucgag infection model in female BALB/c mice cannot be used to observe direct effects of mucosal protection and may not suit for evaluating the efficacy of neutralizing antibody, it is a high throughput method to evaluate systemic immune responses, especially for T cell responses.Citation24 These results indicate that alum not only promoted onset and magnitude of MEP1, but also helped MEP1 to generate protective responses, which are partly consistent with previous findings, in which alum is efficient to induce a Th2-biased Ab immune response of an antigen, but does not promote significant Th1 cellular response.Citation26,43 The difference might be related with the characteristic of antigen MEP1 we used in this study, which consists of T cell epitopes instead of B cell eptiopes.
Finally, we observed if MEP1 plus alum could elicit long-term immune responses, as we know, long-term immune responses are important factors for host to protect from pathogen infection. As what we expected, MEP1 with alum elicited a long-term Ab and cellular immune responses persisting at high level at least 44 weeks post immunization, moreover Th1-associated immune responses increased with time. These results are some different from DNA vaccine MEG1, which cellular immune response at 9 months post final immunization was much lower than that on 10 d post final immunization (data unpublished). Data showed that a long-lasting T-cell responses elicited by MRKAd5/HIV-1 gag/pol/nef vaccine targeting at least 3 gag peptides correlates with lower viral loads.Citation37 The long-lasting immune responses induced by MEP1 with alum indicate that MEP1 formulated with alum elicits effective memory T and B cells, which are beneficial for host to defend HIV-1 infection.Citation10,44,45
In summary, we successfully prepared a multi-epitope protein (MEP1) with good immunogenicity and efficacy, and alum promotes onset, magnitude, breadth and duration of immune responses elicited by MEP1, demonstrating that MEP1 is potential to develop as an effective preventive and therapeutic vaccine candidate.
Materials and methods
Mice
Female 6–8-week-old BALB/c mice, purchased from Beijing Experimental Animal Center, were used for the mouse experiments. The animal protocols were approved by the IACUC of the Laboratory Animal Center, State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology (Permit number: PBS-05–2014–016).
Virus
Recombinant virus rTTV-lucgag, which expresses HIV-1 gag (strain CN54) and firefly luciferase fusion protein based on Tiantan strain (an attenuated but replication-competent poxvirus), was kindly provided by Professor Jianqing Xu from Fudan University Medical College, Shanghai; and was propagated in CEF (chick embryo fibroblast) cellsCitation24 to evaluate the protection efficacy of recombinant protein vaccine MEP1. Luciferase expression of rTTV-lucgag in vivo positively correlated with p24 level and virus titer, and replicated most efficiently in ovaries, cervix and uterus in female BALB/c mice.
Expression and purification of the recombinant protein MEP1
The amino acid sequence of the multi-epitope protein MEP1 is the same as DNA vaccine pJW4303-MEG1 reported previously.Citation23 In brief, it contains 7 fragments which was connected with a flexible space sequence “GGGS” between each fragment. The plasmid pQE30-MEG1.E was constructed by inserting the E. coli codon optimized sequence MEG1.E into prokaryotic vector pQE30, and identified using restricted enzyme analysis (BamH I and Hind III) and sequencing (SinoGenoMax).The recombinant E. coli containing the plasmid pQE30-MEG1.E was obtained by transforming pQE30-MEG1.E into host cell (E.coli strain M15), and IPTG at final concentration of 1 mM was added when OD value is 0.4∼0.6, bacteria cells were harvested after 2–4 h post induction by centrifugation, and identified by SDS-PAGE and Western blot. The protein was purified by Ni-NTA chromatography as described previously,Citation43 and further identified by SDS-PAGE and Western blot. The sterile and endotoxin-negative recombinant protein MEP1 was stored at −80°C for further experiments.
Immunization
To evaluate the immunogenicity of the recombinant protein MEP1, mice were intramuscularly injected with 10 μg of protein MEP1 either dissolved in 200 μl PBS (GIBCO, C10010500BT), or 10 μg MEP1 in 180 μl PBS mixed with 20 μl Alhydrogel 2% (InvivoGen, vac-alu-250), or 200 μl PBS only, mice were inoculated on day 0, 28 and 56, respectively; serum samples were collected by tail-vein bleeding before immunization, and 2 weeks after each immunization, and used for antibodies detection by ELISA. Splenocytes were harvested for ELISPOT assay 14 d after the final immunization.
To evaluate the early immune responses induced by MEP1, mice were immunized with MEP1 plus alum or MEP1 alone were killed 10 d later. Splenocytes were harvested for cellular immune response measurement.
To observe the long-term immune responses elicited by MEP1 in combination with alum, mice were immunized with MEP1 plus alum 3 times with 4-week interval, sera were collected at specific time point till 44 weeks post first immunization, and splenocytes were harvested at specific time point for evaluation of the cellular immune responses by ELISPOT.
ELISA and ELISPOT Assay
Both ELISA and ELISPOT assay (IFN-γ and IL-4) were performed as described before.Citation23 In brief, ELISA was performed to evaluate the humoral immune responses induced by protein MEP1, specific antibody IgM (Southern Biotech, 1021–05), IgG (Southern Biotech, 1030–05) and its subclasses IgG1 (Southern Biotech, 1070–05) and IgG2a (Southern Biotech, 1080–05) were measured respectively, using a protocol similar to that described previously,Citation23 the purified MEP1 was used as coating antigen at the concentration of 1 μg/ml. ELISPOT assay was performed according to the manufacture's protocol (BD Biosciences, 551083 and 551017) to measure cellular immune responses induced by protein MEP1, IFN-γ- and IL-4-spot forming units were expressed as SFUs per million cells.
Challenge with the recombinant virus rTTV-lucgag
To evaluate the protective efficacy of the recombinant protein MEP1, female BALB/c mice were immunized with MEP1 plus alum 3 times in total followed by intraperitoneally inoculated with total 4 ml virus suspension (1 ml of TCID50 2 × 108 CEF supernatant diluted with 3 ml endotoxin-free PBS). Three days after innoculation, mice were killed, ovary from each mouse was taken, and homogenized immediately in 200 μl luciferase lysing buffer (Promega, E266A) and centrifuged; then, the supernatant mixed with equal-volume luciferase was used to measure luciferase activity.Citation24 The higher intensity of luciferase activity stands for more severe virus infection.
Statistic analysis
Statistical analyses were performed using GraphPad Prism version 5.0. Non-parametric t test was used to analyze the statistic difference for 2 group experiments, and repeated measure 2-way ANOVA was used to analyze the antibody kinetics in the long- term observation of immune responses elicited by MEP1 plus alum. It is regarded as statistic difference when p value less than 0.05.
Abbreviations
| Ab | = | antibody |
| AIDS | = | acquired immunodeficiency syndrome |
| ADCC | = | antibody-dependent cellular cytotoxicity |
| Ad | = | adenovirus |
| BNAbs | = | broadly neutralizing antibodies |
| CEF | = | chick embryo fibroblast |
| CMV | = | cytomegalovirus |
| CRF | = | circulating recombinant form |
| ELISA | = | enzyme-linked immunosorbent assay |
| ELISPOT | = | enzyme-linked immunospot |
| FDA | = | food and drug administration |
| HIV-1 | = | human immunodeficiency virus type 1 |
| HLA | = | human leukocyte antigen |
| IFN | = | interferon |
| IL | = | interleukin |
| IPTG | = | isopropyl β-D-1-thiogalactopyranoside |
| MEP1 | = | multi-epitope protein 1 |
| MHC | = | major histocompatibility complex |
| SFU | = | spot forming unit |
| TTV | = | Tiantan vaccinia |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was partially supported by the NSFC grant #31270975 and SKLPBS grant #1416.
References
- Gray GE, Laher F, Lazarus E, Ensoli B, Corey L. Approaches to preventative and therapeutic HIV vaccines. Curr Opin Virol 2016; 17:104-9; PMID: 26985884; https://doi.org/10.1016/j.coviro.2016.02.010
- Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013; 369(22):2083-92; PMID: 24099601; https://doi.org/10.1056/NEJMoa1310566
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF, rgp HIVVSG. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005; 191(5):654-65; PMID: 15688278; https://doi.org/10.1086/428404
- Karpenko LI, Bazhan SI, Antonets DV, Belyakov IM. Novel approaches in polyepitope T-cell vaccine development against HIV-1. Expert Rev Vaccines 2014; 13(1):155-73; PMID: 24308576; https://doi.org/10.1586/14760584.2014.861748
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372(9653):1881-93; PMID: 19012954; https://doi.org/10.1016/S0140-6736(08)61591-3
- McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 2010; 33(4):542-54; PMID: 21029964; https://doi.org/10.1016/j.immuni.2010.09.011
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361(23):2209-20; PMID: 19843557; https://doi.org/10.1056/NEJMoa0908492
- Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP, Jr., Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 2015; 522(7557):487-91; PMID: 25855300; https://doi.org/10.1038/nature14411
- Richard J, Pacheco B, Gohain N, Veillette M, Ding S, Alsahafi N, Tolbert WD, Prevost J, Chapleau JP, Coutu M, et al. Co-receptor Binding Site Antibodies Enable CD4-Mimetics to Expose Conserved Anti-cluster A ADCC Epitopes on HIV-1 Envelope Glycoproteins. EBioMedicine 2016; 12:208-18; PMID: 27633463; https://doi.org/10.1016/j.ebiom.2016.09.004
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 2009; 15(3):293-9; PMID: 19219024; https://doi.org/10.1038/nm.1935
- Hansen SG, Piatak M, Jr., Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIV infection. Nature 2013; 502(7469):100-4; PMID: 24025770; https://doi.org/10.1038/nature12519
- Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, et al. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med 2011; 3(81):81ra36; PMID: 21543722; https://doi.org/10.1126/scitranslmed.3002351
- Churchyard GJ, Morgan C, Adams E, Hural J, Graham BS, Moodie Z, Grove D, Gray G, Bekker LG, McElrath MJ, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204). PLoS One 2011; 6(8):e21225; PMID: 21857901; https://doi.org/10.1371/journal.pone.0021225
- McMichael AJ, Haynes BF. Lessons learned from HIV-1 vaccine trials: new priorities and directions. Nat Immunol 2012; 13(5):423-7; PMID: 22513323; https://doi.org/10.1038/ni.2264
- Stephenson KE, D'Couto HT, Barouch DH. New concepts in HIV-1 vaccine development. Curr Opin Immunol 2016; 41:39-46; PMID: 27268856; https://doi.org/10.1016/j.coi.2016.05.011
- Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, et al. Broadly targeted CD8(+) T cell responses restricted by major histocompatibility complex E. Science 2016; 351(6274):714-20; PMID: 26797147; https://doi.org/10.1126/science.aac9475
- Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 2013; 340(6135):1237874; PMID: 23704576; https://doi.org/10.1126/science.1237874
- Goepfert PA, Elizaga ML, Seaton K, Tomaras GD, Montefiori DC, Sato A, Hural J, DeRosa SC, Kalams SA, McElrath MJ, et al. Specificity and 6-month durability of immune responses induced by DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2014; 210(1):99-110; PMID: 24403557; https://doi.org/10.1093/infdis/jiu003
- Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol 2007; 178(10):6596-603; PMID: 17475891; https://doi.org/10.4049/jimmunol.178.10.6596
- Nkolola JP, Bricault CA, Cheung A, Shields J, Perry J, Kovacs JM, Giorgi E, van Winsen M, Apetri A, Brinkman-van der Linden EC, et al. Characterization and immunogenicity of a novel mosaic M HIV-1 gp140 trimer. J Virol 2014; 88(17):9538-52; PMID: 24965452; https://doi.org/10.1128/JVI.01739-14
- Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993; 259(5102):1745-9; PMID: 8456302; https://doi.org/10.1126/science.8456302
- Chen Y, Wang S, Lu S. DNA Immunization for HIV Vaccine Development. Vaccines (Basel) 2014; 2(1):138-59; PMID: 26344472; https://doi.org/10.3390/vaccines2010138
- Yang Y, Sun W, Guo J, Zhao G, Sun S, Yu H, Guo Y, Li J, Jin X, Du L, et al. In silico design of a DNA-based HIV-1 multi-epitope vaccine for Chinese populations. Hum Vaccin Immunother 2015; 11(3):795-805; PMID: 25839222; https://doi.org/10.1080/21645515.2015.1012017
- Huang Y, Qiu C, Liu LX, Feng YM, Zhu T, Xu JQ. A mouse model based on replication-competent Tiantan vaccinia expressing luciferase/HIV-1 Gag fusion protein for the evaluation of protective efficacy of HIV vaccine. Chin Med J 2009; 122(14):1655-9; PMID: 19719967; https://doi.org/10.3760/cma.j.issn.0366-6999.2009.14.010
- Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010; 464(7286):217-23; PMID: 20220840; https://doi.org/10.1038/nature08757
- Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, Coban C, Akira S, Ishii KJ, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med 2011; 17(8):996-1002; PMID: 21765404; https://doi.org/10.1038/nm.2403
- Voss JE, Macauley MS, Rogers KA, Villinger F, Duan L, Shang L, Fink EA, Andrabi R, Colantonio AD, Robinson JE, et al. Reproducing SIVDeltanef vaccine correlates of protection: trimeric gp41 antibody concentrated at mucosal front lines. Aids 2016; 30(16):2427-38; PMID: 27428745; https://doi.org/10.1097/QAD.0000000000001199
- McCoy LE, Weiss RA. Neutralizing antibodies to HIV-1 induced by immunization. J Exp Med 2013; 210(2):209-23; PMID: 23401570; https://doi.org/10.1084/jem.20121827
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366(14):1275-86; PMID: 22475592; https://doi.org/10.1056/NEJMoa1113425
- Saez-Cirion A, Sinet M, Shin SY, Urrutia A, Versmisse P, Lacabaratz C, Boufassa F, Avettand-Fenoel V, Rouzioux C, Delfraissy JF, et al. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J Immunol 2009; 182(12):7828-37; PMID: 19494307; https://doi.org/10.4049/jimmunol.0803928
- Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, Barre-Sinoussi F, Delfraissy JF, Sinet M, Pancino G, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A 2007; 104(16):6776-81; PMID: 17428922; https://doi.org/10.1073/pnas.0611244104
- Reguzova A, Antonets D, Karpenko L, Ilyichev A, Maksyutov R, Bazhan S. Design and evaluation of optimized artificial HIV-1 poly-T cell-epitope immunogens. PLoS One 2015; 10(3):e0116412; PMID: 25786238; https://doi.org/10.1371/journal.pone.0116412
- Bazhan SI, Karpenko LI, Ilyicheva TN, Belavin PA, Seregin SV, Danilyuk NK, Antonets DV, Ilyichev AA. Rational design based synthetic polyepitope DNA vaccine for eliciting HIV-specific CD8+ T cell responses. Mol Immunol 2010; 47(7-8):1507-15; PMID: 20189249; https://doi.org/10.1016/j.molimm.2010.01.020
- Haynes BF, Shaw GM, Korber B, Kelsoe G, Sodroski J, Hahn BH, Borrow P, McMichael AJ. HIV-Host Interactions: Implications for Vaccine Design. Cell Host Microbe 2016; 19(3):292-303; PMID: 26922989; https://doi.org/10.1016/j.chom.2016.02.002
- Safety and efficacy study of vaccine schedule with Ad26.Mos.HIV and MVA-Mosaic in human immunodeficiency virus (HIV)-infected adults. Available from: https://clinicaltrials.gov/ct2/show/NCT02919306
- Jin X, Newman MJ, De-Rosa S, Cooper C, Thomas E, Keefer M, Fuchs J, Blattner W, Livingston BD, McKinney DM, et al. A novel HIV T helper epitope-based vaccine elicits cytokine-secreting HIV-specific CD4+ T cells in a Phase I clinical trial in HIV-uninfected adults. Vaccine 2009; 27(50):7080-6; PMID: 19786145; https://doi.org/10.1016/j.vaccine.2009.09.060
- Janes H, Friedrich DP, Krambrink A, Smith RJ, Kallas EG, Horton H, Casimiro DR, Carrington M, Geraghty DE, Gilbert PB, et al. Vaccine-induced gag-specific T cells are associated with reduced viremia after HIV-1 infection. J Infect Dis 2013; 208(8):1231-9; PMID: 23878319; https://doi.org/10.1093/infdis/jit322
- Letourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, Yang H, Dorrell L, Dong T, Korber B, McMichael AJ, et al. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One 2007; 2(10):e984; PMID: 17912361; https://doi.org/10.1371/journal.pone.0000984
- Steers NJ, Currier JR, Jobe O, Tovanabutra S, Ratto-Kim S, Marovich MA, Kim JH, Michael NL, Alving CR, Rao M. Designing the epitope flanking regions for optimal generation of CTL epitopes. Vaccine 2014; 32(28):3509-16; PMID: 24795226; https://doi.org/10.1016/j.vaccine.2014.04.039
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19(12):1597-608; PMID: 24309663; https://doi.org/10.1038/nm.3409
- Park H, Adamson L, Ha T, Mullen K, Hagen SI, Nogueron A, Sylwester AW, Axthelm MK, Legasse A, Piatak M, Jr., et al. Polyinosinic-polycytidylic acid is the most effective TLR adjuvant for SIV Gag protein-induced T cell responses in nonhuman primates. J Immunol 2013; 190(8):4103-15; PMID: 23509365; https://doi.org/10.4049/jimmunol.1202958
- Oleszycka E, Lavelle EC. Immunomodulatory properties of the vaccine adjuvant alum. Curr Opin Immunol 2014; 28:1-5; PMID: 24463269; https://doi.org/10.1016/j.coi.2013.12.007
- Guo J, Yang Y, Xiao W, Sun W, Yu H, Du L, Lustigman S, Jiang S, Kou Z, Zhou Y. A truncated fragment of Ov-ASP-1 consisting of the core pathogenesis-related-1 (PR-1) domain maintains adjuvanticity as the full-length protein. Vaccine 2015; 33(16):1974-80; PMID: 25736195; https://doi.org/10.1016/j.vaccine.2015.02.053
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011; 473(7348):523-7; PMID: 21562493; https://doi.org/10.1038/nature10003
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011; 470(7335):543-7; PMID: 21350488; https://doi.org/10.1038/nature09737
