ABSTRACT
Due to the low uptake, adherence, and completion of vaccination among drug users, and their compromised immune responses to hepatitis B vaccination, the current practice of hepatitis B vaccination may not provide optimal protection. The aim of this study was to evaluate the immunogenicity and safety of 60 µg and 20 µg hepatitis B vaccines among drug users. A randomized, open-labeled, blank-controlled trial was conducted among drug users at 2 drug rehabilitation centers in China. The eligible participants were drug users who were serologically negative for the hepatitis B surface antigen (HBsAg) and the hepatitis B surface antibody (anti-HBs). Participants were randomized in a ratio of 1:1:1 to receive 20 µg (IM20 group) or 60 µg (IM60 group) of hepatitis B vaccine or blank control at months 0, 1, and 6, and followed at months 6, 7, and 12. Seroconversion rates of 94.7% and 92.6% were observed in IM20 and IM60 groups at month 7, and correspondingly decreased to 89.5% and 91.7% respectively at month 12. The IM60 group showed significantly higher geometric mean concentrations (GMCs) of anti-HBs (2022.5 and 676.7 mIU mL–1) than the IM20 group did (909.6 and 470.5 mIU mL–1) at months 7 and 12 (P < 0.05). No safety concerns associated with vaccination were noted. Three-dose intramuscular immunization with hepatitis B vaccines showed good immunogenicity among the drug users.
Introduction
According to the Annual Report on Drug Control in China from 2001 to 2015, the total number of cumulative registered drug users has been on a sharp increase from 850,000 in 2000 to 2.955 million in 2014, and it is estimated that the actual number exceeded 14 million at the end of 2014.Citation1 Drug users, particularly injecting drug users, are at risk of acquiring hepatitis B virus (HBV) infection, due to sharing needles, trading sex for drugs, and high-risk sexual behaviors.Citation2,3 It has been confirmed that the prevalence of HBV infection among drug users, including both intravenous drug users (IVDU) and non-intravenous drug users (non-IVDU), is higher than in the general population in China.Citation4-7 HBV infection among drug users, as one of the most prevalent and preventable health concern, has become an important public health problem in China.
Hepatitis B vaccination is an effective method of preventing HBV infection.Citation8 To prevent HBV infection, hepatitis B vaccination has been recommended for drug users, especially people who inject drugs.Citation2,3,8-10 However, uptake, adherence, and completion of vaccination among current drug users were low,Citation2,11-14 and their immune functionCitation15,16 and immune response to the hepatitis B vaccinationCitation17-20 was also suboptimal, indicating that the current practice of hepatitis B vaccination might not protect drug users from HBV infection. According to the latest guideline issued in China for the prevention and treatment of chronic hepatitis B, a 3-dose regimen with 20 µg hepatitis B vaccines has been recommended for general adults, while higher vaccine doses (60 μg) and/or prolonged vaccination schedules are recommended for the immunocompromised population or non-responders.Citation10 However, whether the immunization with the 60 μg hepatitis B vaccines in drug users could yield a superior protection against hepatitis B infection than did the 20 μg doses remains unclear.
The goal of this study was to evaluate the immunogenicity and safety of the 20 µg and 60 µg recombinant hepatitis B vaccines among drug users.
Results
Enrollment and follow-up of the subjects
This study was conducted between August 2014 and October 2015. A total of 488 drug users at 2 drug rehabilitation centers were screened for eligibility, and 480 were enrolled and randomized (). 158 participants in the 20 µg recombinant hepatitis B vaccine (IM20) group and 156 in the 60 µg recombinant hepatitis B vaccine (IM60) group received the first injection, and 155 in the IM20 group and 150 in the IM60 group completed the whole vaccine series. Blood samples were collected from 151, 151, and 124 participants in the control group, 155, 150, and 133 in the IM20 group, and 150, 149, and 133 in the IM60 group, at months 6, 7, and 12, respectively (). Participants' characteristics among the 3 groups were comparable at enrollment (). No significant differences in demographic, drug addiction, and rehabilitation characteristics were noted between the participants who completed the entire study and those who failed to follow-up (P > 0.05).
Figure 1. Participant flow in the hepatitis B vaccination randomized open-label, blank-controlled trial among drug users.
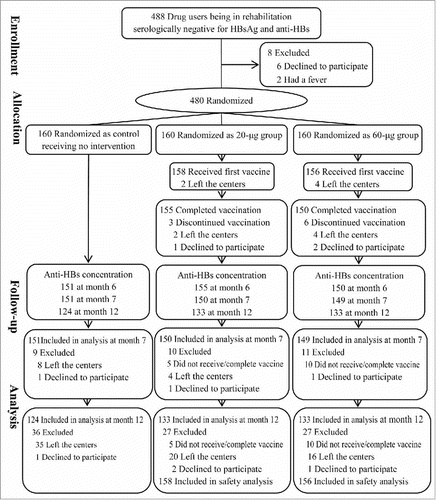
Table 1. Characteristics of drug users at the enrollment.
Immunogenicity
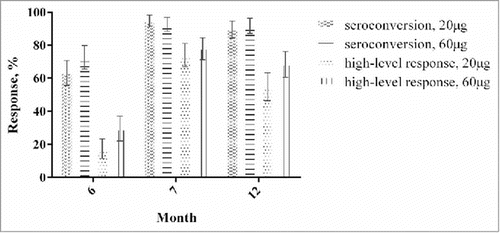
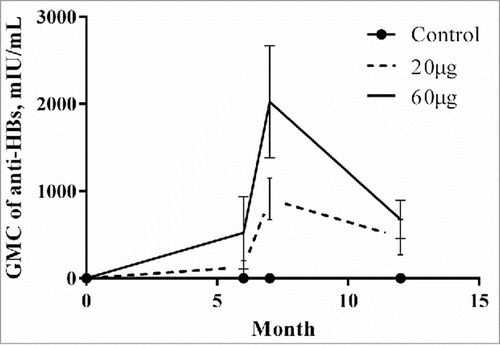
One month after the completion of the 3-dose vaccination (month 7), the seroconversion rates in the treatment groups were similarly high, with a proportion of 94.7% in the IM20 group and 92.6% in the IM60 group (P = 0.47). After then, the vaccine-elicited antibody responses decreased during the following months, and showed a seroconversion rate of 89.5% and 91.7% in IM20 and IM60 groups at month 12, respectively (P = 0.53) (, ). No significant differences remained between different dose vaccinations after adjusting for demographic, drug addiction and rehabilitation characteristics (). The IM60 group had significantly higher geometric mean concentrations (GMCs) of hepatitis B surface antibody (anti-HBs) (2022.5 and 676.7 mIU mL–1) than the IM20 group (909.6 and 470.5 mIU mL–1) at months 7 and 12 (P < 0.05), in spite of the decreased GMCs of anti-HBs among both groups from months 7 to 12 (, ). A significantly higher proportion of participants with high-level responses post-vaccination was also found in the IM60 group compared with that in the IM20 group at month 12 (, ). No seroconversion was noted among the participants in the control group.
Table 2. Response of drug users with different vaccination schedules at months 6, 7 and 12.
We also measured the anti-HBs level at month 6 before the third injection: the GMCs of anti-HBs (126.3 and 524.5 mIU mL–1), seroconversion rate (63.2% and 72.7%), and high-level response rate (17.4% and 29.3%) at month 6 were all lower than those with all 3-dose vaccinations at month 7 for both IM20 and IM60 groups. Contrary to what we expected, the GMC of anti-HBs, seroconversion rate, and high-level response rate in the IM60 group at month 6 before the third injection were lower than in the IM20 group at month 7. At month 6 before the third injection, the IM60 group had a higher anti-HBs level and high-level response than the IM20 group (P < 0.05). However, there was no significant difference in seroconversion rate between the vaccination groups at month 6 before the third injection ().
Safety
Three hundred and fourteen participants received at least one injection of vaccine and safety measures were conducted. In the IM20 group, ten (6.3%), eight (5.1%), four (2.5%), three (1.9%) and two (1.3%) individuals reported pain, erythema, edema, induration, and fever within 7 d after vaccination, and the corresponding number was nine (5.8%), nine (5.8%), five (3.2%), two (1.3%) and two (1.3%) in the IM60 group, which were not significantly different (P > 0.05) (). There were 1 (0.6%) and 1 (0.6%) in the IM20 group and 1 (0.6%) and 2 (1.3%) in the IM60 group reporting fever and headache, respectively, within 28 d after vaccination (P > 0.05) (). All the reported adverse reactions were minor. No serious adverse events were reported. During follow-up, no participants in the 3 study groups had become hepatitis B surface antigen (HBsAg) positive.
Table 3. Summary of solicited adverse reactions, and unsolicited adverse reactions occurred within 28 d after vaccination.
Discussion
Of previous studies focusing on the seroconversion of hepatitis B vaccination among drug users, with 10 μg or 20 μg hepatitis B vaccine in 0, 1, 2-month or 0, 1, 6-month schedule,Citation17,18 only 3 small studies with sample sizes ranging from 9 to 34 have shown seroconversion rates >90%.Citation17,18 However, in our study a high seroconversion rate (94.7%–92.6%) was achieved in the drug users, which was similar to that found in the general population (90–95%).Citation9,21-23 The main possible reason for a significant higher seroconversion rate achieved in our study is the good compliance and high completion of the 3-dose regimen, while, a relatively high drop outs were noted in other studies involving in illegal drug users or those on methadone maintenance.Citation17-19 Moreover, during drug rehabilitation, the immune function of drug users gradually recovered.Citation24,25
Although the high dose (60 μg) vaccine did not show superiority in terms of the seroconversion rate over the 20 μg vaccine in this study, higher GMC of anti-HBs and proportion of participants with high-level responses were noted in the 60 μg recipients than in those who received 20 μg. The higher peak anti-HBs level achieved in the 60 μg group may be associated with a longer lasting immunity to HBV.Citation26,27
In one previous study, the high seroconversion rates (93–96%) among healthy young adults with 60 µg 2-dose vaccinations on 0–1 schedule were observed.Citation28 Thus, we planned an antibody test at month 6 before the injection of the third dose, hoping that the 60 µg vaccine may provide an alternative shorter immunization regimen against HBV infection for the drug users, since these people generally had poor adherence and difficulty completing the 3-dose vaccination.Citation13,14,17,18 However, in our study, the 60 µg 2-dose vaccinations showed low seroconversion rates in the drug users (63–72%), which were lower than those with the 3-dose vaccination. Therefore, even with the 60 μg vaccine, the importance of 3-dose vaccination among drug users should still be emphasized.
One limitation of our study is the generalizability of the results, since all the participants involved in this study were male, and recruited from 2 drug rehabilitation centers. The rehabilitation centers could provide drug users with a relative stable environment to withdraw from using drugs, maintain follow-up and access to vaccination. But, for most drug users in the society, they may be less likely to complete a 6-month hepatitis B vaccination schedule due to social instability and poor access to health care,Citation2 with a completion rate varying between 23–73%.Citation29-32 The US Centers for Disease Control and Prevention recommends HBV vaccination in juvenile and adult correctional facilities, where a high percentage of drug users are present.Citation33 Similarly, we believe that drug rehabilitation centers are an ideal setting for administering vaccination to drug users, for the complete vaccination rate in this study was as high as 95.3% (305/320).
In this study, one blank control group was set to show the levels of anti-HBs in the drug users without a vaccination intervention during the study period. The serological testing results also showed that no participants in the control group had been infected during the study period.However, considering all these anti-HBs negative drug users were still potentially vulnerable to HBV infection, we administered the 20 μg hepatitis B vaccine intramuscularly according to a 0, 1, 6-month schedule in all these participants in the blank control group who received no vaccination after the end of the study.
To the best of our knowledge, this represents the first study focusing on the immunogenicity of hepatitis B vaccination among drug users at drug rehabilitation centers, not limited to IVDU.Citation34 The results from this study indicated that both 20 μg and 60 μg hepatitis B vaccines administrated with a 3-dose regimen in the drug users could induce good immune responses. However, a higher antibody level in terms of GMC and the proportion of high-level response elicited by 60 μg hepatitis B vaccines may result in more durable protection.
Materials and methods
Participants and study design
This is a randomized, open-labeled, blank-controlled trial, conducted among drug users who were not infected with HIV at 2 drug rehabilitation centers in Yongji, Shanxi, China. Eligible participants were drug users including both IVDUs and non-IVDUs, who were aged between 18 and 70 y at enrollment, currently using illicit drug before drug rehabilitation, negative for HBsAg and anti-HBs at enrollment, and having spent acute physiological detoxification phase. The exclusion criteria included any intolerance or allergy to any component of the vaccine, ongoing opportunistic infections, liver disease, hemopathy, cancer, and unexplained fever in the last week before the recruitment. The risk of the trial had been carefully evaluated and the protocol was approved by the Ethics Committee of Shanxi Medical University before the recruitment. Participants must provide written informed consent before the screening for eligibility. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The clinical trial was registered on http://www.clinicaltrials.gov/ under the identifier NCT02959775.
Randomization
A list was generated by a computer-generated randomization. Participants were randomized in a ratio of 1:1:1 and allocated to the IM20 group, IM60 group, or blank control group, respectively. The personnel involved in the randomization were not allowed to participate in any other procedure of the trial. The participants, the study investigators involved in the vaccination and data collection were aware of the treatment allocation. But the laboratory staff who detected the antibodies in serum and assessed the outcomes were blinded to the allocation of participants.
Procedure
Participants in the IM20 group received 3 intramuscular injections of 20 µg recombinant hepatitis B vaccine, while those in the IM60 group received 3 intramuscular injections of 60 µg at months 0, 1 and 6, respectively. Both the 20 µg and 60 µg recombinant hepatitis B vaccines used in this study were produced by Shenzhen Kangtai Biological Products Co., Ltd., China (Batch No. 201308009 and No. A201301002). Participants allocated to the blank control group received no vaccination during the study period. Currently, providing a serum detection for HBV infection is routine for the drug users before admittance into rehabilitation centers in China, but the HBV vaccination is usually not provided in the rehabilitation center. During their stay at the rehabilitation center, no drug or risky sexual behavior is allowed, so these drug users are considered to be at a minimal risk of HBV infection. Drug addiction and rehabilitation history were collected through face-to-face interviews and abstraction of medical records, with socio-demographic data. Demographic information including age, gender, race, occupation, education level, and drug usage information including age of first drug abuse, route of drug abuse, drug category, history of hepatitis B vaccination, and the rehabilitation status were collected.
Safety assessment
We watched participants for immediate adverse reactions for 30 minutes after vaccination and followed them for any solicited adverse reactions occurring within 7 d post-vaccination and unsolicited adverse events within 28 d. The solicited injection-site reactions included erythema, edema, pain, induration, pruritus, discoloration, and nodule and systematic reactions included fever, headache, asthenia, cutaneous eruption, vertigo, malaise, nausea, vomiting, abdominal pain, diarrhea, myalgia, and arthralgia. Serious adverse events occurring throughout the whole study period were documented. The severity grading of the adverse events was performed by the investigators according to the standard guidelines issued by China Food and Drug Administration.Citation35
Laboratory assays
Blood samples, collected at baseline before the vaccination, months 6 (before the third injection), 7 and 12, were tested for HBsAg and anti-HBs using Chemiluminescent Microparticle ImmunoAssay (ARCHITECT HBsAg/Anti-HBs Reagent Kit; Abbot Ireland Diagnostics Division, Sligo, Ireland). Samples were tested by trained technical personnel masked to group allocation, and those with concentrations of more than the upper detection limit were retested after being diluted. Non-response was defined as post-vaccination anti-HBs concentrations of < 10 mIU mL–1. Seroconversion and high-level response were defined as post-vaccination anti-HBs concentrations of ≥ 10 and ≥ 100 mIU mL–1, respectively. The primary end point of the immunogenicity was the percentage of participants with a seroconversion at month 7 after the first vaccination.
Statistical analysis
To detect a 12% difference in seroconversion rate between 20 μg and 60 μg doses of recombinant hepatitis B vaccine (the 20 μg dose of recombinant hepatitis B vaccine conferred a 78% protection rate vs 90% in the 60 μg dose group), with 80% power at the 2-sided significance level of α = 0.05, a sample size of 146 per group was calculated. Considering a dropout rate of 15% during the study, 160 patients needed to be enrolled in each group. All the participants who enrolled and received at least one shot of vaccine were involved in the safety analysis cohort, while only those who completed the 3-dose vaccination and the 7-month follow-up were included in the per-protocol cohort for the immunogenicity analysis.
Data were double entered using EpiData, and analyzed using SAS version 9.3 (SAS Institute, Cary, NC, USA). We used Chi-square tests or Fisher's exact tests to compare baseline categorical data and one-way ANOVA (analysis of variance) to analyze baseline continuous data. GMCs and the corresponding 95% confidence intervals (CI) were calculated for anti-HBs levels, and were compared using Student's t-test. Proportions were compared using Chi-square test or Fisher's exact test. For different vaccination doses, unconditional logistic regression was used to calculate odds ratio (OR) and 95% CI. P < 0.05 was regarded as significant for all analyses.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We gratefully acknowledge the contribution from the dedicated students and the staff members of the Center for Disease Control and Prevention and the drug rehabilitation centers.
Funding
This research was supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (2012ZX10002001), Research Project Supported by Shanxi Scholarship Council of China (2013–056) and Construction Project of Characteristic Key Disciplines for Universities of Shanxi Province.
References
- China National Narcotics Control Commission. The annual report on drug control in China, 2000, 2015. Available from: http://www.nncc626.com/index/ndbg.html
- WHO. Guidance on prevention of viral hepatitis B and C among people who inject drugs. Geneva, Switzerland: WHO; 2012. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23805439
- Centers for Disease Control and Prevention (CDC). Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: Summary guidance from CDC and the U.S. Department of Health and Human Services. MMWR Recommendations Rep 2012; 61(RR-5):1–40; PMID:23135062
- Wu J, Huang J, Xu D, Lu C, Deng X, Zhou X. Infection status and risk factors of HIV, HBV, HCV, and syphilis among drug users in Guangdong, China–a cross-sectional study. BMC Public Health 2010; 10:657; PMID:21040549; https://doi.org/10.1186/1471-2458-10-657
- Wu Q, Zu J, Wei X, You L, Kou L, Li H, Zhuang G. Survey of Hepatitis B infection and vaccination status among drug users in Xi'an. Zhonghua Yu Fang Yi Xue Za Zhi 2014; 48(10):862-6; PMID:25573123
- Zhou YH, Liu FL, Yao ZH, Duo L, Li H, Sun Y, Zheng YT. Comparison of HIV-, HBV-, HCV- and co-infection prevalence between Chinese and Burmese intravenous drug users of the China-Myanmar border region. PloS One 2011; 6(1):e16349; PMID:21283696; https://doi.org/10.1371/journal.pone.0016349
- Zhou YH, Yao ZH, Liu FL, Li H, Jiang L, Zhu JW, Zheng YT. High prevalence of HIV, HCV, HBV and co-infection and associated risk factors among injecting drug users in Yunnan province, China. PloS One 2012; 7(8):e42937; PMID:22916185; https://doi.org/10.1371/journal.pone.0042937
- WHO. Guidelines for the prevention, care and treatment of persons with Chronic Hepatitis B infection. Geneva, Switzerland: WHO; 2015. PMID:26225396
- Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, Rodewald LE, Douglas JM, Jr, Janssen RS, Ward JW, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recommendations Rep 2006; 55(RR-16):1-33; quiz CE1-4; PMID:17159833
- Chinese Society of Infectious Diseases CMA, Hou JL. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update. Zhonghua Gan Zang Bing Za Zhi 2015; 23(12):888-905; PMID:26739464; https://doi.org/10.3760/cma.j.issn.1007-3418.2015.12.002
- Winter RJ, Dietze PM, Gouillou M, Hellard ME, Robinson P, Aitken CK. Hepatitis B virus exposure and vaccination in a cohort of people who inject drugs: what has been the impact of targeted free vaccination? J Gastroenterol Hepatol 2013; 28(2):314-22; PMID:23190264; https://doi.org/10.1111/jgh.12063
- Topp L, Day CA, Wand H, Deacon RM, van Beek I, Haber PS, Shanahan M, Rodgers C, Maher L, Hepatitis A, et al. A randomised controlled trial of financial incentives to increase hepatitis B vaccination completion among people who inject drugs in Australia. Preventive Med 2013; 57(4):297-303; PMID:23639625; https://doi.org/10.1016/j.ypmed.2013.04.013
- Weaver T, Metrebian N, Hellier J, Pilling S, Charles V, Little N, Poovendran D, Mitcheson L, Ryan F, Bowden-Jones O, et al. Use of contingency management incentives to improve completion of hepatitis B vaccination in people undergoing treatment for heroin dependence: a cluster randomised trial. Lancet 2014; 384(9938):153-63; PMID:24725468; https://doi.org/10.1016/S0140-6736(14)60196-3
- McGregor J, Marks PJ, Hayward A, Bell Y, Slack RC. Factors influencing hepatitis B vaccine uptake in injecting drug users. J Public Health Med 2003; 25(2):165-70; PMID:12848409
- Riss GL, Chang DI, Wevers C, Westendorf AM, Buer J, Scherbaum N, Hansen W. Opioid maintenance therapy restores CD4+ T cell function by normalizing CD4+CD25(high) regulatory T cell frequencies in heroin user. Brain Behavior Immunity 2012; 26(6):972-8; PMID:22613171; https://doi.org/10.1016/j.bbi.2012.05.008
- Friedman H, Eisenstein TK. Neurological basis of drug dependence and its effects on the immune system. J Neuroimmunol 2004; 147(1–2):106-8; PMID:14741438
- Kamath GR, Shah DP, Hwang LY. Immune response to hepatitis B vaccination in drug using populations: a systematic review and meta-regression analysis. Vaccine 2014; 32(20):2265-74. PMID:24631093; https://doi.org/10.1016/j.vaccine.2014.02.072
- Baral S, Sherman SG, Millson P, Beyrer C. Vaccine immunogenicity in injecting drug users: a systematic review. Lancet Infect Dis 2007; 7(10):667-74; PMID:17897609; https://doi.org/10.1016/S1473-3099(07)70237-2
- Day CA, Shanahan M, Wand H, Topp L, Haber PS, Rodgers C, Deacon R, Walsh N, Kaldor J, van Beek I, et al. Development of immunity following financial incentives for hepatitis B vaccination among people who inject drugs: A randomized controlled trial. J Clin Virol 2016; 74:66-72; PMID:26679830; https://doi.org/10.1016/j.jcv.2015.11.031
- Quaglio G, Lugoboni F, Mezzelani P, Des Jarlais DC, Lechi A. Hepatitis vaccination among drug users. Vaccine 2006; 24(15):2702-9; PMID:16436307; https://doi.org/10.1016/j.vaccine.2005.12.045
- Zuckerman JN. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J Med Virol 2006; 78(2):169-77; PMID:16372285; https://doi.org/10.1002/jmv.20524
- Luo Z, Li L, Ruan B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis 2012; 16(2):e82-8; PMID:22178658; https://doi.org/10.1016/j.ijid.2011.10.009
- Lemon SM, Thomas DL. Vaccines to prevent viral hepatitis. N Eng J Med 1997; 336(3):196-204; PMID:8988900; https://doi.org/10.1056/NEJM199701163360307
- Wang Z, Yang XR, Song H, Cao BR, Yin F, An ZM, Kang L, Li J. Immune function alterations during 12 weeks of abstinence in heroin users. Folia Biologica 2015; 61(6):241-6; PMID:26789146
- Wang W, Zhang R, Xing Y, Zhang S, Hu D, Wu J, He J, Yang X, Fu J, Zhao H, et al. Changes and the implications of CD4(+);CD25(+);CD127(low); regulatory T cells in drug addicts during natural drug withdrawal. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2013; 29(10):1072-5; PMID:24103268
- Gesemann M, Scheiermann N. Quantification of hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. Vaccine 1995; 13(5):443-7; PMID:7639012
- Hadler SC, Francis DP, Maynard JE, Thompson SE, Judson FN, Echenberg DF, Ostrow DG, O'Malley PM, Penley KA, Altman NL, et al. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Eng J Med 1986; 315(4):209-14; PMID:2941687; https://doi.org/10.1056/NEJM198607243150401
- Wang ZZ, Li MQ, Wang P, Yang ZX, Wei L, Zeng Y, Li YP, Yan L, Liu XE, Zhuang H. Comparative immunogenicity of hepatitis B vaccine with different dosages and schedules in healthy young adults in China. Vaccine 2016; 34(8):1034-9; PMID:26801063; https://doi.org/10.1016/j.vaccine.2016.01.018
- Stitzer ML, Polk T, Bowles S, Kosten T. Drug users' adherence to a 6-month vaccination protocol: effects of motivational incentives. Drug Alcohol Dependence 2010; 107(1):76-9; PMID:19828264; https://doi.org/10.1016/j.drugalcdep.2009.09.006
- Altice FL, Bruce RD, Walton MR, Buitrago MI. Adherence to hepatitis B virus vaccination at syringe exchange sites. J Urban Health 2005; 82(1):151-61; PMID:15746385; https://doi.org/10.1093/jurban/jti016
- Hwang LY, Grimes CZ, Tran TQ, Clark A, Xia R, Lai D, Troisi C, Williams M. Accelerated hepatitis B vaccination schedule among drug users: a randomized controlled trial. J Infect Dis 2010; 202(10):1500-9; PMID:20936979; https://doi.org/10.1086/656776
- Seal KH, Kral AH, Lorvick J, McNees A, Gee L, Edlin BR. A randomized controlled trial of monetary incentives vs. outreach to enhance adherence to the hepatitis B vaccine series among injection drug users. Drug Alcohol Dependence 2003; 71(2):127-31; PMID:12927650
- Weinbaum C, Lyerla R, Margolis HS, Centers for Disease C, Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. Centers for Disease Control and Prevention. MMWR Recommendations Rep 2003; 52(RR-1):1-36; quiz CE1-4; PMID:12562146
- Lugoboni F, Migliozzi S, Schiesari F, Pauletto N, Bovo GL, Ciaffoni S, Mezzelani P. Immunoresponse to hepatitis B vaccination and adherence campaign among injecting drug users. Vaccine 1997; 15(9):1014-6; PMID:9261950
- China Food and Drug Administration (CFDA). The standard guidelines for adverse reactions grading of vaccine clinical trials. 2005. Available at: http://www.sda.gov.cn/WS01/CL0844/9350_5.html