ABSTRACT
A split-virion trivalent inactivated influenza vaccine produced according to the Chinese pharmacopeia (Shz-IIV3) has been commercially available in China since 2014. Here, we describe the results of a phase IV open-label trial to describe the immunogenicity and safety of the 2014–2015 Northern Hemisphere formulation of Shz-IIV3 in individuals ≥ 6 months of age. Subjects 6–35 months of age received 2 half-doses of Shz-IIV3 (0.25 ml) 28 d apart, and subjects ≥ 3 y of age received a single full dose (0.5 ml). The study included 602 subjects. Except for the A (H3N2) strain in subjects 3–17 years, geometric mean hemagglutination inhibition titer ratios were ≥ 10 and rates of seroconversion/significant increase in titer were ≥ 78% in all age groups. For the H3N2 strain in subjects 3–17 years, the geometric mean titer ratio was 3.8 and the rate of seroconversion/significant increase was 56%. Post-vaccination seroprotection rates were ≥ 88% for all strains in all age groups. The most common solicited reactions were injection-site pain/tenderness and fever, most of which were grade 1 and resolved within 3 d. Vaccine-related unsolicited adverse events were reported only by subjects 6–23 months, most of which were mild abnormal crying and irritability. No vaccine-related serious adverse events and no deaths were reported. No new safety signals or unexpected safety events occurred, although an immediate anaphylactic skin reaction occurred in one subject. This study confirmed that the 2014–2015 Northern Hemisphere formulation of Shz-IIV3 was well tolerated and highly immunogenic in subjects ≥ 6 months of age.
Introduction
Vaccination is the most effective way of preventing seasonal influenza and its complications.Citation1 The World Health Organization recommends influenza vaccination for individuals at high risk, including those ≥ 65 y of age, those with chronic medical conditions, pregnant women, children <5 years, and especially children <2 y of age.Citation2-4
In China, rates of hospitalization for severe acute respiratory infection due to influenza have been reported to be 61–142 per 100,000Citation5,6 and rates of mortality due to influenza-related respiratory and circulatory diseases from 11.1–12.4 per 100,000.Citation7,8 Seasonal influenza peaks in China at different times in different regions, with a peak in January or February in the temperate zone, 2 peaks in January to February and June to August in mid-latitude regions, and a peak in April to June in the southernmost regions.Citation9,10
Sanofi Pasteur has been producing an inactivated split-virion trivalent influenza vaccine (Vaxigrip®) since 1968.Citation11,12 In compliance with World Health Organization recommendations, Vaxigrip contains 2 variants of influenza subtype A (H1N1 and H3N2) and one variant of type B. Vaxigrip reduces the incidence of influenza infection, decreases workplace absenteeism, and decreases hospitalization and mortality in the elderly and other at-risk populations.Citation12-14 Long-term experience has shown that this trivalent influenza vaccine is well tolerated.Citation12
A split-virion trivalent inactivated influenza vaccine (Shz-IIV3) has been produced by Sanofi Pasteur's affiliate in China since 2013 and has been commercially available since 2014. Shz-IIV3 is made using the same method as Vaxigrip and is a bio-comparative vaccine, although in accordance with the Chinese pharmacopeia, it is produced without hydrocortisone during virus production. Here, we report the results of a phase IV study evaluating the immunogenicity and safety of the 2014–2015 Northern Hemisphere formulation of Shz-IIV3 in individuals ≥ 6 months of age.
Results
Subjects
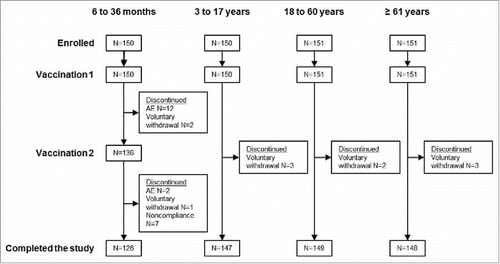
Between September 5 and October 9, 2014, 602 subjects were enrolled (n = 150, 6–35 months; n = 150, 3–17 years; n = 151, 18–60 years; n = 151, ≥ 61 years), and the study ended on December 27, 2014. The study was completed by 84.0% subjects aged 6–35 months, 98.0% subjects aged 3–17 years, 98.7% subjects aged 18–60 years, and 98.0% subjects aged ≥ 61 y (). Just over half of subjects were male in the 6–35 month (54.7%) and 3–17 y (53.3%) age groups, whereas less than half were male in the 18–60 y (24.5%) and ≥ 61 y (47.7%) age groups. None of the subjects were vaccinated the previous year (2013–2014) for seasonal influenza and none had influenza disease diagnosed before study enrolment.
Immunogenicity
Post-vaccination seroprotection rates were ≥ 88.8% for all strains in all age groups (). Geometric mean titer ratios were ≥ 10.9 for all strains in all age groups, except for the H3N2 strain in subjects 3–17 y for whom the ratio was 3.8. Rates of seroconversion/significant increase in hemagglutination inhibition (HAI) titer were ≥ 78.2% for all strains and in all age groups, except for the H3N2 strain in subjects 3–17 y for whom it was 56.2%. No major differences in immunogenicity were found between subjects 3–8 y and 9–17 y of age. Committee for Medicinal Products for Human Use (CHMP) criteria for subjects 18–60 and ≥ 61 y of age were met for all 3 vaccine strains.
Table 1. Humoral immunogenicity.
Solicited injection-site and systemic reactions
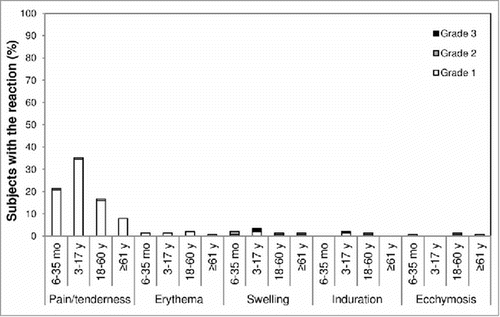
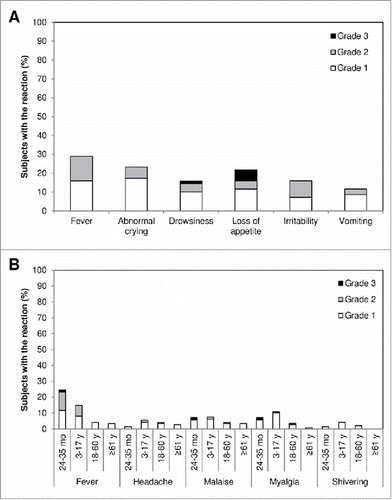
Overall, the most common solicited reactions were pain/tenderness at the injection site and fever (≥ 38.0°C) (). Most solicited reactions resolved within 3 d after vaccination (data not shown). Rates of solicited reactions were highest in the youngest age groups. Grade 3 reactions were reported for 8 subjects: in the 6–23 month age group, 3 subjects had grade 3 loss of appetite and one had grade 3 loss of appetite and drowsiness; in the 24–35 month age group, one subject had grade 3 fever and one had grade 3 malaise; and in the 3–17 y age group, one subject had grade 3 injection-site induration and swelling and one had grade 3 injection-site swelling. No grade 3 reactions were reported for subjects ≥ 18 y of age.
Unsolicited adverse events (AEs)
Proportions of subjects reporting unsolicited AEs were highest for the youngest age groups (). Serious adverse events (SAEs) were reported for 3 subjects, including one who had severe acute bronchitis, one who had severe hyperplasia of the prostate and severe lung infection, and one who had severe hand, foot, and mouth disease. None of these SAEs were considered to be vaccine-related. No deaths were reported.
Table 2. Adverse events within 28 d after vaccination.
Eleven subjects in the youngest age group (6–35 months) had unsolicited AEs considered related to the vaccination. Most of these related events were crying and irritability (n = 6; 4.0%) and decreased appetite (n = 4; 2.7%). Other less common unsolicited AEs considered related to vaccination included diarrhea and vomiting, sleepiness, upper respiratory tract infection, and agitation, all mostly mild (grade 1), and a grade 3 cutaneous anaphylactic reaction. Most of these AEs resolved spontaneously within 8 d. Vaccine-related unsolicited AEs (pruritus and diarrhea), all mild or moderate, were also reported for 3 subjects ≥ 61 y of age. None of the other age groups had vaccine-related AEs, and none of the vaccine-related events were considered serious.
Unsolicited AEs led to discontinuation of the study for 14 subjects in the 6–35 month age group (12 subjects post-dose 1 and 2 subjects post-dose 2). None of the subjects in the other age groups discontinued because of an AE.
Immediate unsolicited AEs
Two subjects in the 6–35 month age group had immediate unsolicited AEs (within 30 min of vaccination). This included one subject who had a grade 3 cutaneous anaphylactic reaction on the face and finger that lasted 24 days, was treated by medication, and led to discontinuation of the study. The other subject had mild abnormal crying that lasted 2 d and resolved spontaneously. Both of these events were considered to be related to the vaccination.
Discussion
This study, conducted at the request of the China Food and Drug Administration, showed that the 2014–2015 Northern Hemisphere formulation of Shz-IIV3 was highly immunogenic and well-tolerated in individuals ≥ 6 months of age. Post-vaccination seroprotection rates were at least 88% for all strains in all age groups, and seroconversion/significant increase rates were at least 78% for all strains in all age groups, except for the H3N2 strain in subjects 3–17 years, for whom the rate was 56%. This is similar to the seroprotection and seroconversion rates reported for Vaxigrip.Citation13 For subjects ≥ 18 y of age, CHMP criteria were met for all 3 vaccine strains.
In this study, immunogenicity was assessed by measuring HAI titers, and seroprotection estimated using the standard definition of a HAI titer ≥ 1:40. This value of this threshold and of specific correlates of protection based on serological responses continue to be debated.Citation15 Accordingly, a specific threshold for estimating protection based on HAI titers is not included in the updated CHMP guidelines that became available in 2016.Citation16 These issues, however, do not change the fact that IIV3 appeared highly immunogenic in all age groups.
Seroconversion/significant increase rates were at least 78% for all strains in all age groups, but the rate was only 56% for the H3N2 strain in subjects 3–17 y. This was due to high baseline titers against this strain–78% had a baseline HAI titer ≥ 1:40 and the geometric mean ratio of post- to pre-vaccination HAI titer was only 3.80. In all other cases, less than 40% had a baseline HAI titer ≥ 1:40 and geometric mean titer ratios were above 10. Because none of the subjects were vaccinated the previous year (2013–2014) for seasonal influenza, the high baseline titers for the H3N2 strain in subjects 3–17 y were probably due to exposure to this strain from a natural infection during a previous year.
Vaccine responses in older adults are typically lower than in younger adults due to aging of the immune system, commonly referred to as immunosenescence.Citation17 This decrease in HAI titers with age can be seen for the A/H1N1 and B strains. The immune response for the A/H3N2 strain, on the other hand, decreased little with age. In all cases, however, seroprotection and seroconversion rates remained relatively high in the oldest subjects and immunosenescence appeared to have only a moderate effect on immunogenicity. This was most likely because none of the subjects were vaccinated for seasonal influenza the previous year.
Overall, the vaccine was well-tolerated with no unexpected events or new safety signals. As found in other studies of influenza vaccines,Citation12,18 reactogenicity and rates of unsolicited AEs were highest in the youngest participants, and the most common solicited reactions were pain/tenderness at the injection site and fever. Fever is known to be associated with trivalent inactivated influenza vaccines but is not considered to be serious and is not associated with complications.Citation19 One subject 24–35 months of age had an immediate cutaneous anaphylactic reaction on the face and finger after a first vaccination. Such immediate allergic reactions are rare and can be triggered by the hemagglutinin antigens themselves or other vaccine components.Citation20
A recent analysis found that the economic burden of influenza-associated outpatient and inpatient visits in mainland China is substantial.Citation21 The average total costs were estimated at $155 US for influenza-associated outpatient visits and $1511 for influenza-associated inpatient visits. Costs were highest for children under 5 y of age, adults over 60 y of age, and individuals with underlying medical conditions. Results from other countriesCitation22,23 suggest that vaccination for seasonal influenza will be cost-effective or cost-saving in China, at least for these groups, although comprehensive cost-effectiveness studies have not yet been reported for mainland China. At the same time, influenza vaccination coverage rates in China remain very low, with overall national rates around 10%.Citation24-27 Increasing influenza vaccination rates, using Shz-IIV3 or other seasonal influenza vaccines, would help to further reduce the significant burden of seasonal influenza in China.
Patients and methods
Study design and conduct
This was a phase IV, open-label, descriptive, single-arm study in healthy subjects ≥ 6 months of age conducted at a single center in the Province of Guangxi, China between September and December, 2014 (NCT02228980). The study was conducted to comply with a request from the China Food and Drug Administration. The objective was to describe the immunogenicity and safety of a single dose (subjects ≥ 3 y of age) or 2 doses (subjects 6–35 months of age) of Shz-IIV3. The study was approved by the Guangxi Institutional Review Board before the start of the trial, conducted according to the Declaration of Helsinki, and complied with the International Conference on Harmonization Guidelines for Good Clinical Practice. Written informed consent was obtained from subjects or their legal representatives.
Subjects
The study included subjects ≥ 6 months of age. Subjects aged 6–35 months of age had to have been born at full term of pregnancy (≥ 37 weeks) at a birth weight ≥ 2.5 kg, and they could not have had a previous vaccination against influenza (2 consecutive doses of influenza vaccine having the same seasonal strain composition) any time before study enrollment or have a history of prior exposure to influenza virus through natural infection. Subjects 3–8 y of age had to have previously received at least one dose of influenza vaccine or had to have had a history of prior exposure to influenza virus through natural infection. Subjects were excluded if they had received or planned to receive any vaccine within 2 weeks before the first trial vaccination or 2 weeks after the last trial vaccination; were vaccinated against influenza within the 6 months before the first trial vaccination or planned to receive a non-study influenza vaccine during the study; had received immune globulins, blood, or blood-derived products in the past 3 months that could interfere with assessment of the immune response; had a known or suspected congenital or acquired immunodeficiency or had received immunosuppressive therapy within the preceding 6 months; had received long-term systemic corticosteroid therapy (prednisone or equivalent for more than 2 consecutive weeks within the past 3 months); had thrombocytopenia, a bleeding disorder, or had received anticoagulants in the preceding 3 weeks; had known systemic hypersensitivity to eggs or to any of the vaccine ingredients; or were pregnant.
Vaccination
Shz-IIV3 was a trivalent split virion, inactivated influenza vaccine formulated for the 2014–2015 Northern Hemisphere influenza season. The vaccine was provided in pre-filled syringes of 0.25 ml (half dose containing 7.5 µg hemagglutinin per strain) or 0.5 ml (full dose containing 15 µg hemagglutinin per strain) of A/California/7/2009 (H1N1)pdm09, A/Texas/50/2012 (H3N2), and B/Massachusetts/2/2012. Shz-IIV3 does not contain thimerosol, preservatives, hydrocortisone, or adjuvants. Infants (6–12 months of age) were vaccinated by intramuscular injection in the anterolateral aspect of the thigh. All other subjects were vaccinated by intramuscular injection in the deltoid muscle. Subjects 6–35 months of age received 2 half-doses (0.25 ml) of Shz-IIV3 28 d apart. All other subjects received one full dose (0.5 ml).
Immunogenicity assessments
Immunogenicity was described in all age groups according to the recommendations of the CHMP Note for Guidance on Harmonization of Requirements for Influenza Vaccines.Citation28 Anti-hemagglutinin antibody levels were measured by HAI assay before vaccination (day 0) and 28 d after the last vaccination by the National Institutes for Food and Drug Control in China. Immunogenicity endpoints included HAI titer, post-vaccination/pre-vaccination HAI titer ratio, seroprotection, and seroconversion/significant increase. Seroprotection was defined as a HAI titer ≥ 1:40; seroconversion as a pre-vaccination HAI titer <1:10 and a post-vaccination titer ≥ 1:40; and a significant increase as a pre-vaccination HAI titer ≥ 1:10 and ≥ 4-fold increase in post- vs. pre-vaccination HAI titer. In addition, the immunogenicity vs. all 3 vaccine strains was assessed according to the CHMP Note for Guidance criteria.Citation28 Specifically, for subjects 18–60 y of age, the criteria include a rate of seroconversion or significant increase in post-vaccination HAI titer >40%, a mean geometric increase in HAI titer between pre- and post-vaccination >2.5, and a rate of post-vaccination seroprotection >70%; for subjects ≥ 61 y of age, the criteria include a rate of seroconversion or significant increase in post-vaccination HAI titer >30%, a mean geometric increase in HAI titer between pre- and post-vaccination >2, and a rate of post-vaccination seroprotection >60%
Safety and tolerability
Safety was assessed according to International Conference on Harmonization E2A Guideline for Clinical Safety Data Management: Definitions and Standards for Expedited Reporting.Citation29 Subjects or their parents or legal representatives recorded information about solicited reactions in a diary card for up to 7 d after each vaccination, about unsolicited AEs up to 28 d after each vaccination, and about SAEs throughout the trial. Solicited injection-site reactions included tenderness (6–23 months), pain (≥ 2 years), erythema, swelling, induration, and ecchymosis. Solicited systemic reactions included fever, vomiting, abnormal crying, drowsiness, loss of appetite, and irritability for subjects 6–23 months of age; and fever, headache, malaise, myalgia, shivering for subjects ≥ 2 y of age. The intensity of solicited reactions was graded as mild (1), moderate (2), or severe (3) according to the China Food and Drug Administration scale (). SAEs were collected by investigators throughout the trial. Immediate unsolicited AEs were defined as those occurring within 30 min following vaccination. Investigators assessed unsolicited AEs and SAEs as unrelated or possibly related to the vaccination.
Table 3. Grading of solicited reactions according to the China Food and Drug Administration scale.
Sample size
As required by the China Food and Drug Administration, the sample size was set to have 500 evaluable subjects for safety evaluation at the end of the assessment period. Assuming that 17% of subjects would not be assessable, 600 subjects were enrolled (150 subjects in each group) to ensure that this sample size was met. For the 150 subjects 3–17 y of age, enrollment was stratified so that 75 subjects 3–8 y of age and 75 subjects 9–17 y of age would be enrolled. With 500 evaluable subjects overall, there was a 95% probability of observing an event that had a true incidence of 0.6%, and with 125 evaluable subjects in each age group (150 subjects enrolled), there was a 95% probability of observing an event that had an incidence of 2.4%.
Statistical analysis
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Missing data were not replaced and no search for outliers was performed. All analyses were descriptive. Immunogenicity was analyzed in all eligible subjects who were vaccinated according to protocol and who had valid serology results. Safety was analyzed in all subjects who received the study vaccine. Confidence intervals for single proportions were calculated using the exact binomial method (Clopper-Pearson method). Geometric mean HAI titers, geometric mean post-vaccination/pre-vaccination HAI titer ratios, and their 95% confidence intervals were calculated assuming that log10 transformation of the titers followed a normal distribution.
Abbreviations
| AE | = | adverse event |
| CHMP | = | Committee for Medicinal Products for Human Use |
| HAI | = | hemagglutination inhibition titer |
| SAE | = | serious adverse event |
| Shz-IIV3 | = | bio-comparative split-virion inactivated trivalent influenza vaccine produced by Sanofi Pasteur's affiliate in China |
Disclosure of potential conflicts of interest
X. Liao, K. Go, and N. Lavis are employees of Sanofi Pasteur. The other authors declare no conflicts of interest.
Acknowledgments
Medical writing for this manuscript was provided by Dr. Phillip Leventhal (4Clinics, Paris, France) and paid for by Sanofi Pasteur. The authors also thank Nathalie Cochet (Sanofi Pasteur, Lyon, France) for preparing the protocol and clinical study report and Sarah Li (Sanofi Pasteur, Beijing, China) for clinical trial coordination and budget management.
Funding
This study was funded by Sanofi Pasteur.
References
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1-62; PMID:20689501
- Nicoll A, Ciancio B, Tsolova S, Blank P, Yilmaz C. The scientific basis for offering seasonal influenza immunisation to risk groups in Europe. Euro Surveill 2008; 13(43):19018.
- World Health Assembly. Resolution WHA56.19, tenth plenary meeting. Prevention and control of influenza pandemics and annual epidemics 2003. Geneva: World Health Organization; 2003 May 28. Available from: http://apps.who.int/gb/archive/pdf_files/WHA56/ea56r19.pdf
- World Health Organization. Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec 2012; 87:461-76; PMID:23210147
- Wong CM, Yang L, Chan KP, Leung GM, Chan KH, Guan Y, Lam TH, Hedley AJ, Peiris JS. Influenza-associated hospitalization in a subtropical city. PLoS Med 2006; 3:e121; PMID:16515368; https://doi.org/10.1371/journal.pmed.0030121
- Yu H, Huang J, Huai Y, Guan X, Klena J, Liu S, Peng Y, Yang H, Luo J, Zheng J, et al. The substantial hospitalization burden of influenza in central China: surveillance for severe, acute respiratory infection, and influenza viruses, 2010–2012. Influenza Other Respir Viruses 2014; 8:53-65; PMID:24209711; https://doi.org/10.1111/irv.12205
- Feng L, Shay DK, Jiang Y, Zhou H, Chen X, Zheng Y, Jiang L, Zhang Q, Lin H, Wang S, et al. Influenza-associated mortality in temperate and subtropical Chinese cities, 2003–2008. Bull World Health Organ 2012; 90:279-88B; PMID:22511824; https://doi.org/10.2471/BLT.11.096958
- Yu H, Feng L, Viboud CG, Shay DK, Jiang Y, Zhou H, Zhou M, Xu Z, Hu N, Yang W, et al. Regional variation in mortality impact of the 2009 A(H1N1) influenza pandemic in China. Influenza Other Respir Viruses 2013; 7:1350-60; PMID:23668477; https://doi.org/10.1111/irv.12121
- Feng L, Yang P, Zhang T, Yang J, Fu C, Qin Y, Zhang Y, Ma C, Liu Z, Wang Q, et al. Technical guidelines for the application of seasonal influenza vaccine in China (2014–2015). Hum Vaccin Immunother 2015; 11:2077-101; PMID:26042462; https://doi.org/10.1080/21645515.2015.1027470
- Yu H, Alonso WJ, Feng L, Tan Y, Shu Y, Yang W, Viboud C. Characterization of regional influenza seasonality patterns in China and implications for vaccination strategies: spatio-temporal modeling of surveillance data. PLoS Med 2013; 10:e1001552; PMID:24348203; https://doi.org/10.1371/journal.pmed.1001552
- Proctor GD. “Vaxigrip” influenza vaccine. Med J Aust 1984; 141:136; PMID:6738435
- Delore V, Salamand C, Marsh G, Arnoux S, Pepin S, Saliou P. Long-term clinical trial safety experience with the inactivated split influenza vaccine, Vaxigrip. Vaccine 2006; 24:1586-92; PMID:16271424; https://doi.org/10.1016/j.vaccine.2005.10.008
- Arnoux S, Weinberger C, Gessner BD. Vaccine-preventable influenza disease burden from clinical trials of Vaxigrip - an inactivated split virion influenza vaccine - supports wider vaccine use. Vaccine 2007; 25:7720-31; PMID:17920168; https://doi.org/10.1016/j.vaccine.2007.08.063
- Jianping H, Xin F, Changshun L, Bo Z, Linxiu G, Wei X, Jiande S. Assessment of effectiveness of Vaxigrip. Vaccine 1999; 17(Suppl 1):S57-8; PMID:10471182; https://doi.org/10.1016/S0264-410X(99)00107-3
- Trombetta CM, Montomoli E. Influenza immunology evaluation and correlates of protection: a focus on vaccines. Expert Rev Vaccines 2016; 15:967-76; PMID:26954563; https://doi.org/10.1586/14760584.2016.1164046
- Committee for Medicinal Products for Human Use. Guideline on influenza vaccines. Non-clinical and clinical module. London: European Medicines Agency; 2016 July 21. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211324.pdf
- Haq K, McElhaney JE. Immunosenescence: Influenza vaccination and the elderly. Curr Opin Immunol 2014; 29:38-42; PMID:24769424; https://doi.org/10.1016/j.coi.2014.03.008
- Grohskopf LA, Shay DK, Shimabukuro TT, Sokolow LZ, Keitel WA, Bresee JS, Cox NJ. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices–United States, 2013–2014. MMWR Recomm Rep 2013; 62:1-43; PMID:24048214
- Glanz JM, Newcomer SR, Hambidge SJ, Daley MF, Narwaney KJ, Xu S, Lee GM, Baggs J, Klein NP, Nordin JD, et al. Safety of trivalent inactivated influenza vaccine in children aged 24 to 59 months in the vaccine safety datalink. Arch Pediatr Adolesc Med 2011; 165:749-55; PMID:21810637; https://doi.org/10.1001/archpediatrics.2011.112
- World Health Organization. Information sheet. Observed rate of vaccine reactions. Influenza vaccine. Geneva: World Health Organization; 2012 July. Available from: http://www.who.int/vaccine_safety/initiative/tools/Influenza_Vaccine_rates_information_sheet.pdf
- Yang J, Jit M, Leung KS, Zheng YM, Feng LZ, Wang LP, Lau EH, Wu JT, Yu HJ. The economic burden of influenza-associated outpatient visits and hospitalizations in China: a retrospective survey. Infect Dis Poverty 2015; 4:44; PMID:26445412; https://doi.org/10.1186/s40249-015-0077-6
- de Waure C, Veneziano MA, Cadeddu C, Capizzi S, Specchia ML, Capri S, Ricciardi W. Economic value of influenza vaccination. Hum Vaccin Immunother 2012; 8:119-29; PMID:22251999; https://doi.org/10.4161/hv.8.1.18420
- Nichol KL. Cost-effectiveness and socio-economic aspects of childhood influenza vaccination. Vaccine 2011; 29:7554-8; PMID:21820477; https://doi.org/10.1016/j.vaccine.2011.08.015
- Wagner AL, Montgomery JP, Xu W, Boulton ML. Influenza vaccination of adults with and without high-risk health conditions in China. J Public Health (Oxf) 2016; PMID:27160858; https://doi.org/10.1093/pubmed/fdw041
- Lv M, Fang R, Wu J, Pang X, Deng Y, Lei T, Xie Z. The free vaccination policy of influenza in Beijing, China: The vaccine coverage and its associated factors. Vaccine 2016; 34:2135-40; PMID:26917011; https://doi.org/10.1016/j.vaccine.2016.02.032
- Zhou L, Su Q, Xu Z, Feng A, Jin H, Wang S, Feng Z. Seasonal influenza vaccination coverage rate of target groups in selected cities and provinces in China by season (2009/10 to 2011/12). PLoS One 2013; 8:e73724; PMID:24040041
- Wu S, Yang P, Li H, Ma C, Zhang Y, Wang Q. Influenza vaccination coverage rates among adults before and after the 2009 influenza pandemic and the reasons for non-vaccination in Beijing, China: a cross-sectional study. BMC Public Health 2013; 13:636; PMID:23835253; https://doi.org/10.1186/1471-2458-13-636
- Committee for Proprietary Medicinal Products (CPMP). Note for Guidance on Harmonisation of Requirments for Influenza Vaccines. CPMP/BWP/214/96. London: European Medicines Agency; 1997 Mar 12. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf
- International Conference on Harmonisation. Clinical safety data management: definitions and standards for expedited reporting E2A. Geneva: International Council for Harmonisation; 1994 Oct 27. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf