ABSTRACT
Middle East respiratory syndrome (MERS) continues to raise worldwide concerns due to its pandemic potential. Increased MERS cases and no licensed MERS vaccines highlight the need to develop safe and effective vaccines against MERS. We have previously demonstrated that a receptor-binding domain (RBD) fragment containing residues 377–588 of MERS-coronavirus (MERS-CoV) spike protein is a critical neutralizing domain and an important vaccine target. Nevertheless, its optimal immunogen dosage and immunization interval, key factors for human-used vaccines that induce protective immunity, have never been investigated. In this study, we optimized these criteria using a recombinant MERS-CoV RBD protein fused with Fc (S377–588-Fc) and utilized the optimal immunization schedule to evaluate the protective efficacy of RBD against MERS-CoV infection in human dipeptidyl peptidase 4 transgenic (hDPP4-Tg) mice. Compared with one dose and 2 doses at 1-, 2-, and 3-week intervals, a regimen of 2 doses of this protein separated by an interval of 4 weeks induced the strongest antibody response and neutralizing antibodies against MERS-CoV infection, and maintained at a high level during the detection period. Notably, RBD protein at the optimal dosage and interval protected hDPP4-Tg mice against lethal MERS-CoV challenge, and the protection was positively correlated with serum neutralizing antibodies. Taken together, the optimal immunogen dosage and immunization interval identified in this study will provide useful guidelines for further development of MERS-CoV RBD-based vaccines for human use.
Introduction
Middle East respiratory syndrome (MERS) caused by MERS coronavirus (MERS-CoV) continues to raise global concerns due to its pandemic potential. MERS-CoV was first reported in Saudi Arabia in 2012,Citation1 and it has since infected at least 1,888 individuals worldwide including 670 deaths (mortality rate ∼35%) in a total of 27 countries (http://www.who.int/emergencies/mers-cov/en/). Dromedary camels and bats are believed to be the important sources of zoonotic transmission of MERS-CoV, and the mechanisms driving bat-to-human transmission of MERS-CoV are being extensively explored.Citation2-7 MERS-CoV causes human infections after close contact with sick patients, resulting in illness in family clusters and healthcare workers,Citation8-11 and the virus once had significant spread in South Korea in 2015.Citation12 The continuous threat of MERS-CoV and the absence of licensed vaccines make it a high priority to develop safe and efficacious MERS vaccines.
The spike (S) protein of MERS-CoV plays critical roles in virus infection and pathogenesis. It consists of a globular S1 subunit responsible for cellular tropism and interaction with target cells, and a membrane-proximal S2 subunit which mediates fusion of virus and cell membranes.Citation13-15 Viral receptor-binding domain (RBD) in the S1 subunit binds the cellular receptor, human dipeptidyl peptidase 4 (hDPP4), mediating virus entry into target cells.Citation16-19 Therefore, RBD of MERS-CoV is an attractive target for the development of vaccines against MERS.
Indeed, MERS-CoV RBD induced strong neutralizing antibodies in experimental animals.Citation18,20-22 Several fragments containing different lengths of MERS-CoV S1 subunit are defined as RBD.Citation16-18,22,23 By comparing these fragments, we identified a fragment containing residues 377–588 of MERS-CoV RBD as a critical neutralizing domain (CND),Citation22,24-27 and, hence, a promising MERS vaccine candidate.
Since MERS-CoV does not infect wild-type mice, we have developed a transgenic mouse model expressing MERS-CoV's receptor hDPP4 (hDPP4-Tg).Citation28 The codon-optimized hDPP4 gene is transferred into the wild-type mice, and the generated Tg mice show global expression of hDPP4, including lung, trachea, spleen, kidney, and brain. The Tg mice are fully permissive to MERS-CoV infection, resulting in severe pneumonia and multi-organ damage, with viral replication being detected in lung, kidney, and brain. The MERS-CoV-infected Tg mice also exhibit constant weight loss and death. Therefore, the established hDPP4-expressing Tg mouse model has become an evaluable and convenient tool to evaluate the efficacy of MERS-CoV vaccines and anti-MERS-CoV therapeutic agents.Citation27,29,30
Adjuvants are widely used to improve the efficacy of subunit vaccines. After comparing several commercially available adjuvants, such as Freund's adjuvant, aluminum (alum), Monophosphoryl lipid A, Montanide ISA51 and MF59, we identify MF59 as a potent adjuvant to promote MERS-CoV RBD to induce higher titers of antibody responses, neutralizing antibodies, and protective immunity against MERS-CoV infection.Citation25 MF59 adjuvant has been approved for human use with influenza vaccines (https://www.cdc.gov/flu/protect/vaccine/adjuvant.htm),Citation31-33 and is evaluated in clinical trails with other vaccines against viruses such as HSV, HIV, HBV, and cytomegalovirus (CMV).Citation34-36 In addition to MF59, alum is also effective to trigger MERS-CoV RBD protein to elicit sufficient antibody responses and neutralizing antibodies against MERS-CoV.Citation25 Compared with MF59, alum is used in >80% of licensed human vaccines with a long-term history. Alum has high safety, strong antigen stabilization, and significant augmentation of high and long-lasting antibody titer.Citation34 Thus, MF59 and alum adjuvants are applicable for MERS-CoV RBD vaccines. Since MF59 and alum have been optimized for RBD proteins in wild-type mice and hDPP4-Tg mice, respectively,Citation25,27 they are used in the present study.
To achieve the best effect, optimization of vaccine immunogen dosage is a critical step to reduce dosage and increase population coverage, which will help determine the stockpile size of pandemic vaccines.Citation37 Optimization of immunization schedule is another crucial step since traditional immunization pathways require booster doses to maintain protective antibody concentrations, reinforcing the significance of optimizing immunization dose spacing. In this study, we aimed to optimize the immunization dosage and interval of a MERS-CoV RBD-based subunit vaccine (S377-588-Fc), and then utilize the optimal schedule to evaluate its protective efficacy in our established hDPP4-Tg mouse model.
Results
Two doses of S377-588-Fc separated by an interval of 4 weeks induced the highest IgG antibody responses when compared to mice receiving one or two doses at 1-, 2- or 3-week intervals
To optimize the dosage of immunogen, S377-588-Fc protein, and the interval between two immunizations, we immunized mice as described in Methods (). Mice were boosted at 1-, 2-, 3-, and 4-week intervals, respectively, or not boosted, and sera were collected at 10 and 40 days post-last vaccination to detect IgG antibody responses.
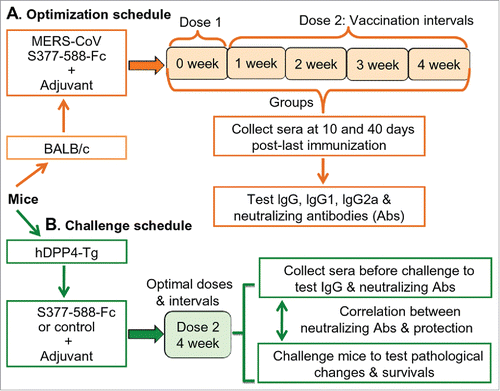
Figure 1. Schematic diagrams of optimization and challenge schedules. (A) Optimization schedule. BALB/c mice (5 groups) were immunized with S377-588-Fc protein and either boosted once at 1-, 2-, 3-, or 4-week intervals, respectively, or not boosted, as described in Materials and Methods. Sera were collected from the immunized mice at 10 and 40 days after the last immunization and tested for IgG, IgG1 and Ig2a, as well as neutralizing antibodies. (B) Challenge schedule. The hDPP4-Tg mice were first immunized with S377-588-Fc protein, or PBS control, at the optimal doses and interval. Next, sera were collected before challenge for testing neutralizing antibodies and the challenged mice were evaluated for pathological effects and survival, as described in Materials and Methods.
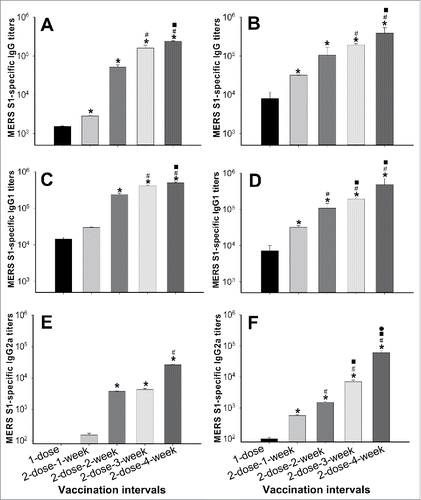
In general, two doses of S377-588-Fc induced significantly higher titers of IgG antibodies than those induced with one dose (). When two doses were administered, IgG antibodies elicited at an interval of 1 week were lower, or significantly lower, than those elicited at an interval of 2, 3 or 4 weeks, respectively. Correspondingly, IgG antibodies elicited at an interval of two weeks were significantly lower than those elicited at an interval of four weeks. Importantly, the 4-week interval induced the highest IgG titers in the sera collected at 10 days post-last immunization (). Under this regimen, the potency of MERS-CoV S1-specific IgG antibody continuously increased and was maintained at high levels for at least 40 days post-last immunization. Among all groups, IgG antibodies elicited at an interval of 4 weeks were the highest and significantly higher than those induced at intervals of 1 and 2 weeks (). Therefore, induction of the highest antibody responses in mice can be achieved by immunization consisting of two doses of S377-588-Fc protein spaced four weeks apart.
Figure 2. Antibody responses induced by S377-588-Fc at different doses and immunization intervals. Sera of mice immunized with S377-588-Fc were collected at 10 (A, C, E) and 40 (B, D, F) days post-last immunization and detected for MERS-CoV S1-specific IgG (A, B), IgG1 (C, D), and IgG2a (E, F) antibodies by ELISA. The antibody titers are presented as the endpoint dilutions that remain positively detectable, and the data are shown as mean titers ± standard deviation (SD) from five mice in each group. For (A) and (B), significant differences were revealed between 1-dose and four 2-dose groups, respectively (*); between 2-dose-1-week and 2-dose-3- or 4-week, respectively (#); or between 2-dose-2-week and 2-dose-4-week (▪). For (C), significant differences were observed between 1-dose or 2-dose-1-week and 2-dose-2-, 3- or 4-week, respectively (*); between 2-dose-2-week and 2-dose-3- or 4-week, respectively (#); or between 2-dose-3-week and 2-dose-4-week (▪). For (D) and (F), significant differences were revealed between 1-dose and the four 2-dose groups, respectively (*); between 2-dose-1-week and 2-dose-2-, 3- or 4-week, respectively (#); or between 2-dose-2-week and 2-dose-3- or 4-week, respectively (▪). For (E), significant differences were observed between 1-dose or 2-dose-1-week and 2-dose-2-, 3- or 4-week, respectively (*); or between 2-dose-2- or 3-week and 2-dose-4-week, respectively (#). For (F), significant differences were also observed between 2-dose-3-week and 2-dose-4-week (•).
Two doses of S377-588-Fc spaced 4 weeks apart induced the highest subtype antibody response among the groups receiving one and two doses at intervals of 1, 2, or 3 weeks
We tested IgG1 (T-helper 2: Th2) and IgG2a (Th1) subtypes in mouse sera. S377-588-Fc-induced IgG1 antibodies were higher, or significantly higher, when immunization was carried out in two doses, rather than one, reaching the highest level at an interval of 4 weeks. Additionally, significantly higher titers of IgG1 were revealed in sera when two doses were administered 3 or 4 weeks apart compared to those detected when two doses were administered 1 or 2 weeks apart. At an interval of 4 weeks, IgG1 titers were significantly higher than those detected when two doses were administered after 3 weeks (). MERS-CoV S1-specific IgG2a antibodies raised against S377-588-Fc basically followed a similar trend. Two doses of S377-588-Fc administered 4 weeks apart elicited the highest titer of IgG2a antibody compared to the other groups administered one dose, or two doses 1, 2, or 3 weeks apart, respectively (). High subtype antibodies were maintained for at least 40 days after the last immunization (). Thus, a S377-588-Fc booster at 4 weeks is ideal since it allows the protein to induce strong Th1 (IgG2a)- and Th2 (IgG1)-mediated antibody responses.
Two doses of S377-588-Fc spaced 4 weeks apart induced the highest neutralizing antibodies among the groups receiving one or two doses at intervals of 1, 2, or 3 weeks
We first performed a MERS pseudovirus neutralization assay in hDPP4-expressing Huh-7 cells using sera collected at 10 and 40 days, respectively, post-last immunization. This method is safe, rapid and efficient, and does not require live MERS-CoV or BSL-3 laboratories.Citation38 As shown in , S377-588-Fc at one dose failed to induce sufficient neutralizing antibodies against pseudotyped MERS-CoV expressing S protein, whereas such antibodies induced at two doses 4 weeks apart were the highest against infection of MERS pseudovirus, and significantly higher than those detected in all other groups. Significantly higher pseudovirus neutralizing antibodies were also manifested in mouse sera at an interval of 3 weeks with two doses when compared to one or two doses at intervals of 1 or 2 weeks, respectively, in the sera of mice collected at 10 days post-last immunization (). Neutralizing antibodies increased dramatically following the regimen of two doses spaced 3 weeks apart, reached the highest level at 4 weeks apart, and maintained this level for at least 40 days post-last immunization ().
Figure 3. Neutralizing antibodies induced by S377-588-Fc at different immunization doses and intervals. Neutralizing antibodies against MERS pseudovirus (A, B) and live MERS-CoV (C, D) infection in Huh-7 and Vero E6 cells, respectively, in mouse sera from 10 (A, C) and 40 (B, D) days post-last immunization. Pseudovirus neutralizing antibodies are expressed as 50% neutralizing antibody titers (NT50), and live neutralizing antibody titers are expressed as the reciprocal of the highest dilution of sera that resulted in complete inhibition of virus-induced cytopathic effect (CPE) in at least 50% of the wells (NT50). The data are presented as mean ± SD from five mice in each group. For (A) and (C), significant differences were revealed between 1-dose and 2-dose-2-, 3- or 4-week, or 2-dose-1-week and 2-dose-3- or 4-week, respectively (*); or between 2-dose-2-week and 2-dose-3- or 4-week, respectively (#). For (A), significant differences were also revealed between 2-dose-3-week and 2-dose-4-week (▪). For (B), significant differences were observed between 1-dose and the four 2-dose groups, respectively (*); between 2-dose-1-week and 2-dose-2-, 3- or 4-week, respectively (#); between 2-dose-2-week and 2-dose-3- or 4-week, respectively (▪); or between 2-dose-3-week and 2-dose-4-week (•). For (D), significant differences were observed between 1-dose and the four 2-dose groups, respectively (*); or between 2-dose-1-week or 2-dose-2-week and 2-dose-3- or 4-week, respectively (#).
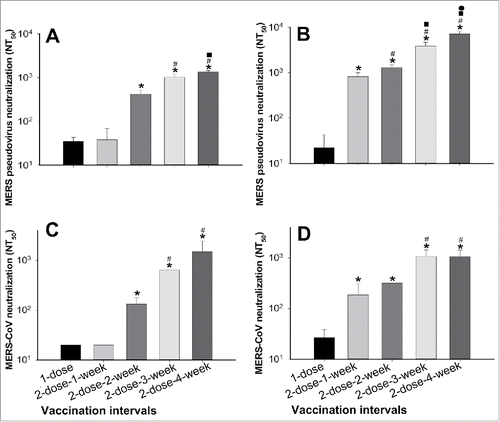
A live MERS-CoV-based neutralization assay was then performed in Vero E6 cells to confirm the above results. As shown in , while one dose of S377-588-Fc was unable to induce MERS-CoV-specific neutralizing antibodies, two doses of this protein spaced 4 weeks apart elicited the highest titer of such antibodies against MERS-CoV infection, which were significantly higher than those induced at one or two doses at intervals of 1 or 2 weeks, respectively. Significant differences were also revealed among the groups receiving 2 doses spaced 3 weeks apart and groups receiving one or two doses at intervals of 1 or 2 weeks, respectively, in the sera at 10 days post-last immunization (). As expected, for an immunization regimen consisting of 2 doses spaced 3 or 4 weeks apart, high titers of neutralizing antibodies were maintained for at least 40 days post-last immunization ().
Therefore, two doses of S377-588-Fc boosted at a 4-week interval induced the highest titer of neutralizing antibodies against MERS-CoV infection.
Two doses of S377-588-Fc spaced 4 weeks apart protected hDPP4-Tg mice from lethal challenge of MERS-CoV
We therefore established the 2-dose-4-week interval of RBD immunization as an optimal regimen inducing the highest neutralizing antibody responses. We then tested this schedule using hDPP4-Tg mice (). As expected, S377-588-Fc protected mice against MERS-CoV infection, with slightly thickened alveolar walls in the lung tissues (), as compared with those of normal control mice (). In contrast, after MERS-CoV challenge, mice in the PBS control had significant lung damage with serious inflammatory cell infiltration, focal exudation and hemorrhage, as well as severe tissue edema ().
Figure 4. Protective efficacy of S377-588-Fc in hDPP4-Tg mice with optimal immunization doses and intervals. (A) Detection of pathological changes in challenged mice. Mice were immunized with S377-588-Fc protein or PBS control, boosted once at a 4-week interval, challenged with MERS-CoV at 12 weeks post-boost, and then observed for pathological changes in lung tissues (Aa). Lung tissues from normal mice (Ab) and those injected with PBS and challenged with MERS-CoV (Ac) were included as controls. Shown are representative images of lung tissue sections stained with hematoxylin and eosin (H&E) and observed under light microscopy (100× magnification). (B) Detection of IgG antibody titers of mouse sera before MERS-CoV challenge. The antibody titers are presented as the endpoint dilutions that remain positively detectable. (C) Detection of neutralizing antibody titers (NT50) of mouse sera against live MERS-CoV infection before MERS-CoV challenge. (D) Calculation of mouse survivals at 21 days after MERS-CoV challenge. For (B)-(D), M1-M6 indicates mouse numbers in each group.
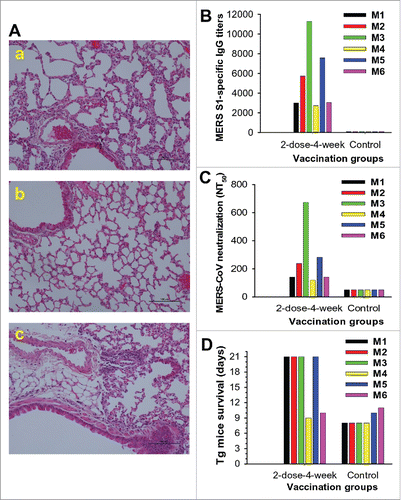
Neutralizing antibodies induced by severe acute respiratory syndrome coronavirus (SARS-CoV) RBD protein were shown to protect against SARS-CoV infection.Citation39-41 Here we tested the association between MERS-CoV RBD-induced neutralizing antibodies and protection (). Four S377-588-Fc-immunized mice that had higher IgG () and neutralizing antibody () titers (≥ 1:141) against MERS-CoV infection in their sera survived MERS-CoV challenge (), while two of these mice with lower levels of IgG and neutralizing antibodies died (). In contrast, control mice having only a background level of IgG and neutralizing antibodies did not survive (). Therefore, MERS-CoV RBD-induced neutralizing antibodies are positively correlated with protection against MERS-CoV infection.
Discussion
MERS-CoV is an emerging infectious respiratory tract disease causing serious public health problems. Since vaccination is one of the most effective strategies to prevent and treat virus infection, exploration of a safe and efficacious vaccine is required to prevent further spread of MERS-CoV and its potential pandemic. We and others have proven that MERS-CoV RBD is an important target for vaccine development.Citation20-24 Our previous studies have demonstrated that a recombinant protein containing residues 377–588 of MERS-CoV RBD fused with human IgG-Fc (S377-588-Fc) induced potent neutralizing antibodies against MERS-CoV infection,Citation22,24-26 suggesting that vaccines based on this critical neutralizing domain fragment could be further developed as an ideal candidate to prevent MERS-CoV infection.
A key step in vaccine development involves the identification of an ideal immunogen dosage and immunization interval. A vaccine candidate capable of inducing high titers of antibody responses with minimal dosage is preferable for further development as it will significantly reduce the total cost. In addition, certain vaccines, such as adult tetanus-diphtheria toxoid [Td], pediatric diphtheria-tetanus toxoid [DT], and tetanus toxoid, may produce increased local or systemic reactions in some recipients when administered too frequently by the formation of antigen-antibody complexes.Citation42,43 Thus, administration based on an optimal schedule can decrease the incidence of such reactions without adversely affecting immune responses elicited by vaccines. According to the Advisory Committee for Immunization Practices (ACIP) General Recommendations on Immunization, most vaccines, such as hepatitis B, DTap, Hib, and influenza, have recommended vaccination intervals. Generally speaking, an immunization interval of 4 weeks is widely used in clinical trials of vaccines to prevent diseases. For example, individuals with measles usually receive an extra administration of MMR (measles, mumps and rubella) vaccine, separated by at least 28 days (http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5102a1.htm). We have previously demonstrated the immunogenicity and/or protection of MERS-CoV RBD protein in mice and/or rabbits after three doses of immunization at a 3-week interval.Citation24-27 The present study further identified the immunogenicity of RBD vaccine in one dose and two doses at intervals of 1-, 2-, 3-, and 4-weeks. Here we aimed to develop a MERS vaccine able to elicit strong neutralizing antibody responses with minimal dosage (2 doses), long interval (4-weeks), yet be safe for individuals in all age groups who are susceptible to MERS-CoV infection. In the current status of no approved MERS vaccines for human use and the high cost and impracticality for conducting such optimization directly in humans, the optimized schedule in mice will be helpful for future clinical trials of MERS vaccines in terms of selection of appropriate antigen doses and immunization intervals, thus saving time and antigens for the vaccination and selection processes.
Our data demonstrated that two doses of MERS-CoV RBD (S377-588-Fc), including a boost dose at 4 weeks, induced strong IgG antibody responses. In comparison, a single dose of such vaccine could not elicit sufficient IgG antibodies. It is noted that the MERS-CoV S-specific IgG antibody titer kept increasing from day 10 to day 40 post-boost. Similar to humans, which show four subclasses of IgG (e.g., IgG1, IgG2, IgG3, IgG4), the mouse IgG subclasses are categorized as IgG1, IgG2a, IgG3, and IgG2b, correspondingly.Citation44 Both Th1 and Th2 cells promote humoral immune responses to vaccines. In mice, Th1 cells secret IL-2, INF-γ and TNF-β cytokines to produce IgG2a antibody, thus mediating cellular immunity, while Th2 cells secrete IL-4, IL-5, and IL-10 cytokines to facilitate B cells to produce IgG1 antibody, thus eliciting humoral immunity and regulating Th1 responses to restrain cellular immunity.Citation45 The IgG subtypes are largely influenced by the Th1/Th2 immune responses, and the ratio of IgG1 and IgG2a may reflect which Th type is predominant. However, it might be not appropriate to simply define each immune response as only one type (Th1 or Th2) or one type being superior to the other since some cytokines associated with Th1 or Th2 cells may function differently in different cases.Citation45 Here we noted that in addition to IgG, significantly higher titers of Th2-associated IgG1 and Th1-associated IgG2a antibodies were also induced in mice immunized with two doses of MERS-CoV RBD vaccine at an interval of 4 weeks compared to those subtype titers in mice immunized with one or two doses at intervals of 1, 2 or 3 weeks, respectively (), suggesting the importance of antibody responses in MERS-CoV RBD-based vaccines.
Table 1. Summary of antibody responses elicited by S377-588-Fc in BALB/c mice at different immunogen doses and immunization intervals.
Instead of utilizing 3 doses of immunizations,Citation26,29 our findings revealed that a satisfactory neutralizing antibody response could be achieved for a regimen consisting of 2 doses of MERS-CoV RBD protein at a 4-week interval, similar to the typical regimen of clinically trialed vaccines against other viral diseases.Citation46,47 These neutralizing antibodies were able to keep at similar, or higher, levels after 40 days post-last immunization to potently neutralize infections from MERS pseudovirus and live MERS-CoV (). Importantly, the reduction of immunogen doses and extension of vaccination interval have afforded MERS-CoV RBD protein the ability to induce suitable neutralizing antibodies that were maintained for at least 4 months during the detection period to protect aged hDPP4-Tg mice against lethal MERS-CoV infection. This will be particularly beneficial not only to the young and adults but also to the elders who are infected with MERS-CoV. Currently, there is no defined threshold of neutralizing antibody titers in protection against MERS-CoV infection. While neutralizing antibody titers (NT50) at 1:40 or 1:119 can be protective (unpublished data),Citation26 here we found that two mice at NT50 ≤ 1:141 did not survive from lethal MERS-CoV challenge. This could be due partially to the different doses of infectious viruses used for the challenge as well as other factors such as the role of cellular immune responses in the protection. Nevertheless, data from this study demonstrate that neutralizing antibody titers are, in general, positively correlated with the protection against MERS-CoV infection, a phenomenon also revealed in other MERS-CoV vaccines,Citation27,48 and SARS-CoV RBD-based subunit vaccines.Citation49,50 Future studies should be carried out to increase RBD's neutralizing ability to improve protective efficacy by modifying its sequences using structure-based vaccine design,Citation30 or adjusting the RBD to form native conformational structures, as in other viral glycoproteins.Citation51 Further studies could also be conducted to investigate RBD's protective efficacy after a boost dose of longer than 4 weeks.
MERS-CoV infects cells through two major steps: receptor binding and viral entry. We have demonstrated that MERS-CoV RBD-induced antibodies may efficiently block the binding of MERS-CoV RBD to the viral receptor DPP4, and that mutations of the key residues in the receptor-binding motif of the RBD reduce the production of antibody responses, including neutralizing antibodies, and significantly diminish the RBD-DPP4 binding.Citation18,26 Since the RBD with an optimal dosage and immunization interval identified in this study induced in the immunized mice a higher antibody titer with stronger neutralizing activity, demonstrating a great potential of these antibodies in inhibiting RBD-receptor binding, thus protecting against MERS-CoV infection.
To summarize, this study emphasizes the importance of immunogen doses and vaccination intervals in determining an appropriate immunization schedule, identifies an ideal interval and suitable dose for MERS-CoV RBD vaccines to induce effective neutralizing antibody responses in protecting against lethal MERS-CoV infection, and elucidates the potential for developing MERS vaccines targeting viral RBD as a promising subunit vaccine candidate. Taken together, the data in this report provide a solid scientific foundation and technical guidance for the design, optimization, and application of a safe and effective MERS subunit vaccine for clinical trials. The identified optimal immunization schedule (2-dose-4-week interval) may also be applied to vaccination against other enveloped viruses with class I glycoproteins.
Materials and methods
Ethics statement
Female BALB/c mice at 6–8 weeks and male and female hDPP4-Tg mice at 4 months were used. Animal studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal protocols were approved by the Committee on the Ethics of Animal Experiments of the New York Blood Center (Approval number: 194.17) and Beijing Institute of Microbiology and Epidemiology (Permit Number: PMB15-0012).
Construction, expression and purification of recombinant proteins
Construction, expression and purification of recombinant MERS-CoV RBD and S1 proteins were described previously.Citation18,22,24,25 Briefly, S377-588-Fc was constructed by fusion of MERS-CoV RBD (residues 377–588) with human IgG-Fc, expressed in 293T cell culture supernatant, and purified by Protein A affinity chromatography (GE Healthcare). MERS-CoV S1 (residues 18–725) containing a C-terminal His6 (S1-His) was expressed in the 293T cell culture supernatant and purified by Ni-NTA Superflow (Qiagen).
Mouse immunization and sample collection
This was performed following our previously described protocols with some modifications.Citation18,22,24,25 Briefly, BALB/c mice were s.c. immunized with S377-588-Fc (10 μg/100 μl/mouse) in the presence of MF59 adjuvant and boosted either once at 1-, 2-, 3-, or 4-week intervals, respectively, or not boosted. Sera were collected at 10 and 40 days post-last vaccination and measured for MERS-CoV S1-specific IgG, subtypes, and neutralizing antibodies.
ELISA
ELISA was performed to evaluate MERS-CoV S1-specific antibody responses in mouse sera as previously described.Citation18,22,24,25 Briefly, ELISA plates were coated with MERS-CoV S1-His protein overnight at 4°C and blocked with 2% non-fat milk for 2 h at 37°C. Serially diluted mouse sera were added to the plates and incubated for 1 h at 37°C, followed by four washes with PBST. Horseradish peroxidase (HRP)-conjugated anti-mouse IgG (1:3,000, GE Healthcare), IgG1 (1:2,000) or IgG2a (1:5,000) (Invitrogen) antibodies were added to the plates and incubated for 1 h at 37°C. The substrate 3,3′,5,5′-tetramethylbenzidine (TMB, Invitrogen) was added to the plates, and the reaction was stopped by H2SO4 (1 N). The absorbance at 450 nm (A450) was measured by ELISA plate reader (Tecan).
Pseudovirus neutralization assay
Neutralizing antibodies were detected in mouse sera using a pseudovirus neutralization assay as previously described.Citation38,52,53 Briefly, 293T cells were co-transfected with a plasmid encoding Env-defective, luciferase-expressing HIV-1 genome (pNL4-3.luc.RE) and a plasmid encoding MERS-CoV (EMC-2012 strain) S protein using the Calcium Phosphate Method. Supernatants containing MERS pseudovirus were harvested 72 h post-transfection and used for single-cycle infection. MERS pseudovirus was incubated with serially diluted mouse sera at 37°C for 1 h before adding to Huh-7 cells, followed by addition of fresh medium 24 h later, and culture of the cells for an additional 48 h. Cells were then lysed using cell lysis buffer (Promega) and transferred to 96-well luminometer plates. Luciferase substrate (Promega) was added to the cell lysates, and relative luciferase activity was measured in an Infinite® 200 PRO Luminator (Tecan). MERS pseudovirus neutralization was calculatedCitation54 and expressed as NT50.
Live MERS-CoV neutralization assay
Neutralizing antibodies were confirmed using a standard microneutralization assay as previously described.Citation22,24,25,38 Briefly, serially diluted mouse sera were incubated for 1 h at room temperature with ∼100 infectious MERS-CoV (EMC-2012 strain) before addition to Vero E6 cells and culture for 72 h at 37°C. Neutralizing antibodies were assessed in mouse sera by determining the presence or absence of CPE. Neutralizing antibody titers were calculated as the reciprocal of the highest dilution of sera that completely inhibited virus-induced CPE in at least 50% of the wells (NT50).
Animal challenge experiments
Using our established MERS-CoV-susceptible small animal model, hDPP4-Tg mice,Citation28 the optimal immunogen dosage and immunization interval identified above were applied for the challenge studies as previously described.Citation27 Briefly, mice were immunized with S377-588-Fc protein (5 μg/mouse) and alum adjuvant, boosted once at 4 weeks, challenged with the lethal dose of MERS-CoV (EMC-2012 strain, 104 TCID50) 12 weeks post-boost, and investigated for 3 weeks to detect pathological changes and survival. The correlation between neutralizing antibodies and mouse survival was compared and recorded.
Statistical analysis
Values are presented as mean with SD. Statistical significance among different immunization groups was calculated by Student's t-test using GraphPad Prism statistical software. P values less than 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by NIH grants (R01AI098775, U01AI124260, R21AI109094, and R21 AI128311) to LD and SJ, the National Program of Infectious Disease Fund of China (2014ZX10004001004) to YZ, and the National Key Research and Development Program of China (2016YFC1201000) to SJ.
References
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814-20; PMID:23075143; https://doi.org/10.1056/NEJMoa1211721; http://www.ncbi.nlm.nih.gov/pubmed/23075143
- Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, Alhakeem R, Durosinloun A, Al Asmari M, Islam A, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis 2013; 19:1819-23; PMID:24206838; https://doi.org/10.3201/eid1911.131172; http://www.ncbi.nlm.nih.gov/pubmed/24206838
- Madani TA, Azhar EI, Hashem AM. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 2014; 371:1360; PMID:25271614; http://www.ncbi.nlm.nih.gov/pubmed/25271614
- Briese T, Mishra N, Jain K, Zalmout IS, Jabado OJ, Karesh WB, Daszak P, Mohammed OB, Alagaili AN, Lipkin WI. Middle East respiratory syndrome coronavirus quasispecies that include homologues of human isolates revealed through whole-genome analysis and virus cultured from dromedary camels in Saudi Arabia. Mbio 2014; 5:e01146-14; PMID:24781747; https://doi.org/10.1128/mBio.01146-14
- Azhar EI, Hashem AM, El-Kafrawy SA, Sohrab SS, Aburizaiza AS, Farraj SA, Hassan AM, Al-Saeed MS, Jamjoom GA, Madani TA. Detection of the Middle East respiratory syndrome coronavirus genome in an air sample originating from a camel barn owned by an infected patient. Mbio 2014; 5:e01450-14; PMID:25053787; https://doi.org/10.1128/mBio.01450-14; http://www.ncbi.nlm.nih.gov/pubmed/25053787
- Yang Y, Du L, Liu C, Wang L, Ma C, Tang J, Baric RS, Jiang S, Li F. Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc Natl Acad Sci U S A 2014; 111:12516-21; PMID:25114257; https://doi.org/10.1073/pnas.1405889111
- Wang Q, Qi J, Yuan Y, Xuan Y, Han P, Wan Y, Ji W, Li Y, Wu Y, Wang J, et al. Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26. Cell Host Microbe 2014; 16:328-37; PMID:25211075; https://doi.org/10.1016/j.chom.2014.08.009; http://www.ncbi.nlm.nih.gov/pubmed/25211075
- Omrani AS, Matin MA, Haddad Q, Al-Nakhli D, Memish ZA, Albarrak AM. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis 2013; 17:e668-72; PMID:23916548; https://doi.org/10.1016/j.ijid.2013.07.001; http://www.ncbi.nlm.nih.gov/pubmed/23916548
- Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med 2013; 369:407-16; PMID:23782161; https://doi.org/10.1056/NEJMoa1306742; http://www.ncbi.nlm.nih.gov/pubmed/23782161
- Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med 2013; 368:2487-94; PMID:23718156; https://doi.org/10.1056/NEJMoa1303729; http://www.ncbi.nlm.nih.gov/pubmed/23718156
- Abroug F, Slim A, Ouanes-Besbes L, Hadj Kacem MA, Dachraoui F, Ouanes I, Lu X, Tao Y, Paden C, Caidi H, et al. Family cluster of Middle East respiratory syndrome coronavirus infections, Tunisia. Emerg Infect Dis 2014; 20:1527-30; PMID:25148113; https://doi.org/10.3201/eid2009.140378; http://www.ncbi.nlm.nih.gov/pubmed/25148113
- Park SH, Kim YS, Jung Y, Choi SY, Cho NH, Jeong HW, Heo JY, Yoon JH, Lee J, Cheon S, et al. Outbreaks of Middle East respiratory syndrome in two hospitals initiated by a single patient in daejeon, South Korea. Infect Chemother 2016; 48:99-107; PMID:27433380; https://doi.org/10.3947/ic.2016.48.2.99; http://www.ncbi.nlm.nih.gov/pubmed/27433380
- Gao J, Lu G, Qi J, Li Y, Wu Y, Deng Y, Geng H, Li H, Wang Q, Xiao H, et al. Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the S protein of Middle East respiratory syndrome coronavirus. J Virol 2013; 87:13134-40; PMID:24067982; https://doi.org/10.1128/JVI.02433-13; http://www.ncbi.nlm.nih.gov/pubmed/24067982
- Lu L, Liu Q, Zhu Y, Chan KH, Qin L, Li Y, Wang Q, Chan JF, Du L, Yu F, et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun 2014; 5:3067; PMID:24473083; http://www.ncbi.nlm.nih.gov/pubmed/24473083
- Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol 2015; 89:1954-64; PMID:25428871; https://doi.org/10.1128/JVI.02615-14
- Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y, Zhang Y, Zhang W, Yuan Y, Bao J, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 2013; 500:227-31; PMID:23831647; https://doi.org/10.1038/nature12328
- Wang N, Shi X, Jiang L, Zhang S, Wang D, Tong P, Guo D, Fu L, Cui Y, Liu X, et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res 2013; 23:986-93; PMID:23835475; https://doi.org/10.1038/cr.2013.92; http://www.ncbi.nlm.nih.gov/pubmed/23835475
- Du L, Kou Z, Ma C, Tao X, Wang L, Zhao G, Chen Y, Yu F, Tseng CT, Zhou Y, et al. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PloS One 2013; 8:e81587; PMID:24324708; https://doi.org/10.1371/journal.pone.0081587; http://www.ncbi.nlm.nih.gov/pubmed/24324708
- Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013; 495:251-4; PMID:23486063; https://doi.org/10.1038/nature12005
- Ma C, Li Y, Wang L, Zhao G, Tao X, Tseng CT, Zhou Y, Du L, Jiang S. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines. Vaccine 2014; 32:2100-8; PMID:24560617; https://doi.org/10.1016/j.vaccine.2014.02.004
- Zhang N, Jiang S, Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev Vaccines 2014; 13:761-74; PMID:24766432; https://doi.org/10.1586/14760584.2014.912134; http://www.ncbi.nlm.nih.gov/pubmed/24766432
- Tang J, Zhang N, Tao X, Zhao G, Guo Y, Tseng CT, Jiang S, Du L, Zhou Y. Optimization of antigen dose for a receptor-binding domain-based subunit vaccine against MERS coronavirus. Hum Vaccin Immunother 2015; 11:1244-50; PMID:25874632; https://doi.org/10.1080/21645515.2015.1021527; http://www.ncbi.nlm.nih.gov/pubmed/25874632
- Mou H, Raj VS, van Kuppeveld FJ, Rottier PJ, Haagmans BL, Bosch BJ. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol 2013; 87:9379-83; PMID:23785207; https://doi.org/10.1128/JVI.01277-13
- Ma C, Wang L, Tao X, Zhang N, Yang Y, Tseng CT, Li F, Zhou Y, Jiang S, Du L. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments–the importance of immunofocusing in subunit vaccine design. Vaccine 2014; 32:6170-6; PMID:25240756; https://doi.org/10.1016/j.vaccine.2014.08.086
- Zhang N, Channappanavar R, Ma C, Wang L, Tang J, Garron T, Tao X, Tasneem S, Lu L, Tseng CT, et al. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol Immunol 2016; 13:180-90; PMID:25640653; https://doi.org/10.1038/cmi.2015.03; http://www.ncbi.nlm.nih.gov/pubmed/25640653
- Tai W, Wang Y, Fett CA, Zhao G, Li F, Perlman S, Jiang S, Zhou Y, L D. Recombinant receptor-binding domains of multiple MERS-coronaviruses induce cross-neutralizing antibodies against divergent human and camel MERS-coronaviruses and antibody-escape mutants. J Virol 2016; 91:pii: e01651-16; https://doi.org/10.1128/JVI.01651-16
- Tai W, Zhao G, Sun S, Guo Y, Wang Y, Tao X, Tseng CK, Li F, Jiang S, Du L, et al. A recombinant receptor-binding domain of MERS-CoV in trimeric form protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. Virology 2016; 499:375-82; PMID:27750111; https://doi.org/10.1016/j.virol.2016.10.005
- Zhao G, Jiang Y, Qiu H, Gao T, Zeng Y, Guo Y, Yu H, Li J, Kou Z, Du L, et al. Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with Middle East respiratory syndrome-coronavirus. PloS One 2015; 10:e0145561; PMID:26701103; https://doi.org/10.1371/journal.pone.0145561
- Tao X, Garron T, Agrawal AS, Algaissi A, Peng BH, Wakamiya M, Chan TS, Lu L, Du L, Jiang S, et al. Characterization and demonstration of the value of a lethal mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol 2015; 90:57-67; PMID:26446606; https://doi.org/10.1128/JVI.02009-15
- Du L, Tai W, Yang Y, Zhao G, Zhu Q, Sun S, Liu C, Tao X, Tseng CK, Perlman S, et al. Introduction of neutralizing immunogenicity index to the rational design of MERS coronavirus subunit vaccines. Nat Commun 2016; 7:13473
- Levin Y, Kochba E, Shukarev G, Rusch S, Herrera-Taracena G, van Damme P. A phase 1, open-label, randomized study to compare the immunogenicity and safety of different administration routes and doses of virosomal influenza vaccine in elderly. Vaccine 2016; 34:5262-72; PMID:27667332; https://doi.org/10.1016/j.vaccine.2016.09.008
- Gasparini R, Schioppa F, Lattanzi M, Barone M, Casula D, Pellegrini M, Veitch K, Gaitatzis N. Impact of prior or concomitant seasonal influenza vaccination on MF59-adjuvanted H1N1v vaccine (Focetria) in adult and elderly subjects. Int J Clin Pract 2010; 64:432-8; PMID:20039974; https://doi.org/10.1111/j.1742-1241.2009.02309.x
- Black S. Safety and effectiveness of MF-59 adjuvanted influenza vaccines in children and adults. Vaccine 2015; 8:062
- Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw 2015; 15:51-7; PMID:25922593; https://doi.org/10.4110/in.2015.15.2.51
- O'Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of MF59(R) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines 2013; 12:13-30; PMID:23256736; https://doi.org/10.1586/erv.12.140
- Fouda GG, Cunningham CK, McFarland EJ, Borkowsky W, Muresan P, Pollara J, Song LY, Liebl BE, Whitaker K, Shen X, et al. Infant HIV type 1 gp120 vaccination elicits robust and durable anti-V1V2 immunoglobulin G responses and only rare envelope-specific immunoglobulin A responses. J Infect Dis 2015; 211:508-17; PMID:25170104; https://doi.org/10.1093/infdis/jiu444
- Riley S, Wu JT, Leung GM. Optimizing the dose of pre-pandemic influenza vaccines to reduce the infection attack rate. PLoS Med 2007; 4:e218; PMID:17579511; https://doi.org/10.1371/journal.pmed.0040218; http://www.ncbi.nlm.nih.gov/pubmed/17579511
- Zhao G, Du L, Ma C, Li Y, Li L, Poon VK, Wang L, Yu F, Zheng BJ, Jiang S, et al. A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV. Virol J 2013; 10:266; PMID:23978242; https://doi.org/10.1186/1743-422X-10-266; http://www.ncbi.nlm.nih.gov/pubmed/23978242
- Du L, He Y, Wang Y, Zhang H, Ma S, Wong CK, Wu SH, Ng F, Huang JD, Yuen KY, et al. Recombinant adeno-associated virus expressing the receptor-binding domain of severe acute respiratory syndrome coronavirus S protein elicits neutralizing antibodies: Implication for developing SARS vaccines. Virology 2006; 353:6-16; PMID:16793110; https://doi.org/10.1016/j.virol.2006.03.049; http://www.ncbi.nlm.nih.gov/pubmed/16793110
- Cao Z, Liu L, Du L, Zhang C, Jiang S, Li T, He Y. Potent and persistent antibody responses against the receptor-binding domain of SARS-CoV spike protein in recovered patients. Virol J 2010; 7:299; PMID:21047436; https://doi.org/10.1186/1743-422X-7-299; http://www.ncbi.nlm.nih.gov/pubmed/21047436
- Sui J, Deming M, Rockx B, Liddington RC, Zhu QK, Baric RS, Marasco WA. Effects of human anti-spike protein receptor binding domain antibodies on severe acute respiratory syndrome coronavirus neutralization escape and fitness. J Virol 2014; 88:13769-80; PMID:25231316; https://doi.org/10.1128/JVI.02232-14; http://www.ncbi.nlm.nih.gov/pubmed/25231316
- Levine L, Edsall G. Tetanus toxoid: what determines reaction proneness? J Infect Dis 1981; 144:376; PMID:7288216; https://doi.org/10.1093/infdis/144.4.376
- Edsall G, Elliott MW, Peebles TC, Eldred MC. Excessive use of tetanus toxoid boosters. JAMA 1967; 202:111-3; PMID:6071998; https://doi.org/10.1001/jama.202.1.111
- Visciano ML, Tagliamonte M, Tornesello ML, Buonaguro FM, Buonaguro L. Effects of adjuvants on IgG subclasses elicited by virus-like particles. J Transl Med 2012; 10:1479-5876; https://doi.org/10.1186/1479-5876-10-4
- Schulte S, Sukhova GK, Libby P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am J Pathol 2008; 172:1500-8; PMID:18467709; https://doi.org/10.2353/ajpath.2008.070776
- Crank MC, Gordon IJ, Yamshchikov GV, Sitar S, Hu Z, Enama ME, Holman LA, Bailer RT, Pearce MB, Koup RA, et al. Phase 1 study of pandemic H1 DNA vaccine in healthy adults. PloS One 2015; 10:e0123969; PMID:25884189; https://doi.org/10.1371/journal.pone.0123969; http://www.ncbi.nlm.nih.gov/pubmed/25884189
- Meyer RG, Britten CM, Siepmann U, Petzold B, Sagban TA, Lehr HA, Weigle B, Schmitz M, Mateo L, Schmidt B, et al. A phase I vaccination study with tyrosinase in patients with stage II melanoma using recombinant modified vaccinia virus Ankara (MVA-hTyr). Cancer Immunol Immunother 2005; 54:453-67; PMID:15627214; https://doi.org/10.1007/s00262-004-0616-7
- Haagmans BL, van den Brand JM, Raj VS, Volz A, Wohlsein P, Smits SL, Schipper D, Bestebroer TM, Okba N, Fux R, et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science 2016; 351:77-81; PMID:26678878; https://doi.org/10.1126/science.aad1283
- Du L, Zhao G, He Y, Guo Y, Zheng BJ, Jiang S, Zhou Y. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine 2007; 25:2832-8; PMID:17092615; https://doi.org/10.1016/j.vaccine.2006.10.031
- Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol 2009; 7:226-36; PMID:19198616; https://doi.org/10.1038/nrmicro2090
- Du L, Zhao G, Sun S, Zhang X, Zhou X, Guo Y, Li Y, Zhou Y, Jiang S. A critical HA1 neutralizing domain of H5N1 influenza in an optimal conformation induces strong cross-protection. PloS One 2013; 8:e53568; PMID:23320093; https://doi.org/10.1371/journal.pone.0053568; http://www.ncbi.nlm.nih.gov/pubmed/23320093
- Du L, Zhao G, Zhang X, Liu Z, Yu H, Zheng BJ, Zhou Y, Jiang S. Development of a safe and convenient neutralization assay for rapid screening of influenza HA-specific neutralizing monoclonal antibodies. Biochem Biophys Res Commun 2010; 397:580-5; PMID:20617558; https://doi.org/10.1016/j.bbrc.2010.05.161; http://www.ncbi.nlm.nih.gov/pubmed/20617558
- Liu C, Tang J, Ma Y, Liang X, Yang Y, Peng G, Qi Q, Jiang S, Li J, Du L, et al. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J Virol 2015; 89:6121-5; PMID:25787280; https://doi.org/10.1128/JVI.00430-15
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58:621-81; PMID:16968952; https://doi.org/10.1124/pr.58.3.10; http://www.ncbi.nlm.nih.gov/pubmed/16968952
