ABSTRACT
Shortages of vaccines such as inactivated poliovirus and yellow fever vaccines have been addressed by administering reduced—or fractional—doses, as recommended by the World Health Organization Strategic Advisory Group of Experts on Immunization, to expand population coverage in countries at risk. We evaluated 3 kinds of vaccine vial stoppers to assess their performance after increased piercing from repeated withdrawal of doses needed when using fractional doses (0.1 mL) from presentations intended for full-dose (0.5 mL) delivery. Self-sealing capacity and fragmentation of the stopper were assessed via modified versions of international standard protocols. All stoppers maintained self-sealing capacity after 100 punctures. The damage to stoppers measured as the fragmentation rate was within the target of ≤ 10% of punctures resulting in a fragment after as many as 50 punctures. We concluded that stopper failure is not likely to be a concern if existing vaccine vials containing up to 10 regular doses are used up to 50 times for fractional dose delivery.
Recently, due to increasing demand for inactivated poliovirus vaccine (IPV) and yellow fever vaccine (YFV) for routine immunization and outbreak response, limited availability of these important vaccines has resulted in a shortage of both products in many countries. On the basis of clinical data, the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization has recommended use of fractional doses of IPV (fIPV; one-fifth of dose delivered intradermally) and YFV (fYFV; one-fifth of dose delivered subcutaneously) to permit introduction and higher coverage for areas in need.Citation1,2 A full dose of each vaccine is 0.5 mL, and the recommended fractional dose is 0.1 mL, which increases the potential number of doses that can be obtained from existing 5- or 10-dose vials to approximately 25 or 50 doses. In 2016, fYFV was used in outbreak response campaigns in the Democratic Republic of Congo, and fIPV was implemented in India, Sri Lanka, and Pakistan and is planned for use in Bangladesh. It is likely that more countries will adopt fractional dosing, because vaccine supplies remain limited.Citation3,4Citation,5
Administering fractional doses increases the number of piercings of IPV and YFV vial stoppers compared with what they were validated for, and this off-label practice could reduce their performance. Possible stopper failures include loss of self-sealing capacity, increasing the risk of contamination or leakage, and fragmentation of the stopper material, increasing the chance of syringe blockage or injection of particles that could result in adverse events following immunization. Harmonized standards for vial stoppers from the United States Pharmacopeia (USP), European Pharmacopoeia, and International Organization for Standardization (ISO) require testing only up to 10 punctures per stopper.Citation6,Citation7,Citation8 Review of the literature found that stopper performance varies by material and needle gauge, and that stoppers used for radiopharmaceuticals maintain performance to at least 100 punctures.Citation9,10 However, no information was available on high-frequency piercing of vaccine vial stoppers or for stopper puncture with the type of needle used for fIPV and fYFV delivery (27 gauge [G], ½ inch). Therefore, we evaluated IPV and YFV stoppers using USP <381> procedures modified to simulate fractional dose vaccine administration.Citation6
Three stoppers (#1, #2, and #3) used in WHO-prequalified IPV and YFV were evaluated: (#1) a 13 mm bromobutyl stopper (Dätwyler, V9024 FM457/0 ISAF1 015 GREY); (#2) a 20 mm chlorobutyl stopper (Aptar Stelmi, C 5324 6320 GS 6 AP2); and (#3) a 20 mm bromobutyl stopper (West Pharmaceutical Services, 1018 PH 4001/45/GREY DB). Stoppers were prepared before testing as in the USP <381> protocol by boiling in deionized, filtered (DF) water for 5 minutes, rinsing in DF water, autoclaving at 121°C for 30 minutes, and air drying.
To evaluate self-sealing after piercing, 10 glass vials were prepared for each stopper type by filling with DF water, inserting a prepared stopper, and crimping an aluminum seal in place with manual crimpers (Chemglass, CV-5700–0013/CG-4930–20). To verify the initial seal, filled vials were submerged upright in a 0.1% methylene blue solution (Alfa Aesar, A18174). The dish with the vials and solution was placed in a vacuum chamber equipped with a mechanical vacuum gauge and the pressure lowered to 27 kPa less than ambient. Vials were exposed to the reduced pressure for 10 minutes, then the pressure was raised to ambient and held for 30 minutes. Vials were then removed from the vacuum chamber, rinsed with tap water, and dried. After drying, vials were visually inspected for the presence of dye in the vial, which would signify a compromised seal. After verification of the initial seal, each stopper was pierced 10 times using a 1 mL Luer-Lok™ syringe (BD, 309628) with a new 27G, ½ inch needle (BD, 305109) attached for each piercing. After 10 punctures, the vials were checked for self-sealing using the same vacuum procedure used for the initial seal verification. Any vials containing blue dye were to be removed from the test. The process of piercing each stopper 10 times and checking the self-sealing was repeated until each stopper had been punctured 100 times or had failed. Experimenters attempted to pierce a new location in the stopper each time, but after many piercings this became unfeasible.
A modified version of the USP <381> protocol was developed to assess the fragmentation rate after piercing. The USP test requires piercing 12 stoppers 4 times each, and a maximum of 5 fragments for all 12 stoppers combined is permitted to pass the test. This is equivalent to a fragmentation rate of just greater than 10% of punctures resulting in a fragment (5 fragments per 48 total piercings), and we used this rate as our benchmark when increasing the number of punctures per stopper. The bottom of each vial was ground off and replaced with a polypropylene receptacle held in place with a rubber ring to permit testing of vial contents at intervals without removing stoppers. All components of the vial assembly were washed and rinsed with DF water to remove particles, and the rinsate was checked to confirm absence of particulate contamination. For each of the 3 stopper types, 12 vial assemblies were prepared for piercing with new 27G, ½ inch needles (BD, 305109) attached to 20 mL syringes (BD, 302830). DF water (1 mL) was injected into the vial assembly with each piercing to flush any stopper fragments or cores from the needle lumen.
After each stopper had been pierced 10 times, the contents of vial assemblies for all 12 stoppers of each type were emptied into the funnel of a glass filtration apparatus (EMD Millipore, XX1004700) and rinsed with DF water. Fragments were collected by filtering the vial contents and rinsate through a 0.45 µm nylon, hydrophilic, 47 mm diameter filter (Pall, 66608). The filters were then examined for particulates via naked eye by 2 technicians in accordance with USP <381>. Any particle visible to the naked eye (> 50 µm in diameter) on the filter was reviewed under an optical microscope (10X to 63X total magnification) to determine whether it was a stopper fragment or other type of particle based on shape, color, transparency, and texture. We repeated the process of piercing, filtering, and counting particles until each individual stopper had been pierced 80 times or until the 10% fragmentation rate was exceeded. For calculation of 95% confidence intervals, the fragmentation rate was assumed to follow a Poisson distribution. A Poisson distribution applies if the probability of a fragment is independent of the number of punctures, and may become less applicable after many punctures.
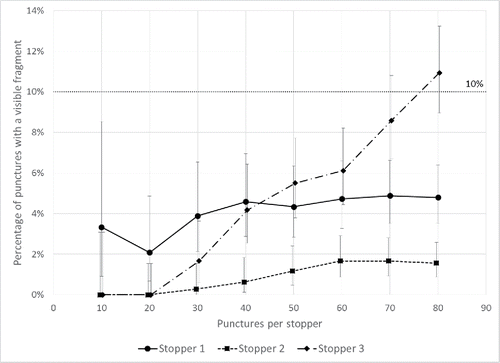
The self-sealing test found no dye ingress in any vials after 10 stoppers of types #1, #2, and #3 were pierced up to 100 times each, indicating that self-sealing capacity would be maintained well beyond the anticipated number of piercings if fractional dosing were used. The fragmentation test found that the fragmentation rate increased with the number of piercings but remained at less than 10% for the anticipated number of punctures for drawing fractional doses from existing 5- and 10-dose vaccine vials (). After 30 punctures, the fragmentation rates were 3.9%, 0.3%, and 1.7% for stoppers #1, #2, and #3, respectively; after 50 punctures, the rates were 4.3%, 1.2%, and 5.5%, respectively. After 80 punctures per stopper, the fragmentation rates for stoppers #1 and #2 remained at less than 10% (4.8% and 1.9%), but the rate for stopper #3 was 10.9%.
Figure 1. Fragmentation rate as a result of damage to vaccine vial stoppers following piercing up to 80 times with new 27 gauge, ½ inch needles in comparison with the calculated USP <381> fragmentation rate of approximately 10%. Contents of 12 vials for each of 3 stopper types were combined, filtered, and examined for fragments after each set of 10 punctures. Error bars denote 95% confidence intervals for the cumulative fragmentation rate, calculated with the assumption that stopper fragmentation follows a Poisson distribution.
We conclude that the evaluated IPV and YFV stoppers perform in compliance with international harmonized standards, maintaining a seal when punctured up to 100 times with new 27G needles, and resisting fragmentation when punctured up to at least 50 times. The 27G, ½ inch needles used for fIPV and fYFV injections are smaller gauge than needles specified for USP- and ISO-required testing (21G, no length specification), which likely contributes to the favorable performance of the stoppers, but the 27G, ½ inch needles were used here to simulate immunization program practices. The clinical risk of stopper fragmentation is unknown. A limitation of this analysis is translation of the maximum number of particles specified in the USP into a target fragmentation rate, which may not be the most relevant indicator of risk in a higher-frequency use setting. Alternative stoppers designed and validated specifically for high-frequency piercing applications are available from manufacturers, but may not be necessary for vials used for fIPV and fYFV delivery, as even a 10-dose vial—the largest that is likely to be used for fractional dosing—would require a maximum of approximately 50 punctures to administer fractional doses of the contents. Our results support the use of existing IPV and YFV vials for fractional dose delivery.
This work was funded by a grant from the Bill & Melinda Gates Foundation. The views expressed herein are solely those of the authors and do not necessarily reflect the views of the Foundation. The authors would like to thank Jacqueline Fournier-Caruana and Alejandro Javier Costa of WHO for their input and assistance; as well as representatives of Dätwyler, Aptar Stelmi, and West Pharmaceutical Services. The authors also thank Jessica Smith for assistance with the testing.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2016 – conclusions and recommendations. Wkly Epidemiol Rec 2016; 91(48):561–82; PMID:27922031
- World Health Organization, Department of Immunization, Vaccines and Biologicals. Fractional dose yellow fever vaccine as a dose-sparing option for outbreak response. WHO Secretariat information paper. Geneva: World Health Organization; 2016
- World Health Organization. Winning the war on yellow fever. Geneva: World Health Organization; 2016 Nov 25 [accessed 2016 Nov 29]. http://www.who.int/features/2016/winning-the-war-against-yellow-fever/en/
- World Health Organization. 15th WHO/UNICEF consultation with OPV & IPV manufacturers and national regulatory authorities. Note for the record. Geneva: World Health Organization; 2016 Jul 28 [accessed 2016 Nov 29]. http://www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/oral_polio_vaccine/WHO_UNICEF_consultation_28July2016_Note_for_the_record.pdf?ua = 1
- Bahl S, Verma H, Bhatnagar P, Haldar P, Satapathy A, Kumar KN, Horton J, Estivariz CF, Anand A, Sutter R. Fractional-dose inactivated poliovirus vaccine immunization campaign - Telangana State, India, June 2016. MMWR Morb Mortal Wkly Rep 2016; 65(33):85963; PMID:27559683; https://doi.org/10.15585/mmwr.mm6533a5
- The United States Pharmacopeial Convention. <381>Elastomeric Closures for Injections. United States Pharmacopeia; Revision Bulletin 2009 May 1 [accessed 2016 Nov 29]. http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/generalChapter381.pdf
- European Pharmacopoeia. 3.2.9 Rubber closures for containers for aqueous parenteral preparations, for powders and for freeze-dried powders. In: European Pharmacopoeia 5.0. European Pharmacopoeia; 2005 [accessed 2016 Nov 29]. http://library.njucm.edu.cn/yaodian/ep/EP5.0/03_materials_and_containers/3.2.__containers/3.2.9.%20Rubber%20closures%20for%20containers%20for%20aqueous%20parenteral%20preparations,%20for%20powders%20and%20for%20freeze-dried%20powders.pdf
- International Organization for Standardization. ISO 8871–5. Elastomeric parts for parenterals and for devices for pharmaceutical use — Part 5: Functional requirements and testing. International Organization for Standardization; 2016 [accessed 2016 Nov 29]. http://www.iso.org/iso/catalogue_detail.htm?csnumber = 68560
- Chanana GD, Guo X, Avis KE, Fleischner AM, Sheth BB. Evaluation of closure integrity after multiple penetrations. J Parenter Sci Technol 1992; 47(1):22–5
- Ponto JA. Self-sealing capacity of vial stoppers after multiple needle punctures. J Am Pharm Assoc 2013; 53(1):58–60; https://doi.org/https://doi.org/10.1331/JAPhA.2013.12064
