ABSTRACT
Malaria is a severe infectious disease with relatively high mortality, thus having been a scourge of humanity. There are a few candidate malaria vaccines that have shown a protective efficacy in humans against malaria. One of the candidate human malaria vaccines, which is based on human malaria sporozoites and called PfSPZ Vaccine, has been shown to protect a significant proportion of vaccine recipients from getting malaria. PfSPZ Vaccine elicits a potent response of hepatic CD8+ T cells that are specific for malaria antigens in non-human primates. To further characterize hepatic CD8+ T cells induced by the sporozoite-based malaria vaccine in a mouse model, we have used a cutting-edge Single-cell Barcode (SCBC) assay, a recently emerged approach/method for investigating the nature of T-cells responses during infection or cancer. Using the SCBC technology, we have identified a population of hepatic CD8+ T cells that are polyfunctional at a single cell level only in a group of vaccinated mice upon malaria challenge. The cytokines/chemokines secreted by these polyfunctional CD8+ T-cell subsets include MIP-1α, RANTES, IFN-γ, and/or IL-17A, which have shown to be associated with protective T-cell responses against certain pathogens. Therefore, a successful induction of such polyfunctional hepatic CD8+ T cells may be a key to the development of effective human malaria vaccine. In addition, the SCBC technology could provide a new level of diagnostic that will allow for a more accurate determination of vaccine efficacy.
Malaria still ranks among the most prevalent infectious disease globally particularly in tropical countries. Approximately 200 million people become infected yearly, with relatively high rates of morbidity and mortality. Severe morbidity and mortality occur particularly in young children living in a malaria endemic area and also in adults who travels to the endemic area without prior exposure to malaria. The WHO estimates that more than a half million children die of malaria every year in Africa alone.Citation1 The widespread occurrence and the increasing incidence of malaria in many countries are caused by drug-resistant parasites (Plasmodium falciparum, recently also P. vivax), insecticide-resistant vectors (Anopheles mosquitoes), and economic/political deterioration in many African countries. These underscore the need for developing new methods for the control of this disease.
The concept that a malaria vaccine is feasible was shown in the late 1960s by the demonstration that the repeated immunization of rodents with irradiated sporozoites (IrSp) led to complete protection against sporozoites challenge.Citation2 This study was followed by a trial, in which human volunteers were immunized with the bite of P. falciparum infected and radiation-attenuated Anopheles mosquitoes.Citation3,4 Vaccination with radiation-attenuated sporozoites (RAS) has been shown to induce complete protection - sterilizing immunity - against malaria infection in experimental animals, as well as in humans,Citation2-5 demonstrating the feasibility of effective vaccination against this disease. In fact, RAS or live non-attenuated sporozoites with chloroquine chemoprophylaxis are the only 2 immunogens that have ever induced high-level (80–90%) and long-lasting (10–25 months) protection against malaria in humans.Citation6,7 Recently, Sanaria Inc. has been able to purify, vial and cryopreserve P. falciparum sporozoites (PfSPZ) from salivary glands of “aseptically-raised” infected Anopheles mosquitoes under cGMPs (Good Manufacturing Practices) for human use. They determined the optimal radiation dose and number of PfSPZ to be given to humans as a vaccine.Citation8 In a recent Phase 1 clinical trial, Sanaria's human RAS vaccine, called PfSPZ Vaccine, was shown to be safeCitation9 and induced sterile protection in all human vaccine recipients.Citation10 Importantly, PfSPZ Vaccine was effective not only against the P. falciparum parasite isolate used for vaccination, but also against parasite isolates from different geographical isolates.Citation11,12
Protection induced by RAS vaccine is multifactorial, that is, mediated by humoral immunity (antibodies) and cell-mediated immunity (T cells). Antibodies neutralize sporozoites before sporozoites enter the hepatocytes, and T cells inhibit the development of parasites within hepatocytes. Although studies in rodents, monkeys and humans demonstrate that antibodies alone may be sufficient to induce protection against malaria, an accumulated number of published evidence underscores the protective role of CD8+ T cells, particularly those reside in the liver, in vaccine-induced immunity against malaria.Citation13-21 It is noteworthy that human CD8+ T cells have been shown to mediate protection against malaria in humanized mice by our most recent study.Citation22 With regards to hepatic CD8+ T cells, it has been observed that mouse RAS vaccine and PfSPZ Vaccine are able to induce a potent hepatic CD8+ T-cell response in mice and monkeys, respectively.Citation9,23,24
Now the important question is what kind of hepatic CD8+ T cells are induced in vaccinated animals that were protected against malaria challenge. Although very high numbers of circulating memory CD8+ T cells are required to maintain protection,Citation25 their effector mechanisms have been shown to depend on the kind of species of Plasmodium used to vaccinate and challenge.Citation26 Murine RAS vaccine has been shown to protect mice from malaria by inducing hepatic CD8+ T cells that secrete IFN- γ9. Furthermore, long-term protection was shown to be induced in mice immunized with mouse RAS vaccine, and the protection correlates with sustained IFN-γ responses of hepatic CD8+ memory T cells.Citation27 However, a few studies have demonstrated that CD8+ T cells of mice receiving other forms of malaria vaccine, namely recombinant viruses expressing a malaria antigen, mediated protective anti-malaria immunity, but independently of IFN-γ.Citation28,29 Therefore, at present there is still no definitive answer regarding whether IFN-γ plays a vital role in mediating anti-liver stages effects of CD8+ T cells. More recently, distinct phenotypic differences have been identified between splenic and liver T cell populations after mouse RAS vaccination, suggesting that liver T cells expressed a unique transcriptional profile.Citation23 A most recent study has highlighted the protective role of liver tissue-resident memory T cells (Trm) induced by a mouse RAS vaccine in mice.Citation24
In view of the imprecise protective role of IFN-γ secreted by hepatic CD8+ T cells, the assessment of polyfunctional T cells, co-secreting multiple cytokines/chemokines by single cells, is emerged as a crucial approach to investigate the nature of T-cell responses against infection or cancer.Citation30-32 Currently, the major commercial technologies for evaluating single T-cell cytokines include flow cytometric analysis, mass cytometric analysis, and ELISpot assay.Citation33-41 To date flow cytometric and mass cytometric analyses have achieved the greatest level of multiplexing of surface markers simultaneously from a single cell, effectively phenotyping the array of patient's cells. However, the number of cytokines that can be simultaneously measured is limited to ∼5 in flow cytometric analysis due to spectral overlap. Mass cytometric analysis, which allows for simultaneous detection of up to 42 parameters per cell, may not be suitable for the measurement of the number of the cells releasing cytokines/chemokines, because of the use of intra-cellular staining that potentially causes non-specific binding. Alternatively, ELISpot assay measures true cytokine secretion by a single T cell, but does not identify T-cell polyfunctionality due to limitations on multiplexing (< 4), which is associated with the functional performance.Citation41
In our current study, we used IsoPlexis' IsoCode single cell technology: the Single Cell Barcode Chip (SCBC). The SCBC platform has the advantage of a high multiplexing capacity with the ability to detect 40+ secreted cytokines/chemokines simultaneously, per single cell, across thousands of patient-derived cells. At the same time, the SCBC is unable to detect a high number of surface markers (∼4 markers maximum), and cannot identify very rare subsets (less than 1 in 1,000 cells) compared with flow cytometric and mass cytometric analyses. This may circumvented by using these cytometric analyses or bead based sorting platforms upstream of the SCBC. Thus, the SCBC has specific value in highly multiplexed functional definition of specific phenotypes of enriched, functionally heterogeneous cell types, such as CD8+ or CD4+ T cells. This uniquely high function multiplexing ability has allowed the SCBC to identify both cellular heterogeneity and novel polyfunctional cellular sub-populations in various cancer immunotherapies.Citation42,43 We used this value in immune functional profiling here to identify hepatic CD8+ T-cell polyfunctionality induced by PfSPZ Vaccine and the potential biomarkers for vaccine efficacy evaluation.
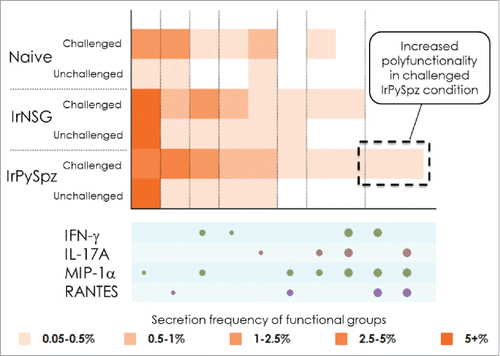
As we and others have previously shown, a single immunization with mouse RAS vaccine, i.e. irradiated P. yoelii sporozoites (IrPySpz), conferred a partial protection to mice against live PySpz challenge.Citation44-47 In this study, we immunized mice intravenously with a single dose of 1 × 105 IrPySpz, followed by challenge with 50 viable PySpz 2 weeks later, and 3 out of 8 immunized mice were protected against PySpz challenge (). Therefore, we sought to analyze hepatic CD8+ T cells from IrPySpz-immunized mice by the IsoPlexis' SCBC to determine if distinct markers of polyfunctional response, which may correlate with anti-malaria protection, can be identified. We compared the hepatic CD8+ T-cell responses of naïve mice with those of IrPySpz-immunized mice or with those of mice received irradiated non-infected mosquitoes' salivary gland extracts (IrNSG). We also compared the responses in IrPySpz-immunized, IrNSG-immunized or naïve mice that were exposed or not to challenge with 1 × 104 live PySpz (). Hepatic CD8+ T cells were isolated as described previously.Citation48 The cells were then stimulated with immobilized anti-CD3 antibody and soluble anti-CD28 antibody, and loaded onto the SCBC chips for single-cell polyfunctional profiling on a 32 plex SCBC panel. As shown in , a fewer percentage of hepatic CD8+ T cells with high polyfunctionality was induced in either naïve mice or IrNSG-immunized mice (which did not confer protection). In contrast, the SCBC identified distinct signatures of polyfunctional response in IrPySpz-immunized, live PySpz-challenged mice, which resulted in conferring a partial protection. The multiple markers, which were unique to hepatic CD8+ T cells derived from IrSpz-immunized and partially protected mice, are identified as a co-secretion of MIP-1α, RANTES, IFN-γ, and/or IL-17A, from a single hepatic CD8+ T cell. These results demonstrate that a group of polyfunctional hepatic CD8+ T cells, having both effector and chemo-attractive functions at a single cell level, may associate with anti-malaria immunity.
Table 1. Anti-plasmodial Protection Induced by IrPySpz immunization.
Figure 1. SCBC single-cell heat map shows unique and highly polyfunctional hepatic CD8+ T-cell subsets with the dominant profile of MIP-1α, RANTES, IFN-γ, and/or IL-17A, which were induced in a group of mice that received IrPySpz immunization and live PySpz challenge.
The role of CD8+ T cells and its mechanism of action are not yet clear in the field of malaria vaccine research. One of the leading candidate vaccines, PfSPZ Vaccine, elicits a potent hepatic CD8+ T cells against malaria in non-human primates. Other studies have also emphasized the role of hepatic CD8+ T cells induced by the sporozoite-based malaria vaccine in a mouse model, although there are still no definitive characteristics of the hepatic CD8+ T cells, which are associated with protective anti-malaria immunity. In this regard, a successful induction of certain polyfunctional hepatic CD8+ T cells could lead to the development of an effective malaria vaccine in humans. The identification of single cell polyfunctional biomarkers by the SCBC technology in the context of an experimental malaria vaccine study setting, may prove useful in designing more effective T cell-based human malaria vaccine in the future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- World Health Organization (WHO): World Malaria Report 2014. http://www.who.int/malaria/publications/world_malaria_report_2014/en/
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature 1967; 216:160-2; PMID:6057225
- Clyde DF, McCarthy VC, Miller RM, Hornick RB. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am J Med Sci 1973; 266:398-403; PMID:4590095
- Herrington D, Davis J, Nardin E, Beier M, Cortese J, Eddy H, Losonsky G, Hollingdale M, Sztein M, Levine M, et al. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg 1991; 45:539-47; PMID:1951863
- Gwadz RW, Cochrane AH, Nussenzweig V, Nussenzweig RS. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull World Health Organ 1979; 57(Suppl 1):165-73; PMID:120766
- Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 2002; 185:1155-64; PMID:11930326
- Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 2009; 361:468-77; PMID:19641203; https://doi.org/10.1056/NEJMoa0805832
- Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, Chakravarty S, Gunasekera A, Chattopadhyay R, Li M, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin 2010; 6:97-106; PMID:19946222
- Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8 T cell immunity. Science 2011; 334:475-80; PMID:21903775; https://doi.org/10.1126/science.1211548
- Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013; 341:1359-65; PMID:23929949; https://doi.org/10.1126/science.1241800
- Epstein JE, Paolino KM, Richie TL, Sedegah M, Singer A, Ruben AJ, Chakravarty S, Stafford A, Ruck RC, Eappen AG, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2017; 2:e89154; PMID:28097230; https://doi.org/10.1172/jci.insight.89154
- Lyke KE, Ishizuka AS, Berry AA, Chakravarty S, DeZure A, Enama ME, James ER, Billingsley PF, Gunasekera A, Manoj A, et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A 2017; 114(10):2711-2716; pii: 201615324; https://doi.org/10.1073/pnas.1615324114
- Rénia L, Rodrigues MM, Nussenzweig V. Intrasplenic immunization with infected hepatocytes: a mouse model for studying protective immunity against malaria pre-erythrocytic stage. Immunology 1994; 82:164-8; PMID:7913914
- Doolan DL, Sedegah M, Hedstrom RC, Hobart P, Charoenvit Y, Hoffman SL. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med 1996; 183:1739-46; PMID:8666931
- White KL, Snyder HL, Krzych U. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J Immunol 1996; 156:3374-81; PMID:8617963
- Rodrigues EG, Zavala F, Eichinger D, Wilson JM, Tsuji M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol 1997; 158:1268-74; PMID:9013969
- Miyahira Y, García-Sastre A, Rodriguez D, Rodriguez JR, Murata K, Tsuji M, Palese P, Esteban M, Zavala F, Nussenzweig RS. Recombinant viruses expressing a human malaria antigen can elicit potentially protective immune CD8+ responses in mice. Proc Natl Acad Sci U S A 1998; 95:3954-9; PMID:9520474
- Jobe O, Lumsden J, Mueller AK, Williams J, Silva-Rivera H, Kappe SH, Schwenk RJ, Matuschewski K, Krzych U. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex Class I-dependent interferon-gamma-producing CD8+ T cells. J Infect Dis 2007; 196:599-607; PMID:17624847
- Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med 2007; 13:1035-41; PMID:17704784
- Jobe O, Donofrio G, Sun G, Liepinsh D, Schwenk R, Krzych U. Immunization with radiation-attenuated Plasmodium berghei sporozoites induces liver cCD8alpha+DC that activate CD8+T cells against liver-stage malaria. PLoS One 2009; 4:e5075; PMID:19347042; https://doi.org/10.1371/journal.pone.0005075
- Tsuji M. A retrospective evaluation of the role of T cells in the development of malaria vaccine. Exp Parasitol 2010; 126:421-5; PMID:19944099; https://doi.org/10.1016/j.exppara.2009.11.009
- Li X, Huang J, Zhang M, Funakoshi R, Sheetij D, Spaccapelo R, Crisanti A, Nussenzweig V, Nussenzweig RS, Tsuji M. Human CD8+ T cells mediate protective immunity induced by a human malaria vaccine in human immune system mice. Vaccine 2016; 34:4501-6; PMID:27502569; https://doi.org/10.1016/j.vaccine.2016.08.006
- Tse SW, Cockburn IA, Zhang H, Scott AL, Zavala F. Unique transcriptional profile of liver-resident memory CD8+ T cells induced by immunization with malaria sporozoites. Genes Immun 2013; 14:302-9; PMID:23594961; https://doi.org/10.1038/gene.2013.20
- Fernandez-Ruiz D, Ng WY, Holz LE, Ma JZ, Zaid A, Wong YC, Lau LS, Mollard V, Cozijnsen A, Collins N, et al. Liver-Resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection. Immunity 2016; 4:889-902; PMID:27692609; https://doi.org/10.1016/j.immuni.2016.08.011
- Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A 2008; 105:14017-22; PMID:18780790; https://doi.org/10.1073/pnas.0805452105
- Butler NS, Schmidt NW, Harty JT. Differential effector pathways regulate memory CD8 T cell immunity against Plasmodium berghei versus P. yoelii sporozoites. J Immunol 2010; 184:2528-38; PMID:20097864; https://doi.org/10.4049/jimmunol.0903529
- Nganou-Makamdop K, van Gemert GJ, Arens T, Hermsen CC, Sauerwein RW. Long term protection after immunization with P. berghei sporozoites correlates with sustained IFNγ responses of hepatic CD8+ memory T cells. PLoS One 2012; 7:e36508; PMID:22563506; https://doi.org/10.1371/journal.pone.0036508
- Rodrigues EG, Claassen J, Lee S, Wilson JM, Nussenzweig RS, Tsuji M. Interferon-gamma-independent CD8+ T cell-mediated protective anti-malaria immunity elicited by recombinant adenovirus. Parasite Immunol 2000; 22:157-60; PMID:10672197
- Chakravarty S, Baldeviano GC, Overstreet MG, Zavala F. Effector CD8+ T lymphocytes against liver stages of Plasmodium yoelii do not require gamma interferon for antiparasite activity. Infect Immun 2008; 76:3628-31; PMID:18519561; https://doi.org/10.1128/IAI.00471-08
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 2008; 8:247-58; PMID:18323851; https://doi.org/10.1038/nri2274
- Haining WN. The numerology of T cell functional diversity. Immunity 2012; 36:10-2; PMID:22284416; https://doi.org/10.1016/j.immuni.2012.01.006
- Anastasopoulou EA, Voutsas IF, Papamichail M, Baxevanis CN, Perez SA. MHC class II tetramer analyses in AE37-vaccinated prostate cancer patients reveal vaccine-specific polyfunctional and long-lasting CD4(+) T-cells. Oncoimmunology 2016; 5:e1178439; PMID:27622033; https://doi.org/10.1080/2162402X.2016.1178439
- Prussin C. Cytokine flow cytometry: understanding cytokine biology at the single-cell level. J Clin Immunol 1997; 17:195-204; PMID:9168399
- Freer G. Intracellular staining and detection of cytokines by fluorescence-activated flow cytometry. Methods Mol Biol 2014; 1172:221-34; PMID:24908309; https://doi.org/10.1007/978-1-4939-0928-5_20
- Chen G, Weng NP. Analyzing the phenotypic and functional complexity of lymphocytes using CyTOF (cytometry by time-of-flight). Cell Mol Immunol 2012; 9:322-3; PMID:22635255; https://doi.org/10.1038/cmi.2012.16
- Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med 2013; 5:208ra145; PMID:24154599; https://doi.org/10.1126/scitranslmed.3006702
- Yao Y, Liu R, Shin MS, Trentalange M, Allore H, Nassar A, Kang I, Pober JS, Montgomery RR. CyTOF supports efficient detection of immune cell subsets from small samples. J Immunol Methods 2014; 415:1-5; PMID:25450003; https://doi.org/10.1016/j.jim.2014.10.010
- Fujihashi K, McGhee JR, Beagley KW, McPherson DT, McPherson SA, Huang CM, Kiyono H. Cytokine-specific ELISPOT assay. Single cell analysis of IL-2, IL-4 and IL-6 producing cells. J Immunol Methods 1993; 160:181-9; PMID:8459105
- Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods 1995; 181:45-54; PMID:7537312
- Boulet S, Ndongala ML, Peretz Y, Boisvert MP, Boulassel MR, Tremblay C, Routy JP, Sekaly RP, Bernard NF. A dual color ELISPOT method for the simultaneous detection of IL-2 and IFN-gamma HIV-specific immune responses. J Immunol Methods 2007; 320:18-29; PMID:17222422
- Anthony DD, Milkovich KA, Zhang W, Rodriguez B, Yonkers NL, Tary-Lehmann M, Lehmann PV. Dissecting the T cell response: proliferation assays vs. cytokine signatures by ELISPOT. Cells 2012; 1:127-40; PMID:24710419; https://doi.org/10.3390/cells1020127
- Ma C, Fan R, Ahmad H, Shi Q, Comin-Anduix B, Chodon T, Koya RC, Liu CC, Kwong GA, Radu CG, et al. A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells. Nat Med 2011; 17:738-43; PMID:21602800; https://doi.org/10.1038/nm.2375
- Ma C, Cheung AF, Chodon T, Koya RC, Wu Z, Ng C, Avramis E, Cochran AJ, Witte ON, Baltimore D, et al. Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discov 2013; 3:418-29; PMID:23519018; https://doi.org/10.1158/2159-8290.CD-12-0383
- Pacheco ND, McConnell E, Beaudoin RL. Duration of immunity following a single vaccination with irradiated sporozoites of Plasmodium berghei. Bull World Health Organ 1979; 57(Suppl 1):159-63; PMID:120765
- Renggli J, Hahne M, Matile H, Betschart B, Tschopp J, Corradin G. Elimination of P. berghei liver stages is independent of Fas (CD95/Apo-I) or perforin-mediated cytotoxicity. Parasite Immunol 1997; 19:145-8; PMID:9106820
- Rodrigues EG, Zavala F, Nussenzweig RS, Wilson JM, Tsuji M. Efficient induction of protective anti-malaria immunity by recombinant adenovirus. Vaccine 1998; 16:1812-7; PMID:9795385
- Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med 2002; 195:617-24; PMID:11877484
- Noe AR, Espinosa D, Li X, Coelho-Dos-Reis JG, Funakoshi R, Giardina S, Jin H, Retallack DM, Haverstock R, Allen JR, et al. A full-length Plasmodium falciparum recombinant circumsporozoite protein expressed by Pseudomonas fluorescens platform as a malaria vaccine candidate. PLoS One 2014; 9:e107764; PMID:25247295; https://doi.org/10.1371/journal.pone.0107764
