ABSTRACT
This phase IV, single blind study assessed the immunogenicity and safety of India-manufactured purified chick embryo cell rabies vaccine (PCECV), compared with a German-manufactured batch obtained by the same production process. A total of 340 participants enrolled at 2 study sites in India were randomized (1:1:1:1) in 4 groups to receive a 5-dose Essen regimen with either 1 of the 3 Indian batches (PCECV-I) or the German batch (PCECV-G), administered on Days (D) 0, 3, 7, 14 and 30. The lot-to-lot consistency of PCECV-I batches in terms of induced immune response at D14 was demonstrated. The immune response elicited by PCECV-I was shown to be non-inferior to that induced by PCECV-G, as the lower limit of the 95% confidence interval for the ratio (PCECV-I/PCECV-G) of rabies virus neutralising antibody (RVNA) geometric mean concentrations was higher than 0.5 at D14. At least 96% of participants developed adequate RVNA concentrations (≥ 0.5 IU/mL) by D14 and all achieved RVNA concentrations ≥ 0.5 IU/mL by D90. RVNA levels were comparable across all groups throughout the entire study. Solicited local and general symptoms had a similar incidence in all groups. Unsolicited adverse events (AEs) were reported by 11% of participants. Only 1 serious AE (leg fracture) was reported and was not related to vaccination. No deaths and no rabies cases were recorded during the 90 days of observation. The study showed that the 3 PCECV-I and the PCECV-G batches induced a similar immune response and had a comparable safety profile when administered according to a 5-dose schedule.
Introduction
Rabies is an acute viral disease, caused by viruses belonging to the Lyssavirus genus within the Rhabdoviridae family. In infected humans, the period of incubation is commonly from 2 weeks to 3 months, and the spreading of the virus to the central nervous system eventually leads to progressive fatal encephalomyelitis, followed by cardiorespiratory arrest within a few days.Citation1
Although rabies is almost eliminated in industrialised countries, this disease is still estimated to cause 59,000 to 60,000 deaths each year in endemic regions, especially in Asia and Africa.Citation2 Due to incomplete reporting, lack of access to medical facilities and misdiagnosis, this figure is likely to be an underestimate of the real burden of rabies. Around 40% of people bitten by animals suspected of having rabies are under 15 years of age,Citation3 and most are male and reside in rural communities.Citation2
Post exposure prophylaxis, including vaccination, is highly effective when administered promptly after contact with the suspected rabid animal. In addition, pre-exposure prophylaxis is also available to prime an immune response against rabies and simplify the post-exposure treatment. Cell culture and embryonated egg-based rabies vaccines (CCEVs), which comply with the World Health Organization (WHO) recommended potency of ≥ 2.5 International Units (IU) per intramuscular dose, are currently used worldwide.Citation4 Post-exposure, vaccines are usually administered according to a 5-dose (Essen) or 4-dose (Zagreb) regimen, and together with correct wound treatment and concomitant administration of rabies immunoglobulins (RIG), they lead to the prevention of the disease. Other post-exposure 4-dose regimens, with intramuscular or intradermal administration of the vaccine, have also been approved by WHO.Citation4
The purified chick embryo cell rabies vaccine (PCECV, Rabipur™, GSK Vaccines) was licensed more than 30 years ago and is currently produced in 2 WHO pre-qualified manufacturing facilities: Marburg, Germany and Ankleshwar, India.Citation5 An almost identical production process is used for both vaccines.
The current study investigated the non-inferiority of 3 PCECV lots manufactured in India (PCECV-I) over a lot manufactured in Germany (PCECV-G) and assessed the consistency of the 3 PCECV-I lots. Participants were vaccinated according to the Essen schedule on days (D) 0, 3, 7, 14 and 30. The primary endpoint was the RVNA geometric mean concentrations (GMCs) on day 14 (after 3 doses of PCECV vaccine).The safety and tolerability of the vaccine lots from the 2 manufacturing sites and the immune response induced at D30 (after 4 doses) and D90 (after 5 doses) were also evaluated.
Results
Demographics
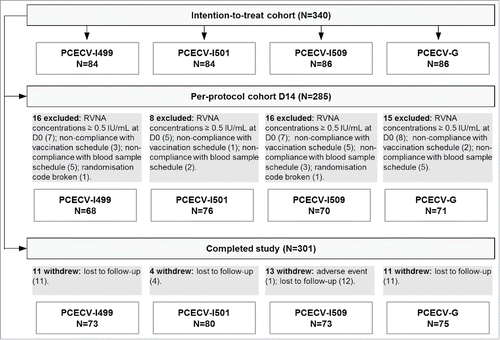
A total of 340 participants were enrolled and 301 completed the study (). In total, 55 individuals were excluded from the per protocol (PP) cohort at D14; the reasons for exclusion for each group are presented in . Minor protocol deviations were reported for 25 other study participants beyond D14 (13 were lost to follow-up and 12 had missing data with respect to vaccine administration or blood sample schedule at D30 or D90), but did not constitute reasons from exclusion from the PP cohort. Out of the 39 individuals withdrawn from the study, 38 were lost to follow-up and 1 withdrew due to an adverse event (AE) which was classified as probably related to vaccination (myalgia) ().
Figure 1. Flow chart for study participants with reasons for exclusion from per-protocol analyses. PCECV, purified chick embryo cell rabies vaccine; N, number of participants in each cohort/group; D, day; RVNA, rabies virus neutralizing antibody; IU, International Units. Batches PCECV-I499, I501 and I509 were manufactured in India, PCEV-G was manufactured in Germany.
The demographic characteristics of the study participants are presented in . Participants were between 9 and 75 years of age, and most of them were male (80%). No statistically significant difference in demographic characteristics was found between vaccinees who received PCECV-I and those receiving PCECV-G. Participants from the 2 trial sites in India differed in terms of prior history of bites from animals with unknown rabies status: 72% of participants vaccinated at trial site 1 and 98% of participants at trial site 2 had been bitten prior to vaccination. One participant in group PCECV-I499 and one in group PCECV-G received equine RIG at the time of the first vaccination, through local infiltration around the wound and injected intramuscularly in the gluteal region.
Table 1. Demographic characteristics for study participants (intention-to-treat cohort).
Immunogenicity
The pooled data for groups receiving PCECV-I was used to demonstrate the primary objective. The calculated GMCPCECV-I/GMCPCECV-G ratio at D14 was 0.83 and the lower limit of the 95% confidence interval (CI) was 0.57, so non-inferiority of PCECV-I over PCECV-G in terms of induced immune response was demonstrated. Lot-to-lot consistency for the 3 Indian-manufactured batches was demonstrated by performing pair-comparisons of D14 antibody GMCs in groups receiving PCECV-I. The 90% CIs for all 3 ratios included 1 and were overlapping, so no evidence suggesting differences among batches was found ().
Table 2. Clinical consistency of the Indian-manufactured PCECV batches (per-protocol cohort).
At D14, 96–99% of the participants in the PCECV-I groups and 99% in the PCECV-G group developed levels of RVNA ≥ 0.5 IU/mL (). Six study participants had lower levels: 3 in the PCECV-I509 group and 1 in each of the other 3 groups. Five of them developed RVNA levels ≥ 0.5 IU/mL by D30, and 1 by D90. One of these individuals (who developed RVNA concentrations of 0.4 IU/mL at D14 and ≥ 0.5 IU/mL by D30) had also received equine RIG concomitantly with the first vaccine dose.
Figure 2. Rabies virus neutralizing antibody geometric mean concentrations for study participants, by timepoint (per-protocol cohort). GMC, geometric mean concentration; IU, international units; PCECV, purified chick embryo cell rabies vaccine. Note: Values above error bars represent percentages of study participants with rabies virus neutralizing antibody concentration ≥ 0.5 IU/mL. Error bars represent 95% confidence intervals. Antibody concentration in all groups were in the 0.025–0.4 (Day 0), 0.025–1510 (Day 14 and Day 30), and 0.025–301 (Day 90) ranges.
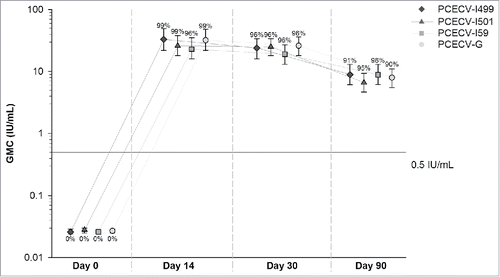
At D90, RVNA levels ≥ 0.5 IU/mL were maintained for at least 91% of participants in each of the PCECV-I groups, and 90% in the PCECV-G group (). All participants included in the PP cohort in all groups had RVNA concentrations≥ 0.5 IU/mL for at least 1 sample across the study period.
RVNA GMCs were comparable between groups at all timepoints. The highest antibody GMC values were observed at D14, and they declined at least 2.5-fold by D90 but were still above ≥ 0.5 IU/mL ().
An analysis of RVNA levels by trial site showed that antibody GMCs at D14 tended to be higher at site 2 compared with site 1 (). Of note, 13 study participants in the PP cohort had detectable RVNA concentrations (although < 0.5 IU/mL) at D0; of these, 12 individuals were enrolled at site 2 (3 in the PCECV-I499 group, 5 in the PCECV-I501 group, and 2 in each of the PCECV-I509 and PCECV-G groups).
Table 3. RVNA GMCs (with 95% CIs and RVNA concentration ranges), by timepoint and by site (per-protocol cohort).
Safety and reactogenicity
Overall, the incidence of solicited local reactions during the 3-day follow-up period post-vaccination was similar in all groups (). The most common local reaction was pain at injection site for both the PCECV-I pooled groups (33% of participants) and the PCECV-G group (33% of participants). Grade 3 local reactions were reported for 3 participants. A 38-year-old woman from the PCECV-I501 group reported an erythema, which occurred 1 day after the second dose and disappeared on the third day without medication. A 30-year-old woman from group PCECV-I501 reported severe pain at the injection site, occurring within 30 minutes from the administration of the second dose. The symptom was resolved by the third day, without medication. A 23-year-old healthy man from the PCECV-I509 group reported severe injection site pain within 6 hours after the second dose, which disappeared after 2 days without medication.
Figure 3. Incidence of local and general solicited symptoms following administration of study vaccine (intention-to-treat cohort). PCECV, purified chick embryo cell rabies vaccine.
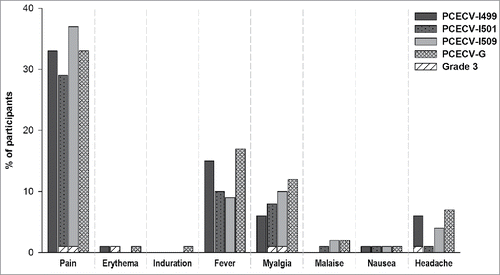
Solicited general symptoms were also reported with a similar occurrence during 3 days post-vaccination in all groups. The most commonly reported general symptom was fever in both the PCECV-G (17% of participants) and pooled PCECV-I groups (11% of participants). Myalgia was reported in 12% of PCECV-G vaccinees vs. 8% of PCECV-I vaccinees. Headache was noted with an incidence of 7% and 4% in the PCECV-G and PCECV-I groups, respectively. Grade 3 general symptoms were reported for 3 participants. One 24-year-old woman from the PCECV-I509 group reported myalgia with an onset of approximately 6 hours after the first dose of PCECV, which became severe on the second day and subsided after 3 days. The participant chose to withdraw from the study. A 30-year-old woman from the PCECV-I501 group reported myalgia within 30 minutes after the second vaccine dose, which disappeared without medication on the fourth day following vaccination. A 13-year-old boy enrolled in the PCECV-I499 group reported severe headache 1 day after the third dose. The symptom was resolved without medication on the third day after vaccination.
A total of 36 vaccinees (11% of study participants) reported unsolicited AEs during the study: 8 (10%) in the PCECV-I499, 7 (8%) in the PCECV-I501, 11 (13%) in the PCECV-I509 and 10 (12%) in the PCECV-G groups, respectively (). In total, 45 AEs were reported. All AEs were resolved by the end of the study, except the only serious AE (SAE) reported (fracture of the right lower leg due to an accident), which was considered unrelated to vaccination. No deaths and no rabies cases were recorded during the study.
Table 4. Number and percentage of participants in each group reporting at least one unsolicited adverse event and serious adverse events from Day 0 to study end (intention-to-treat cohort).
Discussion
In this phase IV study, non-inferiority of Indian-manufactured PCECV commercial batches to a marketed batch from Germany was demonstrated in terms of immune response induced at 14 days after the first dose of a 5-dose Essen vaccination schedule. Furthermore, consistency of the 3 Indian batches was shown. Although the study was conducted more than 15 years ago, full dissemination of the results remains of major importance, seeing that the trial demonstrated equivalence of PCECV batches manufactured in India and Germany, and contributed to the WHO granting of the pre-qualification title to the 2 facilities, in the year 2002.Citation6 Moreover, this is the first study to assess non-inferiority of PCECV batches produced in India to those manufactured in Germany. Our trial predated and had more statistical power than a similar study which demonstrated that PCECV batches from both manufacturing sites had an acceptable safety profile and induced an adequate immune response in a Chinese population, without assessing any non-inferiority endpoints.Citation7
In previous studies, PCECV manufactured at German and Indian facilities have already demonstrated effectiveness against rabies in patients with a confirmed exposure to a rabid animal.Citation4,8,9 The 3 PCECV-I batches assessed in our study were found to be comparable in terms of RVNA levels elicited during the entire study period. For all considered groups, immunogenicity results in terms of percentages of participants with RVNA concentrations ≥ 0.5 IU/ml and total RVNA GMC values were in line with previously reported data.Citation8 In a recent study comparing the Zagreb and Essen schedules of PCECV in India, the RVNA GMCs at D14 were 12 IU/mL for both schedules, which is comparable to the values observed for the PCECV-I and PCECV-G batches in the present study.Citation10
Six slow-responders were identified at D14. The reasons for the delayed acquisition of adequate RVNA levels in this study were not clear in the present study. One of them did not develop RVNA levels ≥ 0.5 IU/mL until D90, while 5 had adequate RVNA concentrations by the next blood draw timepoint at D30. A review of PCECV immunogenicity and safety data covering the last 30 years showed that in almost all clinical trials evaluating the post-exposure Essen schedule in healthy individuals, 100% of participants had RVNA levels ≥ 0.5 IU/mL by D14.Citation8 Recently, 2 studies reported delayed immune responses in populations of healthy Chinese males and females after the first 3 out of 5 doses of purified Vero cell rabies vaccines as post-exposure prophylaxis measure.Citation11,12 In previous studies, immunogenicity of PCECV-I has been shown to be impacted in immunocompromised individuals,Citation13 with lower RVNA GMCs and percentages of participants achieving adequate concentrations at D14.Citation14 A possible explanation for the unexpected number of the slow-responders is that some of them were unknowingly immunocompromised, and this could not be recognized in the anamnesis, although no evidence of this was documented during the study. In our study, no participants were non-responders, as all achieved RVNA concentrations ≥ 0.5 IU/mL by D90 at the latest. Although a defined RVNA concentration at D14 is recommended as a marker of an adequate immune response to vaccination, there is no formal correlate of protection for rabies in humans. Studies demonstrating effectiveness, especially after exposure to a laboratory-confirmed rabid animal, remain of significant value for current and future vaccines. PCECV administered according to different post-exposure intramuscular schedules has demonstrated 100% survival over 7 effectiveness studies which included 1539 patients, of whom 432 had exposure to an animal with confirmed rabies.Citation8
All participants included in the immunogenicity analyses had RVNA concentrations < 0.5 IU/mL at D0; however, RVNA GMCs at D14 tended to be higher at study site 2, which also had the highest exposure in terms of recorded bites for all groups. Of note, 12 study participants with detectable baseline RVNA concentrations were enrolled at this site, and they were likely to achieve higher RVNA levels post-immunisation. The trend of higher RVNA GMCs was not observed beyond the D14 timepoint, and no clear correlation between these findings and the study site was established.
The detection of pre-existing rabies virus neutralising antibodies in the study population was unexpected, seeing that previous rabies vaccination was an exclusion criterion. However, it has already been hypothesized that rare cases of sub-clinical rabies may occur in humans, as RVNAs in unvaccinated populations have been detected in remote communities with no access to medical care or facilities.Citation15,16 Although not demonstrated, any of the 12 study participants could have been previously exposed to rabid animals, and had not followed correct post-exposure prophylaxis measures. It is also possible that some of them had already been vaccinated against rabies, and had no recollection of it. A community-based study conducted in Delhi at the time of our study determined that only 44% of persons bitten by a potentially rabid dog would receive a complete rabies vaccination dose, and even basic measures like washing the wound were not applied,Citation17 despite official recommendations. The situation has slightly improved over the last decade,Citation18,19 but recent studies still indicate a lack of awareness among the general population on transmission and prevention of rabies.Citation10,20,21
The incidence of AEs after vaccination was similar among vaccinees who received the Indian and German lots. Only 6 grade 3 reactions were recorded, and all were resolved in less than 4 days, without medication. No allergic reactions or serious adverse reactions were reported.
Both the Indian and German vaccines had an acceptable safety and tolerability profile. This is in line with the already demonstrated safety profile of the PCECV vaccine.Citation4 Injection site pain was also the most commonly reported local symptom in a 10-year post-surveillance study in India for PCECV.Citation9 In the United States, Vaccine Adverse Event Reporting System data collected for more than 1 million doses of PCECV identified an incidence of 30 AEs per 100,000 doses, with only 7% of the reported AEs considered as serious.Citation22
The strengths of the present study included its design as a multi-center single-blind study and the use of multiple Indian PCECV lots to demonstrate the primary objective. All administered vaccines were from marketed lots, so the results obtained can be extrapolated to vaccine batches reaching the general population. The number of individuals excluded from the PP cohort was higher than expected, leading to a slightly smaller number of participants than needed to meet the primary objective with a power of 90%, and this could be a potential limitation of the study. However, the sample size still allowed for a power of 80%.
The results of the present study showed that the PCECV batches were produced consistently in India and were non-inferior to German batches in terms of induced immune response in individuals of all ages. Vaccines manufactured in the 2 countries had a similar safety and reactogenicity profile.
Materials and methods
Study design and participants
This was a phase IV, randomized, single-blind study carried out in 2 centers in India between February and August 2000.
Study participants were healthy males or females, with or without animal bites, from whom informed consent was obtained. Exclusion criteria for enrolment were: previous history of rabies immunization, history/suspicion of an infectious disease, treatment with anti-malarial drugs, steroids, immunosuppressive or anti-inflammatory drugs within 2 months prior to study enrolment, previous autoimmune diseases, concomitant enrolment in other clinical studies, pregnancy, history of drug or alcohol abuse, planned surgery during the study period, hypersensitivity to substances administered in the study, and conditions that render the participant unable to fully understand the nature of the study.
Study participants were randomized in 4 groups: 3 groups received the PCECV vaccine from batches manufactured in India (groups PCECV-I499, PCECV-I501, and PCECV-I509), and 1 group received the vaccine manufactured in Germany (group PCECV-G). The numbers in the groups' names indicate the number of the manufactured batch. All vaccines were from marketed batches, not specifically developed for the trial.
All participants were vaccinated at D0, 3, 7, 14 and 30 of the study, and blood samples were collected at D0 (before vaccination with the first dose), D14 (before dose 4), D30 (before dose 5) and D90 (60 days after the primary vaccination course) (). In compliance with routine care in India, an injection of tetanus toxoid was administered with the first dose of the anti-rabies vaccine in case of animal bites. Administration of RIG was not mandated per protocol, and was decided by the treating physician. This study was undertaken with the standard of care appropriate at the time of the study and it is important to note that the rabies status of the animals involved in exposures was unknown. The use of RIG is now recommended for all patients, with no history of prior rabies vaccination, who present with a category III exposure.
Figure 4. Study design. B, blood sample; D, day; syringes symbolize one dose (1 mL) of purified chick embryo cell rabies vaccine.
The study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. Informed consent was obtained from all study participants. The study protocol, amendments, and informed consent were reviewed and approved by regional Independent Ethics Committees in India and Germany. The study is registered at EU Clinical Trials Register, document reference 48386; more information is available at http://art45-pediatric-studies.ema.europa.eu/clinicaltrials/details.php?PkID=48386 and the GSK Clinical Study Register, e-track number 205264. In the interest of full transparency and to ensure a wider availability of the data, the current paper presents the complete results of the trial.
Study vaccines
PCECV, a lyophilised vaccine produced from the Flury LEP-25 strain grown in primary cultures of chick fibroblast and inactivated with β-propiolactone, was used in this study. The vaccine has already been described in detail.Citation8,23 Prior to administration, each dose was reconstituted with the water for injection provided with the vaccine according to the manufacturer's instructions. At each vaccination, participants received a dose of 1 mL of PCECV (lot numbers 499, 501, 509, and 244011) with a potency of ≥ 2.5 IU per dose, administered in the deltoid muscle, in alternate arms at each vaccination.
Randomization and masking
Study participants were randomized (1:1:1:1) using a minimization procedure accounting for center, to receive PCECV from one of the 3 Indian investigated batches or the Germany-manufactured reference batch. Treatment allocation at the investigator site was preformed using a randomization list generated by Chiron Biostatistics and Clinical Data Management.
The study was conducted in a single-blind manner; the vaccine recipient and laboratory personnel were unaware of which vaccine was administered.
Study objectives
The primary objective was to assess the non-inferiority of PCECV vaccine lots manufactured in India over a vaccine lot produced in Germany, with respect to RVNA GMCs on D14, following administration of the 3 first doses, on D0, 3 and 7.
Secondary objectives were: (i) to compare the safety and tolerability of the lots manufactured in India and in Germany; (ii) to assess the consistency of the 3 lots produced in India with respect to RVNA GMCs on D14; (iii) to evaluate the RVNA concentrations on D30 (16 days post-dose 4) and D90 (60 days post-dose 5); (iv) to assess the proportion of study participants with RVNA concentrations ≥ 0.5 IU/mL on D14, 30 and 90.
Immunogenicity assessment
Blood samples for immunogenicity assessments were centrifuged and the serum was distributed into 2 aliquots, to be stored at −20°C until sent from the 2 Indian trial sites to an approved laboratory (College of Veterinary Medicine, Kansas State University, United States) for antibody concentration determination.
Serum samples were tested using the rapid fluorescent focus inhibition test.Citation24 RVNA concentrations ≥ 0.5 IU/mL were considered as indicative of an adequate immune response to the vaccine.Citation4
Safety and reactogenicity assessment
All vaccinees were observed for 30 minutes after vaccination for immediate reactions. Solicited local and general symptoms, and unsolicited AEs were collected during an observation period of 3 days after each vaccination. The occurrence of AEs was reported by study participants, or their parents or legal guardians if applicable, and all AEs were followed-up by the investigator.
Solicited local symptoms were pain at injection site, induration, and erythema; solicited general symptoms assessed were fever (≥ 38°C), headache, malaise, myalgia, and nausea.
All AEs were assessed based on 3 criteria: severity, expectedness, and relatedness to vaccine administration. The intensity of all AEs was graded on a scale of 1 (mild) to 3 (severe), where a grade 3 AE was defined as preventing normal activities.
SAEs and AEs with medical attendance, as well as any AE resulting in withdrawal from study were collected through the entire study period (D0–D90). An SAE was defined as any untoward medical occurrence that resulted in death, was life-threatening, required hospitalization or prolonged existing hospitalization, resulted in a congenital anomaly/birth defect, or resulted in disability or incapacity.
Statistical analyses
Allowing for a drop-out rate of 30%, a sample size of 320 study participants was needed to have 80% power to meet the primary objective (1-sided α=0.05).
Demographic variables, medical history, and concomitant medications were tabulated for all study participants.
Immunogenicity analyses
Analyses were performed on the PP cohort, which included participants seronegative at baseline, who received the vaccine at D0, 3 and 7 and had a blood draw at D14.
Antibody concentrations were assessed for each timepoint and group. GMCs were tabulated with calculated scatter factor, median, minimum, maximum, and 95% CIs.
The primary objective was demonstrated if the lower limit of the 95% CI for the ratio GMCPCECV-I/GMCPCECV-G at D14 was higher than 0.5. The data from groups receiving Indian-manufactured doses were pooled. Consistency between Indian batches was demonstrated if the 95% CI of RVNA GMCs ratios between pairs of PCECV-I groups at D14 included 1.
Safety analyses
Safety and reactogenicity assessments were performed in the intention-to-treat cohort, comprising all participants receiving at least 1 study vaccine dose.
The percentage of participants reporting AEs, as well as the number of AEs, were tabulated for each group. A descriptive analysis of the comparison between AEs reported by PCECV-I and PCECV-G vaccinees was performed.
All statistical analyses were performed using SAS version 6.12.
Trademark statement
Rabipur™ is a trademark of GSK group of companies.
Previous publications
Sampath G, Deshpande A, Briggs D, Malerczyk C, Chaves R, Banzhoff A. Comparison of immunogenicity and safety of Purified Chick Embryo Culture Rabies Vaccine (PCECV) manufactured in India with PCECV manufactured in Germany. Proceedings of the XIII International Meeting on Research Advances and Rabies Control in the Americas 2002, November 3rd-8th, Oaxaca City, Mexico (poster presentation).
Abbreviations
| AE | = | adverse event |
| CCEV | = | cell culture and embryonated egg-based rabies vaccines |
| CI | = | confidence interval |
| GMC | = | geometric mean concentration |
| IU | = | international units |
| PCECV | = | purified chick embryo cell rabies vaccine |
| RFFIT | = | Rapid Fluorescent Focus Inhibition Test |
| RIG | = | rabies immunoglobulins |
| RVNA | = | rabies virus neutralizing antibody |
| PP | = | per protocol |
| SAE | = | serious adverse event |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
AB, AKA, CM and SP are employees of GSK group of companies. SP also holds shares and options from the sponsoring company. HV declares that he was Medical Director of Hoechst and Aventis Pharma, the manufacturers of Rabipur in India at the time of the study. GS and AD declares no conflict of interest.
Authors' contribution
AB, HV and AD were involved in the conception or the design of the study. JB and OVDM participated in the data collection. HV, CM, AD and GS participated in the collection or generation of the study data and HV, AD and GS also performed the study/project. SP, AB, AKA, HV, CM and AD were involved in the analysis and interpretation of the data. All authors had full access to the data, were involved with developing this manuscript, gave final approval before submission and are accountable for all aspects of the work.
Acknowledgments
The authors thank the subjects for participating in this trial and Mr. D. Variava (Clinical Research Associate at Chiron) for his support during the study. Authors would like to thank XPE Pharma & Science platform for editorial assistance and manuscript coordination, on behalf of GSK Vaccines. Petronela M. Petrar and Claire Verbelen provided medical writing support and Angeles Ceregido and Susana Montenegro Gouveia coordinated manuscript development and editorial support.
Funding
This study was sponsored and funded by Chiron Vaccines which transitioned to Novartis Vaccines and Diagnostics, now GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA took charge of all costs associated with developing and publishing the manuscript.
References
- Yousaf MZ, Qasim M, Zia S, Khan M, Ashfaq UA, Khan S. Rabies molecular virology, diagnosis, prevention and treatment. Virol J 2012; 9:50; PMID:22348291; https://doi.org/10.1186/1743-422X-9-50
- World Health Organization. WHO Expert Consultation on Rabies. Second report. World Health Organ Tech Rep Ser 2013; (982):1-139, back cover
- World Health Organization. Rabies fact sheet. WHO technical report series 931. Updated March 2016 and 2017. Available at http://www.who.int/mediacentre/factsheets/fs099/en/
- World Health Organization. Rabies vaccines: WHO position paper. Weekly Epidemiological Record 2010; 85(32):309-20
- World Health Organization. WHO prequalified vaccines. Updated September 2016. Available at http://www.who.int/immunization_standards/vaccine_quality/Web_List_Help2016.pdf?ua=1
- Sampath G, Deshpande A, Briggs D, Malerczyk C, Chaves R, Banzhoff A. Comparison of immunogenicity and safety of Purified Chick Embryo Culture Rabies Vaccine (PCECV) manufactured in India with PCECV manufactured in Germany. Proceedings of the XIII International Meeting on Research Advances and Rabies Control in the Americas (poster presentation). Oaxaca City, Mexico, 3-8 November 2002
- Zhu FC, Wu CH, Meng FY. Safety and immunogenicity of rabies vaccine (chick embryo cell) for human use produced in Germany and India. Zhongguo Yi Miao He Mian Yi 2009; 15(5):447-50; PMID:20084974
- Giesen A, Gniel D, Malerczyk C. 30 Years of rabies vaccination with Rabipur: a sum mary of clinical data and global experience. Expert Rev Vaccines 2015; 14(3):351-67; PMID:25683583; https://doi.org/10.1586/14760584.2015.1011134
- Sehgal S, Bhattacharya D, Bhardwaj M. Ten year longitudinal study of efficacy and safety of purified chick embryo cell vaccine for pre- and post-exposure prophylaxis of rabies in Indian population. J Commun Dis 1995; 27(1):36-43; PMID:7636151
- Mahendra BJ, Narayana DA, Agarkhedkar S, Ravish HS, Harish BR, Agarkhedkar S, Madhusudana SN, Belludi A, Ahmed K, Jonnalagedda R, et al. Comparative study on the immunogenicity and safety of a purified chick embryo cell rabies vaccine (PCECV) administered according to two different simulated post exposure intramuscular regimens (Zagreb versus Essen). Hum Vaccin Immunother 2015; 11(2):428-34; PMID:25692792; https://doi.org/10.4161/21645515.2014.995059
- Li R, Huang L, Li J, Mo Z, He B, Wang Y, Wu X, Minutello M, Guinet-Morlot F, Pichon S. A next-generation, serum-free, highly purified Vero cell rabies vaccine is safe and as immunogenic as the reference vaccine Verorab(R) when administered according to a post-exposure regimen in healthy children and adults in China. Vaccine 2013; 31(50):5940-7; PMID:24148575; https://doi.org/10.1016/j.vaccine.2013.10.043
- Wang LY, Sun MP, Zhang XC, Suo LD, Xu RH, Zou YJ, Zuo LB, Qi H. Safety and immunogenicity of two freeze-dried Vero cell rabies vaccines for human use in post-exposure prophylaxis. Vaccine 2011; 29(15):2679-81; PMID:21296694; https://doi.org/10.1016/j.vaccine.2011.01.053
- Jaijaroensup W, Tantawichien T, Khawplod P, Tepsumethanon S, Wilde H. Postexposure rabies vaccination in patients infected with human immunodeficiency virus. Clin Infect Dis 1999; 28(4):913-4; PMID:10825063; https://doi.org/10.1086/517241
- Deshpande A, Briggs DJ, Banzhoff A. Investigation of immune response to purified chick embryo cell tissue culture rabies vaccine (PCECV) in HIV infected individuals using a simulated post-exposure regimen. Proc XIII Int AIDS Conference, Durban, South Africa, 9-14 July 2000:163-8
- Gilbert AT, Petersen BW, Recuenco S, Niezgoda M, Gomez J, Laguna-Torres VA, Rupprecht C. Evidence of rabies virus exposure among humans in the Peruvian Amazon. Am J Trop Med Hyg 2012; 87(2):206-15; PMID:22855749; https://doi.org/10.4269/ajtmh.2012.11-0689
- Lopez RA, Miranda PP, Tejada VE, Fishbein D. Outbreak of human rabies in the Peruvian jungle. Lancet 1992; 339(8790):408-11; PMID:1346669; https://doi.org/10.1016/0140-6736(92)90088-K
- Lai P, Rawat A, Sagar A, Tiwari K. Prevalence of dog-bites in Delhi: knowledge and practices of residents regarding prevention and control of rabies. Health Population Perspectives Issues 2005; 28(2):50-7; PMCID:PMC4866364
- Sudarshan MK, Mahendra BJ, Madhusudana SN, Ashwoath Narayana DH, Rahman A, Rao NS, F XM, Lobo D, Ravikumar K, Gangaboraiah . An epidemiological study of animal bites in India: results of a WHO sponsored national multi-centric rabies survey. J Commun Dis 2006; 38(1):32-9; PMID:17370688
- Menezes R. Rabies in India. CMAJ 2008; 178(5):564-6; PMID:18299543; https://doi.org/10.1503/cmaj.071488
- Kole AK, Roy R, Kole DC. Human rabies in India: a problem needing more attention. Bull World Health Organ 2014; 92(4):230; PMID:24700986; https://doi.org/10.2471/BLT.14.136044
- Mani RS, Anand AM, Madhusudana SN. Human rabies in India: an audit from a rabies diagnostic laboratory. Trop Med Int Health 2016; 21(4):556-63; PMID:26799375; https://doi.org/10.1111/tmi.12669
- Dobardzic A, Izurieta H, Woo EJ, Iskander J, Shadomy S, Rupprecht C, Ball R, Braun MM. Safety review of the purified chick embryo cell rabies vaccine: Data from the Vaccine Adverse Event Reporting System (VAERS), 1997–2005. Vaccine 2007; 25(21):4244-51; PMID:17382435; https://doi.org/10.1016/j.vaccine.2007.02.075
- Malerczyk C, Müller H. Purified chick embryo cell vaccine. In: Rupprecht CE, Nagarajan , ed. Current laboratory techniques in rabies diagnosis, research and prevention. New York, Amsterdam: Elsevier; 2015:251-60
- Smith JS, Yager PA, Baer GM. A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ 1973; 48(5):535-41; PMID:4544144
