ABSTRACT
T cell immunity is critical in controlling human cytomegalovirus (HCMV) infection in transplant recipients, and T cells targeting viral immediate early proteins such as IE1, IE2 and pp65 have been speculated to be more effective against reactivation. Here we report efforts to construct replication incompetent adenovirus 6 vectors expressing these viral antigens as vaccine candidates. To reduce the potential liabilities of these viral proteins as vaccine antigens, we introduced mutations to inactivate their reported functions including their nuclear localization signals. The modifications greatly reduced their localization to the nuclei, thus limiting their interactions with cellular proteins important for cell cycle modulation and transactivation. The immunogenicity of modified pp65, IE1 and IE2 vaccines was comparable to their wild-type counterparts in mice and the immunogenicity of the modified antigens was demonstrated in non-human primates.
Introduction
Human cytomegalovirus (HCMV) is a prototype human β-herpes virus, prevalent in over 50% of the adult population worldwide. The infection in healthy subjects rarely causes any discernible clinical symptoms and the virus is persistent in the host for life.Citation1 When the host immune system is weakened as in transplant recipients under immunosuppression, HCMV infection, either as a primary infection or reactivation from latency, can cause serious and even life threatening disease. In addition, frequent viral episodes in these transplant recipients are often associated with poor clinical outcomes including mortality and engraftment failure.Citation2,3 Although the use of antiviral agents has substantially improved the overall outcomes in these patients, it is recognized that the long term control of viral reactivation is ultimately dependent on the host immune system, especially T cell mediated immune responses. The early recovery of host T cell responses to HCMV, especially those mediated by CD4+ T cells, is associated with protection against viral episodes in transplant recipients.Citation4,5 Thus, developing vaccine candidates that are safe and effective in inducing or enhancing host anti-HCMV T cells is considered an attractive approach to address the need to control HCMV infection in transplant recipients.
HCMV is a double stranded DNA virus with a genome size greater than 235 Kb, capable of encoding more than 160 ORFs.Citation6 The expression of HCMV viral genes follows distinct kinetics, i.e., immediately early, early and late phases. Many viral proteins can be targeted by host T cells. A comprehensive survey of a cohort of 33 HCMV seropositive donors with diverse HLA backgrounds revealed that more than 70% of viral antigens were recognized by host T cells.Citation7 Immediate early (IE) gene products are 2–4 times more frequently targeted by both CD4+ and CD8+ T cells compared to those expressed in early, early/late and late replication phases. In addition, in a murine CMV (MCMV) viral reactivation model,Citation8 Simon et al showed that the control of viral reactivation, measured by detection of viral immediate early and early gene transcripts, is dependent on the CD8+ T-cells specific to an immunodominant epitope in viral immediate early protein; abrogation of the T cell response by mutating this epitope leads to more frequent viral reactivations.Citation9 These lines of evidence suggest that T cells specific to the proteins expressed in the immediate early and early phases of viral replication would more effectively control viral reactivation than those specific to proteins expressed in later phases of the viral replication cycles.
HCMV IE1 (UL123) and IE2 (UL122) proteins are detectable in cells within hours following HCMV infection. They are abundant in the viral lytic cycle and together with pp65 (UL83) are among the most frequently recognized antigens by human CD4+ and CD8+ T cells, and are thus of interest as targets for vaccine design. However, these proteins carry risks as vaccine antigens if expressed without any modification. Viral protein pp65 is a major tegument protein with reported kinase functions,Citation10 and may also inhibit interferon activity.Citation11 IE1 and IE2 proteins have been reported to have functions in modulating cell cycles by blocking apoptosis,Citation12 and can disrupt PML-associated nuclear bodies including ND10.Citation13 In this study, we genetically modified these proteins to nullify their undesired functions, making them potentially safe as vaccine antigens for clinical development. The modified antigens and their wild-type counterparts were cloned into recombinant replication incompetent adenovirus 6 (Ad6) vectors, and evaluated for their immunogenicity in mice. A mixture of Ad6 vectors expressing the modified antigens was then further evaluated in rhesus macaques. Our results suggest that these modified antigens maintain their immunogenicity in preclinical animal models and could be further developed for clinical evaluation.
Results
Vaccine design
Viral proteins pp65, IE1 and IE2 are important antigens for vaccines aimed at eliciting or enhancing host anti-HCMV T cell immunity. Viral protein pp65 of 561 amino acids contains casein kinase II phosphorylation sites (residues 426–498) and has serine/threonine kinase activity in vitro.Citation10 There is a putative kinase domain of ATP binding motifs with a highly conserved lysine at residue 436. In addition, pp65 contains a bipartite nuclear localization signal (NLS).Citation14,15 Thus, we hypothesized that pp65 could be functionally nullified by deletion and/or modification of the bipartite NLS, and with a substitution of the conserved lysine at position 436 with a glycine.Citation16 The vaccine construct, named modified pp65 (mpp65), and its amino acid sequence in comparison with the original pp65 is provided in Figure S1A.
Transcription of IE1 and IE2 is driven by the major immediate early promoter (MIEP) through alternative splicing.Citation1 The IE1 transcript contains exons 1, 2, 3 and 4, while the IE2 transcript contains exons 1, 2, 3 and 5. The two proteins share the first 85 amino acids encoded by exons 2 and 3. Both IE1 (491 amino acids) and IE2 (579 amino acids) are nuclear proteins with well-defined bipartite NLSs.Citation13,17,18 They are important in viral gene regulation, with IE1 augmenting MIEP activity while IE2 inhibiting MIEP activity.Citation1 In addition, both proteins have been shown to be capable of modulating host cell cycles, possibly through their interactions with Rb family proteins including p107 for IE1, and p53 and Rb for IE2.Citation19-22
To nullify these functions in IE1 and IE2, we first removed the NLSs to limit the proteins to the cytoplasm, thus reducing the probability of their interaction with cell cycle modulation proteins, such as p53, Rb and p107, and cellular transcriptional activation factors, and also limiting their ability to disrupt ND10. Secondly, we deleted both exons 2 and 3 to eliminate the probability of these proteins to activate latent HCMV.Citation23 Exons 2 and 3 contain a structure that is important for binding to p107,Citation19 and a mutant HCMV virus with a deletion in its genome corresponding to amino acids 30 to 77 of IE1 and IE2 showed severely impaired growth kinetics in fibroblast cells, even at high multiplicity of infection.Citation23 Thus, this truncation in IE1 and IE2 further attenuated their functions in modulating host cell cycles. These constructs are named as mIE1 and mIE2, respectively. In addition, since all constructed antigens were expressed through MIEP and IE2 is known to negatively regulate MIEP activity, we designed two more IE2 constructs, IE2(H2A) and mIE2(H2A), in which two alanine substitutions were introduced to replace two histidines at positions 446 and 452.Citation24,25 The alignments for the IE1 and IE2 constructs are provided in Figures S1B and S1C, respectively.
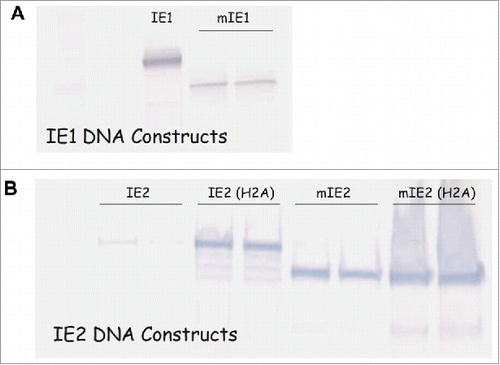
Since all constructs were driven by MIEP, we assessed the expression of modified IE1 and IE2 proteins by Western blot analysis. HEK 293 cells were transfected with DNA constructs, and the cell lysate samples were then blotted with mAbs specific for IE1 or IE2. The expression level of mIE1 seemed to be substantially lower than its wild type counterpart (IE1) (), indicating that the modification limited the IE1s ability to augment MIEP activity, due to the removal of NLS. It is also possible that the modification might have compromised the protein stability. Expression from the IE2 DNA constructs is shown in . As expected, the IE2 construct with two alanine substitutions (H2A) was expressed at much higher levels than wild type IE2. Interestingly, the mIE2 construct expressed at similar levels as IE2(H2A), suggesting that the removal of the NLS was also effective in restricting the access of IE2 protein to the nucleus where it can affect MIEP transcriptional activity. The expression level of mIE2(H2A) was comparable to that of mIE2.
Figure 1. Modification altered expression levels of IE1 and IE2. Western blot analysis of HEK293 cells transfected with DNA plasmids expressing wild-type IE1 or modified IE1 (A); wild-type IE2, IE2 with two alanine substitutions (IE2(H2A)), modified IE2 (mIE2), or modified IE2 with alanine substitutions (mIE2(H2A)) (B).
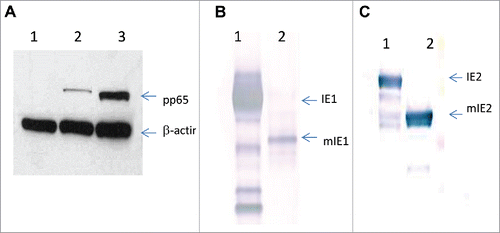
Based on these data, we selected three pairs of HCMV antigens, as summarized in , for the construction of replication incompetent adenovirus 6 (Ad6) vaccines.Citation26 Transgene expressions of the Ad6 vaccines were confirmed by Western blot analysis (). Although the expression level of β-actin was comparable in cells transduced with mock, Ad6-pp65 and Ad6-mpp65, enhanced expression was observed with mpp65 as compared to its unmodified counterpart (). The expression levels of IE1 versus mIE1 and IE2(H2A) versus mIE2 were similar to those seen in DNA transfected cell cultures.
Table 1. Summary of CMV antigen constructs.
Figure 2. Expression of wild-type versus modified HCMV antigens by Ad6 vectors. Western blot analysis of Per.C6 cell lysates from Ad6-Mock, Ad6-pp65 and Ad6-mpp65 (A: lane 1, 2 and 3, respectively); Ad6-IE1 and Ad6-mIE1 (B: lane 1 and 2, respectively); Ad6-IE2 and Ad6-mIE2 (C: lane 1 and 2, respectively).
Subcellular localization of modified HCMV antigens
To confirm the effect of removal of NLS on subcellular localization of these antigens, we conducted indirect immunofluorescent staining on MRC-5 cells transduced with the Ad6 vectors. Cells were stained in chamber slides with antigen specific antibodies as well as a mAb against Sp100, a prominent structural protein of ND10.Citation27 The nuclei were stained with DAPI. The slides were examined using confocal microscopy and the images were overlaid. As shown in and , while wild type pp65 was predominantly localized to the nucleus, as expected, mpp65 was evenly distributed between the cytoplasm and the nucleus. Thus, the modification of pp65 to eliminate the bipartite NLS sequence changed the cellular distribution pattern of pp65 from exclusively nuclear to both nuclear and cytoplasmic. Also as expected, the expression of pp65, or mpp65 did not affect the integrity of ND10, which appeared as punctuate staining within the nucleus by using mAb specific to the Sp100 protein.
Figure 3. Subcellular localization of wild-type versus modified HCMV antigens by indirect immunofluorescent staining. MRC-5 cells infected with Ad6-pp65 (A), Ad6-mpp65 (B), Ad6-IE1 (C), Ad6-mIE1 (D), Ad6-IE2 (E) and Ad6-IE2 (F) were stained with antigen-specific antibodies and antibody specific to Sp100. Cells were also nucleus stained with DAPI. Pictures were acquired for individual staining and overlaying using confocal microscopy as described in Materials and methods.
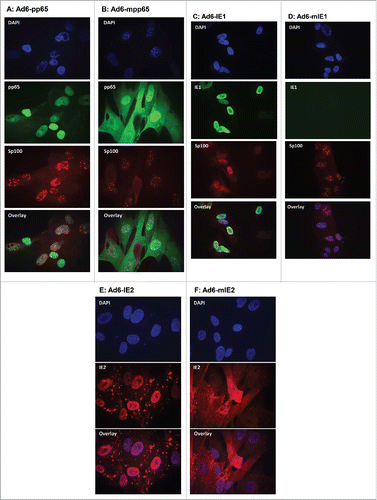
While IE1 stained brightly in the nucleus of the MRC-5 cells transduced with Ad6-IE1, there was little staining seen in the Ad6-mIE1 transduced cells (). This result was consistent with the immunoblot analysis () showing that mIE1 had a lower expression level than IE1 in transiently transfected cell cultures. In addition, the Sp100 staining was visibly different between the cells expressing IE1 and those expressing mIE1. The Sp100 staining in cells expressing IE1 was diffuse in the nucleus, confirming the ability of IE1 to disperse the ND10 structure.Citation27 Such Sp100 staining patterns were not detected in cells expressing mIE1, indicating that the removal of NLS in IE1 was able to reduce its interaction with ND10 ().
As for the IE2 constructs, we found that IE2(H2A) was localized in the nucleus, and mIE2 was detected predominantly in the cell cytoplasm. These results confirmed that removal of NLS for these proteins was effective in changing their subcellular localization from nuclei to cytoplasm.
Immunogenicity evaluation
To confirm that the modified HCMV antigens were comparably immunogenic to their wild-type counterparts, we immunized C57Bl/6 × Balb/c F1 mice with Ad6 vectored vaccines with dose titrations. Spleens from four mice per group were harvested four weeks post vaccination and the splenocytes were pooled for evaluation in IFN-γ ELISPOT assays using 15-mer peptide pools of corresponding antigens as stimulants. While DMSO, the solvent used to prepare the peptide pools, did not generate any detectable T cell responses, antigen-specific responses were demonstrated for all Ad6 vectored vaccines. Both Ad6-pp65 and Ad6-mpp65 were highly immunogenic, eliciting a response that was close to 1000 SFC/106 spleen cells at 106 viral particles (vp) per dose in mice (). Regarding the IE1 and IE2 Ad6 vectored vaccines, both wild-type and modified versions were comparably immunogenic and T cell responses seemed to peak at around 3 × 106 to 1 × 107 vp per dose (). However, their peak responses were about 2–3-fold lower compared to those seen in pp65 or mpp65 vaccination.
To make sure that mixing three Ad6 vectored vaccines would not interfere with vaccine immunogenicity in vivo, we first tested the mixture of Ad6 vectored mpp65, mIE1 and mIE2 vaccines in mice. We immunized C57Bl/6 × Balb/c F1 mice with a mixture of Ad6-mpp65, Ad6-mIE1 and Ad6-mIE2 with dose titration, and assessed the immune response to all three antigens in IFN-γ ELISPOT assay. Similar data were obtained as seen in mice vaccinated with individual vaccines, and this result suggested that the delivery of all three vaccines as a mixture would not compromise their immunogenicity in mice (Figure S2).
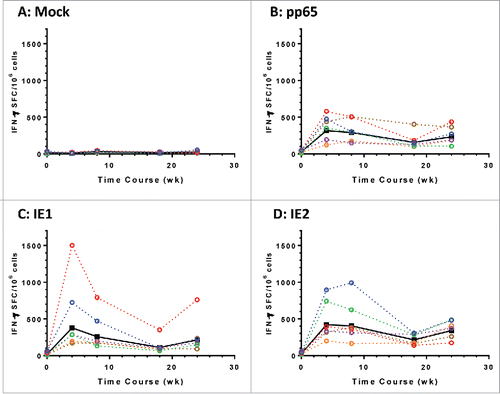
We next immunized six rhesus macaques with the mixture of Ad6-mpp65, Ad6-mIE1 and Ad6-mIE2 at 1 × 1010 vp for each construct. Vaccines were given at weeks 0 and 20, and PBMCs were collected for IFN-γ ELISPOT assays at multiple time points. The response to each antigen was graphed longitudinally for each macaque, with the geometric mean values of the group plotted in solid black lines (). A single vaccination with the vaccine mixture was effective in eliciting T cell responses to pp65, IE1 and IE2. The responses to pp65, IE1 and IE2 were evenly distributed among the three antigens, different from those observed in mice, in which mpp65 seemed more immunogenic than mIE1 and mIE2 (Fig. S2). In addition, the T cell responses seemed to peak after the first vaccination, and the second vaccination at week 20 didn't produce responses of the same magnitude at week 24. The overall T cell responses to three antigens totaled between 500 to 1000 SFC/106 cells for each animal. The data confirmed that the modified pp65, IE1 and IE2 antigens were immunogenic in nonhuman primates when delivered as Ad6 vectored vaccines.
Figure 4. Comparable immunogenicity of wild-type versus modified HCMV antigens in mice. C57BL/6 × Balb/c F1 mice (n = 10) were immunized with the indicated vaccine and dose, and the spleen cells from four mice were pooled and assessed for IFN-γ secretion in response to re-stimulation with corresponding antigen pp65 (A), IE1 (B) and IE2(C) peptide pools in ELISPOT assays. Open circles in plots represent vaccines with wild-type antigens and open triangle represent vaccines with modified antigens. DMSO at the same concentration as in peptide pools was used as negative control (dashed lines). Number of spot forming cells (SFC) on y axis was plotted against vaccine doses of viral particles (vp) on x axis.
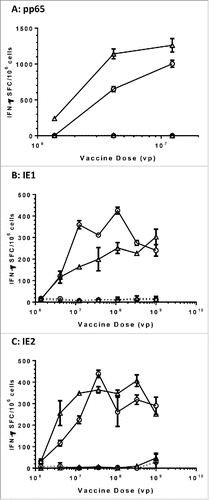
Figure 5. Modified HCMV antigens immunogenic in nonhuman primates. T cell responses in six rhesus macaques immunized with a mixture of Ad6 vector vaccines at day 0 and wk 20. The mixture contains Ad6-mpp65, Ad6-mIE1 and Ad6-mIE2 at 1 × 1010 vp per construct. PBMCs were assessed for IFN-γ secretion pre-vaccination and at weeks 4, 8, 18 and 24 post vaccination in ELISPOT assay. PBMCs were stimulated with DMSO control (A), pp65 peptide pool (B), IE1 peptide pool (C) or IE2 peptide pool (D). IFN-γ secreting cell numbers per 1 × 106 PBMCs for each monkey were plotted in colored dashed lines and geometric mean values from all monkeys were plotted in solid black line. Number of spot forming cells (SFC) on y axis was plotted against time points of PBMC sampling.
Discussion
HCMV infection in transplant recipients remains an important cause for morbidity and mortality despite the availability of small molecule antiviral drugs. The long term control of HCMV infection from viral reactivation is dependent on the recovery or reconstitution of host T cell immunity, and vaccines remain an attractive approach for this therapeutic goal. Here, we explored Ad6 vectored vaccines for eliciting T cell responses to the dominant HCMV viral antigens pp65, IE1 and IE2. These three antigens were chosen based on the following reasons. First, these antigens are expressed or present abundantly during the immediate early phase of viral infection, and T cells targeting these antigens are likely more effective in blocking viral gene expression cascades which lead to full blown viral reactivation. Although conclusive evidence to support this notion is yet to be demonstrated, an early work on surveying host T cell responses to HCMV antigens has shown that the antigens expressed in the immediate early phase are 2–4 times more frequently targeted by host CD4+ and CD8+ T cells than those expressed in other viral replication kinetic phases.Citation7 Second, these antigens are essential or important for viral replication and are highly conserved in sequence among known clinical virus isolates, making it less likely for the virus to generate mutations to escape vaccine-induced immune responses. Lastly, these antigens are highly immunogenic in healthy HCMV seropositive human subjects with diverse genetic backgrounds.Citation7 More importantly, T cells targeting IE1 and pp65 have been shown to be effective in control of HCMV infection when adoptively transferred in transplant recipients.Citation28,29
We further addressed the concern about potential safety liabilities related with in vivo expression of pp65, IE1 and IE2 by modifying the antigens as outlined in . Our key strategy focused on removal of the well-defined bipartite NLS in these antigens. By relocating these antigens from nuclei to cytoplasm, the chances for these proteins to interact with host proteins involving cell cycle regulations or transcription activation were greatly reduced. The effect of changing subcellular localization by removal of NLS was visualized by immunofluorescent staining. Supporting this design, we also observed that the auto-regulation of IE2 on MIEP activity was abrogated when its bipartite NLS was removed (). Based on the literature, we also mutated the highly conserved lysine at residue 436 of pp65,Citation16 which is located in a putative kinase domain of ATP binding motifs, and deleted the exons 2 and 3 in both IE1 and IE2 proteins to eliminate the transactivation activities of these antigens while still retaining important immune epitopes.Citation23 Although it is difficult to quantitatively measure the effects of these modifications including the deletions on overall vaccine immunogenicity, our mouse study showed minimal difference between the immune responses induced by wild-type versus modified antigens (), suggesting that T cell epitopes were largely preserved in the modified antigens.
Replication incompetent adenovirus vaccines are highly effective in inducing T cell responses, and have been studied extensively for HIV-1 vaccines.Citation30 One of the key limitations of the technology is that the recombinant adenovirus is highly sensitive to the serum neutralizing antibodies specific to its serotype.Citation31,32 The preexisting host T-cell responses to adenovirus could also negatively affect vaccine immunogenicity; however, the effect by the T-cells to adenovirus antigens on vaccine immunogenicity has not been well characterized. The serotype specific effect has been shown in previous HIV-1 vaccine studies where Ad5 vectored HIV-1 vaccines were less immunogenic in subjects with seroneutralizing titers greater than 200 as compared to those with the lower or negative titers.Citation33 Our rhesus data also suggested this effect as the second vaccination at week 20 did not further boost the host T cell responses beyond the previous peak levels at week 4. To overcome the hurdle of preexisting serum neutralizing antibodies against the vector, higher doses are generally required as shown in rhesus macaque studies.Citation34 Another approach is to utilize adenovirus vectors with lower seroprevalence in the general population. Ad5 and Ad6 are both group C viruses, and share high sequence homology except E3 region, hexon and fiber,Citation35 and many platforms for recombinant vaccine construction and production are interchangeable. Additionally, a previous work has surveyed the worldwide seroprevalence of Ad5 and Ad6,Citation36 and the prevalence rate for seroneutralizing titers greater than 200 against Ad6 in North America and Europe is 17% and 8%, respectively, compared to 35% and 39% of those against Ad5. This observation confirms that Ad6 is a better vector choice than the traditional Ad5 vector.
In summary, we described here an effort to engineer HCMV viral proteins to nullify their potential functions in regulating cell cycles or interacting with cell transcriptional factors. The modifications were intended to address the potential issues of expressing these proteins as T cell antigens in recombinant adenovirus vectors. We further showed that the modifications did not affect the ability of these antigens to induce T cell responses when compared with their wild-type counterparts, and three Ad6 vectored HCMV vaccines administered together as a mixture in nonhuman primates were able to induce T cell responses to all antigens. These vaccines could be further evaluated in additional animal models including those transgenic mice with defined elements of immunodeficiency prior to clinical evaluations in transplant recipients.
Materials and methods
Cells and reagents
HEK293 (ATCC CRL 1573) and MRC5 (ATCC CCL-171) cells were acquired from ATCC (Manassas, VA, USA), and maintained in Eagle's Minimum Essential Medium (EMEM) supplemented with 10% fetal bovine serum (FBS). AD169 virus from ATCC was propagated in MRC5 cells as previously described.Citation37 Peptides of 15-mer overlapping by 11 amino acids covering entire open reading frames of HCMV pp65, IE1 and IE2 proteins were synthesized by SynPep Corp (Dublin, CA, USA). Peptide pools were made as previously described.Citation38
Mouse monoclonal antibody (mAb) for HCMV pp65 was purchased from US Biologicals (Salem, MA, USA), and mouse mAbs for HCMV IE1 and IE2, recognizing exon 4 (IE1) and exon 5 (IE2), respectively, were purchased from Vancouver Biotech LTD (Vancouver, Canada). Mouse anti-human CMV IE1 mAb, clone L-14, was acquired from ATCC. Anti-IE2 poly-serum used for indirect immunofluorescent staining was generated in house. Briefly, rabbits were immunized with Ad6-IE2 at 1 × 1010 viral particles at week 0 and 3, followed by vaccination with synthetic peptide (DPDNPDEGPSSKVPRPETPC) conjugated to Keyhole limpet hemocyanin at 100 µg at week 11. Rabbit immune sera were collected at week 15. Rabbit anti-human Sp100 (ND10) polyclonal sera were acquired from Chemicon International, Inc. (Temecula, CA, USA). Alexa Fluor 594 chicken anti-rabbit IgG and Alexa Fluor 488 chicken anti-mouse IgG were purchased from Invitrogen (Carlsbad, CA, USA).
Vaccine constructs
DNA constructs containing the open reading frame (ORF) of pp65, IE1 and IE2 derived from AD169 virus and their corresponding modified forms were codon optimized for mammalian expression. The synthetic genes were cloned into V1Jns plasmid vector as described previously.Citation39 The authenticity of the genes was confirmed by sequencing, and expression was confirmed by Western blot analysis (see below). Selected constructs were cloned into a replication-incompetent Ad6 vector under the HCMV major immediate early promoter, and the Ad6 virus vaccines were expanded in PER.C6 cells (Janssen Vaccines, Leiden, Netherlands) and the CsCl gradient purified vaccine stocks were quantified by optical density method as described.Citation26
Vaccinations
All animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Institutional Animal Care and Use Committee of Merck & Co., Inc., Kenilworth, NJ, USA, and New Iberia Research Center, New Iberia, LA, USA, when applicable. Groups of ten four-six weeks old pathogen-free female C57Bl/6 × Balb/c F1 mice (Jackson Laboratory, Bar Harbor, ME, USA) were immunized with Ad6 constructs intramuscularly (i.m.) at week 0. The vaccines were administered in a total volume of 100 μL containing between 105 to 108 viral particles (vp) with 50 μL injected in each quadriceps without anesthesia. The nonhuman primate study was conducted at New Iberia Research Center. Rhesus macaques were pre-screened for their baseline responses to selected HCMV antigens in ELISPOT assay, and six animals with minimal background responses were chosen and immunized with a mixture of three Ad6 vector vaccines (1 × 1010 vp per construct) i.m. in 0.5mL volume under anesthesia.
Western blot
Cell lysates were prepared from 293 cells transfected with pV1Jns vectored IE1, or IE2 and their corresponding modified genes, or from Per.C6 cells infected with Ad6 vectored vaccines. The cell lysates were denatured in reducing sample buffer (Invitrogen), and separated on a 4–20% SDS-PAGE (Invitrogen). The proteins were transferred to nitrocellulose membranes (Invitrogen) and blotted with mouse mAb specific to the CMV antigens. The blot was developed using the WesternBreeze Chromogenic Kit (Invitrogen).
ELISPOT assays
The enzyme-linked immunospot (ELISPOT) assays used to enumerate antigen specific gamma interferon (IFN-γ)-secreting cells from mouse spleens and rhesus macaque PBMC were conducted as previously described,Citation40 using peptide pools of 15-mer peptides overlapping by 11 amino acids representing HCMV proteins pp65, IE1 and IE2 as stimulating antigens. Media containing the same amount of DMSO as in the peptide pools was used as controls.
Indirect immunofluorescence staining and confocal microscopy
Subcellular localization of HCMV antigens was examined by immunofluorescent confocal microscopy. Briefly, MRC-5 cells (1 × 104 cells/well) in DMEM/10% FBS were plated in 4-well Lab-Tek II Chamber Slides (Nalgen Nunc International, Penfield, NY, USA) and cultured at 37°C, 5% CO2 for 48h. Cells were then infected with Ad6 vector virus expressing mock antigen or modified pp65, IE1, IE2 at 1,000 viral particles/cell overnight. After washing, cells were fixed with 2% paraformaldehyde in PBS at room temperature for 30 min and then permeabilized with 0.2% Triton X-100/0.2% BSA at room temperature for 10 min. Afterwards, slides were first stained with the following antibodies: mouse anti-human CMV IE1 mAb, clone L-14 (1 µg/mL); rabbit anti-human CMV IE1/2 immune serum (1:500 dilution); mouse anti-CMV pp65 antibody (1:50 dilution); or rabbit anti-human Sp100 (ND10) polyclonal antibody (1:100 dilution) at room temperature for 60 min and followed by staining with Alexa Fluor 594 chicken anti-rabbit IgG (1:1,000 dilution) or Alexa Fluor 488 chicken anti-mouse IgG (1:1,000 dilution) at room temperature for 60 min. Chambers were then removed and slides dried briefly at room temperature. Nuclei were stained with Vectashield Mounting Medium with DAPI (Vector laboratories, Burlingame, CA, USA) and slides were then covered with coverslips and sealed with Nail Polish. Images of the cells were taken with a confocal microscope (Nikon Eclipse TE2000-U with the PerkinElmer Ultraview ERS Rapid Confocal Imager system). The scanning procedure itself illuminates the specimen through a Nipkow spinning disc with specific laser emissions at the following wavelengths: 405 nm, 488 nm, 568 nm, and 640 nm.
Disclosure of potential conflicts of interest
All authors were employees of Merck & Co., Inc. at the time of the study, and had no conflict of interest to disclose.
Supplemental_Material.pptx
Download MS Power Point (141.6 KB)Acknowledgments
We thank all animal care staff at Merck & Co., Inc., West Point, PA, USA and New Iberia Research Center, New Iberia, LA, USA for their technical supports in animal studies. We also acknowledge the critical review by Amy S. Espeseth, and the technical assistance provided by Kiersten Anderson and Jeffrey Sadler of Merck & Co., Inc., West Point, PA, USA.
References
- Mocarski ES, Shenk T, Pass RF. Cytomegaloviruses. In Knipes DM, Howley PM, eds. Fields Virology. Lippincott Williams & Wilkins, 2007:2701-72.
- Razonable RR, Brown RA, Humar A, Covington E, Alecock E, Paya CV. Herpesvirus infections in solid organ transplant patients at high risk of primary cytomegalovirus disease. J Infect Dis 2005; 192:1331-9; PMID:16170749; https://doi.org/10.1086/466529
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007; 357:2601-14; PMID:18094380; https://doi.org/10.1056/NEJMra064928
- Gerna G, Lilleri D, Fornara C, Comolli G, Lozza L, Campana C, Pellegrini C, Meloni F, Rampino T. Monitoring of human cytomegalovirus-specific CD4 and CD8 T-cell immunity in patients receiving solid organ transplantation. Am J Transplant 2006; 6:2356-64; PMID:16889599; https://doi.org/10.1111/j.1600-6143.2006.01488.x
- Gabanti E, Lilleri D, Ripamonti F, Bruno F, Zelini P, Furione M, Colombo AA, Alessandrino EP, Gerna G. Reconstitution of human cytomegalovirus-specific CD4+ T cells is critical for control of virus reactivation in hematopoietic stem cell transplant recipients but does not prevent organ infection. Biol Blood Marrow Transplant 2015; 21:2192-202; PMID:26260678; https://doi.org/10.1016/j.bbmt.2015.08.002
- Murphy E, Yu D, Grimwood J, Schmutz J, Dickson M, Jarvis MA, Hahn G, Nelson JA, Myers RM, Shenk TE. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc Natl Acad Sci U S A 2003; 100:14976-81; PMID:14657367; https://doi.org/10.1073/pnas.2136652100
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202:673-85; PMID:16147978; https://doi.org/10.1084/jem.20050882
- Reddehase MJ, Simon CO, Seckert CK, Lemmermann N, Grzimek NK. Murine model of cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol 2008; 325:315-31; PMID:18637514
- Simon CO, Holtappels R, Tervo HM, Bohm V, Daubner T, Oehrlein-Karpi SA, Kühnapfel B, Renzaho A, Strand D, Podlech J, et al. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J Virol 2006; 80:10436-56; PMID:16928768; https://doi.org/10.1128/JVI.01248-06
- Somogyi T, Michelson S, Masse MJ. Genomic location of a human cytomegalovirus protein with protein kinase activity (PK68). Virology 1990; 174:276-85; PMID:2152994; https://doi.org/10.1016/0042-6822(90)90075-3
- Browne EP, Shenk T. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc Natl Acad Sci U S A 2003; 100:11439-44; PMID:12972646; https://doi.org/10.1073/pnas.1534570100
- Zhu H, Shen Y, Shenk T. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J Virol 1995; 69:7960-70; PMID:7494309
- Wilkinson GW, Kelly C, Sinclair JH, Rickards C. Disruption of PML-associated nuclear bodies mediated by the human cytomegalovirus major immediate early gene product. J Gen Virol 1998; 79(Pt 5):1233-45; PMID:9603339; https://doi.org/10.1099/0022-1317-79-5-1233
- Gallina A, Percivalle E, Simoncini L, Revello MG, Gerna G, Milanesi G. Human cytomegalovirus pp65 lower matrix phosphoprotein harbours two transplantable nuclear localization signals. J Gen Virol 1996; 77(Pt 6):1151-7; PMID:8683200; https://doi.org/10.1099/0022-1317-77-6-1151
- Schmolke S, Drescher P, Jahn G, Plachter B. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J Virol 1995; 69:1071-8; PMID:7815485
- Yao ZQ, Gallez-Hawkins G, Lomeli NA, Li X, Molinder KM, Diamond DJ, Zaia JA. Site-directed mutation in a conserved kinase domain of human cytomegalovirus-pp65 with preservation of cytotoxic T lymphocyte targeting. Vaccine 2001; 19:1628-35; PMID:11166885; https://doi.org/10.1016/S0264-410X(00)00423-0
- Pizzorno MC, Mullen MA, Chang YN, Hayward GS. The functionally active IE2 immediate-early regulatory protein of human cytomegalovirus is an 80-kgdalton polypeptide that contains two distinct activator domains and a duplicated nuclear localization signal. J Virol 1991; 65:3839-52; PMID:1645794
- Delmas S, Martin L, Baron M, Nelson JA, Streblow DN, Davignon JL. Optimization of CD4+ T lymphocyte response to human cytomegalovirus nuclear IE1 protein through modifications of both size and cellular localization. J Immunol 2005; 175:6812-9; PMID:16272338; https://doi.org/10.4049/jimmunol.175.10.6812
- Johnson RA, Yurochko AD, Poma EE, Zhu L, Huang ES. Domain mapping of the human cytomegalovirus IE1-72 and cellular p107 protein-protein interaction and the possible functional consequences. J Gen Virol 1999; 80(Pt 5):1293-303; PMID:10355776; https://doi.org/10.1099/0022-1317-80-5-1293
- Hagemeier C, Caswell R, Hayhurst G, Sinclair J, Kouzarides T. Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J 1994; 13:2897-903; PMID:8026474
- Hsu CH, Chang MD, Tai KY, Yang YT, Wang PS, Chen CJ, Wang YH, Lee SC, Wu CW, Juan LJ. HCMV IE2-mediated inhibition of HAT activity downregulates p53 function. EMBO J 2004; 23:2269-80; PMID:15141169; https://doi.org/10.1038/sj.emboj.7600239
- Castillo JP, Kowalik TF. Human cytomegalovirus immediate early proteins and cell growth control. Gene 2002; 290:19-34; PMID:12062798; https://doi.org/10.1016/S0378-1119(02)00566-8
- White EA, Spector DH. Exon 3 of the human cytomegalovirus major immediate-early region is required for efficient viral gene expression and for cellular cyclin modulation. J Virol 2005; 79:7438-52; PMID:15919900; https://doi.org/10.1128/JVI.79.12.7438-7452.2005
- Macias MP, Stinski MF. An in vitro system for human cytomegalovirus immediate early 2 protein (IE2)-mediated site-dependent repression of transcription and direct binding of IE2 to the major immediate early promoter. Proc Natl Acad Sci U S A 1993; 90:707-11; PMID:8380646; https://doi.org/10.1073/pnas.90.2.707
- Petrik DT, Schmitt KP, Stinski MF. The autoregulatory and transactivating functions of the human cytomegalovirus IE86 protein use independent mechanisms for promoter binding. J Virol 2007; 81:5807-18; PMID:17376893; https://doi.org/10.1128/JVI.02437-06
- Capone S, Meola A, Ercole BB, Vitelli A, Pezzanera M, Ruggeri L, Davies ME, Tafi R, Santini C, Luzzago A, et al. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J Virol 2006; 80:1688-99; PMID:16439526; https://doi.org/10.1128/JVI.80.4.1688-1699.2006
- Maul GG. Initiation of cytomegalovirus infection at ND10. Curr Top Microbiol Immunol 2008; 325:117-32; PMID:18637503
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med 1995; 333:1038-44; PMID:7675046; https://doi.org/10.1056/NEJM199510193331603
- Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, Proesch S, Reinke P, Volk HD, Lehmkuhl H, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med 2005; 201:1031-6; PMID:15795239; https://doi.org/10.1084/jem.20042384
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 2002; 415:331-5; PMID:11797011; https://doi.org/10.1038/415331a
- Hanke T. STEP trial and HIV-1 vaccines inducing T-cell responses. Expert Rev Vaccines 2008; 7:303-9; PMID:18393600; https://doi.org/10.1586/14760584.7.3.303
- Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther 2005; 16:149-56; PMID:15761255; https://doi.org/10.1089/hum.2005.16.149
- Harro C, Sun X, Stek JE, Leavitt RY, Mehrotra DV, Wang F, Bett AJ, Casimiro DR, Shiver JW, DiNubile MJ, et al. Safety and immunogenicity of the Merck adenovirus serotype 5 (MRKAd5) and MRKAd6 human immunodeficiency virus type 1 trigene vaccines alone and in combination in healthy adults. Clin Vaccine Immunol 2009; 16:1285-92; PMID:19605598; https://doi.org/10.1128/CVI.00144-09
- Casimiro DR, Bett AJ, Fu TM, Davies ME, Tang A, Wilson KA, Chen M, Long R, McKelvey T, Chastain M, et al. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J Virol 2004; 78:11434-8; PMID:15452269; https://doi.org/10.1128/JVI.78.20.11434-11438.2004
- Walsh MP, Seto J, Liu EB, Dehghan S, Hudson NR, Lukashev AN, Ivanova O, Chodosh J, Dyer DW, Jones MS, et al. Computational analysis of two species C human adenoviruses provides evidence of a novel virus. J Clin Microbiol 2011; 49:3482-90; PMID:21849694; https://doi.org/10.1128/JCM.00156-11
- Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: Correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 2010; 28:950-7; PMID:19925902; https://doi.org/10.1016/j.vaccine.2009.10.145
- Tang A, Li F, Freed DC, Finnefrock AC, Casimiro DR, Wang D, Fu TM. A novel high-throughput neutralization assay for supporting clinical evaluations of human cytomegalovirus vaccines. Vaccine 2011; 29:8350-6; PMID:21888937; https://doi.org/10.1016/j.vaccine.2011.08.086
- Dubey S, Clair J, Fu TM, Guan L, Long R, Mogg R, Anderson K, Collins KB, Gaunt C, Fernandez VR, et al. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J Acquir Immune Defic Syndr 2007; 45:20-7; PMID:17310936; https://doi.org/10.1097/QAI.0b013e3180377b5b
- Fu TM, Friedman A, Ulmer JB, Liu MA, Donnelly JJ. Protective cellular immunity: cytotoxic T-lymphocyte responses against dominant and recessive epitopes of influenza virus nucleoprotein induced by DNA immunization. J Virol 1997; 71:2715-21; PMID:9060624
- Casimiro DR, Tang A, Perry HC, Long RS, Chen M, Heidecker GJ, Davies ME, Freed DC, Persaud NV, Dubey S, et al. Vaccine-induced immune responses in rodents and nonhuman primates by use of a humanized human immunodeficiency virus type 1 pol gene. J Virol 2002; 76:185-94; PMID:11739684; https://doi.org/10.1128/JVI.76.1.185-194.2002
