ABSTRACT
To assess the relationship between human papillomavirus (HPV) vaccination and occurrence of optic neuritis (ON) and to evaluate a claims-based algorithm for identification of ON. Females of 9–26 year olds in the HealthCore's Integrated Research Database (HIRDSM) with and without claims evidence of HPV vaccination between 2007 and 2012 were included in this study. Potential ON cases were identified using the claims-based algorithm, positive predictive value (PPV) was determined using medical chart review. For the claims analysis, two study designs, a self-controlled temporal scan statistic and a retrospective matched cohort analysis, were used. ON was defined based on an algorithm developed using diagnosis and procedure codes from the medical claims. The PPV for ON cases using charts that had enough information for reviewers to make a determination was 62.5% (95% CI: 49.5%–74.3%). With the self-controlled temporal scan statistic, the primary analysis restricting on recommended vaccination schedule timing showed an increased risk of potential ON after second dose (RR = 3.39; p = 0.03), this finding was not confirmed for any of the additional analyses performed for individual or combined doses. With the cohort design, there was no increased risk of potential ON following vaccination in either individual or combined dose analyses. The risk of potential ON was higher among participants with a history of prior autoimmune diseases. In conclusion, identifying confirmed ON cases through administrative claims data proved challenging. The claims-based analysis in this study did not provide evidence for an association of ON with HPV vaccination.
Introduction
Human papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States.Citation1 Most HPV infections cause no symptoms and are self-limited, as the body's immune system clears off 90% of the virus naturally within two years.Citation1 However, persistent genital HPV infections can cause cervical cancer in womenCitation2-5 and other types of anogenital cancers or genital warts in both men and women.Citation3,4
The CDC recommends HPV vaccination for all teen girls and women through age 26 and young men through age 21.Citation1 Three HPV vaccines are licensed in the U.S.: a nine-valent (HPV9; Gardasil 9, Merck and Co, Inc.), licensed by FDA in 2014,Citation6 a quadrivalent vaccine (HPV4; Gardasil, Merck and Co, Inc.), licensed in 2006, and a bivalent vaccine (HPV2; Cervarix, GlaxoSmithKline), licensed in 2009.Citation7-9 This study focused on Gardasil and Cervarix, the two HPV vaccines marketed during the study period.
Large clinical trials, with over 18,000 females aged 15 to 25 years for Cervarix, and over 20,000 females aged 16 to 26 for Gardasil, have demonstrated high levels of efficacy for both vaccines.Citation10-13 During Gardasil clinical trials, about 2.3% of vaccinated girls and women 9 to 26 years of age had evidence of new onset systemic autoimmune disorder with a majority (1%) having arthritis/arthralgia/arthropathy; the rate of autoimmune disorders among female placebo recipients was similar, also around 2.3%.Citation14
In the largest Cervarix randomized, controlled trial, which enrolled females 15 through 25 years of age and included active surveillance for potential new onset autoimmune diseases (NOADS), the incidence of potential NOADs was 0.8% among subjects who received Cervarix (78/9,319) and 0.8% among subjects in the comparison group, who received the Hepatitis A vaccine instead.Citation15
Over the past decade, reports of serious adverse events (AEs) including autoimmune diseases among vaccine recipients have heightened the public's concern regarding safety of the new vaccines.Citation16-19 Some AEs temporally associated with HPV vaccinesCitation20-23 have caused debate among providers and the general public, although there are no data to support a causal association. Based on results from clinical trials, post-licensure surveillance, and observational studies, FDA and CDC consider the HPV vaccines safe and effective. There is, however, continued post-licensure monitoring of adverse event reports, as is done for all vaccines, including for autoimmune diseases occurring after HPV vaccination.Citation24-26 Nonetheless, case reports of optic neuritis after HPV vaccines have been made.Citation27 While these case reports document temporal association, they cannot establish causality and could be coincidental.Citation27
Population-based studies have also investigated the potential association between vaccination and autoimmune diseases. An epidemiologic study, which used an integrated safety database, examined potential autoimmune diseases following administration of adjuvanted vaccines (e.g., HPV, HBV, and HSV), and did not show statistically significant differences in event rates between the vaccinated and control groups.Citation25,28,29 A recent case-control study conducted in France to investigate the association between Gardasil and risk of autoimmune disorders showed no evidence of increased risk following HPV vaccination.Citation30 Also, a recently published study from 2 Scandinavian countries, using nationwide registries, followed a cohort of almost 4 million females for up to seven years and found no association between the quadrivalent HPV vaccine and multiple sclerosis or other demyelinating diseases.Citation31
Although temporal associations of adverse events with vaccination will always occur, temporal associations alone do not prove causality.Citation26,32 Because HPV vaccines are administered to young females who are more likely to develop autoimmune diseases irrespective of vaccination, these events could occur by chance alone. To evaluate such associations, safety surveillance systems must be able to analyze large-scale databases to rapidly identify and properly evaluate rare potential AEs.Citation33,34 A critical first step to using such data is to demonstrate that the automated claims-based case definitions used represent true clinical disease. If the predictive values are high and consistent, then such algorithms can be efficiently used for near real-time surveillance of outcomes of interest.
The objectives of this study were (i) to develop a claims-based algorithm for identification of incident optic neuritis (ON) and to evaluate the algorithm's positive predictive value (PPV) based on the medical chart review, and (ii) to evaluate the association between HPV vaccination and occurrence of ON.
Results
Chart validation
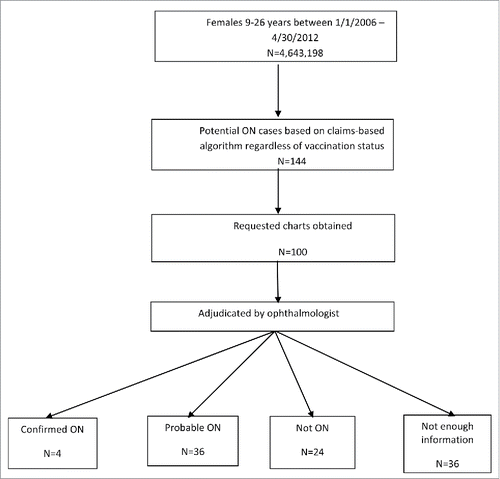
Overall, 144 patients were identified as potential ON cases using the automated claims-based algorithm, regardless of vaccination status, and 100 (69%) of the requested charts were obtained. The remaining 44 charts were not available from the provider. On adjudication by the ophthalmologist, 4 were confirmed as ON cases, 36 were considered probable ON cases, 24 were determined not to be ON, 31 charts did not have enough information to make a determination whether the patient had ON or not, and 5 charts did not have any information relevant to ON ().
Based on the claims-based algorithm developed for ON, the PPV for confirmed or probable ON cases was calculated as 40.0% (95% CI: 30.3%–50.3%) utilizing the 100 charts obtained as the denominator. In addition, the PPV was also calculated using as denominator the 64 charts that had enough information for the reviewers to make a determination, resulting in a corrected estimated PPV of 62.5% (95% CI: 49.5%–74.3%).
Vaccine safety analysis
Self-controlled temporal scan statistic
The number of exposures to Cervarix (2,614 individuals) was small, and no outcomes of interest were identified after Cervarix vaccination. Hence, although we found no evidence of Cervarix causing ON in our data, the limited data we had were not conclusive to rule out an association. Further, only 1,104 members (0.25%) were exposed to both Gardasil and Cervarix vaccinations. If ON occurs in these participants, it would be difficult to attribute the outcome to one vaccine or the other and hence they were excluded. Therefore, only outcomes after Gardasil vaccination were evaluated using formal statistical analyses.
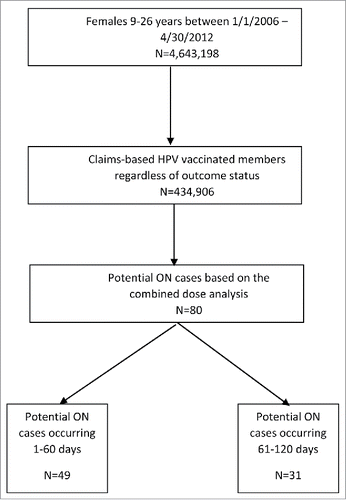
The administrative claims data identified a total of 434,906 members receiving at least one dose of HPV (Gardasil) vaccination. The mean age of this population was 16.2 ± 4 years with a median of 16 years. Overall, 29% of the vaccinated cohort lived in the South of the U.S., followed by 26% in North-East, 24% in Mid-West and 20% in the West. Among them, 315,611 and 185,523 had claims evidence of receiving at least two and three HPV vaccine doses, respectively.
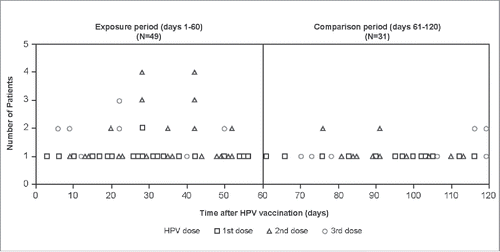
In total, prior to chart confirmation, there were 80 potential ON cases with onset within 120 days after any of the three doses, with 49 occurring in the 1–60 days and 31 in 61–120 days post vaccination (). The data were also represented in a stacked strip plot ().
Table 1. HPV vaccination doses and occurrence of potential ON in the self-controlled temporal scan statistic.
For potential ON cases, temporal scan analysis was conducted separately for each of the three doses (). In the primary analysis, for which the only restriction was the timing of the vaccination schedule, the most likely cluster of 4 potential cases was identified 26–28 days after the first dose, with a statistically non-significant increased risk of potential ON (RR = 5.38; p = 0.25). After second dose, there was a statistically significant increased risk of potential ON with a cluster of 18 cases identified 20–52 days after vaccination (RR = 3.39; p = 0.03). No statistically significant increased risk of potential ON was identified after the third dose (6–22 days; RR = 4.04; p = 0.26) or in the combined (all doses) analysis (9–56 days; RR = 2.03; p = 0.06) ().
Table 2. Association between HPV vaccination and occurrence of ON using temporal scan statistic.
The secondary analysis (which applied additional restrictions on time intervals between the three HPV doses) showed no statistically significant clusters for any of the individual doses or the combined all doses analysis. The individual dose as well as the combined all doses secondary analyses did not show any significant increased risk of potential ON (). Temporal scan analysis was not conducted on the chart-confirmed or probable ON cases as only four of the chart-confirmed ON cases had claims evidence of HPV vaccination: two of the ON outcomes occurred prior to HPV vaccination, one occurred 366 days after vaccination, and one ON case occurred 20 days after vaccination.
Matched cohort design
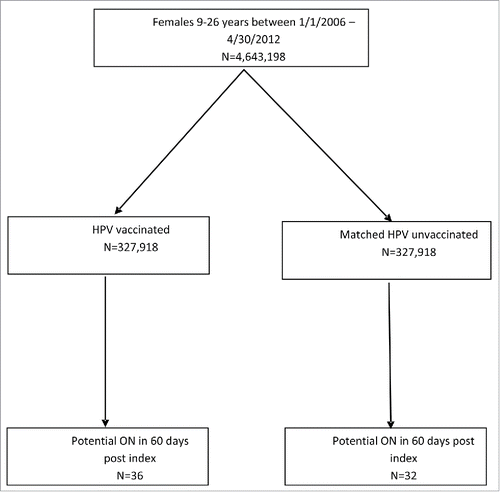
Among the 327,918 HPV vaccinated (exposed) and the matched HPV unvaccinated/well-child visit (unexposed) individuals, about 2% of the exposed had a prior history of other autoimmune diseases other than ON, as opposed to 1% of the unexposed members (). All the exposed members had history of other vaccinations including the routine vaccinations. About 9.5% of the exposed and 8.4% of the unexposed members had higher enhanced Deyo-Charlson comorbidity index. There were almost equal proportions of exposed and unexposed members in the North, East, and South of the U.S., with slightly higher exposed members in the Mid-West and slightly higher unexposed in the West ().
Table 3. Baseline characteristics for HPV vaccinated patients and matched HPV unvaccinated patients.
Overall, there were 68 potential ON cases identified using the automated claims-based algorithm with onset in the 60 days after index date in either the exposed or unexposed cohorts. Of these, 36 cases occurred in the exposed cohort and 32 in the unexposed cohort. There were 16, 17 and 3 potential cases in the 60 days after first, second and third doses, respectively, for the exposed cohort, and 16, 13 and 3 cases in the 60 days after the first, second and third dose, respectively, for the unexposed cohort ().
Table 4. Occurrence of potential ON in the HPV vaccinated and matched HPV unvaccinated groups in the 60 days following index dateFootnote* in the matched cohort design.
The unadjusted analysis showed no statistically significant increased risk of potential ON with any dose in either the individual and combined all doses analyses (). In the adjusted analysis, there was no statistically significant increased risk of potential ON following any dose both in the individual and combined all doses analyses (). Adjusted analysis was not done for the third dose as the models did not converge due to the small number (6) of potential ON outcomes.
Table 5. Association between HPV vaccination and occurrence of potential ON in the matched cohort design.
There was a consistent statistically significant increased risk of potential ON observed for all members with a history of other prior autoimmune conditions. The risk of potential ON in the 60 days post-index date was higher among those who had a history of autoimmune diseases than among those who had no such history, regardless of whether they belonged to the HPV vaccinated or the unexposed cohort. This trend consistently persisted with the first, second dose and the combined all doses analysis ().
Discussion
To our knowledge, this study was among the first to utilize a large administrative claims database to identify and validate algorithms for autoimmune diseases using medical chart review, and analyze the association between HPV vaccination and autoimmune diseases of interest.Citation25,31,37 The extensive claims-based algorithm developed based on the process of medical care was able to identify potential cases of ON from the claims data, but the PPV for case adjudication was low (40% using all charts obtained and 62.5% using as denominator the 64 charts that had enough information for the reviewers to make a determination). In our validation study, among 144 patients with optic neuritis, medical charts were unobtainable for 30.6% (44 patients) due to various reasons such as providers reporting that no records were available or there was no record of the patient. In some instances, providers were not identifiable from the facility. Also, some providers required local IRB approval and hence the chart was not obtained. For the 100 patients with charts available versus the other 44 patients for whom charts were unavailable, we compared the baseline demographics and risk factors including HPV vaccination status, age at first HPV vaccination, age at first onset of optic neuritis, region of residence, baseline Charlson comorbidity index and other vaccinations or adverse events in the baseline period, and did not find any statistically significant differences between the groups. Based on these comparisons, and from the fact that, in previous studies where medical charts were requested, chart missingness appeared mainly to be associated with characteristics of the institution providing the chart and not with specific characteristics of the patients themselves, it seemed likely that patients with and without charts represented the same source population. Nevertheless, the low PPV found highlights the difficulties in using claims-based algorithms for case confirmation of a complex autoimmune disease such as ON.
Because the risk window for ON could not be specifically defined a priori, we conducted a self-controlled temporal scan analysis to determine the most likely risk window, adjusted for the multiple testing inherent in the many potential risk windows evaluated. Despite the large database size, there was only one chart confirmed case with onset 20 days after vaccination, which was not sufficient to perform the temporal scan analysis. This illustrates the difficulty of studying such a rare event. The temporal scan analysis, performed only for potential ON cases, did not show statistically significant association with HPV vaccination, except following the second dose in the primary analysis. In the secondary temporary scan analysis, which respected the product indication with respect to timing between doses, there was no significant increase in claims-based ON risk for any individual dose or for the combined doses. We also conducted the sensitivity analysis by excluding chart confirmed non-ON cases and obtained similar results; the increased risk of potential ON after the second dose became not statistically significant with a smaller relative risk. Because a second dose vaccination could occur during the control period for the first dose (days 61–120), cases occurring during this period could be assigned to the control window for the first dose. If an association with second dose existed, this would create a situation in which an AE potentially caused by the second dose vaccination would appear in the control period for the first dose, increasing the relative incidence in the unexposed period, thereby making it less likely to be detected in association with dose one. Nonetheless, the matched cohort study, conducted using a 60-day post-vaccination risk window, did not show statistically significant associations between any HPV vaccination and potential ON.
Our study utilized medical claims databases that had inherent limitations, including potential under- or misrecording of autoimmune diseases of interest and limited availability of medical records for diagnosis verification. We expect the capture of HPV vaccination status through the claims data to be accurate since this is an expensive vaccine. Though the study period spanned over 5 years, the total number of ON cases was relatively small. Validation of the ON algorithm was difficult because only a subset of the medical charts was obtained and an even smaller subset had information useful for case adjudication. The difficulties in implementation of the claims-based algorithm, and the low PPV obtained for ON, highlights the difficulties inherent to the determination of such complex diagnosis using claims-based algorithms. Also, the need for medical charts with complete information regarding multiple contacts with the health system multiplied the difficulty of performing case confirmation following identification of probable cases from our claims-based system. There was a consistent statistically significant increased risk of potential ON for participants with prior history of other autoimmune conditions compared to those who did not have such history in all analyses, regardless of whether they belonged to the HPV vaccinated or unexposed cohorts. This finding would need further investigation in an appropriate setting.
In summary, identifying confirmed ON cases through administrative claims data proved challenging. Although this claims-based analysis did not provide evidence for an association of ON with HPV vaccination, additional data from larger studies, including case confirmation by medical chart review, will be needed to confirm or refute our findings. Also, future studies are needed to revise the claims-based algorithm to identify true cases of ON and other autoimmune diseases, so claims-based studies can be performed in the future to rapidly identify suspected associations between HPV and other vaccinations and autoimmune diseases.
Methods
Data source and study population
This study used the HealthCore Integrated Research Database (HIRDSM), which is a broad, clinically rich, and geographically diverse spectrum of longitudinal medical claims, pharmacy claims, enrollment, and electronic outpatient laboratory results data from commercially insured health plan members in primarily 14 different states. The HIRDSM covers about 60 million researchable lives with medical eligibility. Our study population included all females 9–26 year olds in the HIRDSM with and without claims evidence of HPV vaccination between January 2007 and April 2012.
Vaccine exposure and outcomes
HPV vaccination status was assessed using CPT and NDC codes from medical and pharmacy claims, respectively.
Optic neuritis (ON) was defined based on an algorithm developed using administrative claims data including medical claims. This outcome was chosen because the acute nature of the condition makes it possible to identify onset time using administrative data. The algorithm evaluated occurrence of incident ON diagnosis associated with evaluation and management (E&M) codes using CPT codes diagnosed by a neurologist or an ophthalmologist along with claims evidence of an MRI within four weeks of the ON diagnosis.
Other covariates of interest included demographics such as age, region of residence, Enhanced Charlson comorbidity index in the one year prior to vaccination, history of other vaccinations in the 90 days prior and 30 days post vaccination, and history of other autoimmune diseases in the year prior to vaccination, including but not limited to autoimmune thyroid diseases (Hashimoto's thyroiditis, Graves' disease, chronic fibrous thyroiditis), autoimmune demyelinating diseases (multiple sclerosis, neuromyelitis optica, Guillain Barre syndrome). The covariates were determined using beneficiary enrollment data and medical and pharmacy claims data.
Study design and statistical analysis
Chart validation
Potential ON cases were identified from the administrative claims data using claims-based algorithms described earlier by initial screen of automated claims data to create a list of potential ON patients identified using ICD-9-CM diagnosis, ICD-9-CM procedure, CPT, and HCPCS codes, irrespective of the HPV vaccination status. Chronological review of the automated claims data was conducted to identify the most appropriate facility or providers for chart retrieval. Reviews of the redacted medical charts were conducted by clinical reviewers to confirm case status based on the clinical criteria available.
The ophthalmologist reviewed the medical chart and finalized the case status; if there was uncertainty regarding the determination, the ophthalmologist discussed the case with two neurologists to make a final determination by consensus. True ON cases were defined as those who met the international headache society case definition (confirmed cases) or had medical chart evidence of a specialist diagnosis (probable cases).
Following determination of ON cases and non-cases, a PPV was calculated by dividing the number of patients identified by medical record review as true positives (true cases) divided by the sum of the number of patients identified as true positives plus the number of cases identified by chart review as false positives (non-cases).
Patient consent was not required for this study because there was no direct contact with the patient and data were gathered from claims database and chart review. In addition, the Quorum Review IRB has granted a human subjects research waiver. Patient records/information was anonymized and de-identified prior to analysis.
Vaccine safety analysis
Descriptive tables compared baseline characteristics of the vaccinated and non-vaccinated groups by demographics and other risk factors. For the vaccine safety analysis, we employed two study designs, a self-controlled temporal scan statistic as the primary design and a retrospective matched cohort analysis as the secondary design.
Self-controlled temporal scan statistic
Since the risk window for ON following HPV vaccination has not been established, we used a self-controlled temporal scan statistic to review various subsets of the 1–60 days period after HPV vaccination as potential risk windows to identify clusters of cases and compare them to the non-risk 61–120 days post-vaccination window.Citation35,36 This method adjusts for the multiple testing inherent in the many potential risk windows evaluated. The potential risk windows evaluated started between 1 and 30 days after vaccination, ended between 1 and 60 days after vaccination, and were at least 3 days long. For this analysis, we excluded individuals with less than one year of continuous eligibility prior to the first claims evidence of vaccination in the study period, and individuals who had received both Gardasil and Cervarix HPV vaccinations.
We conducted a primary and a secondary analysis. The primary analysis used the data with restrictions only regarding timing of the recommended vaccination schedule (the second dose should occur at least 60 days after the first dose, and the third dose should occur at least 180 days after the first dose). The secondary analysis used the data after applying additional restrictions based on the time intervals between the three doses. The secondary analysis only included those with second dose at least 60 days and at most 180 days after the first dose, third dose at least 180 days and at most 2 years after the first dose and at least 60 days after the second dose.
For both the primary and secondary analyses, the association between HPV vaccination and ON was explored based on individual vaccine dose and for multiple doses. Since routine vaccination is administered as three doses, risk and non-risk windows were evaluated after each dose. For the combined analysis, in addition to the restrictions stated earlier, an additional limitation that the third dose should be given at least 60 days after the second dose was also applied for the primary analysis. Sensitivity analysis was conducted after excluding the cases confirmed by chart review as non-ON cases from the potential ON cases identified using claims-based algorithms described earlier. In addition, Bonferroni correction was done to account for multiple testing. The free SaTScan software (www.satscan.org) was used to conduct the temporal scan statistic analyses.
Matched cohort design
The cohort design included two groups of subjects ages 9 to 26 years: one exposed to HPV vaccination (exposed group), and the other, unexposed to HPV vaccination but exposed to vaccines other than HPV (unexposed group). We only included exposed members with at least one year of continuous eligibility prior to HPV vaccination date. We matched the exposed to the unexposed on age within a year. To ensure that the unexposed members had a healthcare encounter around the same time as the HPV vaccinated members, the unexposed member whose other vaccine administration date was within a month of the corresponding exposed member was chosen as a potential match. When there were no sufficient unexposed individuals to match, we looked for a well-child visit within a month of the HPV vaccination date of the exposed member. We only included unexposed members that had at least one year continuous eligibility prior to their index date (either a vaccine other than HPV or a well-child visit). We repeated this process until matches were obtained for all exposed (HPV vaccinated) members.
The incidence of potential ON identified using claims-based algorithms within the pre-specified risk window (1–60 days after the HPV vaccination date or the corresponding other vaccination/well child visit date) for the 2 groups was compared at the cohort level using conditional Poisson regression, after adjusting for potential confounders. For consistency among the different analytical approaches, we did not evaluate the risk of ON beyond 60 days post vaccination or after the corresponding other vaccination/well child visit date. All analyses were conducted using SAS version 9.4, and all tests were two-sided.
Ethical statements
The Quorum Review IRB has granted a human subjects research waiver. Patient consent was not required for this study because there was no direct contact with the patient and data were gathered from claims database and chart review; Patient records/information was anonymized and de-identified prior to analysis.
Role of the sponsor
The sponsor participated in the design and conduct of the study; interpretation of the data; and preparation, review and approval of the manuscript.
Disclosure of potential conflicts of interest
There are no conflicts of interest for any parties involved with this research.
Author contributions
Drs. Sridhar and Tian had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Izurieta, Sutherland, Forshee, Sridhar, Tian, Selvam,
Acquisition of data: Sridhar, Tian, Selvam
Analysis and interpretation of data: Sridhar, Tian, Selvam, Kulldorff, Forshee, Izurieta, Sutherland, Bryan, Barone, Xu
Drafting of the manuscript: Sridhar, Izurieta, Forshee, Tian
Critical revision of the manuscript for important intellectual content: Izurieta, Forshee, Kulldorff, Sridhar, Tian, Sutherland, Selvam, Bryan, Barone, Xu
Statistical analysis: Tian, Sridhar, Kulldorff, Izurieta, Forshee
Obtaining funding: Selvam, Sridhar, Sutherland, Izurieta, Forshee
Administrative, technical or material support: Sridhar, Tian, Selvam, Forshee, Izurieta
Study supervision: Selvam, Sridhar, Izurieta, Forshee
Funding
This study was funded by the U.S. Food and Drug Administration, Center for Biologics Evaluation and Research.
References
- Centers for Disease Control and Prevention. Incidence, prevalence, and cost of sexually transmitted infections in the United States. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention; 2013
- Parkin DM, Bray F. The burden of HPV-related cancers. Vaccine 2006; 24:S11-25; https://doi.org/10.1016/j.vaccine.2006.05.111
- Watson M, Saraiya M, Wu X. Update of HPV-associated female genital cancers in the United States, 1999–2004. J Womens Health (Larchmt) 2009; 18:1731-8; PMID:19951205; https://doi.org/10.1089/jwh.2009.1570
- Gillison ML. Human papillomavirus-related diseases: oropharynx cancers and potential implications for adolescent HPV vaccination. J Adolesc Health 2008; 43:S52-60; PMID:18809146; https://doi.org/10.1016/j.jadohealth.2008.07.002
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Muñoz N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008; 26:K1-16; PMID:18847553; https://doi.org/10.1016/j.vaccine.2008.05.064
- FDA. Gardasil 9
- Markowitz LE, Dunne E, Saraiya M, Lawson H, Chesson H, Unger E. Quadrivalent human papillomavirus vaccine. MMWR Morb Mortal Wkly Rep 2007; 56:1-24; PMID:17218934
- FDA. Complete List of Vaccines Licensed for Immunization and Distribution in the US
- Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 2010; 59(20):626.
- Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, Puchalski D, Giacoletti KE, Sings HL, Lukac S. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: A randomized controlled trial. Pediatr Infect Dis J 2007; 26:201-9; PMID:17484215; https://doi.org/10.1097/01.inf.0000253970.29190.5a
- Brown DR, Kjaer SK, Sigurdsson K, Iversen O-E, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis 2009; 199:926-35; PMID:19236279; https://doi.org/10.1086/597307
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin 2009; 5:705-19; PMID:19684472; https://doi.org/10.4161/hv.5.10.9518
- Paavonen J, Naud P, Salmeron J, Wheeler C, Chow S, Apter D, Kitchener H, Castellsague X, Teixeira J, Skinner S. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301-14; https://doi.org/10.1016/S0140-6736(09)61248-4
- FDA. Gardasil package insert
- FDA. Cervarix package insert
- Shoenfeld Y, Aron-Maor A. Vaccination and autoimmunity—‘vaccinosis’: A dangerous liaison? J Autoimmun 2000; 14:1-10; PMID:10648110; https://doi.org/10.1006/jaut.1999.0346
- Siegrist C-A, Lewis EM, Eskola J, Evans SJ, Black SB. Human papilloma virus immunization in adolescent and young adults: a cohort study to illustrate what events might be mistaken for adverse reactions. Pediatr Infect Dis J 2007; 26:979-84; PMID:17984802; https://doi.org/10.1097/INF.0b013e318149dfea
- Orbach H, Agmon-Levin N, Zandman-Goddard G. Vaccines and autoimmune diseases of the adult. Discov Med 2010; 9:90-7; PMID:20193633
- Salemi S, D'Amelio R. Could autoimmunity be induced by vaccination? Int Rev Immunol 2010; 29:247-69; PMID:20521925; https://doi.org/10.3109/08830181003746304
- Debeer P, De Munter P, Bruyninckx F, Devlieger R. Brachial plexus neuritis following HPV vaccination. Vaccine 2008; 26:4417-9; PMID:18602437; https://doi.org/10.1016/j.vaccine.2008.06.074
- Borja-Hart NL, Benavides S, Christensen C. Human papillomavirus vaccine safety in pediatric patients: an evaluation of the vaccine adverse event reporting system. Ann Pharmacother 2009; 43:356-9; PMID:19155346; https://doi.org/10.1345/aph.1L492
- Macartney KK, Chiu C, Georgousakis M, Brotherton JM. Safety of human papillomavirus vaccines: A review. Drug Saf 2013; 36:393-412; PMID:23637071; https://doi.org/10.1007/s40264-013-0039-5
- Pellegrino P, Carnovale C, Pozzi M, Antoniazzi S, Perrone V, Salvati D, Gentili M, Brusadelli T, Clementi E, Radice S. On the relationship between human papilloma virus vaccine and autoimmune diseases. Autoimmun Rev 2014; 13:736-41; PMID:24468416; https://doi.org/10.1016/j.autrev.2014.01.054
- Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, Izurieta HS, Ball R, Miller N, Braun MM. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750-7; PMID:19690307; https://doi.org/10.1001/jama.2009.1201
- Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, Kulldorff M, Lewis E, Fireman B, Daley MF. Monitoring the safety of quadrivalent human papillomavirus vaccine: Findings from the vaccine safety datalink. Vaccine 2011; 29:8279-84; PMID:21907257; https://doi.org/10.1016/j.vaccine.2011.08.106
- Slade BA, Gee J, Broder KR, Vellozzi C. Comment on the contribution by Souayah et al.,“Guillain–Barré syndrome after Gardasil vaccinati: Data from vaccine adverse event reporting system 2006–2009”. Vaccine 2011; 29:865-6; PMID:21111783; https://doi.org/10.1016/j.vaccine.2010.11.031
- Sutton I, Lahoria R, Tan I, Clouston P, Barnett M. CNS demyelination and quadrivalent HPV vaccination. Mult Scler 2009; 15:116-9; PMID:18805844; https://doi.org/10.1177/1352458508096868
- Verstraeten T, Descamps D, David M-P, Zahaf T, Hardt K, Izurieta P, Dubin G, Breuer T. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008; 26:6630-8; PMID:18845199; https://doi.org/10.1016/j.vaccine.2008.09.049
- Naleway AL, Gold R, Drew L, Riedlinger K, Henninger ML, Gee J. Reported adverse events in young women following quadrivalent human papillomavirus vaccination. J Womens Health (Larchmt) 2012; 21:425-32; PMID:22229713; https://doi.org/10.1089/jwh.2011.2895
- Grimaldi-Bensouda L, Guillemot D, Godeau B, Bénichou J, Lebrun-Frenay C, Papeix C, Labauge P, Berquin P, Penfornis A, Benhamou PY. Autoimmune disorders and quadrivalent human papillomavirus vaccination of young female subjects. J Intern Med 2014; 275:398-408; PMID:24206418; https://doi.org/10.1111/joim.12155
- Scheller NM, Svanström H, Pasternak B, Arnheim-Dahlström L, Sundström K, Fink K, Hviid A. Quadrivalent HPV vaccination and risk of multiple sclerosis and other demyelinating diseases of the central nervous system. JAMA 2015; 313:54-61; PMID:25562266; https://doi.org/10.1001/jama.2014.16946
- Souayah N, Michas-Martin P, Nasar A, Krivitskaya N, Yacoub HA, Khan H, Qureshi AI. Guillain–Barré syndrome after Gardasil vaccination: data from vaccine adverse event reporting system 2006–2009. Vaccine 2011; 29:886-9; PMID:20869467; https://doi.org/10.1016/j.vaccine.2010.09.020
- Chen RT, Glasser JW, Rhodes PH, Davis RL, Barlow WE, Thompson RS, Mullooly JP, Black SB, Shinefield HR, Vadheim CM. Vaccine safety datalink project: A new tool for improving vaccine safety monitoring in the United States. Pediatrics 1997; 99:765-73; PMID:9164767; https://doi.org/10.1542/peds.99.6.765
- Izurieta HS, Zuber P, Bonhoeffer J, Chen RT, Sankohg O, Laserson KF, Sturkenboom M, Loucq C, Weibel D, Dodd C. Roadmap for the international collaborative epidemiologic monitoring of safety and effectiveness of new high priority vaccines. Vaccine 2013; 31:3623-7; PMID:23707171; https://doi.org/10.1016/j.vaccine.2013.05.027
- Whitaker HJ, Paddy Farrington C, Spiessens B, Musonda P. Tutorial in biostatistics: The self-controlled case series method. Stat Med 2006; 25:1768-97; PMID:16220518; https://doi.org/10.1002/sim.2302
- Kulldorff M. SaTScan user guide for version 9.0. 2011
- Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, Naleway A, Klein NP, Baxter R, Belongia E. The vaccine safety datalink: A model for monitoring immunization safety. Pediatrics 2011; 127:S45-S53; PMID:21502240; https://doi.org/10.1542/peds.2010-1722H