ABSTRACT
The contribution of influenza B to the seasonal influenza burden varies from year-to-year. Although 2 antigenically distinct influenza B virus lineages have co-circulated since 2001, trivalent influenza vaccines (TIVs) contain antigens from only one influenza B virus. B-mismatch or co-circulation of both B lineages results in increased morbidity and mortality attributable to the B lineage absent from the vaccine. Quadrivalent vaccines (QIVs) contain both influenza B lineages. We reviewed currently licensed QIVs and their value by focusing on the preventable disease burden. Modeling studies support that QIVs are expected to prevent more influenza cases, hospitalisations and deaths than TIVs, although estimates of the case numbers prevented vary according to local specificities. The value of QIVs is demonstrated by their capacity to broaden the immune response and reduce the likelihood of a B-mismatched season. Some health authorities have preferentially recommended QIVs over TIVs in their influenza prevention programmes.
Introduction
Influenza viruses are enveloped negative-strand RNA viruses that are divided into 3 genera: type A, B and C. The vast majority of human disease is caused by types A and B, which are genetically and structurally similar, but differ in biology, evolutionary and epidemiological framework.Citation1-3 Influenza A viruses are subtyped according to 2 surface glycoproteins: haemagglutinin (H) and neuraminidase (N) whereas influenza B viruses form a homogenous group segregated according to 2 antigenically distinguishable lineages (B/Victoria and B/Yamagata).
Influenza viruses undergo constant mutation which enables evasion of existing host immunity leading to recurrent infections that manifest as annual outbreaks.Citation4 Periodically, new A subtypes emerge, resulting in a pandemic; the emergent subtype may replace or less frequently co-circulate with the earlier A subtype (). Currently, 2 influenza A subtypes, A/H1N1 and A/H3N2, and the 2 influenza B lineages circulate globally each year.Citation5,6 In any influenza season, several influenza types, A-subtypes, or B-lineages may co-circulate, such that the annual influenza burden differs unpredictably from year-to-year, potentially fluctuating from age-group to age-group, and from region-to-region.Citation4
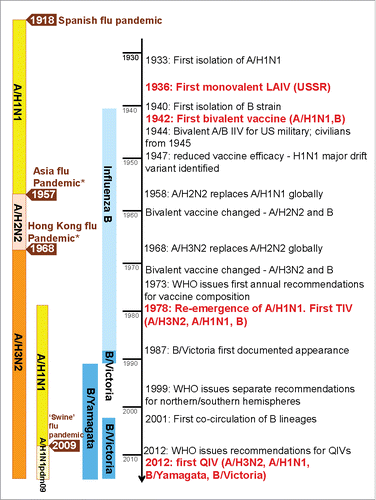
Figure 1. The evaluation of influenza viruses and vaccine development. Information from Hannoun et al.Citation8 and McCullers et al.Citation97 *Asian influenza pandemic caused by the shifted A/H2N2 strain and Hong Kong influenza pandemic caused by the shifted A/H3N2 strain: both examples of A-strain shift through reassortment (sharing) of genetic material. LAIV = live-attenuated trivalent influenza vaccine; TIV = trivalent influenza vaccine; QIV = quadrivalent influenza vaccine; WHO = World Health Organization.
Influenza affects all age-groups and while most deaths occur in older adults, influenza deaths also occur in children. In the United States (US), there were 830 reported laboratory-confirmed influenza deaths between 2004–2012 in children < 18 y of age.Citation7 Of these, 25% were children aged < 24 months.
Since the development of the first monovalent A/H1N1 influenza vaccines more than 75 y ago, influenza vaccines have always required adaptation in response to changes to provide protection against the predominant circulating influenza viruses (). After influenza B was first isolated in 1940, bivalent (A/H1N1, B) vaccines were developed. The vaccine influenza A subtype was changed in 1958 and in 1969 in response to influenza A shifts that triggered severe pandemics (). Seasonal trivalent influenza vaccines (TIVs) containing 2 influenza A -subtype viruses and one B virus were first produced in 1978 after the re-appearance and co-circulation of A/H1N1 with A/H3N2 viruses ().Citation8 Two antigenically distinct lineages of influenza B viruses have circulated globally since 1985 and have co-circulated since 2001.Citation9 The influenza B-lineage vaccine strains induce little or no cross-reactive protection against the alternate B-lineage,Citation10,11 such that for TIVs, protection against the circulating influenza B lineage relies on correctly predicting the B-lineage likely to predominate in the upcoming season. The degree of similarity or difference between the circulating viruses and the strains included in the vaccines is often referred to as “vaccine match” or “vaccine mismatch.” A review of influenza B in 26 countries concluded that the type B lineage selected for inclusion in the annual vaccine differs from the predominant circulating lineage in around 25% of seasons.Citation12 In a mismatched season, influenza vaccine effectiveness may be suboptimal against influenza B epidemics, potentially leading to an increased public health burden during those seasons.Citation13-18 This observation led to international cooperation among the scientific community, leading to the development of quadrivalent seasonal influenza vaccines (QIVs) that included both of the circulating influenza B lineages.Citation10,19 In this work we reviewed the development of currently licensed QIVs and provide an overview of their societal value by focusing on the preventable disease burden associated with vaccination. We identified modeling studies estimating the potential impact of QIVs compared with TIVs in terms of clinical outcomes prevented.
Clinical evaluation and characteristics of currently licensed of QIVs
The first QIV was licensed in 2012 and currently 3 manufacturers produce QIV in various forms (inactivated [IIV4] or live-attenuated [LAIV4]), with new vaccines under development.Citation20,21 Seasonal influenza vaccines induce antibody responses against the head of the haemagglutinin glycoprotein. An haemagglutinin inhibition (HI) titer of ≥ 1:40 is generally accepted as indicative of clinical benefit since it has been associated with protection from influenza illness in up to 50% of subjects.Citation22,23
Cumulative evidence for the immunogenicity and safety of QIVs was obtained through studies designed to demonstrate non-inferiority in terms of the HI geometric mean antibody titres (GMTs) and seroconversion rates (SCRs) to the common 3 strains in the candidate QIVs compared with licensed TIVs, and to demonstrate superiority in terms of the HI GMT and SCR of the added influenza type B lineage compared with licensed TIVs containing either the B/Yamagata or B/Victoria lineages. Safety and reactogenicity of QIVs versus TIVs were also assessed.
IIV4s manufactured by GSK
GSK produces IIV4s that are identical in antigen content but manufactured and licensed separately: FluLaval Quadrivalent (Q-IIV4) is manufactured in Quebec, Canada, and Fluarix Quadrivalent (D-IIV4) is manufactured in Dresden, Germany.Citation24 Both vaccines are approved for use in individuals from 3 y of age. Q-IIV4 is also licensed from 6 months of age in Canada, the US, and Mexico. Individuals from the age of 6 months through to adults > 65 y were enrolled in the clinical development program.Citation24 The results of key studies have been recently reviewed.Citation24-26
Fluarix Quadrivalent (Fluarix Tetra, Influsplit Tetra and Alpharix Tetra): In studies in adults, adolescents and children (≥ 3 y of age), HI GMTs and SCRs following D-IIV4 were non-inferior to licensed D-IIV3s for common strains, and superior in terms of the HI GMT and SCR for the additional type B lineage. Addition of the fourth strain had no impact on the reactogenicity and safety profile of the vaccine.Citation27,28 Efficacy of D-IIV4 is supported by the demonstrated efficacy of trivalent Fluarix in healthy adults aged 18–64 y; since both vaccines use the same manufacturing processes. In adults, Fluarix demonstrated statistically significant efficacy of 66.9% against culture-confirmed antigenically-matched influenza A and/or B (95% confidence interval [CI] 51.9–77.4).Citation29
FluLaval Quadrivalent (Flulaval Tetra): In studies in adults, adolescents and children (≥ 3 y of age), the immunogenicity of Q-IIV4 was non-inferior to that of licensed IIV3s for common strains, and superior for the additional type B strain from the alternate lineage in terms of HI GMTs and SCRs. Similar to D-IIV4, addition of the fourth strain did not impact the reactogenicity and the safety profile of Q-IIV4 as compared with IIV3s.Citation30,31
In children 6 to 35 months of age, Q-IIV4 (containing 15 μg of each virus strain) was non-inferior to Fluzone Quadrivalent (F-IIV4, Sanofi Pasteur) (containing 7.5 μg of each virus strain) for each vaccine strain, and superior in terms of the immune response to both influenza B strains in 6–17 month old children and unprimed children of any age.Citation32
Efficacy of Q-IIV4 was demonstrated in children aged 3−8 y (N = 5,220) who were randomized to receive either Q-IIV4 or inactivated hepatitis A vaccine as control.Citation33 Efficacy of Q-IIV4 in preventing influenza of any severity was 55.4% (95% CI 39.1–67.3), and efficacy against moderate-to-severe influenza (defined as a body temperature > 39°C, acute otitis media, lower respiratory tract illness, or serious extra-pulmonary complications) was 73.1% (97.5% CI 47.1–86.3).Citation33 Influenza cases in the Q-IIV4-vaccinated group tended to be of mild clinical severity and were associated with substantially lower numbers of medical visits, hospitalisations, school absences and parental absences from work than episodes of influenza in the control group.Citation33
IIV4s manufactured by Sanofi Pasteur
Sanofi Pasteur manufacturers 2 IIV4s administered either intramuscularly (F-IIV4 licensed for use from 6 months of age) or intradermally (Fluzone Intradermal Quadrivalent [intradermal F-IIV4] licensed for individuals between 18–64 y of age).
Fluzone Quadrivalent: F-IIV4 was evaluated in 3 clinical trials enrolling > 5,500 participants aged 6 months-9 y, ≥ 18 y and ≥ 65 y, respectively.Citation34-36 Non-inferiority was demonstrated between F-IIV4 and IIV3s in terms of HI GMTs and SCRs for common strains in all of these age-groups; except for the SCR for the H1N1 strain in subjects aged ≥ 65 y. The failure to meet this non-inferiority criterion may be related to a high baseline prevalence of seropositivity against this strain.Citation34 Superiority of the immune response to the added influenza type B lineage compared with 2 licensed IIV3 formulations in terms of GMTs and SCRs was also demonstrated in all age-groups, except for the GMT ratio for the B/Victoria lineage strain in adults aged ≥ 65 y. At least 73.2–100% of adults and 66.9%–98.8% of children who received F-IIV4 had HI titres ≥ 40 after vaccination. The safety profile of F-IIV4 was similar to that of the studied IIV3s.Citation34
Fluzone Intradermal Quadrivalent: This intradermal vaccine is licensed for use in adults aged 18–64 y and was evaluated in 3360 participants in one study in the US.Citation37 Non-inferiority was demonstrated between the intradermal F-IIV4 and intradermal IIV3 in terms of HI GMTs and SCRs to common strains, and superiority of the immune response to the added influenza type B lineage compared with 2 intradermal IIV3 formulations was also demonstrated. At least 86% of intradermal F-IIV4 recipients had HI titres ≥ 40 after vaccination.Citation37 The safety profile of intradermal F-IIV4 was similar to that of intradermal IIV3s.
LAIV4s manufactured by AstraZeneca
Flumist Quadrivalent (US, Canada) and Fluenz Tetra (European Union) are licensed for use in individuals from 2–59 y internationally and 2–49 y in the US. Two clinical trials conducted in the US evaluated immunogenicity and safety in subjects aged 2–49 y.Citation38-41 Non-inferiority of the HI antibody response to LAIV3s was demonstrated for common strains. Post-hoc analyses demonstrated superiority of the HI antibody response for the added B lineage.Citation38 The safety and reactogenicity of LAIV4s were similar to LAIV3s.
The value of QIVs in reducing the burden of influenza B – review of the literature
Influenza B causes epidemics approximately every 2–4 yearsCitation42,43 that impact all age-groups but proportionally more older children.Citation43-47 Influenza B has been reported to be clinically indistinguishable to influenza A,Citation48-50 and has been linked to severe disease, including encephalitis, myositis, pneumonia and fulminant disease in children.Citation51-54 The majority of subjects with lethal influenza B in a US case series died before they could be hospitalised, highlighting the importance of vaccine prophylaxis in mitigating this risk.Citation54
A systematic review of the literature describing influenza B disease published between 1995 and 2010 concluded that influenza B was more likely to be severe in children than in adults.Citation55 Although influenza B affects all age segments, it is more common among children aged 5–17 y.Citation12,55 Influenza B accounts on average, for approximately 20–30% of influenza isolates from respiratory samples across seasons,Citation12,56 although the reported frequencies vary from year-to-year and from region-to-region, ranging from 0–62.9% in children and 0–48% in adults.Citation55 The Danish 2015/16 influenza season was characterized by a major (88%) B-lineage mismatch and it has been put forward that morbidity during the season may have been lower if QIVs had been used instead of TIVs.Citation18 More recently, a comprehensive review of the influenza B burden in 9 European countries highlighted the scarce attention that the influenza B burden has received over previous decade as compared with influenza A.Citation57 This works also underscores the lack of predictable patterns in strain circulation seen in these countries and thus the continuous risk of mismatch when there is high influenza B circulation.
For QIVs, modeling studies are sometimes used to assess cost-effectiveness and whether the added value of the vaccine is likely to offset the added cost; models may also contribute information about the societal value of QIVs quantifying the number of preventable influenza outcomes compared with TIVs. The reported added value of QIVs comes from its capacity to provide broader immunity against influenza B, thereby reducing the likelihood of a mismatched season.
We systematically queried the PubMed database for papers reporting the potential value of QIVs compared with TIVs in terms of illness, hospitalisations and deaths averted using the search string provided in . Articles were selected by a 3-step selection procedure based on 1) screening of title and abstract, 2) screening of full-text article, and 3) final screening during the data-extraction phase. The titles and abstracts retrieved from the Pubmed database were screened in duplicate by 2 independent researchers. The results were compared and discussed; all selected references from the 2 researchers were included for full text selection.
Figure 2. Results of the literature search (30 October 2016). Search string: “(Quadrivalent OR tetravalent) (influenza vaccine OR flu vaccine) (cost OR burden OR epidemiology OR death OR mortality OR illness OR hospitalisation OR hospitalization)” No limits applied.
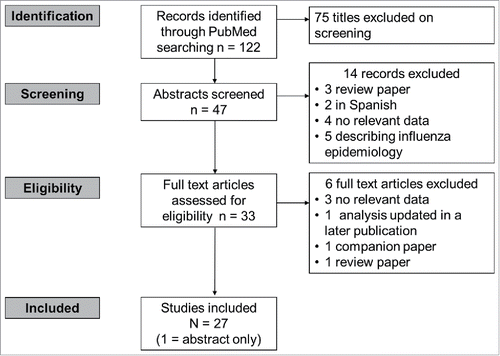
In case of discrepancy or disagreements during the selection, a third researcher was consulted and the study was discussed until consensus was reached. If articles reported on the same study, the most relevant or most complete article was included in this review. If articles complemented each other, both articles were included. The reasons for exclusion of full-text papers were recorded.
We identified 27 eligible studies from 14 countries that used multiple modeling methods and highly variable background assumptions to estimate the potential impact of QIVs over TIVs in various immunisation scenarios (). All of the estimates were highly dependent upon the choice of baseline data, such as the assumed degree of cross-protection afforded by TIVs and the level of mismatch in a given season. In all cases, the results of more conservative static models (i.e., that could not adjust for potential herd protection or reduction in disease transmission) were lower than dynamic models that included herd-protection or disease transmission effects in the model. A summary of current disease burden information and the potential impact of QIVs on the influenza burden from modeling studies is presented for selected countries below.
Table 1. Summary of QIV modeling data in different countries: results from a review of the literature.
United States
A prospective study of medically-attended visits for influenza-like-illness (ILI) in the US from 2009–2013 identified influenza B in 29.0% of influenza-positive respiratory specimens from patients of all ages attending outpatient clinics.Citation56 In each study year the incidence of visits for ILI caused by influenza B was highest in 5–17 year-olds (range 1.0–13.3/1000 population between 2010–2013).Citation56 Retrospective studies reported that around 42% (n = 6,084,951) of annual influenza-attributable office visits in < 65 y olds (2001–2009), and 30% of annual influenza-attributable hospitalisations in all ages (1997–2009), were attributable to influenza B (mortality rate 30/100,000 population).Citation45,47 In 4 of 12 seasons encompassed in one study, 51–95% of all influenza-associated deaths were attributed to influenza B.Citation45
The impact of using QIVs as compared with TIVs in the US was estimated in 7 studies (). Reed et al.,Citation58 estimated that over the 10-year period between 1999 and 2009, QIVs could have prevented 2.7 million more influenza cases, 21,440 more hospitalisations and 1,371 more deaths than TIVs. The preventable burden each year ranged from 2,200–970,000 illnesses, 14–8,200 hospitalisations and 1–485 deaths. The all-age estimate obtained by another static model appeared similar, suggesting that QIVs would prevent 30,251 more influenza cases annually than TIVs.Citation59
Using data from a study that compared the difference between vaccination with QIVs vs. no vaccination and vaccination with TIVs vs. no vaccination, it can be estimated that in one average year, use of QIV rather than TIVs among ≥ 65-year-olds, would prevent 26,701 illnesses, 1,345 hospitalisations and 211 deaths in this age-group.Citation60 A second study that considered adults aged 65 y and older estimated that using QIVs instead of TIVs would prevent 39,136 additional influenza cases, 1,648 additional hospitalisations and 458 additional deaths, annually.Citation61 Differences in the model and in the underlying assumptions likely account for the different estimates.
Four studies used dynamic models that incorporated effects of disease transmission and herd-protection in the model (). As a result, the estimates obtained from these models were higher than those from more conservative static models: Crépey et al.,Citation62 estimated that use of QIVs in the US would have averted 15.8% more influenza B cases, or more than 6.2 million influenza cases (average 482,000 per year), than TIVs over the 13-year study period (2000–2013). Using a similar model, de Boer et al., estimated that replacing TIVs with QIVs in the next 20 y (2014–2034) would reduce influenza B cases by 27.2%, or 16 million cases.Citation63 Mullikin et al.,Citation64 calculated influenza illnesses prevented by TIVs, QIVs (or an adjuvanted TIV in elderly with TIV in other ages) compared with no vaccination, and estimated that in an average season with an average vaccine match (obtained using 1999–2014 US data), vaccination with QIVs would prevent 1,382,509 more illnesses than vaccination with TIVs (an estimate which is higher than the upper range of that from Crépey et al.Citation62), 18,354 hospitalisations and 2,981 deaths. Brogan et al.,Citation65 estimated that over a 10-year period, QIVs instead of TIVs would prevent 1,973,849 additional influenza cases and 1,396 deaths annually. These studies illustrate that although the impact of QIVs may vary in any influenza season, the multi-year cumulative benefit of QIVs over TIVs are predicted to result in substantial societal benefit. This is particularly true for a dynamic model that accounts for herd immunity.
Canada
B-lineage mismatches between the vaccine and circulating strain occurred in 7 out of 15 seasons in Canada between 2001–2015.Citation4 During the 2011/12 influenza season the mismatched influenza B lineage contributed substantially to serious outcomes in adults; the hospital-based Serious Outcomes Surveillance Network in Canada identified influenza B in 65% of adults hospitalised with influenza, of which 68% (205/383) were due to a vaccine mismatched lineage.Citation66 Around 12% of influenza B cases required admission to intensive care. The 30-day mortality was 3% for the vaccine-matched lineage and 10% for the mismatched lineage.
Two studies estimated the potential impact of QIVs over TIVs in Canada: one used a static model and the other a dynamic model. The static model estimated that use of QIV would prevent 2,516 cases, 27 hospitalisations and 5 deaths annually in addition to TIVs.Citation67 The dynamic model estimated that use of QIVs would prevent 135,538 cases, 1,876 hospitalisations and 328 deaths annually in Canada compared in addition to TIVs.Citation68
United Kingdom (UK)
Modeling studies conducted over 12–14 seasons (1995/1996/1997–2009) with results extrapolated to the total UK population estimated seasonal rates for general practitioner visits, hospitalisations and deaths attributable to respiratory disease caused by influenza B of 355/100,000, 2/100,000, and 1/100,000 population respectively.Citation43,69 Around one-third of visits to a general practitioner for otitis media were attributed to influenza type B.Citation43 The number and rate of hospitalisations due to influenza B-attributable respiratory disease was highest in 5–17 year-olds and exceeded that due to influenza A in some seasons.Citation69
Over a 13-year period in Scotland (2000–2012), influenza B detections were close to, or exceeded influenza A detections for 6 out of 13 seasons.Citation70
Two studies in the UK have compared the impact of TIVs and QIVs in those ≥ 65 y of age and clinical at-risk groups, and its implementation in the whole population.Citation68,71 In a static model that considered vaccination of elderly and at-risk groups, QIVs are expected to prevent a total of 183,844 cases, 4,871 hospitalisations and 2,142 deaths due to influenza B over a cumulative 10-year time horizon.Citation71 A separate study using a dynamic model reported that vaccination of all age-groups (current vaccine coverage rates) was estimated to prevent 88,755 cases, 1,050 hospitalisations and 230 deaths due to influenza B annually.Citation68
Germany
In a prospective surveillance study of children ≤16 y of age hospitalised with acute respiratory infection, influenza B was associated with pneumonia in 36% of cases (5/14) and by myositis in 7% (1/14).Citation72 In the same study, influenza A infection was associated with pneumonia in 25% (26/102) of cases, otitis media in 25% (26/102) of cases, anaemia and syncope each in one case.
Two studies used a simulation model that incorporated cross-immunising events and waning/boosting of immunity over a 20-year time horizon.Citation73,74 One study estimated that compared with TIVs, QIVs would prevent 11.2% more influenza B cases. Citation73 Each year this would reduce the annual number of influenza cases by 3.6% in 0–15 y olds, 4.0% in 16–60 y olds, 6.9% in those aged ≥ 61 y and 4.3% (or 395,000 cases) overall.Citation73
The second study estimated that QIVs would prevent 4% more influenza cases, 5.7% more hospitalisations and 6.4% more deaths than TIVs.Citation74
Finland
A dynamic model using data from the 2000 to 2009 influenza seasons estimated that in the Finnish population, QIVs would prevent 40,500 cases, 360 hospitalisations and 54 deaths each year compared with TIV.Citation75 The number of cases was estimated to be even further reduced if the QIV used in 2–18 y olds was LAIV4.
Belgium
Extending the current Belgian influenza immunisation strategy from individuals at-risk (including those aged 50 y or over) to include children 2–17 y of age was assessed in a dynamic model.Citation76 Assuming 50% coverage of QIVs among 2–17 y olds, 2.95 million cases of influenza were calculated to be averted over a 10-year period compared with no vaccination of this age group. Most (63%, or 1,869,582) of these averted cases were due to indirect effects in adults in whom vaccine coverage rates with TIV were assumed to be unchanged. Of a total of 16,968 averted hospitalisations and 1,455 averted deaths, 11,567 and 1,455, respectively, were in adults.
Spain
Switching from TIVs to QIVs in at-risk individuals from 3 y of age and in those aged 65 y and over was estimated to prevent an additional 18,565 influenza cases, 407 hospitalisations and 181 deaths in the first year after implementation.Citation77
Italy
QIVs were introduced in Italy for the 2015/16 season. Assuming that QIVs were used in 9% of the population targeted by the national immunisation program (all individuals at risk and those aged ≥ 65 y), 1,601 additional cases of uncomplicated influenza and 1,031 additional cases of complicated influenza were estimated to be averted compared with TIVs used alone.Citation78
France
By switching from QIVs to TIVs in France during an average epidemic season between 2003 and 2012, it was estimated that 6,214 consultations for influenza, 614 additional hospitalisations and 372 deaths would have been averted.Citation79
European Union
A static model using data from France, Germany, Italy, Spain and the UK, estimated that replacement of TIVs with QIVs during 2002–2013 (2009 season excluded) would have prevented 1,034,727 additional influenza cases, 24,453 hospitalisations and 9,799 deaths.Citation80 Extrapolation to the 27-EU suggested that QIVs would have prevented an estimated 1,624,533 influenza cases, 37,317 hospitalisations and 14,866 deaths. The majority of hospitalisations and almost all deaths prevented would have been in high-risk groups or the elderly.
Hong Kong
Using hospital records to identify laboratory-confirmed influenza cases between 2000–2010, Chan et al.,Citation81 estimated that the average annual incidence of hospital admission (all ages) due to influenza B was 20.6/100,000 population. The highest annual hospitalisations rates were in children < 5 y and 5–9 y of age (median 238/100,000 and 152/100,000, respectively). Both influenza B lineages co-circulated for 9 out of the 10 study years. In the 6 y where a single influenza B lineage predominated (defined as > 80% of identified strains), a mismatch between the predominant B-strain and the vaccine strain occurred in 4. Modeling using Hong Kong death statistics and sentinel laboratory surveillance between 1998–2009 estimated an annual excess mortality of 2.5/100,000 person-years due to influenza B, increasing to 20.3/100,000 person-years in adults aged ≥ 65.Citation82
Static models estimated that compared with TIVs, QIVs used at all ages would prevent 91 influenza illnesses per 100,000 population, 1.8/100,000 hospitalisations and 0.046/100,000 deaths over 1 y ().Citation83 Vaccination of ≥ 65 year-olds with QIV between 2001 and 2009 is estimated to have prevented 191.3 influenza illnesses per 100,000 population in elderly, with the highest reduction in those aged ≥ 80 y (reduction of 451.4 illness per 100,000 population).
Australia
The 2015 influenza season in Australia was dominated by influenza B, which accounted for 62% of all cases notified;Citation84 38% of influenza B isolates were the B/Victoria lineage not included in the 2015 southern hemisphere TIVs. The highest influenza B notification rate was in children 5–9 y of age, followed by 0–4 y and 0–14 y.Citation84
A static model using 2003–2013 data from a single town in Western Australia estimated that compared with TIVs, QIVs would reduce influenza illness by 3.8%, hospitalisations by 2.2% and deaths by 2.1%.Citation85 The authors noted that there was a good match between the vaccine and circulating lineages during the study period in Australia, under which scenario the benefit of QIVs over TIVs is marginal.Citation85 The same study also investigated the impact of QIVs over TIVs in a rural area of South Africa, and reported a greater impact due to a higher degree of mismatch in the seasons studied ().
A second static model estimated that the use of QIVs instead of TIVs in children and adults at-risk for influenza (eligible for free vaccination as defined in the Australian Immunisation handbook) between 2002 and 2012, would have reduced influenza cases, hospitalisations and deaths by 1.02% (68,271), 2.4% (n = 3,522) and 3.7% (n = 683), respectively.Citation86 The highest impact of QIV was in young children and the elderly. In adults aged 65 y and over, QIVs were estimated to prevent an additional 10.1 hospitalisations and 2.1 deaths per 100,000 person-years over TIVs.
Current recommendations for QIVs
Since licensure of the first QIV in 2012, an increasing number of countries use QIVs either permissively (TIVs or QIVs), or preferentially (TIVs available but QIVs preferred) (). However, while several supranational organisations acknowledge that QIVs may improve protection against influenza B strains compared with TIVs, at this time, most health authorities do not preferentially recommend one over the other (). Health authorities are generally concerned that with a trend toward an increased influenza vaccine uptake overall, preferential recommendations may be counterproductive if a preferred product is not sufficiently manufactured and supplied to cover the entire population to be vaccinated.
Table 2. Current recommendations for influenza vaccination using quadrivalent seasonal influenza vaccines (QIVs).
After successful pilot programmes in 2013 and 2014, influenza vaccination using LAIV4 is now offered to 2–4 y old school children in the UK, as well as to at-risk groups from 6 month to 17 y of age.Citation87 This innovative initiative aims to reduce disease incidence in children and transmission to older age-groups, thereby achieving societal benefit at the overall population level.Citation88
Conclusions
Although varying from year-to-year, on average, influenza B causes up to one-third of influenza infections each season. A large body of evidence from numerous countries demonstrates that influenza B accounts for a significant proportion of the overall burden of influenza that inundates healthcare services annually. Once thought to cause predominantly mild illness, numerous studies now indicate that there is little difference in the clinical symptomatology and outcomes of influenza B vs. A. Hospitalisations and mortality attributable to influenza B may have previously been underestimated, with studies reporting higher mortality following influenza B infection than A in some years.Citation13,89-91 In parallel, the 2 influenza B lineages frequently co-circulate, and due to the complexity involved in accurately forecasting which B viruses will circulate, mismatches between the B strain selected for TIVs and circulating strains have occurred in up to one-half of seasons.Citation4,92 Evidence from clinical trials and observational studies suggest that B-mismatched seasons are accompanied by a higher public health burden than well-matched seasons.Citation15,51 Although the risk of breakthrough influenza A from vaccine strain mismatch remains, the risk of breakthrough influenza B from vaccine lineage mismatch can be eliminated by QIV.
Brazil is unique with regards to the pattern of influenza and viral circulating in this heterogeneous climate. A recent review paper highlighted that over a 9 y follow-up period, influenza B lineages circulated in 3 seasons, of which, during one season, there was a high degree of mismatch between the vaccine lineage and the predominant circulating lineage (91.4% [2013]).Citation93 Within tropical and sub-tropical regions such as Brazil, influenza B can have protracted circulation patterns and co-circulation of both B-lineages may not be uncommon, thus further highlighting the added value of QIVs in these countries.Citation94
Modeling the impact of QIVs over TIVs is challenging, not least because of the unpredictable disease burden that differs markedly from year-to-year.Citation95 As might therefore be anticipated, evaluations from different countries all show very large variability in the seasonal impact of QIVs. Analyses that project cumulative effects over multiple seasons based on antecedent virus circulation patterns are therefore most informative. The published dynamic models showed substantially greater improvement in health outcomes based on the use of QIVs as compared with the more conservative static models. However, although dynamic models better reflect the real-world impact of vaccination, dynamic transmission models are inherently more complex and require a greater degree of assumptions in terms of model inputs.
Influenza vaccines typically show reduced efficacy in the elderly due to immune senescence and novel TIVs developed for use in this age-group include an increased antigen dose or adjuvant to overcome this limitation. Modeling studies suggest that the benefits of enhanced TIV formulations in the elderly (high-dose or adjuvanted TIVs) may be as great, or greater than those provided by QIVs.Citation60,61,64 This underscores the fact that in older adults, improvements in vaccine efficacy can be achieved by improving the immune response and by broadening coverage. However, even with high-dose TIV, efficacy against influenza B due to the lineage included in the vaccine is clearly higher than efficacy against influenza B due to the lineage absent from the vaccine.Citation96 An enhanced QIV in this population appears to be the optimal vaccine choice.
Influenza vaccines have been in use since 1936 and the move from TIVs to QIVs is the most recent adaption of seasonal influenza vaccines in response to changes in global circulating influenza strains. Based on the available evidence from clinical trials, epidemiological studies and modeling, several countries have progressively issued recommendations preferentially recommending QIVs over TIVs. To address the co-circulation of B-lineage viruses or B-lineage mismatch, QIVs have been developed and are likely to lead to more stable vaccine effectiveness across seasons, providing broader protection than TIVs and contributing to influenza prevention worldwide. Availability of QIV efficacy data in children and estimates of vaccine effectiveness in coming seasons will provide complementary information on the potential added benefits of QIVs on influenza prevention, which may lead more countries to adopt definitive recommendations for QIVs use.
Trademark statement
FluLaval, Fluarix, Alpharix and Influsplit are trademarks of the GSK group of companies. Fluzone Quadrivalent and Fluzone Intradermal Quadrivalent are trademarks of Sanofi Pasteur. Flumist Quadrivalent and Fluenz Tetra are trademarks of AstraZeneca.
Disclosure of potential conflicts of interest
Riju Ray, Rafik Bekkat-Berkani, Philip O. Buck, Carine Claeys and Gonçalo Matias are employees of the GSK group of companies. Bruce L. Innis reports he was employed by the GSK group of companies at the time of the study and is now an employee of PATH. Riju Ray, Rafik Bekkat-Berkani, Philip O. Buck, Carine Claeys and Bruce L. Innis report ownership of stock options and/or restricted shares in the GSK group of companies. Gaël Dos Santosreports he was employed by Business & Decision Life Sciences (on behalf of GSK) at the time of the study and is now employee of the GSK group of companies.
Authors' contributions
All authors participated in assembling and interpreting the data. All authors reviewed and approved the final manuscript.
Acknowledgments
The authors thank Philippe Buchy for critically reviewing the manuscript. The authors also thank Joanne Wolter (Independent medical writer, on behalf of GSK) for providing writing services, and Bruno Dumont and Veronique Gochet (Business and Decision Life Sciences, on behalf of GSK) for editorial assistance and manuscript coordination.
Funding
GlaxoSmithKline Biologicals SA took in charge all costs associated with the development and publication of the present manuscript.
References
- McCauley J, Hongo S, Kaverin N, Kochs G, Lamb R, et al. Family - orthomyxoviridae. In: King AMQ, Lefkowitz E, Adams MJ, Carstens EB, editors. Virus taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier; 2012; 749-61.
- Chen R, Holmes EC. The evolutionary dynamics of human influenza B virus. J Mol Evol 2008; 66:655-63; PMID:18504518; https://doi.org/10.1007/s00239-008-9119-z
- Tan Y, Guan W, Lam TT-Y, Pan S, Wu S, Zhan Y, Viboud C, Holmes EC, Yang Z. Differing epidemiological dynamics of influenza B virus lineages in Guangzhou, southern China, 2009–2010. J Virol 2013; 87:12447-56; PMID:24027322; https://doi.org/10.1128/JVI.01039-13
- Dos Santos G, Neumeier E, Bekkat-Berkani R. Influenza - Can we cope better with the unpredictable? Hum Vaccin Immunother 2015; 12:699-708; PMID:26360135; https://doi.org/10.1080/21645515.2015.1086047
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1-62
- Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990; 175:59-68; PMID:2309452; https://doi.org/10.1016/0042-6822(90)90186-U
- Wong KK, Jain S, Blanton L, Dhara R, Brammer L, Fry AM, Finelli L. Influenza-associated pediatric deaths in the United States, 2004–2012. Pediatrics 2013; 132:796-804; PMID:24167165; https://doi.org/10.1542/peds.2013-1493
- Hannoun C. The evolving of influenza viruses and influenza vaccines. Expert Rev Vaccines 2013; 12:1085-94; PMID:24024871; https://doi.org/10.1586/14760584.2013.824709
- Shaw MW, Xu X, Li Y, Normand S, Ueki RT, Kunimoto GY, Hall H, Klimov A, Cox NJ, Subbarao K. Reappearance and global spread of variants of influenza B/Victoria/2/87 lineage viruses in the 2000–2001 and 2001–2002 seasons. Virology 2002; 303:1-8; PMID:12482653; https://doi.org/10.1006/viro.2002.1719
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010; 28:Suppl 4:D45-53; PMID:20713260; https://doi.org/10.1016/j.vaccine.2010.08.028
- Skowronski DM, De Serres G, Dickinson J, Petric M, Mak A, Fonseca K, Kwindt TL, Chan T, Bastien N, Charest H, et al. Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006–2007. J Infect Dis 2009; 199:168-79; PMID:19086914; https://doi.org/10.1086/595862
- Caini S, Huang QS, Ciblak MA, Kusznierz G, Owen R, Wangchuk S, Henriques CMP, Njouom R, Fasce RA, Yu H, et al. Epidemiological and virological characteristics of influenza B: Results of the Global Influenza B Study. Influenza Other Respir Viruses 2015; 9:Suppl 1:3-12; PMID:26256290; https://doi.org/10.1111/irv.12319
- Lo Y-C, Chuang J-H, Kuo H-W, Huang W-T, Hsu Y-F, Liu M-T, Chen C-H, Huang H-H, Chang C-H, Chou J-H, et al. Surveillance and vaccine effectiveness of an influenza epidemic predominated by vaccine-mismatched influenza B/Yamagata-lineage viruses in Taiwan, 2011–12 season. PLoS ONE 2013; 8:e58222; PMID:23472161; https://doi.org/10.1371/journal.pone.0058222
- Heikkinen T, Ikonen N, Ziegler T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin Infect Dis 2014; 59:1519-24; PMID:25139969; https://doi.org/10.1093/cid/ciu664
- Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med 2013; 11:153; PMID:23800265; https://doi.org/10.1186/1741-7015-11-153
- Skowronski DM, Janjua NZ, Sabaiduc S, De Serres G, Winter A-L, Gubbay JB, Dickinson JA, Fonseca K, Charest H, Bastien N, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: Cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126-37; PMID:24446529; https://doi.org/10.1093/infdis/jiu048
- Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005–2015. 2015 [Accessed 2015 Apr 26]. http://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm
- Emborg HD, Krause TG, Nielsen L, Thomsen MK, Christiansen CB, Skov MN, Nielsen XC, Weinreich LS, Fischer TK, Rønn J, et al. Influenza vaccine effectiveness in adults 65 years and older, Denmark, 2015/16 - a rapid epidemiological and virological assessment. Euro Surveill 2016; 21:1-6; PMID: 27101732; https://doi.org/10.2807/1560-7917.ES.2016.21.14.30189
- Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother 2012; 8:81-8; PMID:22252006; https://doi.org/10.4161/hv.8.1.17623
- Pillet S, Aubin É, Trépanier S, Bussière D, Dargis M, Poulin J-F, Yassine-Diab B, Ward BJ, Landry N. A Plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clin Immunol 2016; 168:72-87; PMID:26987887; https://doi.org/10.1016/j.clim.2016.03.008
- Tussey L, Strout C, Davis M, Johnson C, Lucksinger G, Umlauf S, Song L, Liu G, Abraham K, White CJ. Phase 1 safety and immunogenicity study of a quadrivalent seasonal flu vaccine comprising recombinant hemagglutinin-flagellin fusion proteins. Open Forum Infect Dis 2016; 3:ofw015; PMID:26925433; https://doi.org/10.1093/ofid/ofw015
- Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004; 103:133-8; PMID:15163501; https://doi.org/10.1016/j.virusres.2004.02.025
- Beyer WE, Palache AM, Baljet M, Masurel N. Antibody induction by influenza vaccines in the elderly: A review of the literature. Vaccine 1989; 7:385-94; PMID:2683459; https://doi.org/10.1016/0264-410X(89)90150-3
- Bekkat-Berkani R, Ray R, Jain VK, Chandrasekaran V, Innis BL. Evidence update: GlaxoSmithKline's inactivated quadrivalent influenza vaccines. Expert Rev Vaccines 2016; 15:201-14; PMID:26641539; https://doi.org/10.1586/14760584.2016.1113878
- McKeage K. Inactivated quadrivalent split-virus seasonal influenza vaccine (Fluarix® quadrivalent): A review of its use in the prevention of disease caused by influenza A and B. Drugs 2013; 73:1587-94; PMID:24022123; https://doi.org/10.1007/s40265-013-0114-3
- Graaf H de, Faust SN. Fluarix quadrivalent vaccine for influenza. Expert Rev Vaccines 2015; 14:1055-63; PMID:26098443; https://doi.org/10.1586/14760584.2015.1057573
- Kieninger D, Sheldon E, Lin W-Y, Yu C-J, Bayas JM, Gabor JJ, Esen M, Fernandez Roure JL, Narejos Perez S, Alvarez Sanchez C, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: A phase III, randomized trial in adults aged ≥ 18 years. BMC Infect Dis 2013; 13:343; PMID:23883186; https://doi.org/10.1186/1471-2334-13-343
- Domachowske JB, Pankow-Culot H, Bautista M, Feng Y, Claeys C, Peeters M, Innis BL, Jain V. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3–17 years. J Infect Dis 2013; 207:1878-87; PMID:23470848; https://doi.org/10.1093/infdis/jit091
- Beran J, Vesikari T, Wertzova V, Karvonen A, Honegr K, Lindblad N, Van Belle P, Peeters M, Innis BL, Devaster J-M. Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: A prospective, randomized, placebo-controlled trial. J Infect Dis 2009; 200:1861-9; PMID:19909082; https://doi.org/10.1086/648406
- Langley JM, Carmona Martinez A, Chatterjee A, Halperin SA, McNeil S, Reisinger KS, Aggarwal N, Huang L-M, Peng C-T, Garcia-Sicilia J, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate: A phase III randomized controlled trial in children. J Infect Dis 2013; 208:544-53; PMID:23847058; https://doi.org/10.1093/infdis/jit263
- Tinoco JC, Pavia-Ruz N, Cruz-Valdez A, Aranza Doniz C, Chandrasekaran V, Dewé W, Liu A, Innis BL, Jain VK. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged ≥ 18 years: A phase III, randomized trial. Vaccine 2014; 32:1480-7; PMID:24486352; https://doi.org/10.1016/j.vaccine.2014.01.022
- Jain VK, Domachowske JB, Wang L, Ofori-Anyinam O, Rodríguez-Weber MA, Leonardi ML, Klein NP, Schlichter G, Jeanfreau R, Haney BL, et al. Time to change dosing 1 of inactivated 2 quadrivalent influenza vaccine in 3 young children: Evidence from a 4 phase III, randomized, controlled trial. J Pediatr Infect Dis Soc 2017; 6(1):9-19.
- Jain VK, Rivera L, Zaman K, Espos RA, Sirivichayakul C, Quiambao BP, Rivera-Medina DM, Kerdpanich P, Ceyhan M, Dinleyici EC, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med 2013; 369:2481-91; PMID:24328444; https://doi.org/10.1056/NEJMoa1215817
- Center for Biologics Evaluation and Research. Approved products - fluzone quadrivalent. [Accessed 2015 Oct 29]. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm356091.htm
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine 2013; 31:770-6; PMID:23228813; https://doi.org/10.1016/j.vaccine.2012.11.074
- Greenberg DP, Robertson CA, Landolfi VA, Bhaumik A, Senders SD, Decker MD. Safety and immunogenicity of an inactivated quadrivalent influenza vaccine in children 6 months through 8 years of age. Pediatr Infect Dis J 2014; 33:630-6; PMID: 24445833; PMID:24445833; https://doi.org/10.1097/INF.0000000000000254
- Gorse GJ, Falsey AR, Ozol-Godfrey A, Landolfi V, Tsang PH. Safety and immunogenicity of a quadrivalent intradermal influenza vaccine in adults. Vaccine 2015; 33:1151-9; PMID:25613721; https://doi.org/10.1016/j.vaccine.2015.01.025
- Toback SL, Levin MJ, Block SL, Belshe RB, Ambrose CS, Falloon J. Quadrivalent Ann Arbor strain live-attenuated influenza vaccine. Expert Rev Vaccines 2012; 11:1293-303; PMID:23151111; https://doi.org/10.1586/erv.12.108
- Block SL, Yi T, Sheldon E, Dubovsky F, Falloon J. A randomized, double-blind noninferiority study of quadrivalent live attenuated influenza vaccine in adults. Vaccine 2011; 29:9391-7; PMID:21983154; https://doi.org/10.1016/j.vaccine.2011.09.109
- Block SL, Falloon J, Hirschfield JA, Krilov LR, Dubovsky F, Yi T, Belshe RB. Immunogenicity and safety of a quadrivalent live attenuated influenza vaccine in children. Pediatr Infect Dis J 2012; 31:745-51; PMID:22466322; https://doi.org/10.1097/INF.0b013e31825687b0
- Sheldon EA, Jeanfreau R, Sliman JA, Charenkavanich S, Rousculp MD, Dubovsky F, Mallory RM. Immunogenicity of a quadrivalent Ann Arbor strain live attenuated influenza vaccine delivered using a blow-fill-seal device in adults: A randomized, active-controlled study. Influenza Other Respir Viruses 2013; 7:1142-50; PMID:23061976; https://doi.org/10.1111/irv.12027
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179-86; PMID:12517228; https://doi.org/10.1001/jama.289.2.179
- Fleming DM, Taylor R, Haguinet F, Schuck-Paim C, Logie J, Webb D, Lustig R, Matias G. Influenza-attributable burden in United Kingdom primary care. Epidemiology and Infection 2016; 144:537-47; PMID:26168005; https://doi.org/10.1017/S0950268815001119
- Paget WJ, Balderston C, Casas I, Donker G, Edelman L, Fleming D, Larrauri A, Meijer A, Puzelli S, Rizzo C, et al. Assessing the burden of paediatric influenza in Europe: The European Paediatric Influenza Analysis (EPIA) project. Eur J Pediatr 2010; 169:997-1008; PMID:20229049; https://doi.org/10.1007/s00431-010-1164-0
- Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of mortality attributable to influenza and RSV in the United States during 1997–2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses 2014; 8:507-15; PMID:24975705; https://doi.org/10.1111/irv.12258
- Fleming DM, Taylor RJ, Lustig RL, Schuck-Paim C, Haguinet F, Webb DJ, Logie J, Matias G, Taylor S. Modelling estimates of the burden of Respiratory Syncytial virus infection in adults and the elderly in the United Kingdom. BMC Infect Dis 2015; 15:443; PMID:26497750; https://doi.org/10.1186/s12879-015-1218-z
- Matias G, Haguinet F, Lustig RL, Edelman L, Chowell G, Taylor RJ. Model estimates of the burden of outpatient visits attributable to influenza in the United States. BMC Infect Dis 2016; 16:641; PMID:27821091; https://doi.org/10.1186/s12879-016-1939-7
- Mosnier A, Caini S, Daviaud I, Nauleau E, Bui TT, Debost E, Bedouret B, Agius G, van der Werf S, Lina B, et al. Clinical characteristics are similar across Type A and B influenza virus infections. PLoS ONE 2015; 10:e0136186; PMID:26325069; https://doi.org/10.1371/journal.pone.0136186
- Silvennoinen H, Huusko T, Vuorinen T, Heikkinen T. Comparative burden of influenza A/H1N1, A/H3N2 and B infections in children treated as outpatients. Pediatr Infect Dis J 2015; 34:1081-5; PMID:26181897; https://doi.org/10.1097/INF.0000000000000814
- Cohen JM, Silva ML, Caini S, Ciblak M, Mosnier A, Daviaud I, Matias G, Badur S, Valette M, Enouf V, et al. Striking similarities in the presentation and duration of illness of influenza A and B in the community: A study based on sentinel surveillance networks in France and Turkey, 2010–2012. PLoS ONE 2015; 10:e0139431; PMID:26426119; https://doi.org/10.1371/journal.pone.0139431
- Li W-C, Shih S-R, Huang Y-C, Chen G-W, Chang S-C, Hsiao M-J, Tsao K-C, Lin T-Y. Clinical and genetic characterization of severe influenza B-associated diseases during an outbreak in Taiwan. J Clin Virol 2008; 42:45-51; PMID:18325832; https://doi.org/10.1016/j.jcv.2007.11.026
- Esposito S, Molteni CG, Daleno C, Valzano A, Fossali E, Da Dalt L, Cecinati V, Bruzzese E, Giacchino R, Giaquinto C, et al. Clinical and socioeconomic impact of different types and subtypes of seasonal influenza viruses in children during influenza seasons 2007/2008 and 2008/2009. BMC Infect Dis 2011; 11:271; PMID:21992699; https://doi.org/10.1186/1471-2334-11-271
- Irving SA, Patel DC, Kieke BA, Donahue JG, Vandermause MF, Shay DK, Belongia EA. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004–2005 through 2007–2008. Influenza Other Respir Viruses 2012; 6:37-43; PMID:21668663; https://doi.org/10.1111/j.1750-2659.2011.00263.x
- Paddock CD, Liu L, Denison AM, Bartlett JH, Holman RC, Deleon-Carnes M, Emery SL, Drew CP, Shieh W-J, Uyeki TM, et al. Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J Infect Dis 2012; 205:895-905; PMID:22291193; https://doi.org/10.1093/infdis/jir861
- Glezen PW, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: A structured literature review. Am J Public Health 2013; 103:e43-51; PMID:23327249; https://doi.org/10.2105/AJPH.2012.301137
- Fowlkes A, Steffens A, Temte J, Lonardo SD, McHugh L, Martin K, Rubino H, Feist M, Davis C, Selzer C, et al. Incidence of medically attended influenza during pandemic and post-pandemic seasons through the Influenza Incidence Surveillance Project, 2009–13. Lancet Respir Med 2015; 3:709-18; PMID:26300111; https://doi.org/10.1016/S2213-2600(15)00278-7
- Tafalla M, Buijssen M, Geets R, Vonk Noordegraaf-Schouten M. A comprehensive review of the epidemiology and disease burden of Influenza B in 9 European countries. Hum Vaccin Immunother 2016; 12:993-1002; PMID:26890005; https://doi.org/10.1080/21645515.2015.1111494
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993-8; PMID:22226861; https://doi.org/10.1016/j.vaccine.2011.12.098
- Clements KM, Meier G, McGarry LJ, Pruttivarasin N, Misurski DA. Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum Vaccin Immunother 2014; 10:1171-80; PMID:24609063; https://doi.org/10.4161/hv.28221
- Chit A, Roiz J, Briquet B, Greenberg DP. Expected cost effectiveness of high-dose trivalent influenza vaccine in US seniors. Vaccine 2015; 33:734-41; PMID:25444791; https://doi.org/10.1016/j.vaccine.2014.10.079
- Raviotta JM, Smith KJ, DePasse J, Brown ST, Shim E, Nowalk MP, Zimmerman RK. Cost-effectiveness and public health effect of influenza vaccine strategies for U.S. Elderly adults. J Am Geriatr Soc 2016; 64:2126-31; PMID:27709600; https://doi.org/10.1111/jgs.14323
- Crépey P, de Boer PT, Postma MJ, Pitman R. Retrospective public health impact of a quadrivalent influenza vaccine in the United States. Influenza Other Respir Viruses 2015; 9 Suppl 1:39-46; PMID:26256294; https://doi.org/10.1111/irv.12318
- de Boer PT, Crépey P, Pitman RJ, Macabeo B, Chit A, Postma MJ. Cost-effectiveness of quadrivalent versus trivalent influenza vaccine in the United States. Value Health 2016; 19:964-75; PMID:27987647; https://doi.org/10.1016/j.jval.2016.05.012
- Mullikin M, Tan L, Jansen JP, Van Ranst M, Farkas N, Petri E. A novel dynamic model for health economic analysis of influenza vaccination in the elderly. Infect Dis Ther 2015; 4:459-87; PMID:26350238; https://doi.org/10.1007/s40121-015-0076-8
- Brogan AJ, Talbird SE, Davis AE, Thommes EW, Meier G. Cost-effectiveness of seasonal quadrivalent versus trivalent influenza vaccination in the United States: A dynamic transmission modeling approach. Hum Vaccin Immunother 2017; 13(3):533-42.
- McNeil S, Ambrose A, Andrews M, Boivin G, Bowie W, Chit A, Elsherif M, Green K, et al. Strain specific influenza burden of disease among hospitalized Canadian adults during the 2011/12 season: A public health agency of Canada/Canadian Institutes of Health Research (PCIRN) serious outcomes surveillance network study. Paper presented at: Options for Control of Influenza VIII; 2013; Cape Town, South Africa.
- Chit A, Roiz J, Aballea S. An assessment of the expected cost-effectiveness of quadrivalent influenza vaccines in Ontario, Canada using a static model. PLoS ONE 2015; 10:e0133606; PMID:26222538; https://doi.org/10.1371/journal.pone.0133606
- Thommes EW, Ismaila A, Chit A, Meier G, Bauch CT. Cost-effectiveness evaluation of quadrivalent influenza vaccines for seasonal influenza prevention: a dynamic modeling study of Canada and the United Kingdom. BMC Infect Dis 2015; 15:465; PMID:26503131; https://doi.org/10.1186/s12879-015-1193-4
- Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Fleming D. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health 2016; 16:481; PMID:27278794; https://doi.org/10.1186/s12889-016-3128-4
- Harvala H, Smith D, Salvatierra K, Gunson R, von Wissmann B, Reynolds A, Frew C, MacLean A, Hunt A, Yirrell D, et al. Burden of influenza B virus infections in Scotland in 2012/13 and epidemiological investigations between 2000 and 2012. Euro Surveill 2014; 19(37):pii: 20903; PMID:25259532; https://doi.org/10.2807/1560-7917.ES2014.19.37.20903
- Meier G, Gregg M, Poulsen Nautrup B. Cost-effectiveness analysis of quadrivalent influenza vaccination in at-risk adults and the elderly: An updated analysis in the UK. J Med Econ 2015; 18:746-61; PMID:25903831; https://doi.org/10.3111/13696998.2015.1044456
- Weigl JAI, Puppe W, Schmitt HJ. The incidence of influenza-associated hospitalizations in children in Germany. Epidemiol Infect 2002; 129:525-33; PMID:12558335; https://doi.org/10.1017/S0950268802007707
- Eichner M, Schwehm M, Hain J, Uphoff H, Salzberger B, Knuf M, Schmidt-Ott R. 4Flu - an individual based simulation tool to study the effects of quadrivalent vaccination on seasonal influenza in Germany. BMC Infect Dis 2014; 14:365; PMID:24993051; https://doi.org/10.1186/1471-2334-14-365
- Dolk C, Eichner M, Welte R, Anastassopoulou A, Van Bellinghen L-A, Poulsen Nautrup B, Van Vlaenderen I, Schmidt-Ott R, Schwehm M, Postma M. Cost-utility of quadrivalent versus trivalent influenza vaccine in Germany, using an individual-based dynamic transmission model. Pharmacoeconomics 2016; 34:1299-308; PMID:27647004; https://doi.org/10.1007/s40273-016-0443-7
- Nagy L, Heikkinen T, Sackeyfio A, Pitman R. The clinical impact and cost effectiveness of quadrivalent versus trivalent influenza vaccination in Finland. Pharmacoeconomics 2016; 34:939-51; PMID:27423657; https://doi.org/10.1007/s40273-016-0430-z
- Gerlier L, Lamotte M, Dos Santos Mendes S, Damm O, Schwehm M, Eichner M. Estimates of the public health impact of a pediatric vaccination program using an intranasal tetravalent live-attenuated influenza vaccine in Belgium. Paediatr Drugs 2016; 18:303-18; PMID:27272706; https://doi.org/10.1007/s40272-016-0180-6
- García A, Ortiz de Lejarazu R, Reina J, Callejo D, Cuervo J, Morano Larragueta R. Cost-effectiveness analysis of quadrivalent influenza vaccine in Spain. Hum Vaccin Immunother 2016; 12:2269-77; PMID:27184622; https://doi.org/10.1080/21645515.2016.1182275
- Pitrelli A. Introduction of a quadrivalent influenza vaccine in Italy: A budget impact analysis. J Prev Med Hyg 2016; 57:E34-40; PMID:27346938
- Duru G, Carrat F, Pribil C, Bricaire F, Pujol P, Robert J, Lafuma A. Cost effectiveness of quadrivalent influenza vaccine over trivalent vaccine in France. Value Health 2014; 17:A678; PMID:27202501; https://doi.org/10.1016/j.jval.2014.08.2525
- Uhart M, Bricout H, Clay E, Largeron N. Public health and economic impact of seasonal influenza vaccination with quadrivalent influenza vaccines compared to trivalent influenza vaccines in Europe. Hum Vaccin Immunother 2016; 12:2259-68; PMID:27166916; https://doi.org/10.1080/21645515.2016.1180490
- Chan PKS, Chan MCW, Cheung JLK, Lee N, Leung TF, Yeung ACM, Wong MCS, Ngai KLK, Nelson EAS, Hui DSC. Influenza B lineage circulation and hospitalization rates in a subtropical city, Hong Kong, 2000–2010. Clin Infect Dis 2013; 56:677-84; PMID:23074315; https://doi.org/10.1093/cid/cis885
- Wu P, Goldstein E, Ho LM, Yang L, Nishiura H, Wu JT, Ip DKM, Chuang S-K, Tsang T, Cowling BJ. Excess mortality associated with influenza A and B virus in Hong Kong, 1998–2009. J Infect Dis 2012; 206:1862-71; PMID:23045622; https://doi.org/10.1093/infdis/jis628
- You JHS, Ming W-K, Chan PKS. Cost-effectiveness of quadrivalent influenza vaccine in Hong Kong - A decision analysis. Hum Vaccin Immunother 2015; 11:564-71; PMID:25714506; https://doi.org/10.1080/21645515.2015.1011016
- Australian Government. Department of health. Australian influenza surveillance report. No. 10 2015. [Accessed 2016 Mar 15]. http://www.health.gov.au/internet/main/publishing.nsf/Content/ozflu-surveil-no10-15.htm
- Milne GJ, Halder N, Kelso JK, Barr IG, Moyes J, Kahn K, Twine R, Cohen C. Trivalent and quadrivalent influenza vaccination effectiveness in Australia and South Africa: Results from a modelling study. Influenza Other Respir Viruses 2016; 10(4):324-32.
- Jamotte A, Chong CF, Manton A, Macabeo B, Toumi M. Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia. BMC Public Health 2016; 16:630; PMID:27449665; https://doi.org/10.1186/s12889-016-3297-1
- Public Health England. The national childhood flu immunisation programme 2015/16 Information for healthcare practitioners. 2015 [Accessed 2016 Jan 30]. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/478996/PHE_Childhood_influenza_programme_2015_16_Nov15b.pdf
- Pebody R, Warburton F, Andrews N, Ellis J, von Wissmann B, Robertson C, Yonova I, Cottrell S, Gallagher N, Green H, et al. Effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 end of season results. Euro Surveill 2015; 20(36); PMID:26535911; https://doi.org/10.2807/1560-7917.ES.2015.20.36.30013
- Chuang J-H, Huang AS, Huang W-T, Liu M-T, Chou J-H, Chang F-Y, Chiu W-T. Nationwide surveillance of influenza during the pandemic (2009–10) and post-pandemic (2010–11) periods in Taiwan. PLoS ONE 2012; 7:e36120; PMID:22545158; https://doi.org/10.1371/journal.pone.0036120
- Song JY, Cheong HJ, Choi SH, Baek JH, Han SB, Wie S-H, So BH, Kim HY, Kim YK, Choi WS, et al. Hospital-based influenza surveillance in Korea: Hospital-based influenza morbidity and mortality study group. J Med Virol 2013; 85:910-7; PMID:23508916; https://doi.org/10.1002/jmv.23548
- Wie S-H, So BH, Song JY, Cheong HJ, Seo YB, Choi SH, Noh JY, Baek JH, Lee JS, Kim HY, et al. A comparison of the clinical and epidemiological characteristics of adult patients with laboratory-confirmed influenza A or B during the 2011–2012 influenza season in Korea: a multi-center study. PLoS ONE 2013; 8:e62685; PMID:23671624; https://doi.org/10.1371/journal.pone.0062685
- European Center for Disease Prevention and Control. Risk assessment influenza 2015/16 - seasonal-influenza-risk-assessment-2015-2016.pdf. 2016 [Accessed 2016 Apr 14]. http://ecdc.europa.eu/en/publications/Publications/seasonal-influenza-risk-assessment-2015-2016.pdf93
- Barros ENC de, Cintra O, Rossetto E, Freitas L, Colindres R. Patterns of influenza B circulation in Brazil and its relevance to seasonal vaccine composition. Braz J Infect Dis 2016; 20:81-90; PMID:26626166; https://doi.org/10.1016/j.bjid.2015.09.009
- Caini S, Andrade W, Badur S, Balmaseda A, Barakat A, Bella A, Bimohuen A, Brammer L, Bresee J, Bruno A, et al. Temporal patterns of influenza A and B in tropical and temperate countries: What are the lessons for influenza vaccination? PLoS ONE 2016; 11:e0152310; PMID:27031105; https://doi.org/10.1371/journal.pone.0152310
- Quinn E, Jit M, Newall AT. Key issues and challenges in estimating the impact and cost-effectiveness of quadrivalent influenza vaccination. Expert Rev Pharmacoecon Outcomes Res 2014; 14:425-35; PMID:24734967; https://doi.org/10.1586/14737167.2014.908713
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635-45; PMID:25119609; https://doi.org/10.1056/NEJMoa1315727
- McCullers JA, Huber VC. Correlates of vaccine protection from influenza and its complications. Hum Vaccin Immunother 2012; 8:34-44; PMID:22252001; https://doi.org/10.4161/hv.8.1.18214
- You JHS, Ming W, Chan PKS. Cost-effectiveness analysis of quadrivalent influenza vaccine versus trivalent influenza vaccine for elderly in Hong Kong. BMC Infect Dis 2014; 14:618; PMID:25420713; https://doi.org/10.1186/s12879-014-0618-9
- You J, Ming W, Chan P. Quadrivalent influenza vaccine in Hong Kong- a cost-effectiveness analysis. Value Health 2014; 17:A678; PMID:27202499; https://doi.org/10.1016/j.jval.2014.08.2523
- Kittikraisak W, Chittaganpitch M, Gregory CJ, Laosiritaworn Y, Thantithaveewat T, Dawood FS, Lindblade KA. Assessment of potential public health impact of a quadrivalent inactivated influenza vaccine in Thailand. Influenza Other Respir Viruses 2015; 10:211-9; PMID:26588892; https://doi.org/10.1111/irv.12361
- Yang M-C, Tan EC-H, Su J-J. Cost-effectiveness analysis of quadrivalent versus trivalent influenza vaccine in Taiwan: A lifetime multi-cohort model. Hum Vaccin Immunother 2017; 13(1):81-89.
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2012–2013 northern hemisphere influenza season. Wkly Epidemiol Rec 2012; 87:83-95; PMID:22462202
- Robert Koch Institut. Wissenschaftliche Begründung für die Änderung der Empfehlung zur Impfung gegen Influenza. Epidemiologisches Bulletin 2013; 36/37:365-70
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices-United States, 2013–2014. MMWR Recomm Rep 2013; 62:1-43; PMID:24048214
- Centre for Health Protection, Department of Health, Hong Kong Special Administrative Region. Scientific committee on vaccine preventable diseases recommendations on seasonal influenza vaccination for the 2013/14 season. 2013 [Accessed 2016 Jan 27]. http://www.chp.gov.hk/files/pdf/recommendations_on_seasonal_influenza_vaccination_for_the_201314_season.pdf
- Public Health Agency of Canada. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). Statement on Seasonal Influenza Vaccine for 2014 – 2015. 2014 [Accessed 2016 Jan 27]. http://www.phac-aspc.gc.ca/naci-ccni/assets/pdf/flu-grippe-eng.pdf
- Ministero della Salute. Prevenzione e controllo dell'influenza: Raccomandazioni per la stagione 2014 – 2015. [Accessed 2016 Jan 27]. http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf;jsessionid = PkVx2wHQuWozSjEDnrNKxA__.sgc4-prd-sal?anno = 0&codLeg = 49871&parte = 1%20&serie
- Haut Conseil de la santé. L'utilisation du vaccin contre la grippe saisonnière FluarixTetra®. 2014 [Accessed 2016 Jan 27]. http://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier = hcspa20140523_placefluarixtetragrippe.pdf
- Haut Conseil de la santé. L'utilisation du vaccin contre la grippe saisonnière Saison hivernale 2015–2016. 2015 [Accessed 2016 Feb 17]. http://www.vaccination-info.be/assets/files/avisCSSgrippe2015.pdf
- Sociedade Brasileira de Imunizações. Vacinas influenza no Brasil em 2015. 2015 [Accessed 2016 Jan 27]. http://www.sbim.org.br/wp-content/uploads/2015/03/nota_tecnica_influenza.pdf
- Joint Committee on Vaccination and Immunisation. Minute of the meeting on 4 June 2014. [Accessed 2016 Jan 27]. https://app.box.com/s/iddfb4ppwkmtjusir2tc/1/2199012147/19052160649/1
- Deutsche Gesellschaft für Tropenmedizin und Internationale Gesundheit e.V.: Grippe. 2015. [Accessed 2016 Jan 27]. http://www.dtg.org/influenzasaisonal.html?&F = 20
- Australian Government Department of Health and Ageing, National Health and Medical Research Council. The Australian Immunisation Handbook 10th Edition (updated June 2015). [Accessed 2015 Aug 14]. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook10-home
