ABSTRACT
HZ/Su, branded as ‘Shingrix’, is one of the newest vaccines to be submitted for multi-national regulatory approval. It is targeted to prevent shingles, a global concern with aging populations. A live attenuated vaccine for shingles has been available for over a decade, however it is contraindicated in specific subgroups of people, and there are added concerns regarding long-term immunogenicity. HZ/Su is the first subunit vaccine developed to protect against shingles. This paper provides a critical appraisal of current evidence regarding HZ/Su.
Introduction
Endemic worldwide, varicella zoster virus (VZV) is a highly neurotropic and T cell tropic αherpes virus. Primary infection leads to the common clinical condition, varicella or ‘chickenpox’. Subsequently, VZV remains dormant in sensory dorsal root ganglia, with the potential for reactivation in one or more sensory neurones leading to herpes zoster (HZ) or ‘shingles’, and complications such as postherpetic neuralgia (PHN).Citation1 The incidence of HZ in the United States and Europe increases with advanced age is estimated at 3 to 10 cases per 1000 person-years.Citation2 For unclear reasons this incidence has steadily increased with time.Citation3 A vaccine for varicella has been available since 2002, and production and licensing of a live-attenuated vaccine for HZ occurred in 2006.Citation4 Notably, there are contra-indications to live-attenuated vaccines, such as VZV naive or immunosuppressed patients in whom vaccination may cause disease. We critical appraise published data on a novel subunit vaccine for VZV, HZ/Su or GSK1437173A (GSK).
Virology
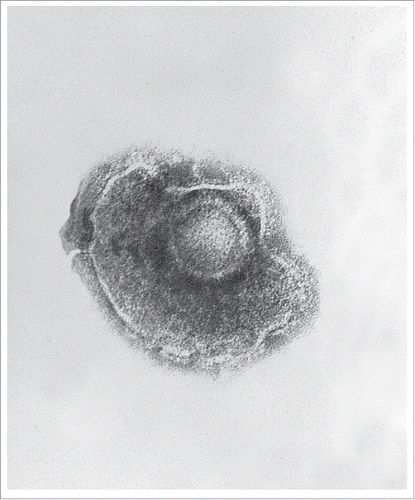
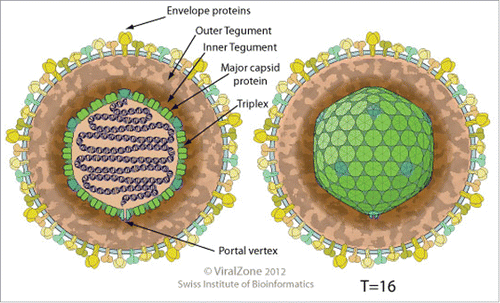
VZV is an enveloped, spherical to pleomorphic double-stranded DNA virus with a monopartite genome approximately 125 kilobases, illustrated in and . The genome encodes at least 71 open reading frames (ORFs) and related promoter sequence. Approximately two thirds of the ORFs are necessary for replication, and this includes 40 genes common among all herpes viruses.Citation5 Glycoprotein E (gE) is a large unique N terminus (amino acids 1–187) essential for replication and T cell entry. This is the most abundant and immunogenic glycoprotein, and the target utilised in the HZ/Su vaccine. summarizes the natural history of the virus, and the basic cell mechanism of viral replication.
Immunology
During primary infection, the initial immune response to VZV involves the innate immune system, with the release of antiviral cytokines and the activation of NK cells.Citation1 This is suggested to control viral replication in the mucosa, as well as triggering adaptive immunity. Memory immunity to VZV involves persistence of VZV IgG and VZV-specific CD4 and CD8 cells, however it is cell-mediated immunity that correlates best with the severity of primary infection.Citation6 The cell-mediated response is crucial for preventing reactivation of latent VZV residing in ganglionic cells. This immunity naturally wanes over time, and this is thought to be the reason HZ chiefly afflicts the elderly.
Delineating this further, a strong VZV-specific CD4 response at the onset of HZ is associated with improved clinical outcomes. Within a cohort of patients who developed HZ in the Shingles Prevention Study (SPS) increased expression of IFN-γ in peripheral blood mononuclear cells (PMBCs) after stimulation with VZV antigen was associated with reduced morbidity and a lower incidence of PHN.Citation7,8 The frequency of VZV-specific memory T cells present predicted development of HZ and severity. Subjects who did not develop HZ during follow up tended to have higher baseline frequencies of VZV-specific T cells when compared with subjects who subsequently developed HZ. Both administration of the attenuated zoster vaccine or an episode of HZ resulted in an increase in VZV-specific T cells in subjects.Citation9 Interestingly levels of anti-VZV antibodies did not correlate with protection against HZ, matching previous observations.Citation10 It is essential therefore that an effective vaccine to prevent HZ elicits a sustained cellular immune response against VZV.
Nature of the disease
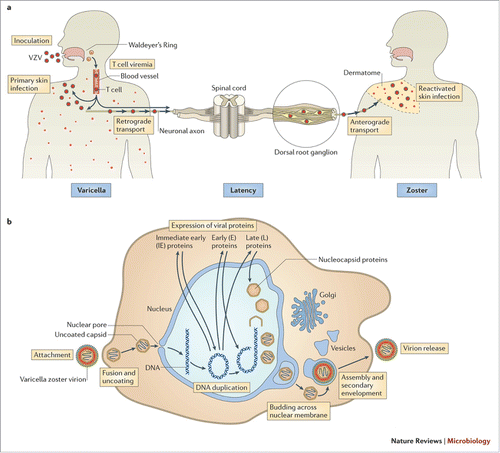
The reactivation of latent VZV residing in the dorsal root ganglion, see , has been demonstrated to involve a decline in cell-mediated immunity.Citation11 Risk factors include older age, cell-mediated immune dysfunction, diabetes, female gender, genetic susceptibility, mechanical trauma, recent psychological stress and white race.Citation12 The clinical presentation involves 3 phases: 1) Prodrome, 2) Infectious rash and 3) Resolution. The prodrome of acute neuralgia may precede the rash by 2–100 d.Citation1 The rash typically involves a single dermatome, with an erythematous macular rash followed by vesicular lesions that ulcerate, scars and heals. Data suggests that complications occur in 12%.Citation13 Persisting pain, or pain which appears more than 90 d after symptom onset is termed postherpetic neuralgia and increases with age. Aside from the severe pain that may be associated with HZ, there are potentially devastating complications: secondary bacterial infection and sepsis, meningoencephalitis, visual and hearing impairment. It is suggested that up to 4% of persons who develop shingles attend secondary care.
Treatment
Antiviral treatment with intravenous or oral Aciclovir, Valaciclovir or Famciclovir administered within 72 hours of symptoms onset, reduce the severity and duration of HZ.Citation14 There are no licensed antivirals to reduce or prevent the onset of PHN, and management depends on supportive care such as analgesia.
Epidemiology and Public Health aspects
The estimated lifetime risk of HZ is 25–30%, and the subsequent risk of developing PHN is approximately 20%.Citation3 In contrast to varicella, there is no seasonal distribution, and cases occur more frequently with increasing age.Citation15 Exogenous exposure to VZV boosts the immune system and prevents reactivation. Undeniably, current epidemiological data has been focused in Europe, USA and Australia and it is in these settings that experience has been gained with the existing live-attenuated HZ vaccine, Zostavax.Citation7
Key issues
| • | Primary VZV infection causes varicella, and reactivation causes shingles/Herpes Zoster. | ||||
| • | Cell-mediated immunity prevents VZV reactivation, and is primed by repeated exogenous exposure to VZV. However, this immunity wanes with increasing age, leading to HZ in the elderly. | ||||
| • | HZ is common, with 3–5 cases/1000 person-years worldwide. Complications include sepsis due to secondary bacterial infection, severe and persistent pain, visual and hearing impairment. | ||||
| • | Persistent pain at least 90 d after the rash has healed, postherpetic neuralgia (PHN), is the commonest complication. This occurs in up to 1 in 5 patients, resulting in considerable morbidity. The incidence and severity of PHN increases with age. | ||||
| • | Antivirals may shorten symptom duration and severity. However, they must be administered within 72 hours of symptom onset, and there is insufficient evidence to demonstrate a benefit in preventing postherpetic neuralgia. | ||||
| • | Immunisation to prevent HZ has existed for over a decade in the form of a live-attenuated vaccine, Zostavax. It is the only licensed HZ vaccine, now licensed in 50 countries. | ||||
| • | The vaccine efficacy of Zostavax against the development of HZ reduces with increasing age of the recipient (from 63.9% in subjects between 60–69 y to 37.6% in subjects aged 70 y or greater in the Shingles Prevention Study). In addition vaccine efficacy against HZ decreased with time. | ||||
| • | Zostavax is contraindicated in immunosuppressed patients, in whom vaccination may lead to disease. | ||||
| • | “HZ/Su” or GSK1437173A varicella zoster vaccine (Shringrix™) is a novel subunit vaccine based on glycoprotein E with AS01 as an adjuvant. | ||||
Zostavax, the existing shingles (HZ) vaccine
The live-attenuated Oka strain was developed in Japan in the 1970s as a vaccine for the prevention of varicella.Citation16 This was derived from VZV isolated from a 3-year-old boy with varicella, and passaged in human embryonic fibroblasts, guinea pig fibroblasts, and then in human diploid fibroblasts. Two decades later the same vaccine at a significantly higher concentration was successfully trialled for the prevention of HZ.Citation17 This was based on Hope-Simpson's theory that an episode of HZ would boost VZV-specific cell-mediated immunity and prevent another episode of HZ.Citation18
The live-attenuated HZ vaccine was first licensed on the 19th May 2006. It is produced by Zostavax Merck/ Sanofi Pasteur for adults over 50 y of age, and costs $100–200 for a single dose.
A recent Cochrane review included 10 randomized controlled trials of Zostavax. 4 studies were deemed to have a low risk for bias but overall, the vaccine reduced the incidence and complications of HZ.Citation19 Vaccine efficacy however decreased with time and the incidence of HZ returned to around baseline levels around year 8 post vaccination. Prevention of burden of illness, a composite measurement of HZ incidence and morbidity, reduced from 66.9% at year 4, to 47.7% at year 7 and 37.9% at year 11. Efficacy in prevention of PHN similarly decreased from 60.7% at year 4 to 11.5% at year 11.Citation20,21 Notably, the external validity of the study was low due to the homogeneity of the population.
52 countries have now licensed Zostavax, and there has been variable introduction in immunisation policies and uptake. In the UK, the vaccine was deemed cost-effective for 70 y olds. The decision was based on the milder symptoms and lower risk of complications in people aged under 70 years, and the limited efficacy above 80 y.
Issues with Zostavax include preclusion of the vaccine to categories of immunosuppressed patients, the limited efficacy demonstrated beyond 5 y or in those patients over 80 y. There are concerns that Zostavax uptake has been low, and suggestions that there is lower confidence in a live-attenuated vaccine. It is for these reasons that an alternative may be necessary.
Design and development of product
The “HZ/su” or GSK1437173A varicella zoster vaccine (Shringrix™) is an investigational subunit vaccine manufactured by GSK containing 50 μg of recombinant VZV glycoprotein E (gE) formulated with AS01B adjuvant.
Glycoprotein E is a 90–98 kDa protein encoded by ORF68 and is the most abundant membrane found on VZV-infected cell membranes and present in high concentrations within the skin during episodes of HZ.Citation22 gE is thought to be essential in facilitating VZV neurotropism and virulence represents a major target in the development of host immunity.Citation5 Early studies demonstrated that primary infection results in development of anti-gE antibodies Citation23 and CD4 responses specific to gECitation24 in human subjects. Monoclonal antibodies raised against gE neutralised infectious virions in vitro with the presence of complement.Citation25 A recombinant, truncated gE molecule lacking the transmembrane anchor and carboxyterminal domains was immunogenic in mice and capable of stimulating production of neutralising antibodies.Citation26 A subsequent gE fusion protein was also capable of stimulating specific humoral and T cell responses in guinea pigsCitation27 supporting it as a potential candidate for subunit vaccine development.
AS01 Adjuvant
AS01 (GSK, Rixensart, Belgium and Antigenics Inc., USA) is a proprietary adjuvant system containing MPL (3-O-desacyl-4′-monophosphoryl lipid A), the saponin derived QS21 combined with dioleoylphosphatidycholine (a phospholipid) and cholesterol. MPL is derived from the Salmonella Minnesota lipopolysaccharide and stimulates antigen presenting cells expressing Toll-Like receptor 4 through activation of the innate immunity. QS21 is a molecule extracted from the South American tree Quillaja saponaria Molina fraction 21 and stimulates innate pathways in monocytes through an unclear mechanism.Citation28 The liposomal formulation is thought to offset the intrinsic hemolytic activity of QS21,Citation29 but may also enhance antigen presentation when compared with its emulsion formulation AS02.Citation30
The synergy between MPL and QS21 resulted in stimulation both classical and monocyte-derived dendritic cells and enhances antigen presentation to T cells. Although within preclinical studies AS01 enhances IFN driven responses, clinical experience has shown that AS01 predominantly induces production of antigen-specific antibodies and specific CD4 cells. AS01 has been used in candidate vaccines in hepatitis B, HIV and malaria with no safety concerns or increased risks of immune-mediated diseases.Citation31 An increased risk of meningitis without clear etiology was reported in the Phase 3 trial in children who received RTS,S/AS01 compared with control but the significance of this was unclear.Citation32
Combination gE / AS01B in animal models
Humoral and cellular mediated immune responses induced by recombinant gE formulated with AS01B adjuvant (‘B’ refers to the dilutions − 50 μg each of MPL and QS21) was investigated through a VZV-primed C57BL/6 mouse model.Citation33 Mice immunized twice with gE formulated with AS01B resulted in significantly higher total frequencies of activated CD4 cells and gE-specific CD4 cells producing IFN-γ and IL-2 when compared with formulation with alum or saline. The geometric mean concentrations of anti-gE antibody at day 30 were higher in mice vaccinated with gE/ AS01B. In direct comparison of AS01B with the oil emulsion-based adjuvant AS02 no significant differences in gE-specific CD4 cells were observed at day 30, although a higher frequency of IFN-γ was observed with ASO1B.
Clinical studies on HZ/Su
Phase I/II trials: Safety and immunogenicity
The safety and immunogenicity of the recombinant gE/AS01 candidate vaccine (HZ/Su) was examined in a phase I/II open-label, randomized study with 155 enrolled subjects.Citation34 Through a staggered recruitment design 20 young adults (aged 18–30 years) and 135 older adults (aged 50–70 years) were randomized to receive 2 doses of: HZ/Su (50 μg recombinant VZV gE with AS01B), or HZ/Su and OKA, (a live attenuated Oka strain VZV vaccine containing approximately 104 PFU per dose injected subcutaneously) or OKA alone on months 0 and 2.
Higher numbers of older subjects who received a regimen containing HZ/Su experienced local symptoms when compared with the OKA only group - 4.4% experienced pain at injection site at grade 3 severity (i.e. preventing normal daily activities) and 20% had > 50 mm of local redness. General reactions such as myalgia and fatigue were also more common in those receiving HZ/Su. No vaccine related serious adverse events (SAEs) or deaths were reported on follow up to month 42.
Humoral immunogenicity measured through geometric mean concentrations (GMC) of serum anti-gE and anti-VZV antibody was higher in subjects immunized with HZ/Su or HZ/Su with OKA compared OKA alone. Antibody levels were highest after the first dose in young adults but after the second dose in the older group and anti-gE specific responses were greater than anti-VZV. At month 42 in those vaccinated with HZ/Su alone there was a decline of both anti-VZV and anti-gE GMCs although this remained higher when compared with baseline.
Cellular immunity was measured through intracellular cytokine staining and T cells considered positive if they expressed at least 2 cytokines induction with VZV lysate or gE. T cell responses were highest after 2 doses of HZ/Su or HZ/Su with OKA compared with 2 doses of OKA alone. The frequency of VZV-specific CD4 cells secreting at least 2 cytokines decreased to levels comparable baseline at month 30. Stimulation of CD8+ cells was not detected in any of the groups.
The authors conclude that HZ/Su was generally tolerated and safe. Although appearing more immunogenic when compared with OKA alone they note that this may not translate into clinical efficacy given that the risks and immunological correlates of protection for HZ are still unclear.Citation21
Phase I/II trials in older subjects, safety in 3 different formulations
Two phase II, randomized multicentre studies funded by GSK Biologicals were conducted in parallel between 2007 and 2011 to investigate the impact of vaccine and adjuvant formulation on the safety and immunogenicity of the HZ/Su vaccine in older adults.
In the antigen dose selectionCitation35 714 patients aged ≥ 60 were recruited to receive 2 doses of trial vaccine 2 months apart. Subjects were randomized to receive 2 doses of 25 μg or 50 μg or 100 μg of gE combined with AS01B 2 months apart, or saline in month 0 followed by one dose of 100 μg gE/ AS01B, or 2 doses of unadjuvanted 100 μg gE 2 months apart. In the adjuvant studyCitation36 410 adults ≥ 50 y were randomized to receive 2 doses of 50 μg gE/ AS01B, or 50 μg gE/ AS01E (a lower dose adjuvant containing 25 μg MPL and 25 μg QS21), or 50 μg gE unadjuvanted or saline 2 months apart.
Similar to the previous study, injection site pain, fatigue, myalgia and headache were the most common reported symptoms and this was attributed to the adjuvant. Subjects in both studies who received a vaccine containing AS01 had a higher incidence of local and general reactions with the majority experiencing pain at injection site. Within the antigen dose selection study < 6.1% of subjects reported a grade 3 local or general reaction; this incidence was higher in subjects aged 60–69 y compared with those over 70. In adjuvant study up to 87% of subjects receiving gE/AS01B experienced local or general symptoms and up to 9.3% were classed as a grade 3 reaction. In contrast 5.3% in saline only group and 2.7% in the gE/saline group experienced grade 3 reactions. Overall the majority of symptoms resolved within 3 d. In addition, solicited reactions were more frequent when the higher dose adjuvant (AS01B) was used when compared with lower dose (AS01E).
At follow up through to 14 months no SAEs or deaths attributable to vaccination occurred in either study. 2 subjects withdrew due to treatment related AEs in the adjuvant formulation study. The authors comment that formulation with AS01 specifically was more immunogenic and induced more adverse reactions in its recipients. However, this did not lead to lower uptake of a second dose of the vaccine, and they conclude AS01 was overall well tolerated under trial conditions.
Subjects who received 2 doses of gE/AS01B had higher anti-gE and anti-VZV titres at month 3 compared with other groups, and those who received either 50 or 100 μg of gE with AS01B had higher GMCs than subjects who received 25 μg. These responses persist through to month 36 and remained higher than pre-vaccination levels. A higher dose adjuvant with AS01B also elicited a stronger humoral response. Notably, based on the anti-VZV antibody criteria used, 38.7% and 17.5% were non-responders with AS01B and AS01E respectively. The significance of this was unclear.
Cellular immune responses were measured through intracellular cytokine staining of stimulated T cells and after adjustment frequencies were higher at month 3 for the 50 and 100 μg gE/ AS01B groups compared 2 doses of 25 μg gE/ AS01B or one dose of 100 μg gE/AS01B or 2 doses of 100 μg gE/Saline groups. In the adjuvant study, cellular responses were significantly higher in AS01B group compared with AS01E. Non-responder rates approximately <12% for gE-specific cellular immunity but 50% according to VZV cellular immunity. In both studies subjects ≥ 70 y old who received gE and AS01 showed similar levels of cellular immunity responses and immunogenicity at month 3 when compared with the other age groups. At 6 y follow up of 119 (out of 166) subjects who received 50 μg gE/ AS01B the gE-specific immunity remained 3.8 times higher than pre-vaccination levels.Citation37
Randomized controlled trials in patient groups older than 50 and 70 y
A multicentre phase 3 randomized placebo controlled trial ZOE-50 (ClinicalTrials.gov number NCT01165177, funded by GSK Biologicals) recruited patients between 2010 and 2011.Citation38 16,160 participants ≥ 50 y who were not immunosuppressed were randomized in a 1:1 ratio to receive either HZ/Su vaccine (consisting of 50 μg gE with AS01B) or placebo (0.9% saline) at months 0 and 2. The investigators and participants were blinded to the intervention administered.
A confirmed case of herpes zoster was defined as unilateral rash with sensory symptoms confirmed by a positive real-time polymerase chain reaction (PCR) result targeting VZV ORF62. Investigators were to examine suspected cases within 48 hours and obtain photographs of the lesions. If the PCR assay was negative for the internal positive control or if samples were unavailable, then diagnosis was established through unanimous agreement among 5 members of a committee through case review.
14,759 (95.8%) of participants were included in the final analysis including exclusion of 749 subjects due to Good Clinical Practice deviations. Most were recruited from Europe and 71.8% were white and 61.2% were female. The mean age of all participants was 62.3 y old.
As with previous studies up to 84.4% participants in the HZ/Su group within the ZOE-50 trial reported symptoms. 17.0% of participants receiving HZ/Su reported grade 3 reactions lasting a median duration of 1 day, including 9.5% reporting local site reactions and 11.4% systemic symptoms. This compared with 3.2% of participants who received placebo reporting grade 3 reactions. In the first 30 d after vaccination 1 HZ/Su and 3 placebo recipients had a SAE related to vaccination. The occurrence and nature of potential immune-mediated diseases was not significantly different in the HZ/Su and placebo group (1.0 and 1.3% respectively throughout the study period). With a mean follow up of 3.5 y the incidences of SAEs and deaths were similar in both the HZ/Su and placebo group.
408 participants reported suspected HZ of which 244 (59.8%) of these cases were confirmed. 33 cases were inconclusive and not included in final evaluation. After exclusion of participants who did not receive 2 doses of vaccine 216 confirmed cases of HZ were analyzed – with 6 occurring in HZ/Su group and 210 in placebo group with a mean follow-up duration of 3.2 y. The overall rate of herpes zoster was 0.3 per 1,000 person-years in the HZ/Su cohort and 9.1 per 1,000 person-years in the placebo cohort. The rate of HZ in the placebo cohort is comparable to population estimates.Citation2
Notably the HZ rate in the HZ/Su group was similar across different age groups and the derived vaccine efficacy was approximately 97.2% for the trial duration (95% confidence interval 93.7 to 99.0%; p <0.001) with no significant difference across all age groups.
A separate phase 3 randomized, placebo controlled multicenter trial ZOE-70 (ClinicalTrials.gov NCT01165229, funded by GSK Biologicals) was performed in parallel with ZOE-50 recruited patients between 2010 and 2011 to examine the efficacy and safety of the HZ/Su vaccine in adults older than 70 y old in reducing the incidence of HZ and PHN.Citation39 14,816 participants were recruited to receive either HZ/Su or placebo in a 1:1 ratio. The protocols for the assessment potential clinical cases of HZ were similar to ZOE-50 and assessment of PHN cases performed through daily questionnaires for 28 d and follow up for at least 90 d after onset of rash.
13,163 (94.7%) participants with a mean age of 75.6 y were included in the final analysis cohort. 22.1% of the cohort were 80 y or older. Around 74.1% of participants who received HZ/Su experienced local reactions, 8.5% of which were of grade 3 severity. The authors observe that adverse reactions occurred less frequently in participants who were older than 80 y old compared with the 70 to 79-year-old group. The overall rate of grade 3 adverse reactions in HZ/Su recipients in turn appear to be lower than that of the younger ZOE-50 cohort which received HZ/Su. With a mean follow up of 4 y the incidence of SAEs including deaths and immune mediated diseases were similar in the HZ/Su and placebo groups.
246 cases of HZ occurred in the final modified vaccinated cohort – 23 in HZ/Su and 223 in placebo patients after a mean duration of 3.7 y. The incidence rate for HZ was 0.9 cases/1000 person-years in the HZ/Su group and 9.2 cases/1,000 person-years in the placebo group. Vaccine efficacy in ZOE-70 was 89.8% (84.2 to 93.7, p <0.001) across the whole efficacy period which did not differ significantly between the 70–79 and ≥ 80-y cohort. In the pooled analysis vaccine efficacy was 87.9% in the fourth year after vaccination.
The calculation of vaccine efficacy against PHN included pooling of participants form the ZOE-50 trial. All patients who developed PHN were over 70 y of age. During a mean follow up of 3.8 y the incidence of PHN in the HZ/Su group was 0.1 cases/1000 person-years and 0.9 cases/1000 person-years in the placebo group deriving a vaccine efficacy against PHN of 88.8% for participants 70 y or older. The incidence of PHN in HZ/Su cohort who developed HZ was not significantly different to that in participants who received placebo.
Phase I/II trials in adults with HIV infection
Patients with HIV have a higher risk of developing HZ. This risk is attenuated but does not return to age-matched population rates after commencement of anti-retroviral therapy (ART) and remains approximately 3–5 times higher.Citation40 Although live attenuated vaccines are thought to be safe in patients with HIV with a CD4 count of greater than 200 mm3 its efficacy is not clear (BHIVA guidelines 2015 use of vaccines in HIV-positive).
The safety and immunogenicity of the HZ/Su vaccine in a cohort of adults infected with human immunodeficiency virus (HIV) was supported through a randomized placebo controlled study.Citation41 Three cohorts of subjects infected with HIV were recruited: 94 subjects established on ART for at least a year and had a CD4 count of ≥ 200 cells/mm3, 14 subjects on ART with a CD4 count of 50–199 cells/mm3, and 15 adults ART-naïve with a CD4 count of ≥ 500. Each group was randomized to receive 3 doses of HZ/Su or 3 doses of saline at months 0, 2, and 6.
123 subjects were enrolled and the majority (91.1%) completed follow up to month 18. Local and systemic adverse events were more common in the HZ/Su group but were short-lived (median duration 1–3 days). Up to 16.4% of subjects receiving HZ/Su and 8.3% receiving saline experienced a grade 3 local or general reaction. Overall administration of HZ/Su had no impact on CD4 cell count, HIV viral load or haematological/biochemical parameters through month 18 of follow up.
The overall magnitude of gE-specific cellular immunity and anti-gE antibody response was higher in the HZ/Su group compared with the saline. This was demonstrated in the combined cohort consisting of ART/high CD4 count and the ART naïve/high CD4 count cohort. The gE-specific cellular immunity did not increment dramatically after the third dose the HZ/Su vaccine and the authors conclude that a 2-dose schedule is likely to be appropriate for HIV infected adults.
The authors comment that although the exact immunological correlates of clinical protection are not defined in HIV infection the HZ/Su vaccine was safe, and immunogenic in subjects with relatively high CD4 count. Because of the low numbers recruited in the ART/low CD4 group there was insufficient statistical power to assess the effects of vaccination within this cohort for which an efficacious subunit vaccine would be of most benefit.
Phase I/II trials in autologous haematopoietic stem cell transplant (HCT) recipients
Patients with haematopoietic stem cell transplants have rates of 15–30% of HZ during first year after transplantationCitation42 and furthermore this is associated with more severe complications such as disseminated infection.
121 adult subjects who underwent autologous HCT within the previous 50–70 d between 2009 and 2012 in the United States were randomized to receive: 3 doses of gE/ AS01B, or 3 doses of gE/ AS01E or 1 dose of saline with 2 doses of gE/ AS01B, or 3 doses of saline on months 0, 1 and 3.Citation43
Subjects who received gE/AS01 vaccines experienced a higher rate of symptoms with up to 17.2% experiencing local reactions rated as grade 3. AS01B tended to be more reactogenic than the lower dose AS01E. One SAE was considered related to vaccination was reported up to month 15 but no deaths or immune mediated inflammatory disorders attributable to vaccination.
At month 4 the overall gE-specific and VZV-specific CD4 cell frequency was higher in all gE/AS01 groups compared with saline. There was no significant difference in the CD4 response between the 3 dose AS01B and the 3 dose AS01E. A similar relationship was also observed in terms of anti-gE antibody titres. Similar to previous studies the increase in immunogenicity after the 3rd dose of gE/AS01B/E was modest.
Notably anti-gE GMCs with AS01B / AS01E did not increase in subjects with NHL B cell lymphoma. This was likely caused by B cell depletion through rituximab administration as part of underlying treatment. Given immune reconstitution and engraftment is brought by HCT and risk of HZ is greatest within 2 y of transplant even a modest short-term protection may be beneficial and should be explored in further clinical studies.
Regulatory approval
In October 2016 GSK submitted a Biologics License Application to the United States Food and Drug Administration for approval of HZ/Su the prevention of herpes zoster and its complications in persons aged 50 y or over. In November 2016 applications were submitted to the European Medicines Agency and Health Canada.
Conclusions
An existing single dose of a live attenuated vaccine to prevent HZ is currently licensed in 52 counties. However, there are understandable concerns about the efficacy of the vaccine, and the fact that it is contraindicated in a sub-section of the population. The results of the novel sub-unit vaccine, HZ/Su, have been eagerly awaited.
HZ/Su has demonstrated immunogenicity in preclinical studies and efficacy in preventing HZ in clinical trials. The vaccine efficacy to prevent development of HZ for up to 4 y follow up was 97% overall in subjects older than 50 y of age, and around 90% in participants older than 70 y old. More importantly the response appears preserved with age. In the ZOE-50 and 70 studies the incidence of HZ within the vaccinated cohort remained similar between the age 60–69 and group ≥ 70-year-old group (0.7 and 0.9 cases per 1,000 person-years respectively). In the SPS incidence of breakthrough HZ in the vaccinated group increased with age (from 3.90 cases per 1,000 person-years in the 60 to 69-year-old group to 7.18 per 1000 person-years in the ≥ 70-year-old group) with comparable incidences of HZ in the placebo groups of both studies.
The main burden of HZ remains PHN. Mainly through reduction in the incidence of HZ HZ/Su appears to be able to protect against PHN. The exact relationship between HZ and risk for PHN remains unclear. The authors note that the efficacy of HZ/Su in preventing PHN in breakthrough cases of HZ was not demonstrated in the study. However, the low numbers of HZ in the vaccine groups make it difficult to draw conclusions.
HZ/Su is reactogenic and local or general reactions are common. The incidence of grade 3 reactions which prevent activities of daily living in addition around 10% may have a disproportionate impact on the willingness for patients outside trial conditions to take up the vaccine. In particular, the target population tends to be frail and older. SAEs and deaths were not increased between trial and placebo groups with follow up at 4 y in the overall pooled studies with no increase in the incidence of immune mediated diseases.
Overall vaccine efficacy for HZ/Su for participants over 50 y of age appeared to decrease with time (from 96.6% at year 1 to 87.9% at year 4), however the authors conclude that the difference was not statistically significant and this will need to be followed up with longer term studies.
Studies in immunocompromised patients unable to receive live attenuated vaccines have been encouraging, with immunogenicity and acceptability demonstrated in patients after HSCT and in HIV infection - although measurements of VZV immunity across Phase I/II trials of HZ/Su have not been standardised. Within the HIV trial the few numbers of patients in the low CD4 group completing the study meant analysis was not possible but the vaccine appeared safe.
Future directions
It has been predicted that the incidence of HZ will rise with the increasing aging of the population. There were concerns that this would be particularly marked for several years after the introduction VZV vaccine in numerous countries. Interestingly, current epidemiological evidence suggests that the incidence of HZ in countries adopting the VZV vaccine has actually fallen.Citation44 It is possible that wider uptake of the VZV vaccine in children will considerably reduce the overall burden of HZ, and subsequent demands for a HZ vaccine.
In terms of the current evidence, the trials have been conducted in areas where the majority of participants were VZV IgG positive in temperate climates. The efficacy of the vaccine in populations with a lower seroprevalence of VZV antibodies, or having previously received the VZV/chickenpox vaccine will need to be evaluated. Given the reactogenicity of HZ/Su studies addressing its management may be important for acceptability outside clinical trial conditions. Although the introduction of routine childhood chickenpox vaccination is thought not to have contributed to the increasing incidence of shinglesCitation44 the changing epidemiology of shingles and the impact of introducing a potentially expensive new vaccine need to be carefully examined.
Clinical trials examining vaccine safety of HZ/Su in renal transplant recipients and vaccine efficacy in HSCT recipients are ongoing (for example, ClinicalTrials.gov NCT02058589 and NCT01610414 respectively). In addition, a direct head-to-head comparison study between Zostavax and HZ/Su in the elderly is underway (ClinicalTrials.gov NCT02114333). The outcomes of these studies may influence future vaccination policy on shingles in different subpopulations.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Fields BN, Knipe DM, Howley PM. Fields virology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
- Johnson RW, Alvarez-Pasquin M-J, Bijl M, Franco E, Gaillat J, Clara JG, Labetoulle M, Michel JP, Naldi L, Sanmarti LS, et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv vaccines [Internet] 2015 [cited 2017 Feb 5]; 3(4):109-20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26478818; https://doi.org/10.1177/2051013615599151
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014; 4(6):e004833; https://doi.org/10.1136/bmjopen-2014-004833
- Leme LEG. Vaccines for preventing herpes zoster in older adults. Sao Paulo Med J [Internet]. 2014; 132(4):255. Available from: http://dev1r2.f1000internal.com/pubmed/ref/25055074; https://doi.org/10.1590/1516-3180.20141324T1
- Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol 2014; 12(3):197-210; PMID:24509782; https://doi.org/10.1038/nrmicro3215
- Arvin AM. Humoral and Cellular Immunity to Varicella‐Zoster Virus: An Overview. J Infect Dis [Internet]. 2008 [cited 2017 Jan 7]; 197(s2):S58-60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18419410; https://doi.org/10.1086/522123
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE et al. A Vaccine to Prevent Herpes Zoster and Postherpetic Neuralgia in Older Adults. N Engl J Med. 2012;367(14):1287-96; PMID:22920912. Available from: http://www.nejm.org/doi/full/10.1056/NEJMoa051016#t=article; https://doi.org/10.1056/NEJMoa1208410
- Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis [Internet] 2009 [cited 2017 Jan 11]; 200(7):1068-77. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19712037; https://doi.org/10.1086/605611
- Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. [Internet]. J Infect Dis 2008 197:825-35. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 4014857&tool = pmcentrez&rendertype = abstract; https://doi.org/10.1086/528696
- Webster A, Grint P, Brenner MK, Prentice HG, Griffiths PD. Titration of IgG antibodies against varicella zoster virus before bone marrow transplantation is not predictive of future zoster. J Med Virol [Internet] 1989 [cited 2017 Mar 28]; 27(2):117-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2537881; https://doi.org/10.1002/jmv.1890270209
- Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, Chan IS, Li DJ, Wang W, Keller PM, et al. Decline in Varicella-Zoster Virus (VZV)–Specific Cell-Mediated Immunity with Increasing Age and Boosting with a High-Dose VZV Vaccine. J Infect Dis 188(9):1336-44; [cited 2017 Mar 28]; Available from: https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/188/9/10.1086/379048/2/188-9-1336.pdf?Expires = 1491009616&Signature = dmhRqhaBOr9sdPInUt9AryTr4YtuDY9liJNqare79M4ySvRkIKfeLzG7cLdJaO∼QqZQjmqUfBNRFQmV6uYR7whnWOktPdjqV1Yj7vP1k8∼ETkBn21BJ∼.
- Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol 2010; 48 Suppl 1:S2-7; https://doi.org/10.1016/S1386-6532(10)70002-0
- Galil K, PW C, JG D, Platt R. The sequelae of herpes zoster. Arch Intern Med 1997; 157(11):1209-13; https://doi.org/10.1001/archinte.1997.00440320105010
- Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Syst Rev 2014; 2:CD006866; https://doi.org/10.1002/14651858.CD006866.pub3
- Lin F, Hadler JL, Hadler J. Epidemiology of Primary Varicella and Herpes Zoster Hospitalizations: The Pre–Varicella Vaccine Era. J Infect Dis 181(6):1897-905; [cited 2017 Mar 28]; Available from: https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/181/6/10.1086/315492/2/181-6-1897.pdf?Expires = 1491010315&Signature = RIB∼EGxxkopg8rYJzTqtKZI7OB0-82DmQRgL9ukCxR281-c8D42kanGUxELT5hF9K1BY01p7ZJioW-dsUz52kAR29n9G43wBmVNgbRT8lV9IROQihuOgybyW2AqYspPHF91l27∼MiZ-fcL44AfvQQNu7j3I0FhCmprcb5Pq89YhZ6IMDHytGLF4UMH32Vw9UILrpuMmYCUyvPPSyizA5Bg5RSkVO2JzhEC4Nt72PrFEqYfkbLs1174CeufKNonl∼emIJxmm2Xb87IY8O1xpL5Da3lZylSr3mm7wd56EaQ6GjqhAJ3DNHEOIV∼h4MYxSoea-cg7K9q-xLOtBjvNF4wA__&Key-Pair-Id = APKAIUCZBIA4LVPAVW3Q.
- Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T, Isomura S. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet 1974; 304(7892):1288-90; https://doi.org/10.1016/S0140-6736(74)90144-5
- Oxman MN. Immunization to reduce the frequency and severity of herpes zoster and its complications. Neurol. 1995; 45(12 Suppl 8):S41-6; https://doi.org/10.1212/WNL.45.12_Suppl_8.S41
- Hope-Simpson RE. The Nature of Herpes Zoster: A Long-term Study and a New Hypothesis. Proc R Soc Med. 1965; 58(1):9-20
- Gagliardi AM, Andriolo BN, Torloni MR, Soares BG. Vaccines for preventing herpes zoster in older adults. In: Gagliardi AM, editor. Cochrane Database of Systematic Reviews [Internet]. Chichester, UK: John Wiley & Sons, Ltd; 2016 [cited 2017 Mar 27]. p. CD008858. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26937872.
- Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, Morrison VA, Gelb L, Guatelli JC, Harbecke R, et al. Persistence of the Efficacy of Zoster Vaccine in the Shingles Prevention Study and the Short-Term Persistence Substudy. Clin Infect Dis [Internet]. 2012 [cited 2017 Mar 27]; 55(10):1320-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22828595; https://doi.org/10.1093/cid/cis638
- Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, Betts R, Gelb L, Guatelli JC, Harbecke R, et al. Long-term Persistence of Zoster Vaccine Efficacy. Clin Infect Dis [Internet] 2015 [cited 2017 Mar 27]; 60(6):900-9; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25416754; https://doi.org/10.1093/cid/ciu918
- Mo C, Lee J, Sommer M, Grose C, Arvin AM. The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology 2002; 304(2):176-86; https://doi.org/10.1006/viro.2002.1556
- Dubey L, Steinberg SP, LaRussa P, Oh P, Gershon AA. Western blot analysis of antibody to varicella-zoster virus. J Infect Dis [Internet] 1988 [cited 2017 Jan 15]; 157(5):882-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2834466; https://doi.org/10.1093/infdis/157.5.882
- Arvin AM, Kinney-Thomas E, Shriver K, Grose C, Koropchak CM, Scranton E, Wittek AE, Diaz PS. Immunity to varicella-zoster viral glycoproteins, gp I (gp 90/58) and gp III (gp 118), and to a nonglycosylated protein, p 170. J Immunol [Internet]. 1986 [cited 2017 Jan 7]; 137(4):1346-51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3016094.
- Grose C. Glycoproteins Encoded by Varicella-Zoster Virus: Biosynthesis, Phosphorylation, and Intracellular Trafficking. Annu Rev Microbiol [Internet] 1990 [cited 2017 Jan 7]; 44(1):59-80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2174668; ; https://doi.org/10.1146/annurev.mi.44.100190.000423
- Haumont MM, Jacquet A, Massaer M, Deleersnyder V, Mazzu P, Bollen A, Jacobs P. Purification, characterization and immunogenicity of recombinant varicella-zoster virus glycoprotein gE secreted by Chinese hamster ovary cells. Virus Res 1996; 40(2):199-204; https://doi.org/10.1016/0168-1702(95)01270-2
- Jacquet A, Haumont M, Massaer M, Garcia L, Mazzu P, Daminet V, Grégoire D, Jacobs P, Bollen A. Immunogenicity of a recombinant varicella-zoster virus gE-IE63 fusion protein, a putative vaccine candidate against primary infection and zoster reactivation. Vaccine 2002; 20(11–12):1593-602; PMID:11858867
- Newman MJ, Wu JY, Gardner BH, Anderson CA, Kensil CR, Recchia J, Coughlin RT, Powell MF. Induction of cross-reactive cytotoxic T-lymphocyte responses specific for HIV-1 gp120 using saponin adjuvant (QS-21) supplemented subunit vaccine formulations. Vaccine [Internet]. 1997 [cited 2017 Jan 15]; 15(9):1001-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9261947; https://doi.org/10.1016/S0264-410X(96)00293-9
- Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines [Internet] 2007 [cited 2017 Jan 15]; 6(5):723-39; Available from: http://www.tandfonline.com/doi/full/10.1586/14760584.6.5.723.
- Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol [Internet] 2007 [cited 2017 Jan 15]; 5(7):505-17. Available from: http://www.nature.com/doifinder/10.1038/nrmicro1681; https://doi.org/10.1038/nrmicro1681
- Stassijns J, Bollaerts K, Baay M, Verstraeten T. A systematic review and meta-analysis on the safety of newly adjuvanted vaccines among children. Vaccine [Internet]. 2016 Feb 3 [cited 2017 Jan 15];34(6):714-22. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0264410×1501796X; https://doi.org/10.1016/j.vaccine.2015.12.024
- RTS, S Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. Krishna S, editor. PLoS Med [Internet] 2014 [cited 2017 Jan 15]; 11(7):e1001685. Available from: http://dx.plos.org/10.1371/journal.pmed.1001685.
- Dendouga N, Fochesato M, Lockman L, Mossman S, Giannini SL. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine 2012; 30:3126-35; PMID:22326899; https://doi.org/10.1016/j.vaccine.2012.01.088
- Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapelière P, Vassilev V, Ledent E, Heineman TC. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J Infect Dis [Internet] 2012; 206(8):1280-90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22872734; https://doi.org/10.1093/infdis/jis497
- Chlibek R, Smetana J, Pauksens K, Rombo L, Van den Hoek JAR, Richardus JH, Plassmann G, Schwarz TF, Ledent E, Heineman TC. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: A phase II, randomized, controlled study. Vaccine [Internet]. 2014; 32(15):1745-53. Available from: https://doi.org/10.1016/j.vaccine.2014.01.019; https://doi.org/10.1016/j.vaccine.2014.01.019
- Chlibek R, Bayas JM, Collins H, De La Pinta MLR, Ledent E, Mols JF, Heineman TC. Safety and immunogenicity of an ASO1 -adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥ 50 years of age. J Infect Dis. 2013; 208(12):1953-61; PMID:23904292; https://doi.org/10.1093/infdis/jit365
- Chlibek R, Pauksens K, Rombo L, van Rijckevorsel G, Richardus JH, Plassmann G, Schwarz TF, Catteau G, Lal H, Heineman TC. Long-term immunogenicity and safety of an investigational herpes zoster subunit vaccine in older adults. Vaccine [Internet] 2016; 34(6):863-8. Available from: https://doi.org/10.1016/j.vaccine.2015.09.073; https://doi.org/10.1016/j.vaccine.2015.09.073
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N Engl J Med [Internet] 2015; 372(22):2087-96. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1501184%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/25916341; https://doi.org/10.1056/NEJMoa1501184
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang S-J, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med [Internet] 2016; 375(11):1019-32. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1603800; https://doi.org/10.1056/NEJMoa1603800
- Moanna A, Rimland D. Decreasing incidence of herpes zoster in the highly active antiretroviral therapy era. Clin Infect Dis [Internet] 2013 [cited 2017 Jan 15]; 57(1):122-5. Available from: http://cid.oxfordjournals.org/lookup/doi/10.1093/cid/cit165; https://doi.org/10.1093/cid/cit165
- Berkowitz EM, Moyle G, Stellbrink HJ, Schürmann D, Kegg S, Stoll M, El Idrissi M, Oostvogels L, Heineman TC; Zoster-015 HZ/su Study Group. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: A phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211(8):1279-87; PMID:25371534
- Leung TF, Chik KW, Li CK, Lai H, Shing MM, Chan PK, Lee V, Yuen PM. Incidence, risk factors and outcome of varicella-zoster virus infection in children after haematopoietic stem cell transplantation. Bone Marrow Transplant [Internet] 2000 [cited 2017 Jan 15]; 25(2):167-72. Available from: http://www.nature.com/doifinder/10.1038/sj.bmt.1702119; https://doi.org/10.1038/sj.bmt.1702119
- Stadtmauer EA, Sullivan KM, Marty FM, Dadwal SS, Papanicolaou GA, Shea TC, Mossad SB, Andreadis C, Young JA, Buadi FK, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood [Internet]. 2014 [cited 2017 Jan 15]; 124(19):2921-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25237196; https://doi.org/10.1182/blood-2014-04-573048
- Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of Herpes Zoster, Before and After Varicella‐Vaccination–Associated Decreases in the Incidence of Varicella, 1992–2002. J Infect Dis [Internet]. 2005 [cited 2017 Feb 5]; 191(12):2002-7. Available from: https://academic.oup.com/jid/article-lookup/doi/10.1086/430325; https://doi.org/10.1086/430325