ABSTRACT
Varicella or chickenpox is a highly contagious disease with a high secondary attack rate. Almost 30% of Indian adolescents lack protective antibodies against varicella, emphasizing the need of routine varicella immunization. The Oka VZV is a well-established, safe and efficacious vaccine strain that is highly immunogenic and produces lifelong protective immunity. The present multicentric, open label, randomized, controlled Phase II/III study, compared the Bio Pox™ (indigenous investigational vaccine) with a licensed vaccine, Varivax™Footnote[a]
[a] Please note that this article refers to the product named VARIVAX as manufactured by Changchun Keygen Biological Products Ltd., China and marketed in India by VHB Life Sciences Limited, Mumbai, and not the product VARIVAX® owned by Merck Sharp & Dohme Corp., Rahway, New Jersey, USA. Merck Sharp & Dohme Corp. have asked us to make clear that the product manufactured by Changchun Keygen Biological Products Ltd. is unrelated to and is not sponsored, endorsed or otherwise authorised by Merck Sharp & Dohme Corp.
, for its safety and immunogenicity profile in 252 healthy subjects in the age group of 1–12 y (cohort I: 6–12 years, II:1–6 years) in 3 tertiary medical institutions. Antibodies were measured by VZV Glycoprotein Enzyme Linked Immunoassay (IgG ELISA) kit. Seroconversion percentage in children having pre-vaccination anti VZV IgG titer <10 mIU/mL (< 5 gp ELISA units/mL) were 80% for Bio Pox™ and 77% for Varivax™ (p = 0.692). The seroconversion rate in the group receiving Bio Pox™ was non-inferior to the group that received Varivax™. There were mild local reactions for both the vaccines; none of the patient had fever or required hospitalization or medication. The Bio Pox™ was found to be safe and immunogenic in children against VZV infection.Introduction
Varicella, commonly known as chickenpox, is a highly contagious, acute and common disease of childhood that is prevalent globally.Citation1-3 Varicella is usually a self-limiting disease that affects healthy individuals who are younger than 15 y of age.Citation4 The disease spreads by airborne route and carries high morbidity and mortality in infants and in individuals with impaired immunity. The disease is associated with direct medical costs and indirect costs due to work absenteeism, which result in a substantial economic burden on the community.Citation5 A high susceptibility to varicella infection has been reported across tropical countries unlike temperate countries. The seroprevalence of varicella antibodies among Indian children was reported as 29% in the age group of 1–5 years, 51.1% in 5–10 y and 71.7% in 11–15 years.Citation6
The Oka strain of VZV was developed by Takahashi et al., from vesicular fluid of a child patient by passaging in human lung cells and guinea pig fibroblast cells and finally in WI-38 human diploid cells. After licensure in the USA in 1995, Advisory Committee on Immunization Practice (ACIP), American Academy of Paediatrics, recommended universal use of varicella vaccination. The incidence of varicella and related hospitalizations were reduced by approximately 90% in USA after introduction of the vaccine.Citation7,8 This decline in varicella incidence was seen not only in the vaccinated children but also in unvaccinated population, suggestive of herd effect. Similar effect of varicella vaccine has been noticed in Australia, Canada and European countries.Citation9-11 At present, the vaccine is not included in the National Immunization schedule of India.
The vaccine based on Oka strain manufactured by Biken, Merck and GSK has been widely used globally and found to possess similar safety and immunogenicity.Citation12 A live attenuated varicella vaccine Varivax™ (manufactured by Changchun Keygen Biological Products Ltd., China), is already licensed and marketed in India. However, in the absence of an indigenous varicella vaccine, this was the only available substitute which faced occasional shortage for clinical use. Bio-Med (P) Ltd developed the technology of manufacturing varicella vaccine from Oka strain in India using MRC-5 human diploid cell culture with proprietary name Bio Pox™. The production and development of this vaccine in India shall result in wider availability, better compliance and lowering of cost. The present study was conducted to evaluate and compare the immunogenicity profile of Bio Pox™ (investigational vaccine) with the licensed vaccine (Varivax™) in the study population by estimating the percentage seroconversion in the recipients measured as anti-VZV titres with the help of VZV glycoprotein IgG ELISA Kit. The secondary objective was to compare safety and tolerability of the 2 vaccines by monitoring the side effects up to 42 ± 7 d of the vaccination.
Results
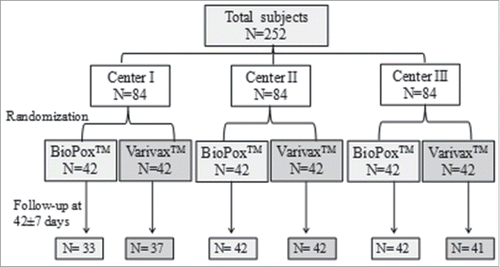
A total number of 252 subjects were included in the study; 84 subjects at each study center who were randomized to receive investigational vaccine or licensed vaccine () Equal number of children were recruited in cohort I (> 6–12 years) and cohort II (1–6 years) at each center. There was a drop-out rate of 5.9% (15/252). The weight and gender of the ‘vaccinees’ were comparable in all the groups and cohorts. The mean age of the vaccinees was also comparable between investigational vaccine and licensed vaccine (cohort I-8.35 ± 1.54yr, 8.39 ± 1.66yr; cohort II- 3.40 ± 1.40yr, 3.26 ± 1.43yr), p>0.05 respectively. At the end of the study, total post immunization blood samples were obtained in 116 (92.06%) and 121 (96.03%) children from cohort I and cohort II, respectively, at all the 3 study centers.
Evaluation of immunogenicity
In cohort I, out of 116 paired serum samples, 114 samples were analyzed as 2 serum samples were haemolysed. The pre-immune anti-VZV titres were <10 mIU/mL in 47.36% (n = 54/114) and 79.33% (n = 96/121) children in cohort I and cohort II, respectively, indicating their susceptibility to varicella. The seroconversion rates were similar for both vaccines in cohort II (Bio Pox™: 82%, Varivax™: 83%) and cohort I (Bio Pox™: 77%, Varivax™: 68%) as shown in , and the difference was not significant (p>0.05). The seroconversion rate in the group receiving Bio Pox™ was non-inferior to the group that received Varivax™. In both the study arms, there was a significant increase (p<0.001) in the pre immune anti varicella titres from <10mIU/mL to 20.1 mIU/mL (IQR 13.9–26.0) and 21.1mIU/mL (IQR 10–35.9) after immunization with Bio Pox™ and Varivax™ respectivelyin the children of 2 age cohorts.
Table 1. Seroconversion: Comparative analysis and non inferiority analysis for subjects with pre-immune titer <10 mIU/mL cohort I and II receiving Bio Pox™ and Varivax™ at all study centers.
The increase in the post-immune median values was similar between both vaccine arms in both cohorts; cohort I (p = 0.713), and cohort II (p = 0.360) and combined cohort I and II (p = 0.275). Therefore, the immunogenicity of Bio Pox™ and Varivax™ was comparable ().
Evaluation of safety
Of the 252 subjects recruited, 237 completed the full study protocol for safety. Fifteen subjects did not come for evaluation on day 42 ± 7 post vaccination. The observations are summarized in . The reactions were categorized by adverse reaction scale on 3 different safety levels in both the groups, self-reported by the vaccines (). Skin reactions (papules/vesicles) at >5 d post vaccination was noted in one subject vaccinated with Varivax™. Papules or vesicles were systemic and the number was about 40–50. The local and systemic side effects of both the varicella vaccines were mild and self-limiting without any sequel. None of the subjects had any systemic reaction such as fever and febrile reactions or required hospitalization.
Table 2. Adverse events (local, systemic and skin reactions) and post-immunization safety profile recorded for combined cohort I (> 6 to 12 years) and cohort II (1 to 6 years) at different time intervals of post vaccination for Bio Pox™ and Varivax™ at all study centers.
Evaluating the lot to lot consistency of Bio Pox™
There was no statistically significant difference (p>0.05) in the levels of the post immune anti varicella titres between the participating centers, in which 3 different batch of BioPox™ was used. This finding was indicative of the lot to lot consistency of BioPox™.
Discussion
Seroconversion in tropical countries is reported at a later age than in temperate countries. As a consequence, the younger age group (12 months to 12 years) is more susceptible to infection which is associated with high morbidity and mortality.Citation13 Immunization during this period of life provides maximum protection against VZV.Citation14 In view of this information, study subjects were enrolled between 12 months to 12 y to compare the safety and immunogenicity between an indigenous investigational vaccine Bio Pox™ and Varivax™ (licensed vaccine). Bio Pox™ was found non-inferior to Varivax™ in the present trial.
A large number of clinical trials globally have proved good immunogenicity, safety and tolerability of Oka strain varicella vaccines in susceptible children (including immune compromised) and adults.Citation15,16 Studies conducted in the United States using Varivax™ (Merck), reported seroconversion rate up to 96% among children from 12 months to 17 y of age with residual seroprotection in 99% subjects at end of first year.Citation17 The vaccine efficacy with single dose has been reported between 70–81% for all forms of varicella and 98–100% against severe varicella.Citation18,19 However, despite a reasonable protection against varicella with a single dose, 2 doses of varicella vaccine are recommended to interrupt transmission, prevent outbreaks in settings with high contact rates and prevent disease in the adults.Citation20
Overall, vaccines using Oka strain have been found to be extremely safe in children and adults.Citation2 In the present study, a low percentage of unsolicited AEs were observed during 6 weeks of post vaccination period. The incidence of AEs within 2 hours of administration of either vaccine was low, of very mild nature and was comparable between 2 study arms. Varicella like rashes were observed in one child vaccinated with Varivax™, similar to earlier reports in the literature.Citation2
The method of determination of specific immune response to varicella vaccine is important. In the present study serological estimation was performed using a highly reliable gp ELISA-kit VaccZyme™.Citation21,22 A 6 week postimmunization antibody titer of at least 5 gp ELISA units/mL has been proposed as a reasonable correlate of sero-protection.Citation23,24 The proportion of children who achieved postimmunization titres of ≥ 5 gp ELISA units after single dose of varicella has been reported to vary from 76–90%.Citation17,21 Li et al. have reported the efficacy of a single dose varicella vaccine at 6 weeks postimmunization as 95.5% among children with an antibody titres of ≥ 5 gp ELISA unit/mL, compared to 83.5% among children with an antibody titres of < 5 gp ELISA units/mL.Citation23 In the present study the investigational vaccine achieved seroconversion rate of 77% and 82% in the cohort I and cohort II, respectively, similar to comparator vaccine. In a recent study from India, Mitra et al. reported seroconversion in 85% children aged 12 months to 12 years, 6 weeks after administering varicella (Oka-RIT strain) vaccine. The incidence and severity of AEs were also similar to our observations.Citation21
In our study, a small proportion of younger children (20.7%, n = 25/121) were found to have pre immune gp ELISA titres of ≥ 10 mIU/mL as compared with older children (52.6%, n = 60/114). Lokeshwar et al. reported varicella sero-positivity rate of > 70% [95% CI; 50.9–91.3] between the ages of 11–15 years in the Indian children, which increased to nearly 90% (95% CI: 71.1–88.7) at the age of 30 years.Citation6 Bartoloni et al. reported sero-positivity rate of 21.2% in 1–4 year, 56.9% in 5–9 years and 83.7% in 10–14 years old children.Citation25 Similar observation has been made by other authors.Citation26,27
The trial had certain limitations like (a) statistical analysis of postimmune titres were not performed in those children who were seropositive at the time of enrolment (b) side effects were recorded based on patient self reporting after first day and (c) the sample size based on the anticipated seroconversion rate seemed insufficient. However, minimum loss of vaccinees to follow up, and estimation of anti VZV antibodies by standard ELISA kit calibrated against international standard for Varicella Zoster immunoglobulin (1987) code W1044, are the strengths of the study.
Conclusion
Bio Pox™, a live attenuated Oka strain varicella vaccine, is safe, immunogenic and is well tolerated by children aged 1–12years.
Materials and methods
This was an open label, controlled and randomized trial (phase II/III) conducted at 3 centers across India (Maulana Azad Medical College and Lok Nayak Hospital, New Delhi (MAMC), University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi (UCMS) and Institute of Child Health, Kolkata (ICH)). The subjects were enrolled from March to June 2015 at all 3 centers. The study was performed after approval from the Drug Controller General of India (DCGI) (CT-01/2014 dated 21/02/2014) and registration with the Clinical Trial Registry, India (CTRI/2014/03/004445 dated 05/03/2014). The ethical committee permission was obtained at all the study centers.
Inclusion/exclusion criteria
Healthy Indian children between the ages of 1–12 years who were siblings of subjects attending out-patient department were screened. They were enrolled after parent(s)/guardian(s) consented to give written and audio-visual informed consent and would comply with all the study related procedures. Subjects with a previous history of chicken pox disease or varicella immunization, history of adverse or allergic reactions during previous vaccinations, history of immunodeficiency or any auto-immune disease, history of intake of steroids, antimicrobials or immunosuppressive drugs and those having any signs of acute infection were excluded. Subjects participating in any other study at present or during past 3 months were also not included in the study.
Vaccine
Bio Pox™, varicella live vaccine, is a lyophilized preparation of attenuated ‘Oka’ strain of VZV. The vaccine contains ≥ 2000 PFU of attenuated ‘Oka’ strain varicella virus propagated in MRC-5 human diploid cells. Bio Pox™ was found to be safe in Phase I, open, unicentric clinical trial (CTRI/2012/11/003156 dated 29/11/2012) in adult subjects.Citation28 Three consecutive batches of Bio Pox™ were used in the clinical trial. Each center was randomly assigned one batch of Bio Pox™. These batches were found to be of standard quality at Central Drugs Laboratory, Kasauli, Himachal Pradesh, India.
Vaccination and patient safety
Randomization of the subjects into 2 groups was done by computer generated random numbers. Allocation concealment was performed by a secret code number to the subject given by a third person who was not associated with the study. The appearance of the investigational and comparator vaccines were different, double blinding was not feasible. However, laboratory personnel who measured the VZV antibodies in the serum samples were double blinded.
Physical examination and medical history was recorded for every subject in the case report forms (CRFs) at enrolment. Pre-immunization blood sample was collected on day ‘0’ and the child was immunized with the reconstituted vaccine using a dose of 0.5 mL subcutaneously in the left upper arm. Post-immunization blood sample was collected on day 42 ± 7. The recruitment and vaccination of the children was done in a sequential manner; first in cohort I (age >6 to 12 years) followed by cohort II (age 1 to 6 years). The younger group (cohort II) was recruited after the older group (cohort I) had completed the study and safety audit was reviewed before the vaccine was administered to cohort II.
The subjects were observed for the development of any local or systemic adverse events (AEs; pain, erythema, inflammation/induration (swelling), fever, febrile reactions, papules) and skin vesicles. The observations were noted for 30, 60, 90 and 120 minutes after vaccination. Subject diaries were maintained to record any adverse event following immunization (AEFI) and any treatment if taken. Papules and vesicular eruptions on the skin were monitored from 5th till 14 days post vaccination. Telephonic calls were made to all participants after 2 weeks of immunization for active surveillance of any side effects. The PI and team scrutinized the subject diaries after completion of observation period of 42 ± 7 days post vaccination. The safety levels were assessed as excellent [local reaction (redness, induration) ≤ 5 mm in diameter and axillary temperature ≤ 38.0°C lasting less than 72 hrs], good (local reaction of > 5–20 mm in diameter and axillary temperature of 38.1–39°C lasting ≤ 4 days), fair (local reaction >20 mm in diameter and axillary temperature >38.1–39°C for >5 days) and bad (local reaction >20 mm in diameter and axillary temperature >39°C for >5 days, papule formation ≥ 5 numbers, vesicle formation ≥ 5 numbers in >5 days post vaccination.
Evaluation of immunogenicity
Blood samples were drawn at each center, serum was separated, coded, transported and stored at −20°C or below in the Department of Microbiology, U.C.M.S. and G.T.B. Hospital, Delhi. Pre immune and post immune sera were tested for anti-VZV antibody titres. IgG antibodies against VZV in coded serum samples were quantified using VZV glycoprotein IgG low level enzyme immunoassay kit (VaccZyme™).Citation22,29,30 This kit has been used previously for vaccine immunogenicity and safety in clinical trial CTRI/2014/08/004858. The assay was performed by the standard procedure of enzyme immunoassay (as detailed by the manufacturer of ELISA kit; The Binding Site Group Birmingham, UK). It was calibrated against the first international standard for Varicella Zoster immunoglobulin (1987) code W1044, with a stated target value of 50 mIU/mL, supplied by the National Institute for Biological Standards and Control (NIBSC: www.nibsc.ac.uk).
The ELISA kit was having a measuring range of 10 mIU/mL to 810 mIU/mL. For the purpose of statistical analysis, immune titres <10 mIU/mL were considered as 5 mIU/mL and values >810 mIU/mL were taken as 810 mIU/mL. Since subjects with ≥ 10 mIU/mL titres were considered as already immune due to natural exposure, the data of subjects with pre immune titer <10 mIU/mL were statistically analyzed. A titer of 5 gp ELISA units/mL (equivalent to10 mIU/mL, by the International reference standard) at 6 weeks after immunization is associated with a high degree of protection against breakthrough infection during 7 years of follow up.Citation23 Hence 10 mIU/mL anti-VZV IgG titer was taken as positive seroconversion in this study. The vaccine codes were decoded and matched against the coded serum samples only after the results of the antibody titres were communicated to all 3 clinical centers.
Statistical analysis
As per the “Drug & Cosmetics Rules, 1945” for a drug which is already marketed in other countries, the minimum number of subjects for Phase III clinical trial should be atleast100 distributed over 3–4 centers primarily to confirm the efficacy and safety of the drug. Considering the same, 252 subjects were enrolled. Anticipated seroconversion rate in the candidate vaccine was taken as 90% or more, the number of subjects per group required to detect a change in seroconversion rates of at least 9%, with an alfa-error 5% and 80% power, was 121.
Therefore, based on non-inferiority testing, 84 subjects (42 subjects for Bio Pox™ vaccine and 42 subjects for Varivax™ vaccine; 21 subjects each in cohort I and cohort II) were recruited at each of the 3 study centers.
Age and weight of the subjects were summarized as mean ± SD. Student's t-test was used to compare age and weight distribution of subjects between 2 arms of the study. For immunogenicity profile, pre and post immune antibody titer values for each of the 2 study arms were summarized as median and inter quartile range (IQR). Comparison of immune titres between 2 study arms was done using Wilcoxon rank sum test. Categorical variable was summarized by frequency (%) and Chi-square test/Fisher's exact test was used. Positive seroconversion for non-inferiority of Bio Pox™ as compared with Varivax™ was done by computing effect size (test group minus reference group) and its 95% confidence interval (CI). To evaluate consistency of Bio Pox™ between the 3 centers, Kruskal Wallis test was used to compare the post immune titres. Stata 10.0 statistical software was used for statistical analysis. A p value <0.05 was considered as statistically significant.
Clinical trials registry
CTRI/2014/03/004445 dated 05/03/2014
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
Authorship
APD, MMAF, MM (Mitra) and IRK conceptualized the study. APD, MMAF, MM (Mitra), IRK, AD, JC, MM and DM contributed in designing the study, acquisition and analysis of data. All authors participated in drafting the article and approved the final submission.
Acknowledgment
The authors acknowledge Knowledge Isotopes Pvt. Ltd. (www.knowledgeisotopes.com) for their medical writing support.
Funding
The work was supported by Bio-Med (P) Ltd, India under grant number BM/CT/VAR/2014.
Notes
[a] Please note that this article refers to the product named VARIVAX as manufactured by Changchun Keygen Biological Products Ltd., China and marketed in India by VHB Life Sciences Limited, Mumbai, and not the product VARIVAX® owned by Merck Sharp & Dohme Corp., Rahway, New Jersey, USA. Merck Sharp & Dohme Corp. have asked us to make clear that the product manufactured by Changchun Keygen Biological Products Ltd. is unrelated to and is not sponsored, endorsed or otherwise authorised by Merck Sharp & Dohme Corp.
References
- Sengupta N, Breuer J. A global perspective of the epidemiology and burden of varicella-zoster virus. Curr Pediatr Rev 2009; 5:207-28; https://doi.org/10.2174/157339609791317315
- Gershon AA, Takahashi M, Seward J. Live attenuated varicella vaccine. In: Plotkin S, Orenstein W, editors. Vaccines. 5th ed. Fourth Edition Elsevers Inc. U.S.A; 2004.
- Gould D. Varicella zoster virus: chickenpox and shingles. Nurs Stand 2014; 28:52-8; PMID:24734838; https://doi.org/10.7748/ns2014.04.28.33.52.e8249
- Boelle PY, Hanslik T. Varicella in non-immune persons: incidence, hospitalization and mortality rates. Epidemiol Infect 2002; 129:599-606; PMID:12558344; https://doi.org/10.1017/S0950268802007720
- Jean-Jasmin LM, Lynette SP, Stefan M, Kai CS, Chew FT, Wah LB. Economic burden of varicella in Singapore–a cost benefit estimate of implementation of a routine varicella vaccination. Southeast Asian J Trop Med Public Health 2004; 35:693-6; PMID:15689089
- Lokeshwar MR, Agrawal A, Subbarao SD, Chakraborty MS, Ram Prasad AV, Weil J, Bock HL, Kanwal S, Shah RC, Shah N. Age related seroprevalence of antibodies to varicella in India. Indian Pediatr 2000; 37:714-9; PMID:10906803
- Baxter R, Tran TN, Ray P, Lewis E, Fireman B, Black S, Shinefield HR, Coplan PM, Saddier P. Impact of vaccination on the epidemiology of varicella: 1995–2009. Pediatrics 2014; 134:24-30; PMID:24913796; https://doi.org/10.1542/peds.2013-4251
- Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS, Gargiullo P, Perella D, Civen R, Seward JF. Changing varicella epidemiology in active surveillance sites—United States, 1995–2005. J Infect Dis 2008; 197 Suppl 2:S71-5; PMID:18419413; https://doi.org/10.1086/522156
- Carville KS, Riddell MA, Kelly HA. A decline in varicella but an uncertain impact on zoster following varicella vaccination in Victoria, Australia. Vaccine 2010; 28:2532-8; PMID:20117265; https://doi.org/10.1016/j.vaccine.2010.01.036
- Tan B, Bettinger J, McConnell A, Scheifele D, Halperin S, Vaudry W, Law B. The effect of funded varicella immunization programs on varicella-related hospitalizations in IMPACT centers, Canada, 2000–2008. Pediatr Infect Dis J 2012; 31:956-63; PMID:22647896; https://doi.org/10.1097/INF.0b013e318260cc4d
- Pozza F, Piovesan C, Russo F, Bella A, Pezzotti P, Emberti Gialloreti L. Impact of universal vaccination on the epidemiology of varicella in Veneto, Italy. Vaccine 2011; 29:9480-7; PMID:22015389; https://doi.org/10.1016/j.vaccine.2011.10.022
- Galea SA, Sweet A, Beninger P, Steinberg SP, Larussa PS, Gershon AA, Sharrar RG. The safety profile of varicella vaccine: a 10-year review. J Infect Dis 2008; 197 Suppl 2:S165-9; PMID:18419392; https://doi.org/10.1086/522125
- Lee BW. Review of varicella zoster seroepidemiology in India and Southeast Asia. Trop Med Int Health 1998; 3:886-90; PMID:9855401; https://doi.org/10.1046/j.1365-3156.1998.00316.x
- Marin M, Watson TL, Chaves SS, Civen R, Watson BM, Zhang JX, Perella D, Mascola L, Seward JF. Varicella among adults: data from an active surveillance project, 1995–2005. J Infect Dis 2008; 197 Suppl 2:S94-S100; PMID:18419417; https://doi.org/10.1086/522155
- Diaz-Mitoma F, Halperin SA, Scheifele D. Reactogenicity to a live attenuated varicella vaccine in Canadian children. Can J Infect Dis 2000; 11:97-101; PMID:18159273; https://doi.org/10.1155/2000/647245
- Doerr HW. Progress in VZV vaccination? Some concerns. Med Microbiol Immunol 2013; 202:257-8; PMID:23649706; https://doi.org/10.1007/s00430-013-0298-x
- White CJ, Kuter BJ, Hildebrand CS, Isganitis KL, Matthews H, Miller WJ, Provost PJ, Ellis RW, Gerety RJ, Calandra GB. Varicella vaccine (VARIVAX) in healthy children and adolescents: results from clinical trials, 1987 to 1989. Pediatrics 1991; 87:604-10.
- Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global Varicella Vaccine Effectiveness: A Meta-analysis. Pediatrics 2016; 137:e20153741; PMID:26908671; https://doi.org/10.1542/peds.2015-3741
- Seward JF, Marin M, Vazquez M. Varicella vaccine effectiveness in the US vaccination program: a review. J Infect Dis 2008; 197 Suppl 2:S82-9; PMID:18419415; https://doi.org/10.1086/522145
- Kuter BJ, Ngai A, Patterson CM, Staehle BO, Cho I, Matthews H, Provost PJ, White CJ. Safety, tolerability, and immunogenicity of two regimens of Oka/Merck varicella vaccine (Varivax) in healthy adolescents and adults. Oka/Merck Varicella Vaccine Study Group. Vaccine 1995; 13:967-72.
- Mitra M, Faridi M, Ghosh A, Shah N, Shah R, Chaterjee S, Narang M, Bhattacharya N, Bhat G, Choudhury H et al. Safety and immunogenicity of single dose live attenuated varicella vaccine (VR 795 Oka strain) in healthy Indian children: a randomized controlled study. Hum Vaccin Immunother 2015; 11:443-9; PMID:25692656; https://doi.org/10.4161/21645515.2014.979646 10.1080/21645515.2014.1004031
- Maple PA, Gray J, Breuer J, Kafatos G, Parker S, Brown D. Performance of a time-resolved fluorescence immunoassay for measuring varicella-zoster virus immunoglobulin G levels in adults and comparison with commercial enzyme immunoassays and Merck glycoprotein enzyme immunoassay. Clin Vaccine Immunol 2006; 13:214-8; PMID:16467328; https://doi.org/10.1128/CVI.13.2.214-218.2006
- Li S, Chan IS, Matthews H, Heyse JF, Chan CY, Kuter BJ, Kaplan KM, Vessey SJ, Sadoff JC. Inverse relationship between six week postvaccination varicella antibody response to vaccine and likelihood of long term breakthrough infection. Pediatr Infect Dis J 2002; 21:337-42; PMID:12075766; https://doi.org/10.1097/00006454-200204000-00014
- Saurbrei A, Wutzler P. Serological detection of varicella-zoster virus-specific immunoglobulin G by an enzyme-linked immunosorbent assay using glycoprotein antigen. J Clin Microbiol 2006; 44:3094-7; PMID:16954232; https://doi.org/10.1128/JCM.00719-06
- Bartoloni A, Bartalesi F, Roselli M, Mantella A, Dini F, Carballo ES, Barron VP, Paradisi F. Seroprevalence of varicella zoster and rubella antibodies among rural populations of the Chaco region, south-eastern Bolivia. Trop Med Int Health 2002; 7:512-7; PMID:12031073; https://doi.org/10.1046/j.1365-3156.2002.00852.x
- Allami A, Mohammadi N. Varicella immunity in Iran: an age-stratified systematic review and meta-analysis. Iran J Microbiol 2014; 6:372-81; PMID:25926953
- Kudesia G, Partridge S, Farrington CP, Soltanpoor N. Changes in age related seroprevalence of antibody to varicella zoster virus: impact on vaccine strategy. J Clin Pathol 2002; 55:154-5; PMID:11865016; https://doi.org/10.1136/jcp.55.2.154
- Garg P, Garg SP, Sharma MK, Sahani S. Phase-I, open, unicentric clinical trial for evaluation of safety of varicella vaccine, live (I.P.) (Oka strain) in adults. Biotechnology International 2014; 7:26-34.
- Provost PJ, Krah DL, Kuter BJ, Morton DH, Schofield TL, Wasmuth EH, White CJ, Miller WJ, Ellis RW. Antibody assays suitable for assessing immune responses to live varicella vaccine. Vaccine 1991; 9:111-6; PMID:1647574
- Maple PA, Breuer J, Quinlivan M, Kafatos G, Brown KE. Comparison of a commercial Varicella Zoster glycoprotein IgG enzyme immunoassay with a reference time resolved fluorescence immunoassay (VZV TRFIA) for measuring VZV IgG in sera from pregnant women, sera sent for confirmatory testing and pre and post vOka vaccination sera from healthcare workers. J Clin Virol 2012; 53:201-7; PMID:22261123