ABSTRACT
Invasive pneumococcal disease (IPD) remains a significant public health problem in Manitoba, Canada although publically-funded pneumococcal conjugate (PCV7 and PCV13) and polysaccharide (PPV23) vaccination programs exist. We analyzed routine surveillance and administrative health data to examine trends in IPD rates as these vaccines were introduced. Data on all individuals with a laboratory-confirmed diagnosis of IPD between 2001 and 2014 were obtained from the provincial Communicable Diseases Surveillance database and linked with Manitoba's provincial immunization registry and physician and hospital databases. We calculated IPD incidence rates overall, by serotype and for different population subgroups defined by socio-demographic and clinical (e.g., chronic diseases, immune status) characteristics. Annual IPD incidence (95%CI) was 8.6 (8.2–9.1)/100,000 people during the study period (n = 1092), and rates were higher in recent years and in regions with predominately indigenous populations. Reduction in the incidence of serotypes included in PCV7 have been offset by rising rates of PCV13-only serotypes in children, and more recently by rising rates of PPV-only serotypes and non-vaccine serotypes among young children and older adults (≥ 65 years). Rates were 3 times higher in those with a chronic disease and highest (> 175-fold) among alcoholics, organ-transplant, and chronic kidney failure patients. The case fatality rate was 12.0% within 30 d of diagnosis. Despite the introduction of several vaccination programs, overall rates of IPD have not declined in Manitoba in the last decade, due to increase in incidence of non-PCV7 serotypes. A disproportionately high burden of disease impacts indigenous communities and people with chronic disease.
Introduction
Systemic infection with Streptococcus pneumoniae, known as invasive pneumococcal disease (IPD), remains a significant cause of severe illness and death in Canada.Citation1 All Canadian provinces have implemented publicly funded vaccination programs to prevent infection with pneumococcal strains commonly associated with IPD.Citation2 In Manitoba, a 23-valent pneumococcal polysaccharide vaccine (PPV23) has been recommended since 2001 for persons at higher risk due to comorbidities or old age. In 2004, a heptavalent conjugate vaccine (PCV7) was added to the province's routine infant immunization schedule to be replaced in 2010 with a 13-valent conjugate vaccine (PCV13).
Jurisdictions that implemented similar programs witnessed significant decline in IPD incidence rates especially among children.Citation3-6 In Manitoba, recent outbreaks of IPD among the urban homeless and other disadvantaged populations have raised concerns that long-term disease trends remain unfavourable.Citation7 However, IPD's burden of illness and long-term trends have not been examined, particularly among those with vaccine indications such as chronic diseases.Citation7,8 This information is essential for planning and evaluating vaccination programs and other disease control measures.
We used Manitoba's comprehensive population-based surveillance and administrative health databases to measure the incidence rates of IPD, and associated clinical outcomes, among all Manitobans and among those with chronic conditions. We also examined trends in disease rates due to different serotypes following the introduction and widespread use of several polyvalent vaccines against S. pneumoniae.
Results
Between 2001 and 2014, 1,457 cases of IPD were reported among Manitobans, representing an annual incidence rate (95% CI) of 8.6 (8.2 – 9.1) per 100,000 people (). Age-standardized annual rates fluctuated over the study period, but overall, they were higher in recent years reaching a peak of 13.2 (8.3–18.0)/100,000 in 2010 when inner city parts of Winnipeg had a large outbreak of 12F serotype infections.Citation7
Table 1. Age-standardized and age-specific incidence rates (95% CI) of invasive pneumococcal disease in Manitoba, Canada, 2001 to 2014.
Children under 2 y had the highest incidence over the study period, at 37.6 (32.1–43.9), followed by adults 65 y and older at 15.9 (14.4–17.6)/100,000. Rates were much lower in other age groups and lowest among 5–17 year-olds at 2.2 (1.8–2.8)/100,000. Rates among adults 18–64 y of age trended higher in recent years although they were still lower than rates observed at the extremes of age. Rates among under 2 year-olds trended lower since 2010, the year PCV13 was introduced into routine childhood vaccination.
Among the cases, about 53% were male and 59% lived in lower income areas (). Age-standardized rates were 3-fold higher in lower income areas being 12.7 compared with 4.8/100,000 in the most affluent areas. The highest rate (15.4/100,000) was in the unknown income category which included persons living in personal care homes and other long-term care facilities. Age-standardized rates were about 5 times higher in northern rural regions (32.3) compared with southern rural regions (6.3) and Winnipeg (7.7/100,000). Within Winnipeg, which is the main urban center of Manitoba with 70% of the population, rates were 3-fold higher in inner city neighborhoods (19.8) than in the northern (6.5) and southern suburbs (4.5/100,000).
Table 2. Number of cases and incidence rate of invasive pneumococcal disease by demographic characteristics and chronic disease risk factors, Manitoba, Canada, 2001–2014.
About 55% of IPD patients had one or more pre-existing chronic disease (), most commonly diabetes (22.5%), chronic obstructive pulmonary disease (21.2%), cancer (19.4%) and ischemic heart disease (16.3%). IPD incidence rates were about 3 times higher in those with a chronic disease (26.4 [22.4 – 30.3]) than in the overall population and about 5 times higher in immunosuppressed patients (41.6 [33.8 – 49.4]/100,000). Incidence rates were highest (> 175/100,000) among organ transplant recipients, those with HIV/AIDS or chronic kidney failure and among those with a history of alcohol abuse.
Over the entire study period, 8% of IPD cases were caused by a serotype included in the PCV7, 29% by a PCV13 serotype, and 55% by a PPV23 serotype of which 89% were PPV23 serotypes not present in PCV7 (). In total, about 47% of IPD cases had received at least one pneumococcal vaccine (). Of adults ≥ 65 years, 70% had received the PPV23 while 61% of children 0–4 y had received at least one dose of a conjugate vaccine (mostly PCV7). Over 70% of cases were hospitalized at least once during the 5-y period preceding diagnosis ().
Table 3. Number of invasive pneumococcal disease cases by clinical risk factors in Manitoba residents (n = 1457), 2001 to 2014.
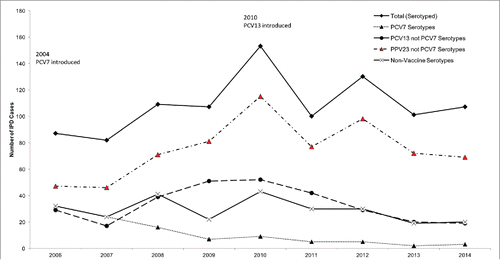
shows trends in number of vaccine serotypes since 2006. Before 2006, serotyping was not performed consistently. Number of IPD cases caused by PCV7 serotypes decreased dramatically from 32 in 2006 to 3 in 2014, whereas the number of PCV13 serotypes not included in PCV7 (PCV13 only) almost doubled before it started to decline in 2011, one year after the switch to PCV13. Thus, the number of all PCV13 serotypes remained constant averaging about 54 cases/year until 2011 before dropping to a minimum of 22 in 2013 and 2014. PPV23 serotypes not included in PCV7 remained stable except for spikes in the 12F outbreak years (2010 and 2012).
Figure 1. Annual number of invasive pneumococcal disease cases by vaccine serotype, Manitoba, Canada, 2001–2014.
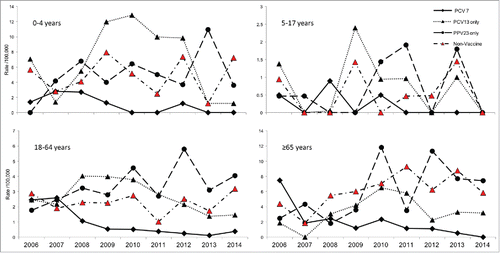
shows trends in the incidence of different vaccine serotypes by age group. The annual incidence of PCV7 serotypes decreased to ≤ 1/100,000 in all age groups while that of PCV13-only serotypes increased among young children (≤ 4 years) and to lesser extent among adults, peaking in 2010 before starting a steep decline reaching rates comparable to PCV7 serotypes. Incidence of PPV-only serotypes and non-vaccine serotypes steadily rose especially among young children and older adults (≥ 65 years).
Figure 2. Annual incidence rate of invasive pneumococcal disease cases by vaccine serotype and age group, Manitoba, Canada, 2001–2014.
A total of 173 patients died (from any cause) within 30 d of diagnosis resulting in a case-fatality rate of 12%. Another 8% of patients died within 1 y (). In multivariate analyses, independent predictors of 30-day mortality were older age (≥ 65 years), residence in an institution, pre-existing diagnosis of chronic renal failure or cardiovascular disease and history of admission to hospital in the past 5 y (). Income and region of residence were not related to mortality risk. Similar patterns were observed for risk of death within one year of diagnosis.
Table 4. Hazard ratios (HR) from adjusted multivariateFootnote* survival analysis of risk factors for adult invasive pneumococcal disease patients in Manitoba who died within 30 d (n = 173), 60 d (n = 202), and one year (n = 298) after diagnosis between 2001 and 2014.
Discussion
We found that the overall incidence of IPD has not declined in Manitoba in the last decade (2001–2014) despite the introduction of several publically funded pneumococcal vaccination programs. A substantial drop in rates of IPD due to PCV7 serotypes was offset by rising rates of PCV13-only serotypes in children, and more recently by rising rates of PPV-only serotypes and non-vaccine serotypes among young children and older adults (≥ 65 years).
The decline in IPD caused by PCV7 serotypes in Manitoba among both children and adults following the introduction of infant PCV7 vaccination in 2004 is impressive especially since rates of IPD were on the rise, particularly among children, in the years before the program began. Although we did not design this ecological analysis to provide direct evidence supporting a causal link, it is hard to explain our findings without assuming such a link given the temporal relationship and the specificity of the effect, i.e., the decline being restricted to PCV7 serotypes. Other jurisdictions that implemented similar programs saw similar trends, and the association is consistent with reports of the effectiveness of PCV7 among vaccinated children and its indirect herd immunity effects among the unvaccinated.Citation9 In several studies, PCVs prevented nasopharyngeal carriage and reduced spread of vaccine serotypes from children to adult caregivers.Citation10,11
In 2010, Manitoba switched to using PCV13 in routine infant vaccination, coinciding with a decrease in rates of disease caused by PCV13 serotypes especially among children. This is particularly promising given that the rates of PCV13-only serotypes were rising rapidly and had effectively replaced the PCV-7 types as the most important cause of IPD in this age group. However, it is too early to determine whether this is a sign of program success.
On the other hand, our results did not support the effectiveness of PPV23 vaccination in reducing IPD rates among older adults targeted by the PPV23 program. In this group, the overall incidence remained unchanged over the study period despite coverage with PPV23 among Manitoba's seniors reaching >65%. Nearly 70% of our cases in this age group had previously received the PPV23, suggesting low PPV vaccine effectiveness. Among older adults, declining rates of PCV7 serotypes, presumably due to herd immunity, were offset by rising rates of PPV-only and non-vaccine serotypes that began in 2007. Similar trends elsewhere were explained in terms of serotype replacement.Citation12,13 While plausible, other factors including changes in antimicrobial resistance and the varying nature of pneumococcal serotype should be considered.Citation14,15
We do not fully understand why IPD rates rose among 18–64 year-olds in recent years, but it could be related to local spread of PPV23-only serotypes among Manitoba's urban disadvantaged communities culminating in the 2010 and 2012 12F outbreaks in Winnipeg; the largest documented 12F outbreak in a community setting.Citation7 Homelessness and substance abuse, as opposed to preexisting chronic conditions, were the most commonly documented risk factors among the 2010 outbreak patients who were predominately 18–64 years-old. This pattern was observed in similar outbreaks in other jurisdictions.Citation16 However, the Winnipeg outbreak was caused by a rapidly evolving novel 12F clone, ST218, that acquired both macrolide and fluoroquinolone resistance via recombination with a 5.3 kb mega gene cassette harboring msrD and mefE as well as several mutations.Citation17 The Manitoba clone appears to have spread to neighboring Canadian and US jurisdictions replacing drug-sensitive strains.Citation17
Our study confirmed that the burden of IPD in Manitoba remained high among groups previously identified at higher risk including the poor and those with chronic diseases. Manitoba's overall rates were in line with Canadian rates, whereas the rates reported in northern Manitoba and inner city Winnipeg, both communities that are socio-economically disadvantaged and pre-dominantly of indigenous ethnicity, were significantly higher. Differences in IPD rates among people of different ethnicities have been previously reportedCitation18 and studies have identified a greater burden of IPD in indigenous peoples.Citation19,20 Poverty, homelessness, and a greater prevalence of chronic medical conditions and substance abuse are likely explanations for this disparity in IPD incidence.
Rates were particularly higher among those with chronic renal failure, HIV/AIDS and organ transplant patients. All these conditions are associated with suppressed immunity.Citation21,22 While these links are well-known,Citation23 it is not known whether increased incidence of IPD may be attributable to an inability to fight off infection or vaccine failure in these individuals as suggested by some studies.Citation24
Fortunately, survival analysis of all IPD cases reported in Manitoba showed that region of residence does not significantly impact survival outcomes. While the burden of disease caused by IPD is substantial in certain parts of Manitoba the case fatality rate is similar to that of other provinces of Canada and developed countries.Citation14
Limitations
To identify IPD cases, we relied on data from Manitoba's routine passive surveillance system, which has several of the well-known limitations of passive surveillance.Citation25 However, we do not believe that laboratory-diagnosed IPD cases were under-reported, because in Manitoba all laboratories are mandated to report test results indicative of IPD, and because positive tests are reported automatically by the laboratory information systems. IPD results in severe illness, so laboratory testing is often not discretionary, and to our knowledge testing guidelines and practices have not changed over the study period. There were also no significant changes in testing practices, except that serotyping of isolated S. pneumoniae was not routinely done before 2006, which is the reason we excluded that period from our serotype-specific trend analyses. Generally, changes in serotype distribution are unlikely to be affected by changes in testing or reporting guidelines. Limited access to healthcare services can theoretically result in under-reporting of cases among disadvantaged populations, so the high IPD rates we found among these populations might still under-estimate their disease burden. Passive surveillance systems are also limited in the amount and quality of data they collect, which is the reason why we used Manitoba's administrative data sets to fill in important information gaps such as vaccination history, pre-existing illnesses and mortality. The combined use of laboratory-confirmed case definitions and additional clinical data from rich administrative data sets distinguishes our approach from trend analyses that relied on a single data source.
Conclusions
Despite the introduction of pneumococcal polysaccharide and conjugate vaccination programs, overall rates of IPD have not declined in Manitoba in the last decade. Reduction in incidence of cases caused by PCV7 serotypes have been offset by rising rates of PCV13-only serotypes in children, and more recently by rising rates of PPV-only serotypes and non-vaccine serotypes among young children and older adults (≥ 65 years). Higher rates of IPD persist among those with vaccine indications including older adults and those with comorbidities. In addition, a disproportionately high burden of disease impacts those living in northern rural regions of Manitoba and the inner-city neighborhoods of Winnipeg compared with other regions. Continued surveillance will be needed to determine the impact of PCV13 serotypes on the overall incidence rate of IPD over the next decade. Future studies on serotype circulation among asymptomatic carriers of S. pneumoniae are needed to further understand the impact and effectiveness of pneumococcal vaccination on IPD rates in Manitoba.
Methods
We conducted a historical cohort study using data obtained from the Communicable Disease Surveillance database (CDS) and other Manitoba Health (MH) administrative and clinical registries, housed at the Manitoba Centre for Health Policy. The study was approved by the governmental Health Information Privacy Committee and the Research Ethics Board of the University of Manitoba.
Data sources
MH is a publicly funded agency that provides comprehensive health insurance, including coverage for laboratory, hospital and ambulatory care services, to the province's 1.3 million residents. Coverage is universal, without regard to age or income.Citation26 For administrative purposes, MH maintains several centralized electronic databases that can be linked using a unique health services number (PHIN). Known for their completeness and accuracyCitation27,28 we and other investigators have extensively used these databases in studies of infectious disease surveillance and vaccine safety and effectiveness.Citation26,29-32
We used the CDS database to identify all persons diagnosed with IPD in Manitoba between 2001 and 2014 (the study period). The database records all cases of notifiable diseases reported by clinicians and clinical laboratories to MH. Under the Manitoba Public Health Act, clinicians must report all cases and deaths due to IPD while clinical laboratories must also report the results of any laboratory testing suggestive of IPD, e.g., blood or CSF cultures positive for S. pneumoniae. In addition to patient identifiers including the PHIN, the CDS database stores information on gender, date of birth and address as well as information on specimen type, collection date and culture results including serotype. Serotyping is performed at Cadham Provincial Laboratory, the sole public health reference laboratory in Manitoba.
Case definition
According to national guidelines,Citation33 we defined IPD as clinical evidence of invasive disease with isolation of S. pneumoniae or demonstration of S. pneumoniae nucleic acid by nucleic acid amplification test (NAAT) from a normally sterile site, e.g., blood or cerebrospinal, synovial or peritoneal fluid. All included patients were residents of Manitoba and had MH coverage when they were diagnosed. To define eligibility, we used MH's Population Registry, a continuously updated database that stores demographic information on all ensured Manitobans, and tracks dates and reasons for initiating and ending coverage, e.g., birth, migration or death.
Covariates
Individuals were assigned to a neighborhood of residence (neighborhood clusters within the capital city of Winnipeg and regional health authorities in the rest of the province) based on their postal code as recorded in MH's Population Registry. Household income quintiles, measured at the level of Census Dissemination areas, were determined using 2006 Canadian census data.
Information on health care utilization (number of hospitalizations and physician visits) and on relevant pre-existing health conditions was obtained from MH's hospital and physician claims databases. Since 1971, information on virtually all hospital admissions and day surgeries in the province has been captured by the Hospital Abstracts Database.Citation28 Available data include diagnosis and treatment information, coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) before April, 2004, and the ICD-10-CACitation34 (Canadian adaptation of the ICD-10Citation35) and the Canadian Classification of Health Interventions (CCI)Citation36 afterwards. The Medical Services Database, also in operation since 1971, collects similar information on services provided by physicians in offices, hospitals and outpatient departments across the province.Citation28 Each record includes tariff codes specific to each service provided and a single 3-digit ICD-9 code which identifies the principal diagnosis or main reason for the visit.
We used previously validated algorithms, based on the frequency of certain disease and procedure codes, to identify people with chronic diseases.Citation37,38 Immunosuppression was defined as a diagnosis of HIV/AIDS, other immune deficiency disorders or cancer (other than non-melanoma skin cancer), or use of immunosuppressive drugs.Citation39 Information on immunosuppressants was obtained from the electronic database of the provincial Drug Program Information Network, the comprehensive database of all out-of-hospital prescriptions dispensed in Manitoba since 1995. Information on use of pneumococcal and influenza vaccines was obtained from the Manitoba Immunization Monitoring System (MIMS), a population-based province-wide registry of all immunizations administered in Manitoba since 1988.Citation29
Statistical analysis
We calculated crude and age-standardized annual incidence rates of IPD using population denominators obtained from MH's Population Registry, and the 2006 Canada census population as the standard population. In addition, we calculated incidence rates, age-standardized when appropriate, for different subsets of the population defined by socio-demographic (e.g., income, neighborhood of residence) and clinical (e.g., pre-existing chronic diseases, immune status) characteristics. When sufficient numbers were available, we also calculated age-standardized rates of IPD among subgroups with specific conditions, e.g., diabetes, chronic kidney failure, etc. Confidence intervals (95%CI) for crude and age-standardized rates were estimated assuming a Poisson distribution.
We assessed trends in the distribution of S. pneumoniae serotypes by measuring changes in the proportion of IPD cases caused by each common serotype over the study period. We repeated the same analysis after grouping cases according to whether the causative serotype is included in the PPV23, PCV7 or PCV13.
We also measured the cumulative probability of dying within 1 month following diagnosis, overall and for specific subsets of cases (e.g., people with chronic conditions). The source population for this analysis was all IPD cases reported in Manitoba over the study period. We used survival analysis methods to evaluate predictors of surviving the illness. Survival time was measured as the time from the date of diagnosis to either the date of death or date of censoring (1 month after diagnosis). We used multivariate Cox proportional hazard models to estimate relative risks (hazards ratios) associated with the presence and type of chronic diseases while adjusting for several potential confounding variables including age, gender, area of residence, income, as well as vaccination and frequency of contact with health care as a proxy for health seeking behavior. In addition, we repeated these analyses for deaths occurring within 60 d and 1 y following diagnosis.
Disclosure of potential conflicts of interest
Salaheddin M. Mahmud received unrestricted grant funding from GSK, Sanofi Pasteur and Merck for unrelated studies. All other authors declare that they have no conflict of interest.
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository under project “2012–048” (HIPC# 2012/2013–31). The results and conclusions are those of the authors. No official endorsement by Manitoba Health, Seniors and Active Living is intended or should be inferred.
Funding
This work was supported by unrestricted research from Pfizer under grant number WS2379184. Salaheddin M. Mahmud is a Canada Research Chair in Pharmaco-epidemiology and Vaccine Evaluation.
References
- Deng X, Church D, Vanderkooi OG, Low DE, Pillai DR. Streptococcus pneumoniae infection: a Canadian perspective. Exp Rev Anti Infect Ther 2013; 11:781-91; PMID:23977934; https://doi.org/10.1586/14787210.2013.814831
- National Advisory Committee on Immunization (NACI). Statement on the use of conjugate pneumococcal vaccine - 13 Valent in Adults (Pneu-C-13). Can Commun Dis Rep 2013; 39:1-50
- Rudnick W, Liu Z, Shigayeva A, Low DE, Green K, Plevneshi A, Devlin R, Downey J, Katz K, Kitai I, et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995-2011. Vaccine 2013; 31:5863-71; PMID:24099873; https://doi.org/10.1016/j.vaccine.2013.09.049
- Bettinger JA, Scheifele DW, Kellner JD, Halperin SA, Vaudry W, Law B, Tyrrell G; Canadian Immunization Monitoring Program, Active (IMPACT). The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000–2007. Vaccine 2010; 28:2130-6; PMID:20044050; https://doi.org/10.1016/j.vaccine.2009.12.026
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11:760-8; PMID:21621466; https://doi.org/10.1016/S1473-3099(11)70090-1
- Paulus S, David S, Tang W, Winters M, Buxton J, Henry B. Incidence of invasive pneumococcal disease after introduction of the universal infant immunization program, British Columbia (2002–2005). Can Commun Dis Rep 2006; 32:157-61; PMID:16869067
- Schillberg E, Isaac M, Deng X, Peirano G, Wylie JL, Van Caeseele P, Pillai DR, Sinnock H, Mahmud SM. Outbreak of invasive Streptococcus pneumoniae Serotype 12F among a marginalized inner-city population in Winnipeg, Canada (2009–2011). Clin Infect Dis 2014; 59(5):651-7; PMID:24842908
- Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res 2002; 55:506-19; PMID:12160947; https://doi.org/10.1016/S0008-6363(02)00414-5
- Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2012; 31:501-8; PMID:22327872; https://doi.org/10.1097/INF.0b013e31824de9f6
- Ricketson LJ, Wood ML, Vanderkooi OG, MacDonald JC, Martin IE, Demczuk WH, Kellner JD; Calgary Streptococcus pneumoniae Epidemiology Research (CASPER) investigators. Trends in asymptomatic nasopharyngeal colonization with streptococcus pneumoniae after introduction of the 13-valent pneumococcal conjugate vaccine in Calgary, Canada. Pediatr Infect Dis J 2014; 33:724-30; PMID:24463806; https://doi.org/10.1097/INF.0000000000000267
- Davis SM, Deloria-Knoll M, Kassa HT, O'Brien KL. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine 2013; 32:133-45; PMID:23684824; https://doi.org/10.1016/j.vaccine.2013.05.005
- Leal J, Vanderkooi OG, Church DL, Macdonald J, Tyrrell GJ, Kellner JD. Eradication of invasive pneumococcal disease due to the seven-valent pneumococcal conjugate vaccine serotypes in Calgary, Alberta. Pediatr Infect Dis J 2012; 31:e169-75; PMID:22673137; https://doi.org/10.1097/INF.0b013e3182624a40
- Weinberger DM, Bruden DT, Grant LR, Lipsitch M, O'Brien KL, Pelton SI, Sanders EA, Feikin DR. Using pneumococcal carriage data to monitor postvaccination changes in invasive disease. Am J Epidemiol 2013; 178:1488-95; PMID:24013204; https://doi.org/10.1093/aje/kwt156
- Lynch J 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med 2009; 30:189-209; PMID:19296419; https://doi.org/10.1055/s-0029-1202938
- Black S. The volatile nature of pneumococcal serotype epidemiology: potential for misinterpretation. Pediatr Infect Dis J 2010; 29:301-3; PMID:19935444
- Vanderkooi OG, Church DL, MacDonald J, Zucol F, Kellner JD. Community-based outbreaks in vulnerable populations of invasive infections caused by Streptococcus pneumoniae serotypes 5 and 8 in Calgary, Canada. PloS One 2011; 6:e28547-e; PMID:22216100; https://doi.org/10.1371/journal.pone.0028547
- Deng X, Peirano G, Schillberg E, Mazzulli T, Gray-Owen SD, Wylie JL, Robinson DA, Mahmud SM, Pillai DR. Whole-genome sequencing reveals the origin and rapid evolution of an emerging outbreak strain of Streptococcus pneumoniae 12F. Clin Infect Dis 2016; 62(9):1126-32
- Kyaw MH, Rose CE Jr, Fry AM, Singleton JA, Moore Z, Zell ER, Whitney CG; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis 2005; 192:377-86; PMID:15995950; https://doi.org/10.1086/431521
- Helferty M, Rotondo JL, Martin I, Desai S. The epidemiology of invasive pneumococcal disease in the Canadian North from 1999 to 2010. Int J Circumpolar Health 2013; 72:21606; https://doi.org/10.3402/ijch.v72i0.21606
- Weatherholtz R, Millar EV, Moulton LH, Reid R, Rudolph K, Santosham M, O'Brien KL. Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis 2010; 50:1238-46; PMID:20367225; https://doi.org/10.1086/651680
- Rachdaoui N, Sarkar DK. Effects of alcohol on the endocrine system. Endocrinol Metab Clin North Am 2013; 42:593-615; PMID:24011889; https://doi.org/10.1016/j.ecl.2013.05.008
- Choudhury D, Luna-Salazar C. Preventive health care in chronic kidney disease and end-stage renal disease. Nat Clin Pract Nephrol 2008; 4:194-206; PMID:18285747; https://doi.org/10.1038/ncpneph0762
- Wotton CJ, Goldacre MJ. Risk of invasive pneumococcal disease in people admitted to hospital with selected immune-mediated diseases: record linkage cohort analyses. J Epidemiol Community Health 2012; 66:1177-81; PMID:22493476; https://doi.org/10.1136/jech-2011-200168
- Siemieniuk RA, Gregson DB, Gill MJ. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study. BMC Infect Dis 2011; 11:314; PMID:22078162; https://doi.org/10.1186/1471-2334-11-314
- Parrish I, McDonnell SM. Sources of health related information. Principles Practice Public Health Surveillance 2000; 2:76-94
- Singh H, Mahmud SM, Turner D, Xue L, Demers AA, Bernstein CN. Long-term use of statins and risk of colorectal cancer: a population-based study. Am J Gastroenterol 2009; 104:3015-23; PMID:19809413; https://doi.org/10.1038/ajg.2009.574
- Robinson JR, Young TK, Roos LL, Gelskey DE. Estimating the burden of disease: comparing administrative data and self-reports. Med Care 1997; 35:932-47; PMID:9298082; https://doi.org/10.1097/00005650-199709000-00006
- Roos L, Mustard C, Nicol J, McLerran D, Malenka D, Young T, Cohen MM. Registries and administrative data: organization and accuracy. Med Care 1993; 31:201-12; PMID:8450678; https://doi.org/10.1097/00005650-199303000-00002
- Roberts J, Roos L, Poffenroth L, Hassard T, Bebchuk J, Carter A, Law B. Surveillance of vaccine-related adverse events in the first year of life: a Manitoba cohort study. J Clin Epidemiol 1996; 49:51; PMID:8598511; https://doi.org/10.1016/0895-4356(95)00522-6
- Mahmud SM, Van Caeseele P, Hammond G, Kurbis C, Hilderman T, Elliott L. No association between 2008–09 influenza vaccine and influenza a (H1N1) pdm09 virus infection, Manitoba, Canada, 2009. Emerg Infect Dis 2012; 18:801; PMID:22516189; https://doi.org/10.3201/eid1805.111596
- Mahmud SM, Kliewer EV, Lambert P, Bozat-Emre S, Demers AA. Effectiveness of the quadrivalent human papillomavirus vaccine against cervical dysplasia in Manitoba, Canada. J Clin Oncol 2014; 32(5):438-43
- Kliewer EV, Mahmud SM, Demers AA, Lambert P. Human papillomavirus vaccination and Pap testing profile in Manitoba, Canada. Vaccine 2013; 32:33-8; PMID:24211170; https://doi.org/10.1016/j.vaccine.2013.10.082
- Canada TPHAo. Case definitions for diseases under national surveillance: invasive pneumococcal disease. CCDR 2009; 35:34-35
- Canadian Institute for Health Information. ICD-10-CA International statistical classification of diseases and related health problems, Tenth Revision. Ottawa, Ontario, Canada, 2009
- World Health Organization. International statistical classification of diseases and related health problems, Tenth Revision. World Health Organization Geneva, Switzerland, 1993
- Canadian Institute for Health Information. Canadian Classification of Health Interventions. Ottawa, Ontario, Canada, 2006
- Lix L, Yogendran M, Burchill C, Metge C, McKeen N, Moore D, et al. Defining and Validating Chronic Diseases: An Administrative Data Approach. Winnipeg: Manitoba Centre for Health Policy, 2006
- Brownell MD, Yogendran MS. Attention-deficit hyperactivity disorder in manitoba children: medical diagnosis and psychostimulant treatment rates. Can J Psychiatry 2001; 46:264-72; PMID:11320681; https://doi.org/10.1177/070674370104600307
- Dublin S, Jackson ML, Nelson JC, Weiss NS, Larson EB, Jackson LA. Statin use and risk of community acquired pneumonia in older people: population based case-control study. BMJ 2009; 338:b2137; PMID:19531550; https://doi.org/10.1136/bmj.b2137
