ABSTRACT
Allergen-specific immunotherapy is the only curative treatment of honeybee venom (HBV) allergy, which is able to protect against further anaphylactic sting reactions. Recent analyses on a molecular level have demonstrated that HBV represents a complex allergen source that contains more relevant major allergens than formerly anticipated. Moreover, allergic patients show very diverse sensitization profiles with the different allergens. HBV-specific immunotherapy is conducted with HBV extracts which are derived from pure venom. The allergen content of these therapeutic extracts might differ due to natural variations of the source material or different down-stream processing strategies of the manufacturers. Since variations of the allergen content of therapeutic HBV extracts might be associated with therapeutic failure, we adressed the component-resolved allergen composition of different therapeutic grade HBV extracts which are approved for immunotherapy in numerous countries. The extracts were analyzed for their content of the major allergens Api m 1, Api m 2, Api m 3, Api m 5 and Api m 10. Using allergen-specific antibodies we were able to demonstrate the underrepresentation of relevant major allergens such as Api m 3, Api m 5 and Api m 10 in particular therapeutic extracts. Taken together, standardization of therapeutic extracts by determination of the total allergenic potency might imply the intrinsic pitfall of losing information about particular major allergens. Moreover, the variable allergen composition of different therapeutic HBV extracts might have an impact on therapy outcome and the clinical management of HBV-allergic patients with specific IgE to particular allergens.
Introduction
Stings of Hymenoptera such as honeybees or vespids can cause severe and even fatal anaphylaxis in allergic individuals. The only curative treatment which is effective in reducing the risk of subsequent systemic reactions is venom-specific immunotherapy (VIT). VIT is effective in 75% to 98% of patients in preventing sting anaphylaxis. Citation1 However, therapy failures occur more often in honeybee venom (HBV) compared with yellow jacket venom (YJV) allergy. Citation2
HBV represents a complex mixture of various substances such as low-molecular weight components (e.g. histamine, noradrenalin, serotonin and dopamine), peptides (e.g., melittin, apamin, kinins and mast cell degranulating peptide) and a plethora of proteins from which several are allergens. Citation3 VIT is performed with venom extracts which are administered either as aqueous or aluminum hydroxide-adsorbed extracts (depot preparations). The latter are used in the conventional build-up and maintenance phases, while the aqueous extracts are used in ultra-rush, rush, clustered and maintenance phases. Citation4 Interestingly, in Europe many specialists switch from aqueous extracts to depot preparations after up-dosing. Citation5,Citation6
All therapeutic HBV extracts are derived from pure venom, which is usually collected by electrostimulation, a procedure which leads to a relatively pure venom. Another possibility for obtaining venom extract is the dissection of whole venom glands and venom sacs, a method yielding less pure extract since in addition to the venom components, also proteins from the surrounding tissue are contained in the extract. However, only scarce information is available about how the venom is further processed by different manufacturers to produce therapeutic grade venom extracts. Although this classification is a little misleading in the literature, aqueous venom extracts are sometimes classified as “purified” and “non-purified” extracts. Citation4,Citation7,Citation8 This terminology results from the fact that, even though, all manufacturers surely undertake purification steps of the pure venom for injection purposes, some companies claim to offer an ultrapure venom extract for therapy which does not contain vasoactive amines and a reduced content of small peptides. Citation4 In the commonly used licensed depot preparation, the “purified” extract is adsorbed onto aluminum hydroxide. In comparative trials, the purified aqueous and the purified aluminum hydroxide-adsorbed extracts appear to be better tolerated than non-purified extracts, especially in terms of severe large local reactions. Citation7,Citation8
Although the production of therapeutic allergen extracts has to be highly standardized in terms of the production process and of the total allergenic potency, Citation9 the lack of information about a broader range of clinically relevant allergens and of appropriate molecular tools for their assessment hampers the generation of highly reliable venom extracts with a more favorable overall therapeutic efficacy.
Especially HBV might represent a challenge for the preparation of therapeutic extracts including all relevant allergens in adequate amounts, since over 60% of its dry-weight is made up by the allergens Api m 1 (12%) and Api m 4 (50%). Citation10 While Api m 1 (phospholipase A2) represents a well-established major allergen, Api m 4 (melittin) is a minor allergen with restricted clinical relevance. Citation3 Recently it was demonstrated that HBV contains many more additional important major allergens, namely Api m 2 (hyaluronidase), Api m 3 (acid phosphatase), Api m 5 (dipeptidyl peptidase IV) and Api m 10 (icarapin) which exhibit sIgE reactivity with 47.9–52.2%, 49.6–50%, 58.3–61.7% and 61.8–72.2% of allergic patient's sera, respectively. Citation11,Citation12 Compared to Api m 1 and Api m 4, all these allergens are present in the venom in only minimal amounts. Citation13,Citation14 This might implicate that especially the amount of these allergens in therapeutic extracts, might be easily affected by natural variations of the source material, different work-up strategies of the manufacturers or even by degradation of particular components.
In a former study we used monoclonal antibodies and demonstrated that, compared with crude HBV, the allergens Api m 3 and Api m 10 are underrepresented or even missing in particular therapeutic HBV extracts which are commonly used for VIT. Citation13 Very recently, another study correlated treatment failures of HBV VIT with a predominant Api m 10 sensitization and demonstrated the lack or underrepresentation of Api m 10 in different therapeutic HBV extracts. Citation11
Such data might be of major importance for the clinical management of HBV-allergic patients with specific IgE to particular allergens. Therefore, in this study we extended former analyses and generated highly specific and sensitive antibodies for the detection of the major allergens Api m 2, Api m 3, Api m 5 and Api m 10 and compared different therapeutic HBV extracts regarding their allergen content. Thereby, we were able to demonstrate the underrepresentation of relevant major allergens in particular therapeutic extracts. Moreover, compared with another study, Citation11 we found dramatically different results concerning the Api m 3 and Api m 10 content of particular products, a fact that is of major importance for clinical decisions on the selection of licensed immunotherapeutic products in Europe. Additionally, our findings on Api m 10 stability might have an impact on the immunotherapeutic procedure for venom allergy, both in Europe and in the United States.
Results
Generation of allergen-specific antibodies
Polyclonal antibodies with specificity for the major allergens Api m 2, Api m 3 and Api m 10 were generated by the immunization of rabbits with the individual purified and cross-reactive carbohydrate determinat (CCD-)-free recombinant allergens. Citation12,Citation13,Citation15,Citation16 For the detection of Api m 5 we used a recombinant monoclonal IgE antibody. Citation17
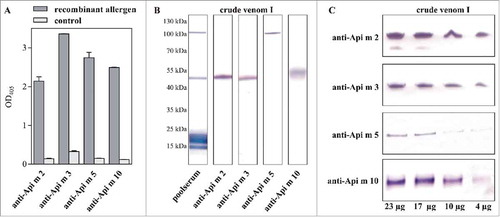
All rabbit antisera that resulted from the immunizations with the individual allergens as well as the monoclonal Api m 5-specific antibody showed excellent reactivity with the recombinant allergens (). Moreover, all antibodies proved to be highly specific for the natural allergens in the crude HBV and detected single allergen bands of the expected molecular weight in immunoblots (). The sensitivity of the detection was assessesd using dilution series of the crude venom and all antibodies showed adequate detection of their target allergens within 4 µg of whole venom ().
Figure 1. Specificity and sensitivity of allergen-specific antibodies. A, Reactivity of the antibodies with their corresponding recombinant target allergens in ELISA. B, Reactivity of the antibodies with their native target allergens in crude honeybee venom. In comparison, the IgE-reactivity of a poolserum from honeybee venom-allergic patients is shown. Since all investigated allergens represent glycoproteins, the molecular weights do not correspond to that of the calculated weights of the protein portions only, which are stated in some databases. C, Detection of the particular allergens in serial dilutions of crude honeybee venom to assess the sensitivity of the allergen-specific antibodies in immunoblot.
Allergen content of therapeutic HBV extracts
We addressed the major allergen content of 4 different aqueous therapeutic HBV extracts which are approved for immunotherapy in different countries world-wide: Venomil (Allergy Therapeutics, Worthing, UK), Reless (ouside Germany also known as Pharmalgen; ALK-Abelló, Hamburg, Germany), ALK lyophylisiert SQ (ouside Germany also known as Aquagen SQ; ALK-Abelló) and Venomenhal (HAL Allergy, Leiden, Netherlands). Furthermore, the allergen content of these therapeutic HBV extracts was compared with 2 commercially available crude HBV extracts (I: Sigma-Aldrich, Taufkirchen, Germany: II: Latoxan, Portes-lès-Valence, France).
For the analyses, all lyophilized extracts were reconstituted with ddH2O and the freshly reconstituted extracts were applied for immunoblotting. Staining of the major allergen Api m 1 served as loading control to ensure that all extracts were present on the immunoblot at equal amounts (representative stainings are shown in ). Api m 1 was present in all therapeutic extracts as well as in the crude venoms in equal and high amounts. Additionally, Api m 2 was well detectable in all therapeutic extracts (). Api m 3, Api m 5 and Api m 10 were detectable in comparable amounts in Venomil, Reless (Pharmalgen) and Venomenhal, although, to a slightly lesser extent than in the crude HBV (). However, all 3 allergens were strongly underrepresented and only barely detectable in ALK lyophylisiert SQ (Aquagen SQ) ().
Figure 2. Allergen content of therapeutic honeybee venom extracts. A, Allergen content of therapeutic venom extracts compared with crude venom as assessed by the use of polyclonal (Api m 2, Api m 3 and Api m 10) and monoclonal (Api m 5) antibodies. Representative results of 5 batches of Venomil (Allergy Therapeutics, Worthing, UK), 2 batches Reless (Pharmalgen) (ALK-Abelló, Hamburg, Germany), 2 batches ALK lyophylisiert SQ (Aquagen SQ) (ALK-Abelló) and 2 batches of Venomenhal (HAL Allergy, Leiden, Netherlands) are shown. Ponceau S staining of Api m 1 served as loading control (a representative staining is shown). B, Api m 3 and Api m 10 content of 3 independent batches (B1-B3) of Venomenhal.
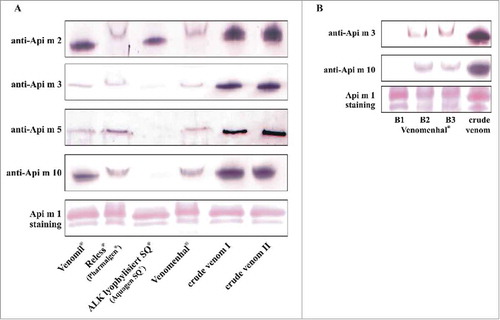
Intriguingly, while these results were reproducible for 5 batches of Venomil, 2 batches of Reless and 2 batches of ALK lyophylisiert SQ, in another batch of Venomenhal, Api m 3 and Api m 10 were not detectable. The direct comparison of the 3 batches of this product showed that both allergens were well detectable in 2 batches but undetectable in a third batch (), while the content of the other investigated allergens was comparable for all batches (data not shown).
In a recent study a comparable Api m 3-specific antiserum was used and a clear reactivity of all therapeutic HBV extracts, comparable to that of crude HBV, was postulated. Due to these divergent results we repeated the analysis using an already published monoclonal Api m 3-specific antibody. Citation13 Thereby, we were able to completely confirm our previous results using the polyclonal antiserum, namely, a lesser Api m 3 content of all therapeutic extracts compared with the crude venom and an underrepresentation of Api m 3 in ALK lyophylisiert SQ (Aquagen SQ) compared with the other products (Fig. S1).
Stability of Api m 10
To address the variable Api m 10 content of different therapeutic products and the observed discrepant results for particular products compared with the recently published study by Frick et al., Citation11 we evaluated the stability of this relevant allergen. Interestingly, applying recombinantly produced and purified Api m 10 in a comparable concentration as found in the crude venom, our analyses demonstrated rapid degradation of the allergen within 3 days, when stored in solution at +4° C ().
Figure 3. Stability of the major allergen Api m 10. A, Stability of recombinant purified Api m 10 produced in insect cells. Api m 10, in a concentration comparable to that detected in crude venom, was stored for 3 d at + 4° C. B, Stability of Api m 10 in crude honeybee venom reconstituted either with HSA-containing diluent for injection (Allergy Therapeutics) or PBS upon storage at + 4° C for 4 weeks. C, Detection of Api m 10 in water-resolved Venomil (no HSA in the lyophylisate) and Reless (HSA in the lyophylisate) stored for 4 weeks at either - 20° C or + 4° C.
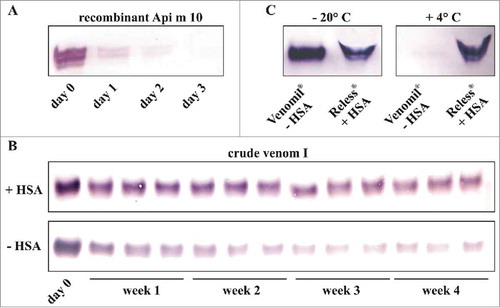
In our analyses we always used freshly reconstituted therapeutic venom extracts. However, the observed instability of Api m 10 could critically influence immunoblot-based analyses in the laboratory. For laboratory purposes the freeze-dried therapeutic venom extracts are routinely solved in ddH2O or buffers like PBS and not in the supplied albumin-containing saline diluent for injection purposes. This implies that some PBS-reconstituted products such as Reless (Pharmalgen) or Venomenhal contain human serum albumin (HSA) since it is already contained in the freeze-dried venom. In contrast, for Venomil the HSA is added together with the diluent in clinical practice, so that ddH2O- or buffer-reconstituted Venomil contains no HSA. The stabilizing effect of HSA on Api m 10 in crude venom, stored in solution at +4° C, is shown in . Moreover, these results indicate that laboratory analyses of therapeutic venom extracts might be influenced by the reagents used for their reconstitution and by the subsequent storage of the solubilized extracts, as demonstrated by the different results for ddH2O-reconstituted Venomil and Reless stored at either -20°C or +4° C (). However, in clinical practice this fact does not matter since all analyzed products contain HSA after reconstitution with the supplied diluent.
Discussion
In this study we established tools that allow a component-resolved analysis of therapeutic HBV extracts. Four therapeutic HBV extracts commonly used for VIT were analyzed for their content of the major allergens Api m 1, Api m 2, Api m 3, Api m 5 and Api m 10. Intriguingly, numerous differences could be demonstrated for the particular products. The observed varying major allergen content of therapeutic HBV extracts might have a high impact on clinical practice and on the handling of patients with particular sensitization profiles.
Although obtained by methods, yielding relatively pure venom, the analyzed therapeutic HBV extracts showed a diverse content of important major allergens. This degree of variation, as also demonstrated for other allergen extracts, Citation18-Citation22 indicates that different strategies for down-stream processing of the pure venom for the production of therapeutic grade venom extracts, can substantially affect the representation of major venom allergens, resulting in the potential loss of particular allergens with high clinical relevance. Moreover, the geographical origin or seasonal variations might additionally affect the composition of the source material. Citation23
During the last years, studies demonstrated that in addition to Api m 1 also Api m 2, Api m 3, Api m 5 and Api m 10 represent major allergens of HBV. Citation12,Citation13,Citation15,Citation17,Citation24,Citation25 Additionally, 39 different sensitization profiles were identified in 144 patients with HVB allergy, applying 6 different allergens (Api m 1–5 and 10). Citation12 Interestingly, in HBV allergy these 6 allergens are necessary to reach a diagnostic sensitivity of approximately 95%, a fact that indicates a more complex allergen composition of HBV compared with YJV. In YJV allergy the 2 major allergens Ves v 1 and 5 are sufficient to reach the same diagnostic sensitivity. Citation26 Both of the 2 important allergens are present in YJV in substantial and equimolar amounts (Ves v 1 with 6–14% and Ves v 5 with 5–10% of the venom dry weight). Citation14 In contrast, in HBV Api m 1 is the only major allergen that is present in substantial amounts and all other relevant allergens make up only 0.6–2% of the venom dry weight. Citation13,Citation14 Therefore, it could be speculated that these differences between the 2 venoms might be a reason for the higher success rate of YJV-specific immunotherapy.
Currently, it is a matter of debate whether particular sensitization profiles are linked to the outcome of VIT with HBV. Very recently, a study correlated treatment failures of HBV VIT with a predominant Api m 10 sensitization. Citation11 The same study demonstrated the lack or underrepresentation of Api m 10 in different therapeutic HBV extracts commonly used for VIT. As a consequence of the variable Api m 10 content, it was suggested that patients with predominant Api m 10 sensitization should be treated with a HBV extract containing a relevant amount of the allergen. To our opinion this is a major step forward toward a personalized medicine approach in VIT and, surely, will influence the use of VIT products. Likewise, it might be an option to treat patients without Api m 10 sensitization with a product that lacks the allergen.
In this study, the content of 5 relevant major allergens in 4 aqueous HBV extracts which are commonly used for immunotherapy was addressed for the first time. Thereby, we were able to demonstrate comparable allergen contents for Api m 1 and Api m 2. However, substantial differences were demonstrated for the other allergens. While Api m 3, Api m 5 and Api m 10 could be reproducibly detected in different batches of Venomil and Reless (Pharmalgen), all 3 allergens were clearly underrepresented in ALK lyophylisiert SQ (Aquagen SQ). An underrepresentation of Api m 3 and Api m 10 was observed for one but not for 2 other batches of Venomenhal. Only one batch of the product was available on the market at a time and the underrepresentation was observed only for the earliest purchased batch. Therefore, the differences in the Api m 3 and Api m 10 content could either be due to batch to batch variations or to a modified production process.
Whether the underrepresentation of the 3 major allergens, which was observed for ALK lyophylisiert SQ (Aquagen SQ), also holds true for the related depot preparation (ALK-depot SQ or Alutard SQ), can only be speculated at this time point since the analysis of aluminum-adsorbed venom extracts by the here applied methods is very challenging. Recently, the presence of Api m 10-derived peptides was demonstrated for Aquagen SQ (and Alutard SQ) by mass spectrometry (MS) analyses. Citation27 This is in accordance with our immunoblot analyses which were also able to detect minimal amounts of intact Api m 10 in ALK lyophylisiert SQ (Aquagen SQ). Nevertheless, our results demonstrate obvious differences in the amount of full-length Api m 10 in this particular product compared with other aqueous extracts. Moreover, while our analyses address the content of the full-length protein, the applied MS analyses are not able to discriminate between full-length Api m 10 and Api m 10-derived degradation products. Additionally, the used MS analyses were not quantitative. However, so far it is not known whether the intact allergen and derived degradation products thereof exhibit the same potency in inducing a tolerogenic immune response. Definitely, further studies are needed, which address, if small amounts of Api m 10 or Api m 10-derived peptides in the therapeutic extracts are sufficient to induce tolerance in the majority of patients. Nevertheless, our analyses suggest that intensive purification and processing steps of the crude venom might strongly influence the content of full-length Api m 10 (as well as of Api m 3 and Api m 5), and since underrepresentation was most pronounced in ALK-lyophylisiert SQ (Aquagen SQ), it's processing that removes low molecular weight substances and reduces the amount of bioactive peptides, may be relevant here. Citation4,Citation7,Citation8
A recent study demonstrated the lack or underrepresentation of Api m 10 in Venomil, Venomenhal and Aquagen SQ as well as its presence in Pharmalgen. Citation11 Although in our analyses we applied the same methods, intriguingly, we found dramatically different results in part, a fact that is of major importance for clinical decisions on the selection of licensed immunotherapeutic products in Europe. Our study was able to confirm the underrepresentation of Api m 10 in Aquagen SQ (ALK lyophylisiert SQ) and its presence in Pharmalgen (Reless). However, in strong contrast to that study, we were able to detect comparable amounts of Api m 10 in 5 independent batches of Venomil. For Venomenhal our analyses demonstrated batch to batch variations ranging from Api m 10 content comparable to Venomil and Pharmalgen to undetectable content. These are facts that might be of importance for the handling of patients with Api m 10 sensitization.
Regarding the reasons for these different results, it can only be speculated. The analyses were reproducibly performed with different independent batches in both studies. The sensitivity of detection is not able to give a reasonable explanation for the observed discrepancy, since the evaluated detection limit of our experimental setup is significantly higher than the postulated detection limit by Frick et al.: Whereas 4–10 µg of crude venom was necessary to achieve adequate detection of Api m 10 in our immunoblots, Frick et al. show effective detection when applying 0.5–1.5 µg of crude venom. Most likely, the observed discrepancies might be explained by the rapid degradation of the allergen after solubilizing the lyophilized therapeutic extracts and storage at + 4° C. Unfortunately, Frick et al. do not state how venom extracts were handled in the laboratory after solubilization. Nevertheless, these data clearly demonstrate the need for standardized operation procedures for quality-control of therapeutic extracts in different laboratories.
In clinical practice in Europe, the observed Api m 10 instability might be limited by the fact that all of the licensed products contain potentially stabilizing human serum albumin after reconstitution of the lyophilized extract. However, our results might implicate that the use of small pharmaceutical phials that contain therapeutic extracts for one injection might be superior over larger ones that are stored for several weeks after reconstitution. However, the obtained results may not only influence clinical decisions for the selection of immunotherapeutic products in Europe. Due to the observed instability of Api m 10, they might also have an impact on the immunotherapeutic procedure in the United States, where the clinical routine practice involves the formulation of patient-specific preparations for immunotherapy based on stock solutions of venom extract stored at + 4° C, exactly the conditions that lead to rapid Api m 10 degradation.
In contrast to a former analysis in which we used an Api m 3-specific monoclonal antibody for detection, Citation13 the study by Frick et al. Citation11 demonstrated the presence of Api m 3 in all assessed therapeutic products in comparable amounts to that in crude HBV by applying polyclonal antibodies. Our analyses shown here, also used polyclonal rabbit antibodies that were generated against full-length Api m 3 produced in insect cells. Surprisingly, these analyses showed clearly lesser amounts of Api m 3 in all therapeutic extracts, compared with crude HBV as well as an underrepresentation in ALK lyophylisiert SQ (Aquagen SQ) and in 1 out of 3 batches of Venomenhal compared with the other therapeutic extracts. Although, both studies used polyclonal rabbit antibodies, these differences might be explained by slight differences in sensitivity or specificity of detection. In contrast to the antibodies used in our study, which detected a single band of Api m 3, the antibodies used by Frick et al. resulted in multiple bands in some of the analyzed products. Moreover, our results are in full accordance with the data obtained in a former study Citation13 and with the results obtained using the monoclonal Api m 3-specific antibody in this study.
Considering the complex sensitization profiles of HBV-allergic patients, Citation12 it might be speculated that some of the therapeutic extracts could be associated with therapeutic failure in patients with particular sensitization profiles, an issue that should be addressed in future studies. Potential candidates might be patients who are polysensitized to several allergens or who are exclusively sensitized to allergens such as Api m 3 and/or Api m 10 (4.8% of patients Citation12 ), which are not or only barely detectable in particular therapeutic HBV extracts, and against which, only minimal IgG4 is induced during VIT in contrast to other allergens that are present in substantial amounts. Citation12 Moreover, the immune response to minimal amounts of allergen in particular HBV extracts might differ strongly in individual patients. Notably, HBV in general might represent a particularly challenging allergen source for the preparation of therapeutic extracts, containing all major allergens in sufficient amounts. Studies of other therapeutic vaccines for allergen immunotherapy demonstrated that maintenance doses of 3 to 20 µg of major allergen are associated with clinical improvement after immunotherapy. Citation28 Taken a maintenance dose of 100 µg, these amounts are only reached for Api m 1 and none of the other major allergens of HBV, even within crude venom. Therefore, the mechanism of tolerance induction against low amounts of major allergens in the majority of HBV-allergic patients clearly should be a focus of future research. The limitation of our study is represented by the fact that at this time no connection between therapeutic outcome, allergen content of products used for therapy and IgE sensitization profiles of patients can be revealed. Therefore, our results clearly demonstrate the need for extended prospective clinical studies focusing on this relationship.
Nevertheless, our results might have implications for i) the clinical management of HBV-allergic patients particular sensitization profiles worldwide, ii) the quality control and regulatory process for patient-named and licensed products used for VIT procedures, and iii) all major stakeholders (doctors, patients, regulators, reimbursement systems/insurance companies) in the affected health markets, helping them to make the right decisions in the emerging era of precision medicine.
Taken together, our data demonstrate obvious differences in the quality of therapeutic HBV extracts in terms of the content of important allergens, a fact that might be of major importance at least for patients with particular sensitization profiles. Moreover, standardization of therapeutic venom extracts by determination of the total allergenic potency might imply the intrinsic pitfall of losing information about particular major allergens. Allergen-specific antibodies represent valuable tools that allow component-resolved analyses of therapeutic extracts on a molecular level that cope with the advanced knowledge of the composition of relevant allergens.
Materials and methods
Allergen-specific antibodies and recombinant allergens
The recombinant CCD-free allergens were produced in Spodoptera frugiperda (Sf9) insect cells and purified as described previously. Citation12,Citation13,Citation15-Citation17 Polyclonal antibodies were generated by immunization of rabbits (Davids Biotechnology, Regensburg, Germany) with either recombinant Api m 2, Api m 3 or Api m 10 according to established protocols. The monoclonal Api m 3- and Api m 5-specific IgE antibodies were generated as described previously. Citation13,Citation17,Citation29
Immunoblotting
For immunoblotting, lyophilized HBV extracts were dissolved in ddH2O to a stock concentration of 1.3 mg/mL. Immediately after dissolving 23 µg/lane (or less for sensitivity testing of the antibodies) were separated by SDS-PAGE under reducing conditions and immobilized onto nitrocellulose membranes (Thermo Scientific, 88018). Blot membranes were blocked with 40 mg/mL nonfat dry milk powder (AppliChem, A0830) in PBS (Life Technologies, 70011051). Polyclonal allergen-specific rabbit antisera were diluted 1:1000 with 20 mg/mL nonfat dry milk powder in PBS. Recombinant monoclonal IgE antibodies were used in form of cell culture supernatants (DMEM (Gibco, 31966–021) supplemented with 10% fetal calf serum (Biochrom, SO115)) of antibody-producing HEK293 cells. All antibodies were applied to the corresponding Western blots and incubated over night at 4° C. After washing for 3 times with PBS, bound allergen-specific antibodies were detected for 1 hour at room temperature via polyclonal goat anti-rabbit IgG (Sigma-Aldrich, SAB3700854) or monoclonal mouse anti-human IgE (BD Biosciences, 555859) antibody, conjugated to alkaline phosphatase, diluted 1:5000 or 1:1000 with 20 mg/mL nonfat dry milk powder in PBS, respectively. After washing for 3 times with PBS bound antibodies were visualized using nitrotetrazolium blue chloride (AppliChem, A1243)/5-bromo-4-chloro-3-indoyl phosphate (AppliChem, A1117) according to the recommendations of the manufacturer. Ponceau S (Sigma-Aldrich, P7170) staining of immobilized Api m 1 served as loading control.
Elisa
F96 maxisorp Nunc-immuno plates (Thermo Scientific, 439454) were coated with recombinant allergens (10 µg/mL) over night at 4° C and blocked with 10 mg/mL BSA (AppliChem, A1391) in PBS (Life Technologies, 70011051). Allergen-specific polyclonal rabbit antisera were diluted 1:5000 and monoclonal recombinant antibody cell culture supernatants 1:2 with 5 mg/mL BSA in PBS, applied to the corresponding wells and incubated for 4 hours at room temperature. After washing 5 times with 0.05% Tween20 (EMD Chemicals, 655204) in PBS, alkaline phosphatase-conjugated polyclonal goat anti-rabbit IgG (Sigma-Aldrich, SAB3700854) diluted 1:5000 or monoclonal mouse anti-human IgE (BD Biosciences, 555859) diluted 1:1000 in 5 mg/mL BSA were added for 1 hour at room temperature. After washing 5 times with 0.05% Tween20 in PBS, detection was performed with 5 mg/mL 4-nitrophenylphosphat disodium salt hexahydrate (AppliChem, A1442) in AP-detection buffer (100 mM Tris, 10 mM MgCl2*6 H2O, 100 mM NaCl, pH 9,5) and signals were read at 405 nm.
Abbreviations
| CCD | = |
cross-reactive carbohydrate determinant |
| HBV | = |
honeybee venom |
| HSA | = |
human serum albumin |
| PBS | = |
phosphate-buffered saline |
| VIT | = |
venom immunotherapy |
| YJV | = |
yellow jacket venom |
Disclosure of potential conflicts of interest
SB has received speaker's honorarium and/or travel support from ALK-Abelló, Bencard and Thermo Fisher Scientific; has received consultancy fees as an advisory board member and research support from Bencard. UD has been speaker, investigator and / or been a member of advisory boards for Allergopharma, ALK-Abelló, Bencard, GSK, Hermal, MEDA, Novartis Pharma, Stallergenes, Stiefel. MS has received travel support from ALK-Abelló. TB has received research funding, speaker's honorarium and consultancy fees from Thermo Fisher Scientific, has received research support from DFG, Novartis and Thermo Fisher Scientific, has received lecture fees from MSD, Novartis, HIPP GmbH & Co, ALK-Abelló, MedComms Ltd and Astellas Pharma GmbH. CBS-W has received grants from Allergopharma, Leti, PLS-Design and Regeneron; is member of the scientific advisory board of Leti and Bencard; has received consultancy fees from Leti, GLG Consultancy and Allergopharma; is board member of the Norwegian Research Council. MO has received consultancy fees from Siemens Healthcare, Hitachi Chemical Diagnostics and Bencard; has received lecture fees from Thermo Fisher Scientific, Bencard and Siemens Healthcare; is co-founder of PLS-Design GmbH. The other authors declare that they have no conflict of interest.
Supplemental_material.doc
Download MS Word (528.5 KB)References
- Golden DB. Insect sting anaphylaxis. Immunol Allergy Clin North Am 2007; 27:261-72, vii; PMID:17493502; https://doi.org/10.1016/j.iac.2007.03.008
- Rueff F , Przybilla B , Bilo MB , Muller U , Scheipl F , Seitz MJ , Aberer W , Bodzenta-Lukaszyk A , Bonifazi F , Campi P , et al. Clinical effectiveness of hymenoptera venom immunotherapy: a prospective observational multicenter study of the European academy of allergology and clinical immunology interest group on insect venom hypersensitivity. PloS one 2013; 8:e63233; PMID:23700415; https://doi.org/10.1371/journal.pone.0063233
- Ollert M , Blank S . Anaphylaxis to Insect Venom Allergens: Role of Molecular Diagnostics. Curr Allergy Asthma Rep 2015; 15:527; https://doi.org/10.1007/s11882-015-0527-z
- Bilo MB , Cinti B , Brianzoni MF , Braschi MC , Bonifazi M , Antonicelli L . Honeybee venom immunotherapy: a comparative study using purified and nonpurified aqueous extracts in patients with normal Basal serum tryptase concentrations. J Allergy 2012; 2012:869243; PMID:22287975; https://doi.org/10.1155/2012/869243
- Bilo BM , Bonifazi F . Hymenoptera venom immunotherapy. Immunotherapy 2011; 3:229-46; PMID:21322761; https://doi.org/10.2217/imt.10.88
- Van Vaerenbergh M , Debyser G , Devreese B , de Graaf DC . Exploring the hidden honeybee (Apis mellifera) venom proteome by integrating a combinatorial peptide ligand library approach with FTMS. J Proteomics 2014; 99:169-78; PMID:24606962; https://doi.org/10.1016/j.jprot.2013.04.039
- Bilo MB , Antonicelli L , Bonifazi F . Purified vs. nonpurified venom immunotherapy. Curr Opin Allergy Clin Immunol 2010; 10:330-6; https://doi.org/10.1097/ACI.0b013e328339f2d1
- Bilo MB , Severino M , Cilia M , Pio A , Casino G , Ferrarini E , Campodonico P , Milani M . The VISYT trial: Venom Immunotherapy Safety and Tolerability with purified vs nonpurified extracts. Ann Allergy Asthma Immunol 2009; 103:57-61; https://doi.org/10.1016/S1081-1206(10)60173-1 10.1016/S1081-1206(10)60144-5 10.1016/S1081-1206(10)60823-X
- Larsen JN , Dreborg S . Standardization of allergen extracts. Methods Mol Med 2008; 138:133-45; PMID:18612605
- Spillner E , Blank S , Jakob T . Hymenoptera allergens: from venom to “venome.” Front Immunol 2014; 5:77; PMID:24616722; https://doi.org/10.3389/fimmu.2014.00077
- Frick M , Fischer J , Helbling A , Rueff F , Wieczorek D , Ollert M , Pfützner W , Müller S , Huss-Marp J , Dorn B , et al. Predominant Api m 10 sensitization as risk factor for treatment failure in honey bee venom immunotherapy. J Allergy Clin Immunol 2016; 138:1663-71 e9; PMID:27372568; https://doi.org/10.1016/j.jaci.2016.04.024
- Kohler J , Blank S , Muller S , Bantleon F , Frick M , Huss-Marp J , Lidholm J , Spillner E , Jakob T . Component resolution reveals additional major allergens in patients with honeybee venom allergy. J Allergy Clin Immunol 2014; 133:1383-9, 9 e1–6
- Blank S , Seismann H , Michel Y , McIntyre M , Cifuentes L , Braren I , Grunwald T , Darsow U , Ring J , Bredehorst R , et al. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Allergy 2011; 66:1322-9
- Müller UR . Insektenstichallergie: Klinik, Diagnostik und Therapie. Stuttgart, New York: Gustav Fischer Verlag, 1988
- Grunwald T , Bockisch B , Spillner E , Ring J , Bredehorst R , Ollert MW . Molecular cloning and expression in insect cells of honeybee venom allergen acid phosphatase (Api m 3). J Allergy Clin Immunol 2006; 117:848-54; PMID:16630944; https://doi.org/10.1016/j.jaci.2005.12.1331
- Seismann H , Blank S , Braren I , Greunke K , Cifuentes L , Grunwald T , Bredehorst R , Ollert M , Spillner E . Dissecting cross-reactivity in hymenoptera venom allergy by circumvention of alpha-1,3-core fucosylation. Mol Immunol 2010; 47:799-808; PMID:19896717; https://doi.org/10.1016/j.molimm.2009.10.005
- Blank S , Seismann H , Bockisch B , Braren I , Cifuentes L , McIntyre M , Rühl D , Ring J , Bredehorst R , Ollert MW , et al. Identification, recombinant expression, and characterization of the 100 kDa high molecular weight hymenoptera venom allergens Api m 5 and Ves v 3. Journal of immunology 2010; 184:5403-13; https://doi.org/10.4049/jimmunol.0803709
- Casset A , Mari A , Purohit A , Resch Y , Weghofer M , Ferrara R , Thomas WR , Alessandri C , Chen KW , de Blay F , et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int Arch Allergy Immunol 2012; 159:253-62; PMID:22722650; https://doi.org/10.1159/000337654
- Curin M , Reininger R , Swoboda I , Focke M , Valenta R , Spitzauer S . Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol 2011; 154:258-63; PMID:20861648; https://doi.org/10.1159/000321113
- Focke M , Marth K , Valenta R . Molecular composition and biological activity of commercial birch pollen allergen extracts. Eur J Clin Invest 2009; 39:429-36; PMID:19302561; https://doi.org/10.1111/j.1365-2362.2009.02109.x
- Kespohl S , Maryska S , Zahradnik E , Sander I , Bruning T , Raulf-Heimsoth M . Biochemical and immunological analysis of mould skin prick test solution: current status of standardization. Clin Exp Allergy 2013; 43:1286-96; PMID:24152161; https://doi.org/10.1111/cea.12186
- Schmidt H , Gelhaus C , Nebendahl M , Janssen O , Petersen A . Characterization of Phleum pratense pollen extracts by 2-D DIGE and allergen immunoreactivity. Proteomics 2010; 10:4352-62; PMID:21136590; https://doi.org/10.1002/pmic.201000451 10.1002/pmic.201000045
- Van Vaerenbergh M , Cardoen D , Formesyn EM , Brunain M , Van Driessche G , Blank S , Spillner E , Verleyen P , Wenseleers T , Schoofs L , et al. Extending the honey bee venome with the antimicrobial peptide apidaecin and a protein resembling wasp antigen 5. Insect Mol Biol 2013; 22:199-210; PMID:23350689; https://doi.org/10.1111/imb.12013
- Frick M , Muller S , Bantleon F , Huss-Marp J , Lidholm J , Spillner E , Jakob T . rApi m 3 and rApi m 10 improve detection of honey bee sensitization in Hymenoptera venom-allergic patients with double sensitization to honey bee and yellow jacket venom. Allergy 2015; 70:1665-8; PMID:26259841; https://doi.org/10.1111/all.12725
- Sturm GJ , Hemmer W , Hawranek T , Lang R , Ollert M , Spillner E , Blank S , Bokanovic D , Aberer W . Detection of IgE to recombinant Api m 1 and rVes v 5 is valuable but not sufficient to distinguish bee from wasp venom allergy. J Allergy Clin Immunol 2011; 128:247-8; author reply 8; PMID:21439627; https://doi.org/10.1016/j.jaci.2011.02.021
- Hofmann SC , Pfender N , Weckesser S , Huss-Marp J , Jakob T . Added value of IgE detection to rApi m 1 and rVes v 5 in patients with Hymenoptera venom allergy. J Allergy Clin Immunol 2011; 127:265-7; PMID:20719373; https://doi.org/10.1016/j.jaci.2010.12.529 10.1016/j.jaci.2010.06.042
- Christensen L , Larsen J , Monsalve R . Identification of allergens in honeybee venom and confirmation of Api m 10 in immunotherapy products as dertermined by LC-MS/MS. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology 2016; 71:71
- Nelson HS . Allergen immunotherapy: where is it now? J Allergy Clin Immunol 2007; 119:769-79; PMID:17337297; https://doi.org/10.1016/j.jaci.2006.08.026 10.1016/j.jaci.2006.12.332 10.1016/j.jaci.2006.12.613 10.1016/j.jaci.2007.01.036 10.1016/j.jaci.2006.11.589
- Braren I , Blank S , Seismann H , Deckers S , Ollert M , Grunwald T , Spillner E . Generation of human monoclonal allergen-specific IgE and IgG antibodies from synthetic antibody libraries. Clin Chem 2007; 53:837-44; PMID:17395713; https://doi.org/10.1373/clinchem.2006.078360
