ABSTRACT
Transforming growth factor-β (TGF-β) regulates cell growth and differentiation, apoptosis, cell motility, extracellular matrix production, angiogenesis, and cellular immunity. It has a paradoxical role in cancer. In the early stages it inhibits cellular transformation and prevents cancer progression. In later stages TGF-β plays a key role in promoting tumor progression through mainly 3 mechanisms: facilitating epithelial to mesenchymal transition, stimulating angiogenesis and inducing immunosuppression. As a result of its opposing tumor promoting and tumor suppressive abilities, TGF-β and its pathway has represented potential opportunities for drug development and several therapies targeting the TGF-β pathway have been identified. This review focuses on identifying the mechanisms through which TGF-β is involved in tumorigenesis and current therapeutics that are under development.
Introduction
Transforming growth factor-β (TGF-β) is a multi-functional cytokine that regulates cell growth and differentiation, apoptosis, cell motility, extracellular matrix production, angiogenesis, and cellular immune responses.Citation1-3 Curiously, TGF-β displays paradoxical activity as it exhibits tumor suppressor activity in the early stages of tumorigenesis; however, in later stages it promotes tumor growth and creates a more hospitable environment for tumor invasion and metastasis.Citation4 This review focuses on the role of TGF-β in tumorigenesis and discusses agents that target TGF-β and its signaling pathways for the treatment of cancer.
TGF-β structure and function
TGF-β is a 25 kDa disulfide-linked dimeric protein that has 3 isoforms: TGF-β1, -β2, and -β3. Functional TGF-β is produced from an inactive precursor (pro-TGF-β) and contains a N-terminal latency-associated peptide (LAP). Transformation of the inactive form begins when the pro-TGF-β precursor undergoes dimerization and furin, a subtilisin-like proprotein convertase, cleaves the TGF-β precursor into C-terminal mature peptides and N-terminal LAP, which is the small latent complex. This is transported to the extracellular matrix (ECM) where it binds to the latent TGF-β binding protein and forms large latent complexes. Large latent complexes interact with proteases in the ECM and triggers activation of TGF-β from large latent complexes. The now activated TGF-β can now participate in cell signaling.
While each isoform has distinctive in vivo functions; in vitro all 3 display overlapping features.Citation3 TGF-β1, the most common isoform is found in cartilage, bone, skin and endochondral tissue highlighting its role in growth and tissue differentiation. TGF-β2 is expressed by neurons and astroglial cells and plays a key role in autonomous cell proliferation. TGF-β3 is expressed in palate and lung tissue and is involved in epithelial-mesenchymal interactions.
Signaling is initiated when activated TGF-β binds to transforming growth factor-β receptor-2 (TβRII) with high affinity. This binding requires the participation of the transforming growth factor-β receptor-3 (TβRIII), also known as βglycan, which causes a conformational change in TβRII that facilitates ligand-receptor binding.Citation5,6 TGF-β receptor-1/ALK-5 (TβR1), a serine/threonine kinase, is then recruited to the TGF-β/TβRII complex and initiates signaling by phosphorylating Smad2 and Smad3, which belong to the receptor-regulated family of Smad proteins. Phosphorylated Smad2 and Smad3 combine to form a heteromeric complex with Smad4Citation7 that translocates to the cell nucleus to interact with various transcriptional factors that ultimately leads to the cellular response.Citation8 Knock out mouse studies for the 3 TGF-β isoforms have been used to further elucidate its specific roles. TGF-β1 suppression led to impaired hematopoiesis and vascular development.Citation9 TGF-β2 deficient mice exhibited numerous developmental defects including skeleton, heart, eyes, ears and uro-genital tract abnormlities leading to death.Citation10 TGF-β3 deficiency mice had impaired development of their pulmonary system along with cleft palates and died shortly after birth.Citation11
TGF-β can signal through intracellular Smad signal transduction proteins and several Smad-independent (non-canonical) pathways including ERK, MAP kinase, PI3K, JNK, p38, and AKT.Citation12-14 The Smad pathway plays a critical role in the antiproliferative properties of TGF-β and alterations such as missense mutations of the Smad system,Citation15,16 or blocking of the phosphorylation process or preventing Smad 2/3 from forming a complex have been shown to play a role in tumor development.Citation17 TGF-β signaling is subjected to negative feedback by 2 inhibitory Smads (I-Smads), Smad6 and Smad7,Citation18 19 and both I-Smads can interfere the phosphorylation of Smad2/3 by interaction with TGF-β RI.
Inactivation of the TGF-β signaling pathway during tumor progression
Paradoxically, TGF-β displays opposing functions. During early stages of tumorigenesis TGF-β can inhibit the proliferation of transformed cells acting as a “tumor suppressor,” but during late stages, TGF-β supports tumor cell proliferation, invasion and metastasis. Ordinarily TGF-β inhibits cell division by arresting healthy cells in the G1 phase through increasing expression of the cyclin dependent kinase (cdk) inhibitors p15 and p21 with subsequent suppression of c-Myc, a multi-functional oncogeneCitation20,21 that has been implicated in numerous human cancers. Tumor cells can evade this process by down-regulating p15 and p21/WAF1/CIP1 via Myc/Smad3 interaction along with activating the PI3K-AKT pathway, which prevents FoxO and Smad3 from complexing.Citation22,23 Activation of the Ras/MAP kinase is also activated which can circumvent TGF-β suppression and induce epithelial-mesenchymal transition (EMT).Citation24,25
Tumor cells can also become refractory to TGF-β's cytostatic activity through mutational inactivatioCitation26n of various components of the receptor-signaling pathway including TβRII, TβRI, Smad2 and Smad4 leading to resistance of the tumor suppressor effects of TGF-β.Citation27,28 The most common gene mutations are observed in TβRII because its coding sequence contains an area of 10 consecutive adenine nucleotides making it a mutational hotspot.Citation29,30 Inactivating mutations of TβRII have been reported in colon,Citation30 breast,Citation31 lung,Citation32 and prostate carcinomas.Citation26 Mutations of TβRI occur less frequently than TβRII mutations, and have been reported most often in ovarian,Citation33 breast,Citation34 and pancreatic cancersCitation35 as well as T cell lymphomas.Citation36
Mutations of Smad proteins have also been implicated in tumorigenesis with mutations occurring more commonly in Smad4 than Smad2. These include missense mutations or loss of heterozygosity on chromosome 18qCitation37 and these have been most frequently observed in pancreatic cancer as well as other malignancies.Citation15,37,38 Mutations of Smad3 associated with cancer have not been identified.
TGF-β as a tumor promoter
Tumor progression occurs when cancer cells can escape the inhibitory effects of TGF-β and instead begin to overexpress TGF-β resulting in increased cell proliferation, invasiveness and enhanced metastatic potential. The 3 most common mechanisms identified that stimulate tumor progression include EMT, increased invasiveness and metastasis, angiogenesis, and immunosuppression. Overexpression of TGF-β has been demonstrated in both animal and human tumor models and is seen clinically in many tumors including cancers of the breast, colon, esophagus, stomach, liver, lung, kidney, pancreas, prostate, brain, and malignant melanoma, as well as certain hematological malignancies.Citation39-46
TGF-β induced epithelial-to-mesenchymal transition (EMT)
EMT is a normal physiological process that is essential for embryonic development, tissue remodeling and repair.Citation24 EMT involves epithelial cells losing their innate characteristics including reduced cell–cell adherence and polarity, increased motility and assuming mesenchymal cell-like properties. Morphologically this is characterized by assumption of a spindle cell shape, decreased expression of E-cadherin and increased expression of mesenchymal markers such as vimentin, fibronectin and N-cadherin along with upregulation of matrix-metalloproteinases (MMP).Citation47-50 These changes result in remodeling of the cytoskeleton and increased cell motility allowing migration and invasion.Citation48
EMT has also been implicated in the stem cell phenotype. Cancer stem cells (CSCs) which are tumor cells capable of differentiation and replicating themselves. Increasing evidence suggests that TGF-β signaling and the transcription factors Snail or Twist may promote generation and maintenance of CSCs.Citation51,52
TGF-β and angiogenesis
TGF-β1 has been shown to enhance angiogenesis, an important component of tumor progression,Citation53 but its mechanism is still not well elucidated. TGF-β exerts an effect on endothelial cells (EC) and up-regulates vascular endothelial growth factor (VEGF) both of which promote angiogenesis.Citation54,55 Furthermore, increased production of VEGF appears to recruit more ECs resulting in sustained angiogenesis.Citation56,57 Studies have demonstrated that Smad-dependent pathways may have some role in TGF-β regulation of VEGF with some showing a repressive effect on angiogenesisCitation58 and others showing that overexpression of Smad3 having a pro-angiogenic effect.Citation59
TGF-β's effects on ECs appear to be contradictory and much of this overall effect on EC is driven by TGF-β's relationship with ALK1 and ALK5 receptors. TGF-β in low concentrations interacts with ALK1 receptors and results in increased expression of the metalloproteases MMP-2 and MMP-9, and enhances the migratory and invasive properties of activated ECs while higher concentrations of TGF-β interacts with ALK5 receptor and can hinder angiogenesis.Citation60,61
TGF-β-mediated tumor immunosuppression
Under normal conditions TGF-β is a key player in controlling immune responses both in the thymus and in the periphery. In the thymus TGF-β is involved development of all lineages of T cells and in the periphery TGF-β promotes survival for low-affinity T cells and inhibits autoreactive T cells.Citation62 The importance of TGF-β in maintaining self-tolerance can be seen through animal studies which have demonstrated that knocking out TGF-β1 results in widespread autoimmunity and inflammation.Citation62,63
In advanced malignancies TGF-β has been shown to suppress the immune system by inhibiting NK-cell activity, decreasing cytokine production, inhibiting dendritic cell maturation, and altering T-cell cytotoxic properties.Citation64-69 TGF-β increases production of regulatory T cells (Tregs), that inactivates cytoxic and helper T cells by inducing transcription factor FoxP3, a regulatory of T cell activity.Citation64 One study showed that tumor cell lines expressing TGF-β generated weaker cytotoxic T-lymphocyte (CTL) responses compared with unmodified cell lines because it inhibited the expression of the high affinity IL-2 receptor by T-lymphocytes.Citation68,69 TGF-β has also been shown to block presentation of antigen to dendritic cells by decreasing MHC expression on their surface.Citation66,67 These properties suggest that TGF-β has both a facilitative and direct role as it directly suppresses the immune system and allows tumor cells to acquire properties to evade the immune system.
Therapeutic potential of inhibiting TGF-β
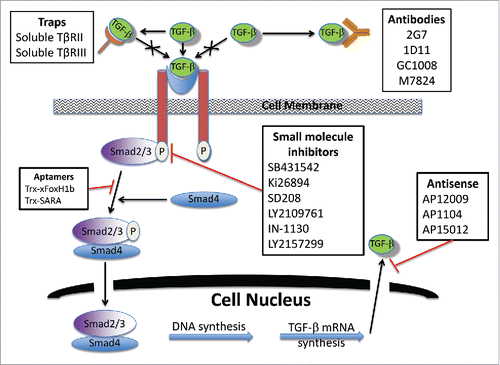
As a result of its tumor promoting abilities, TGF-β and its signaling pathway offer potential opportunities for targeted therapy. A number of agents have been studied or are currently being developed and evaluated in clinical trials that have targeted various components of TGF-β pathway. The most successful agents block this pathway through 3 mechanisms: (1) Direct inhibition of TGF-β synthesis by antisense molecules; (2) Blocking TGF-β and its interaction with its receptor using monoclonal antibodies or soluble TGF-β decoy receptors (traps); or (3) inhibition of the TGF-β signaling pathway by kinase inhibitors or aptamers that interfere with the function of the downstream Smad signaling proteins. Current agents and therapeutic targets under study are summarized in and their mechanism of action indicated in .
Table 1. Approaches inhibiting TGF-b.
Antisense molecules
Antisense oligonucleotides (ASO) are short molecules made up of 13–25 modified nucleotides that downregulate TGF-β synthesis by targeting and interfering with mRNA function.Citation70,71 Development of clinical antisense technology has been faced with limitations that included instability of the ASO molecules, poor uptake into cells and targeting delivery to cancer cells. However, there are several new generation ASOs directed against TGF-β that are currently in pre-clinical and clinical development.
Trabederson AP12009 (Antisense Pharma), a synthetic 18-oligomer phosphorothioate ASO was developed to counter TGF-β overexpression in pancreatic cancer and glioblastomas that is associated with poor outcomes.Citation72,73 Pre-clinical studies by Schlingensiepen et al demonstrated potential clinical efficacy and led to phase I/II trials in recurrent high-grade gliomas.Citation72,74-76 The drug was delivered directly into the tumor using catheters to avoid widespread toxicity associated with systemic administration. It was well tolerated and treated patients demonstrated longer overall survival compared with historical cohorts. Based on these results the phase III SAPPHIRE trial (ClinicalTrials.gov identifier: NCT00761280) was initiated, but was halted due to recruitment issues.
AP11014 and AP15012 are other ASO currently in preclinical development that have reduced TGF-β1 expression in colorectal cancer, lung cancer and metastatic melanoma.Citation77 Oral squamous cell carcinoma has also demonstrated overexpression of TGF-β and animal studies by Kim et al using ASO slowed tumor growth.Citation78
Anti-TGF-β cancer vaccines
TGF-β is an integral regulator of the immunosuppressive state observed in malignancies and this has led to development of vaccines targeting production of TGF-β. The principle of these vaccines is to transfect TGF-β anti-sense molecules into cancer cell lines and reverse the effects of immunosuppression in host cells and increase anti-tumor immunity. Belagenpneumatucel–L (Lucanix ®, NovaRx) is an anticancer vaccine that was developed from non-small cell lung cancer (NSCLC) lines modified to express ASO and blocking expression of TGF-β in host cells.. Belagenpneumatucel–L by inhibiting expression TGF-β in the vaccine cells increased the immunogenicity of the allogeneic lung cancer vaccine cells that were theorized to cross-react with the patient's lung cancer generating an antitumor immune response.
Nemunaitis et al. conducted a Phase I-II trial and patients who received doses >2.5 × 107cells/injection demonstrated improved survival at higher vaccine doses.Citation79,80 These early phase trials also demonstrated that patients who had responses had increased production of tumor necrosis, interferon gamma, and interleukins 4 and 6. This led to a Phase III trial to determine if this vaccine would improve overall survival (OS). The multi-center trial enrolled 532 patients with advanced stage NSCLC (Stage IIIA/B and IV) and randomized patients to receive either belagenpneumatucel or a placebo after frontline chemotherapy. The vaccine did not demonstrate increased survival with a median OS of 20 months with vaccine and 17 months with the placebo (p = 0.594)Citation81 although subsequent sub-analyses showed that prior radiation as well as timing from randomization to treatment both had impact on outcome.Citation82
Monoclonal antibodies directed against TGF-β
Another strategy, monoclonal antibodies that act as ligand traps include 2G7, 1D11, lerdelimumab, metelimumab and fresolimumab (GC1008). 1D11 (Genzyme) neutralizes all 3 active TGF-β isoforms (1, 2 and 3) and produced significant anti-metastatic activity in mouse models of breast cancer.Citation83-86 Tabe et al observed that when 1D11 was combined with cytarabine it prolonged survival in an in vivo leukemia model.Citation87 Another study found that it reduced the incidence of cholangiocarcinoma in a mouse model with hepatic fibrosis.Citation88 Mouse glioma models were treated with 1D11 and although tumor size was not reduced, glioma cells did not invade adjacent unaffected brain tissue.Citation89 Another pan-neutralizing antibody, 2G7 has displayed similar antitumor activity in preclinical models.Citation4,67,90 There are currently no ongoing trials in humans using non-human antibodies due to their immunogenicity and suboptimal pharmacokinetics.
Based on the above preclinical studies, fresolimumab (GC1008, Genzyme/Sanofi) a fully human monoclonal anti-TGF-β antibody that neutralizes all 3 isoforms of TGF-β has been developed. Morris et al studied GC1008 in a phase I trial of 29 patients with advanced melanoma or renal cell cancer that had progressed despite previous therapy.Citation91 One patient in the melanoma group had a partial response, 3 had mixed responses and 1 had stable disease. Stevenson et al conducted a Phase 2 trial with GC1008 on 13 patients with malignant pleural mesothelioma to assess response and laboratory evidence of TGF-β blockade.Citation92 At 3 months 3 patients showed stable disease and those who produced antitumor antibodies had significantly longer survival.
Latent TGF-β targeted therapy
As mentioned above TGF-β exists in its latent form in the extracellular matrix (ECM) and is activated by proteases, heat or acidic pH. While there are no current therapeutic targets that prevents latent TGF-β from becoming activated there is recent pre-clinical data that reported ATRA (active metabolite of vitamin A) was able to remodel the ECM of pancreatic stellate cells (PSCs) in pancreatic cancer cell lines and prevent activation of TGF-β. The study also found that ATRA reverted PSCs into its normal inactive phenotype.Citation93 This study suggests that ATRA maybe a novel therapeutic target that can prevent activation of TGF-β and potentially prevent cancer cell proliferation.
Soluble TβRII and soluble TβRIII (βglycan)
Expressing soluble TβRII and soluble TβRIII (βglycan) through recombinant DNA technology can also function as effective decoy receptors preventing binding of TGF-β.Citation94 Pre-clinical studies demonstrated that antagonizing TGF-β through this method inhibited metastases in mouse models of mesothelioma, liver, breast, and pancreatic cancers.Citation95-98 Zhang et al studied a mouse model of renal cell cancer and administered fused soluble TβRII with dendritic cell vaccines and found enhanced antitumor immunity.Citation99 Others have used oncolytic adenoviruses as a vehicle expressing soluble TBRII and inhibited bone metastases in mouse tumor models.Citation100-102 Soluble TβRIII (βglycan) has also been studied in the preclinical setting and suppressed breast cancer progression in vivo,Citation103,104 reduced tumor growth in mouse models with glioma,Citation105 non-small cell lung cancerCitation106 and breast cancer.Citation107,108 However, studies by Tang et al, and Hang et al have paradoxically demonstrated increased tumor growth using this approach and as such, none of these agents have been developed for clinical use.
TGF-β receptor kinase inhibitors (TRKI)
TGF-β receptor I (ALK-5) signaling is initiated when TGF-β binds to its type II receptor with subsequent phosphorylation of Smad2/Smad3 proteins. This pathway been abundantly investigated with many compounds demonstrating antitumor activity in various cancer cell lines.
SB-431542 (GlaxoSmithKline) is a small molecule selective inhibitor of ALK-5 that inhibited TGF-β mediated transcription of collagen and fibronectin in renal cancer cells, prevented migration and invasion by glioma cells,Citation109,110 suppressed growth by myelomaCitation111 and castrate resistant prostate cancer cell lines.Citation112 Administration of SB-431542 also increased maturation of dendritic cells and activation of CD8+ T lymphocytes.Citation113 The antitumor effects of SB-431542; however, have only been studied in vitro due to its unstable pharmacokinetic properties. Ki26894 (Kirin) another TβRI inhibitor inhibited invasion and development of bone metastases by human breast cancerCitation114 and gastric cancer cell lines in vivo.Citation115
SD208 (Scios, Inc.), a potent oral ATP-competitive TGF-βRI inhibitor has been tested in vivo and inhibited the migration and invasion by glioma cells,Citation116 decreased tumor growth in multiple myeloma,Citation117 promoted hematopoiesis in myelodysplastic syndrome,Citation118 and prevented metastases in breast cancer,Citation119 pancreatic cancer,Citation120,121 and melanoma mouse models.Citation122 It has also shown activity in reducing bone cancer pain in recent animal studies.Citation123
Similarly LY2109761, a dual inhibitor of TβR1 and TβR2 with a stable pharmacokinetic profile displayed in vivo anti-metastatic activity across various tumor models including pancreas,Citation124 colon,Citation125,126 hepatocellular carcinoma (HCC),Citation127-129 glioblastoma,Citation130,131 breastCitation132 and prostate cancer.Citation133 In spite of its anti-proliferative effect in vitro and in vivo, long-term use of LY2109761 resulted in resistance and subsequent tumor progression but this experiment was conducted in immune-suppressed mouse modelsCitation134 and there is emerging evidence that these inhibitors may be more adequately assessed in immune-competent mouse models or patient derived xenografts.Citation135
IN-1130 (In2Gen), a selective ALK-5 inhibitor also inhibits TβR1, ALK-4 and ALK-7 signaling and was found to decrease tumor growth in mice with prostate cancer xenografts,Citation136 inhibit lung metastasis in mouse breast cancer models and increase overall survival of the mice.Citation137
LY2157299 (galunisertib, Eli Lilly) has been shown to inhibit growth of lung and breast cancer cell linesCitation138 and was the first small molecule TGF-β kinase inhibitor that has been studied in clinical trials.Citation139 A first-in-human dose finding study reported by Gueorguieva et al, was evaluated 30 patients with glioblastoma and was found that it was well tolerated.Citation140 Similarly Fujiwara et al, and Rodon et al conducted Phase I studies of LY2157299 in patients with various solid tumors and found it to be safe.Citation141,142 Phase II studies are ongoing in hepatocellular carcinoma (NCT01246986). In addition EW7197 (MedPacto) is another small molecule inhibitor currently being investigated in solid tumors, (NCT02160106).
Peptide aptamers
Peptide aptamers are small designer protein molecules that bind to protein targets and have been used to inhibit TGF-β signaling pathways by blocking Smad2 and Smad3 preventing recruitment of Smad4. Cui et al developed 3 classes of peptide aptamers based on Smad interacting motifs (CBP, FoxH1 and Lef1) that were able to reduce TGF-β expression with the xFoxH1 aptamer.Citation143 Another study used Trx-SARA, an aptamer constructed from Escherichia coli thioredoxin A protein (Trx) and Smad anchor for receptor activation (SARA) which is specifically bound to Smads 2 and 3. It was found that it reduced the levels of Smad2 and Smad3 bound to Smad4. This resulted in TGF-β blockade and inhibited epithelial-mesenchymal transition (EMT) in a murine mammary epithelial cell line.Citation144
Role of TGF-β with PD-1/PD-L1 inhibitors
The programmed death 1 (PD-1) receptor and programmed death ligand-1 (PD-L1) axis has been identified as an important immune-modulatory system that maintains immune homeostasis through self-tolerance. It has been recently discovered that tumor cells use this pathway to escape immune elimination.Citation139 This new understanding has led to a recent approaches pharmacologically targeting this pathway primarily through the use of monoclonal antibodies blocking the interaction of the PD-1 receptor expressed on T cells and PD-L1 on tumor cells ushering in a new era of cancer therapeutics. Targeting of this pathway has produced exceptional tumor responses in some patients, while a significant number of patients have had smaller increments of benefit. Expression of TGF-β has emerged as one possible mechanism for these differing responses. TGF-β has been implicated in immune system homeostasis, but when overexpressed in cancer leads to a state of immune suppression by inhibiting the function of cytotoxic T cells (CTL), natural killer (NK) cells, inhibiting maturation and antigen presentation by dendritic cells, as well as increasing the production of immunosuppressive regulatory T cells (Tregs).Citation62-65 This provides a biological rationale for combining inhibtors of PD-1 with TGF-β inhibitors as they can target both the PD-L1/PD-1 pathway and also inhibit TGF-β, both of which exhibit potent immunopsuppressive effects in the tumor microenvironment. Inhibiting TGF-β impacts regulatory T cell production and can potentially augment the effect of PD-1/PD-L1 inhibitors leading to improved responses. Trials are underway exploring combinations of PD-1 inhibitors and a TGF-β trap (M7824) developed by Merck/EMD Serono (NCT02517398).”
Conclusions
TGF-β plays a crucial role in tumor progression allowing cancer cells to escape immune surveillance, proliferate, invade and metastasize. A further understanding of the paradoxical nature of TGF-β in cancer is still needed. This will aid in developing therapeutics specifically targeting TGF-β and its role in tumor progression and immunosupression. Novel therapeutics that target TGF-β production or block its action are either in pre-clinical trials or early clinical trials and have shown promise. Further clinical trials will help define drugs that target TGF-β activity in cancer treatment.
Abbreviations
| ALK | = | anaplastic lymphoma kinase |
| ASO | = | antisense oligonucleotide |
| cdk | = | cyclin dependent kinase |
| CTL | = | cytotoxic T-lymphocyte |
| EC | = | endothelial cell |
| EMT | = | epithelial-to-mesenchymal transition |
| IL-2 | = | interleukin-2 |
| MMP | = | matrix-metalloproteinases |
| NSCLC | = | non-small cell lung cancer |
| OS | = | overall survival |
| TGF-β | = | transforming growth factor-β |
| TβR | = | transforming growth factor-β receptor |
| VEGF | = | vascular endothelial growth factor |
Disclosure of potential conflicts of interest
The authors report no conflicts of interest.
Funding
JCM is supported in part by the Lcs Foundation.
References
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 2001; 29(2):117-29; PMID:11586292; https://doi.org/10.1038/ng1001-117
- Dumont N, Arteaga CL. Targeting the TGF beta signaling network in human neoplasia. Cancer Cell 2003; 3(6):531-6; PMID:12842082; https://doi.org/10.1016/S1535-6108(03)00135-1
- Massague J. TGF-beta signal transduction. Annu Rev Biochem 1998; 67: 753-91; PMID:9759503; https://doi.org/10.1146/annurev.biochem.67.1.753
- Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression by TGF-beta inhibitors. Invest New Drugs 2003; 21(1):21-32; PMID:12795527; https://doi.org/10.1023/A:1022951824806
- Lopez-Casillas F, Wrana JL, Massague J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell 1993; 73(7):1435-44; PMID:8391934; https://doi.org/10.1016/0092-8674(93)90368-Z
- Sankar S, Mahooti-Brooks N, Centrella M, McCarthy TL, Madri JA. Expression of transforming growth factor type III receptor in vascular endothelial cells increases their responsiveness to transforming growth factor beta 2. J Biol Chem 1995; 270(22):13567-72; PMID:7768960; https://doi.org/10.1074/jbc.270.22.13567
- Savage C, Das P, Finelli AL, et al. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc Natl Acad Sci U S A 1996; 93(2):790-4; PMID:8570636; https://doi.org/10.1073/pnas.93.2.790
- Dou C, Lee J, Liu B, Liu F, Massague J, Xuan S, Lai E. BF-1 interferes with transforming growth factor beta signaling by associating with Smad partners. Mol Cell Biol 2000; 20(17):6201-11; PMID:10938097; https://doi.org/10.1128/MCB.20.17.6201-6211.2000
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 1995; 121(6):1845-54; PMID:7600998
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 1997; 124(13):2659-70; PMID:9217007
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet 1995; 11(4):409-14; PMID:7493021; https://doi.org/10.1038/ng1295-409
- Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci 2002; 115(Pt 15):3193-206; PMID:12118074
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 2000; 275(47):36803-10; PMID:10969078; https://doi.org/10.1074/jbc.M005912200
- Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J 2002; 21(14):3749-59; PMID:12110587; https://doi.org/10.1093/emboj/cdf366
- Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996; 271(5247):350-3; PMID:8553070; https://doi.org/10.1126/science.271.5247.350
- Riggins GJ, Kinzler KW, Vogelstein B, Thiagalingam S. Frequency of Smad gene mutations in human cancers. Cancer Res 1997; 57(13):2578-80; PMID:9205057
- Halder SK, Beauchamp RD, Datta PK. Smad7 induces tumorigenicity by blocking TGF-beta-induced growth inhibition and apoptosis. Exp Cell Res 2005; 307(1):231-46; PMID:15922743; https://doi.org/10.1016/j.yexcr.2005.03.009
- Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-beta superfamily. Nature 1997; 389(6651):622-6; PMID:9335505; https://doi.org/10.1038/39355
- Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 1997; 389(6651):631-5; PMID:9335507; https://doi.org/10.1038/39369
- Alevizopoulos K, Vlach J, Hennecke S, Amati B. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J 1997; 16(17):5322-33; PMID:9311992; https://doi.org/10.1093/emboj/16.17.5322
- Lasorella A, Noseda M, Beyna M, Yokota Y, Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 2000; 407(6804):592-8; PMID:11034201; https://doi.org/10.1038/35036504
- Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, Massague J. TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat Cell Biol 2001; 3(4):400-8; PMID:11283614; https://doi.org/10.1038/35070086
- Wu S, Cetinkaya C, Munoz-Alonso MJ, von der Lehr N, Bahram F, Beuger V, Eilers M, Leon J, Larsson LG. Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene 2003; 22(3):351-60; PMID:12545156; https://doi.org/10.1038/sj.onc.1206145
- Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell 2001; 12(1):27-36; PMID:11160820; https://doi.org/10.1091/mbc.12.1.27
- Hu PP, Shen X, Huang D, Liu Y, Counter C, Wang XF. The MEK pathway is required for stimulation of p21(WAF1/CIP1) by transforming growth factor-beta. J Biol Chem 1999; 274(50):35381-7; PMID:10585406; https://doi.org/10.1074/jbc.274.50.35381
- Wikstrom P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A. Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 1998; 37(1):19-29; PMID:9721065; https://doi.org/10.1002/(SICI)1097-0045(19980915)37:1%3c19::AID-PROS4%3e3.0.CO;2-3
- Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD, Neumann A, Brattain MG, Chang J, Kim SJ, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res 1999; 59(2):320-4; PMID:9927040
- Grady WM, Willis JE, Trobridge P, Romero-Gallo J, Munoz N, Olechnowicz J, Ferguson K, Gautam S, Markowitz SD. Proliferation and Cdk4 expression in microsatellite unstable colon cancers with TGFBR2 mutations. Int J Cancer 2006; 118(3):600-8; PMID:16108056
- Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 1995; 268(5215):1336-8; PMID:7761852
- Parsons R, Myeroff LL, Liu B, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Microsatellite instability and mutations of the transforming growth factor beta type II receptor gene in colorectal cancer. Cancer Res 1995; 55(23):5548-50; PMID:7585632
- Lucke CD, Philpott A, Metcalfe JC, Thompson AM, Hughes-Davies L, Kemp PR, Hesketh R. Inhibiting mutations in the transforming growth factor beta type 2 receptor in recurrent human breast cancer. Cancer Res 2001; 61(2):482-5; PMID:11212236
- de Jonge RR, Garrigue-Antar L, Vellucci VF, Reiss M. Frequent inactivation of the transforming growth factor beta type II receptor in small-cell lung carcinoma cells. Oncol Res 1997; 9(2):89-98; PMID:9167190
- Wang D, Kanuma T, Mizunuma H, Takama F, Ibuki Y, Wake N, Mogi A, Shitara Y, Takenoshita S. Analysis of specific gene mutations in the transforming growth factor-beta signal transduction pathway in human ovarian cancer. Cancer Res 2000; 60(16):4507-12; PMID:10969799
- Chen T, Carter D, Garrigue-Antar L, Reiss M. Transforming growth factor beta type I receptor kinase mutant associated with metastatic breast cancer. Cancer Res 1998; 58(21):4805-10; PMID:9809982
- Goggins M, Shekher M, Turnacioglu K, Yeo CJ, Hruban RH, Kern SE. Genetic alterations of the transforming growth factor beta receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res 1998; 58(23):5329-32; PMID:9850059
- Schiemann WP, Pfeifer WM, Levi E, Kadin ME, Lodish HF. A deletion in the gene for transforming growth factor beta type I receptor abolishes growth regulation by transforming growth factor beta in a cutaneous T-cell lymphoma. Blood 1999; 94(8):2854-61; PMID:10515889
- Schutte M, Hruban RH, Hedrick L, Cho KR, Nadasdy GM, Weinstein CL, Bova GS, Isaacs WB, Cairns P, Nawroz H, et al. DPC4 gene in various tumor types. Cancer Res 1996; 56(11):2527-30; PMID:8653691
- Hahn SA, Hoque AT, Moskaluk CA, da Costa LT, Schutte M, Rozenblum E, Seymour AB, Weinstein CL, Yeo CJ, Hruban RH, et al. Homozygous deletion map at 18q21.1 in pancreatic cancer. Cancer Res 1996; 56(3):490-4; PMID:8564959
- Bruna A, Darken RS, Rojo F, Ocaña A, Peñuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J, et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 2007; 11(2):147-60; PMID:17292826
- Chod J, Zavadova E, Halaska MJ, Strnad P, Fucikova T, Rob L. Preoperative transforming growth factor-beta 1 (TGF-beta 1) plasma levels in operable breast cancer patients. Eur J Gynaecol Oncol 2008; 29(6):613-6; PMID:19115689
- Fischer JR, Darjes H, Lahm H, Schindel M, Drings P, Krammer PH. Constitutive secretion of bioactive transforming growth factor beta 1 by small cell lung cancer cell lines. Eur J Cancer 1994; 30A(14):2125-9; PMID:7857713
- Labidi SI, Menetrier-Caux C, Chabaud S, Chassagne C, Sebban C, Gargi T, Biron P, Blay JY, Ghesquières H. Serum cytokines in follicular lymphoma. Correlation of TGF-beta and VEGF with survival. Ann Hematol 2010; 89(1):25-33; PMID:19582455
- Langenskiold M, Holmdahl L, Falk P, Angenete E, Ivarsson ML. Increased TGF-beta 1 protein expression in patients with advanced colorectal cancer. J Surg Oncol 2008; 97(5):409-15; PMID:18176914
- Shariat SF, Walz J, Roehrborn CG, Zlotta AR, Perrotte P, Suardi N, Saad F, Karakiewicz PI. External validation of a biomarker-based preoperative nomogram predicts biochemical recurrence after radical prostatectomy. J Clin Oncol 2008; 26(9):1526-31; PMID:18349404
- Shirai Y, Kawata S, Tamura S, Ito N, Tsushima H, Takaishi K, Kiso S, Matsuzawa Y. Plasma transforming growth factor-beta 1 in patients with hepatocellular carcinoma. Comparison with chronic liver diseases. Cancer 1994; 73(9):2275-9; PMID:7513247
- von Rahden BH, Stein HJ, Feith M, Pühringer F, Theisen J, Siewert JR, Sarbia M. Overexpression of TGF-beta1 in esophageal (Barrett's) adenocarcinoma is associated with advanced stage of disease and poor prognosis. Mol Carcinog 2006; 45(10):786-94; PMID:16921482
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol 1994; 127(6 Pt 2):2021-36; PMID:7806579
- Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol 1998; 8(23):1243-52; PMID:9822576
- Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev 1996; 10(19):2462-77; PMID:8843198
- Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, Heath JK. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J 1987; 6(7):1899-904; PMID:2820711
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133(4):704-15; PMID:18485877
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 2008; 3(8):e2888; PMID:18682804
- Ito N, Kawata S, Tamura S, Shirai Y, Kiso S, Tsushima H, Matsuzawa Y. Positive correlation of plasma transforming growth factor-beta 1 levels with tumor vascularity in hepatocellular carcinoma. Cancer Lett 1995; 89(1):45-8; PMID:7882301
- Madri JA, Pratt BM, Tucker AM. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol 1988; 106(4):1375-84; PMID:3283153
- Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem 1994; 269(9):6271-4; PMID:8119973
- Petersen M, Pardali E, van der Horst G, Cheung H, van den Hoogen C, van der Pluijm G, Ten Dijke P. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene 2010; 29(9):1351-61; PMID:20010874
- Shin JH, Shim JW, Kim DS, Shim JY. TGF-beta effects on airway smooth muscle cell proliferation, VEGF release and signal transduction pathways. Respirology 2009; 14(3):347-53; PMID:19192227
- Schwarte-Waldhoff I, Volpert OV, Bouck NP, Sipos B, Hahn SA, Klein-Scory S, Lüttges J, Klöppel G, Graeven U, Eilert-Micus C, et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci U S A 2000; 97(17):9624-9; PMID:10944227
- Lu S, Lee J, Revelo M, Wang X, Lu S, Dong Z. Smad3 is overexpressed in advanced human prostate cancer and necessary for progressive growth of prostate cancer cells in nude mice. Clin Cancer Res 2007; 13(19):5692-702; PMID:17908958
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 2002; 21(7):1743-53; PMID:11927558
- Roelen BA, van Rooijen MA, Mummery CL. Expression of ALK-1, a type 1 serine/threonine kinase receptor, coincides with sites of vasculogenesis and angiogenesis in early mouse development. Dev Dyn 1997; 209(4):418-30; PMID:9264265
- Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 2006; 25(3):441-54; PMID:16973387
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 1992; 359(6397):693-9; PMID:1436033
- Chen W, Wahl SM. TGF-beta: the missing link in CD4+CD25+ regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev 2003; 14(2):85-9; PMID:12651220
- Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 2005; 202(8):1075-85; PMID:16230475
- Kao JY, Gong Y, Chen CM, Zheng QD, Chen JJ. Tumor-derived TGF-beta reduces the efficacy of dendritic cell/tumor fusion vaccine. J Immunol 2003; 170(7):3806-11; PMID:12646647
- Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman MK, Whitesell LJ, Ramanathapuram LV, Arteaga CL, Akporiaye ET. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res 2003; 63(8):1860-4; PMID:12702574
- Mule JJ, Schwarz SL, Roberts AB, Sporn MB, Rosenberg SA. Transforming growth factor-beta inhibits the in vitro generation of lymphokine-activated killer cells and cytotoxic T cells. Cancer Immunol Immunother 1988; 26(2):95-100; PMID:2452015
- Ruffini PA, Rivoltini L, Silvani A, Boiardi A, Parmiani G. Factors, including transforming growth factor beta, released in the glioblastoma residual cavity, impair activity of adherent lymphokine-activated killer cells. Cancer Immunol Immunother 1993; 36(6):409-16; PMID:8500113
- Baker BF, Monia BP. Novel mechanisms for antisense-mediated regulation of gene expression. Biochim Biophys Acta 1999; 1489(1):3-18; PMID:10806993
- Crooke ST. Molecular mechanisms of action of antisense drugs. Biochim Biophys Acta 1999; 1489(1):31-44; PMID:10806995
- Schlingensiepen KH, Schlingensiepen R, Steinbrecher A, Hau P, Bogdahn U, Fischer-Blass B, Jachimczak P. Targeted tumor therapy with the TGF-beta 2 antisense compound AP 12009. Cytokine Growth Factor Rev 2006; 17(1-2):129-39
- Schlingensiepen KH, Jaschinski F, Lang SA, Moser C, Geissler EK, Schlitt HJ, Kielmanowicz M, Schneider A. Transforming growth factor-beta 2 gene silencing with trabedersen (AP 12009) in pancreatic cancer. Cancer Sci 2011; 102(6):1193-200; PMID:21366804
- Schlingensiepen R, Goldbrunner M, Szyrach MN, Stauder G, Jachimczak P, Bogdahn U, Schulmeyer F, Hau P, Schlingensiepen KH. Intracerebral and intrathecal infusion of the TGF-beta 2-specific antisense phosphorothioate oligonucleotide AP 12009 in rabbits and primates: toxicology and safety. Oligonucleotides 2005; 15(2):94-104; PMID:15989424
- Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, Balasubramaniam A, Nair S, Oliushine V, Parfenov V, et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol 2011; 13(1):132-42; PMID:20980335
- Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A, Brawanski A, Proescholdt M, Schlaier J, Buchroithner J, et al. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides 2007; 17(2):201-12; PMID:17638524
- Nagaraj NS, Datta PK. Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert Opin Investig Drugs 2010; 19(1):77-91; PMID:20001556
- Kim SG, Song JY. Therapeutic targeting of oncogenic transforming growth factor-beta1 signaling by antisense oligonucleotides in oral squamous cell carcinoma. Oncol Rep 2012; 28(2):539-44; PMID:22581233
- Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, Tong A, Kumar P, Pappen B, Hamilton C, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol 2006; 24(29):4721-30; PMID:16966690
- Nemunaitis J, Nemunaitis M, Senzer N, Snitz P, Bedell C, Kumar P, Pappen B, Maples PB, Shawler D, Fakhrai H. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther 2009; 16(8):620-4; PMID:19287371
- Giaccone G, Bazhenova LA, Nemunaitis J, Tan M, Juhász E, Ramlau R, van den Heuvel MM, Lal R, Kloecker GH, Eaton KD, et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer 2015; 51(16):2321-9; PMID:26283035
- Giaccone G, Bazhenova LA, Nemunaitis J, Tan M, Juhász E, Ramlau R, van den Heuvel MM, Lal R, Kloecker GH, Eaton KD, et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer 2015; 51(16):2321-9; PMID:26283035
- Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, Kohn E, Tang B, Sabzevari H, Anver MR, et al. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res 2008; 68(10):3835-43; PMID:18483268
- Chen X, Yang Y, Zhou Q, Weiss JM, Howard OZ, McPherson JM, Wakefield LM, Oppenheim JJ. Effective chemoimmunotherapy with anti-TGFbeta antibody and cyclophosphamide in a mouse model of breast cancer. PLoS One 2014; 9(1):e85398; PMID:24416401
- Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest 2007; 117(5):1305-13; PMID:17415413
- Ganapathy V, Ge R, Grazioli A, Xie W, Banach-Petrosky W, Kang Y, Lonning S, McPherson J, Yingling JM, Biswas S, et al. Targeting the Transforming Growth Factor-beta pathway inhibits human basal-like breast cancer metastasis. Mol Cancer 2010; 9: 122; PMID:20504320
- Tabe Y, Shi YX, Zeng Z, Jin L, Shikami M, Hatanaka Y, Miida T, Hsu FJ, Andreeff M, Konopleva M. TGF-beta-Neutralizing Antibody 1D11 Enhances Cytarabine-Induced Apoptosis in AML Cells in the Bone Marrow Microenvironment. PLoS One 2013; 8(6):e62785; PMID:23826077
- Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S, Zuk A, Arbeeny C, Ledbetter S. Transforming growth factor beta neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS One 2013; 8(1):e54499; PMID:23349909
- Hulper P, Schulz-Schaeffer W, Dullin C, Hoffmann P, Harper J, Kurtzberg L, Lonning S, Kugler W, Lakomek M, Erdlenbruch B. Tumor localization of an anti-TGF-beta antibody and its effects on gliomas. Int J Oncol 2011; 38(1):51-9; PMID:21109925
- Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. The Journal of clinical investigation 1993; 92(6):2569-76; PMID:7504687
- Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, Hsu FJ, Berzofsky JA, Lawrence DP. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One 2014; 9(3):e90353; PMID:24618589
- Stevenson JP, Kindler HL, Papasavvas E, Sun J, Jacobs-Small M, Hull J, Schwed D, Ranganathan A, Newick K, Heitjan DF, et al. Immunological effects of the TGFbeta-blocking antibody GC1008 in malignant pleural mesothelioma patients. Oncoimmunology 2013; 2(8):e26218; PMID:24179709
- Sarper M, Cortes E, Lieberthal TJ, Del Rio Hernandez A. ATRA modulates mechanical activation of TGF-beta by pancreatic stellate cells. Sci Rep 2016; 6: 27639; PMID:27375161
- Zheng H, Wang J, Koteliansky VE, Gotwals PJ, Hauer-Jensen M. Recombinant soluble transforming growth factor beta type II receptor ameliorates radiation enteropathy in mice. Gastroenterology 2000; 119(5):1286-96; PMID:11054386
- Zhao W, Kobayashi M, Ding W, Yuan L, Seth P, Cornain S, Wang J, Okada F, Hosokawa M. Suppression of in vivo tumorigenicity of rat hepatoma cell line KDH-8 cells by soluble TGF-beta receptor type II. Cancer Immunol Immunother 2002; 51(7):381-8; PMID:; PMID:12192538
- Rowland-Goldsmith MA, Maruyama H, Kusama T, Ralli S, Korc M. Soluble type II transforming growth factor-beta (TGF-beta) receptor inhibits TGF-beta signaling in COLO-357 pancreatic cancer cells in vitro and attenuates tumor formation. Clin Cancer Res 2001; 7(9):2931-40; PMID:11555612
- Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals PJ, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest 2002; 109(12):1551-9; PMID:12070302
- Suzuki E, Kapoor V, Cheung HK, Ling LE, DeLong PA, Kaiser LR, Albelda SM. Soluble type II transforming growth factor-beta receptor inhibits established murine malignant mesothelioma tumor growth by augmenting host antitumor immunity. Clin Cancer Res 2004; 10(17):5907-18; PMID:15355924
- Zhang M, Berndt BE, Chen JJ, Kao JY. Expression of a soluble TGF-beta receptor by tumor cells enhances dendritic cell/tumor fusion vaccine efficacy. J Immunol 2008; 181(5):3690-7; PMID:18714045
- Hu Z, Gerseny H, Zhang Z, Chen YJ, Berg A, Zhang Z, Stock S, Seth P. Oncolytic adenovirus expressing soluble TGFbeta receptor II-Fc-mediated inhibition of established bone metastases: a safe and effective systemic therapeutic approach for breast cancer. Mol Ther 2011; 19(9):1609-18; PMID:21712815
- Hu Z, Gupta J, Zhang Z, Gerseny H, Berg A, Chen YJ, Zhang Z, Du H, Brendler CB, Xiao X, et al. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-beta inhibits established bone metastasis in a prostate cancer mouse model. Hum Gene Ther 2012; 23(8):871-82; PMID:22551458
- Zhang Z, Hu Z, Gupta J, Krimmel JD, Gerseny HM, Berg AF, Robbins JS, Du H, Prabhakar B, Seth P. Intravenous administration of adenoviruses targeting transforming growth factor beta signaling inhibits established bone metastases in 4T1 mouse mammary tumor model in an immunocompetent syngeneic host. Cancer Gene Ther 2012; 19(9):630-6; PMID:22744210
- Dong M, How T, Kirkbride KC, Gordon KJ, Lee JD, Hempel N, Kelly P, Moeller BJ, Marks JR, Blobe GC. The type III TGF-beta receptor suppresses breast cancer progression. J Clin Invest 2007; 117(1):206-17; PMID:17160136
- Lee JD, Hempel N, Lee NY, Blobe GC. The type III TGF-beta receptor suppresses breast cancer progression through GIPC-mediated inhibition of TGF-beta signaling. Carcinogenesis 2010; 31(2):175-83; PMID:19955393
- Naumann U, Maass P, Gleske AK, Aulwurm S, Weller M, Eisele G. Glioma gene therapy with soluble transforming growth factor-beta receptors II and III. Int J Oncol 2008; 33(4):759-65; PMID:18813789
- Finger EC, Turley RS, Dong M, How T, Fields TA, Blobe GC. TbetaRIII suppresses non-small cell lung cancer invasiveness and tumorigenicity. Carcinogenesis 2008; 29(3):528-35; PMID:18174241
- Bandyopadhyay A, Lopez-Casillas F, Malik SN, Montiel JL, Mendoza V, Yang J, Sun LZ. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res 2002; 62(16):4690-5; PMID:12183427
- Bandyopadhyay A, Zhu Y, Cibull ML, Bao L, Chen C, Sun L. A soluble transforming growth factor beta type III receptor suppresses tumorigenicity and metastasis of human breast cancer MDA-MB-231 cells. Cancer Res 1999; 59(19):5041-6; PMID:10519421
- Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol 2002; 62(1):58-64; PMID:12065755
- Lu Y, Jiang F, Zheng X, Katakowski M, Buller B, To SS, Chopp M. TGF-beta1 promotes motility and invasiveness of glioma cells through activation of ADAM17. Oncol Rep 2011; 25(5):1329-35; PMID:21359495
- Takeuchi K, Abe M, Hiasa M, Oda A, Amou H, Kido S, Harada T, Tanaka O, Miki H, Nakamura S, et al. Tgf-Beta inhibition restores terminal osteoblast differentiation to suppress myeloma growth. PLoS One 2010; 5(3):e9870; PMID:20360846
- Miles FL, Tung NS, Aguiar AA, Kurtoglu S, Sikes RA. Increased TGF-beta1-mediated suppression of growth and motility in castrate-resistant prostate cancer cells is consistent with Smad2/3 signaling. Prostate 2012; 72(12):1339-50; PMID:22228025
- Tanaka H, Shinto O, Yashiro M, Yamazoe S, Iwauchi T, Muguruma K, Kubo N, Ohira M, Hirakawa K. Transforming growth factor beta signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances anti-tumor activity. Oncol Rep 2010; 24(6):1637-43; PMID:21042762
- Ehata S, Hanyu A, Fujime M, Katsuno Y, Fukunaga E, Goto K, Ishikawa Y, Nomura K, Yokoo H, Shimizu T, et al. Ki26894, a novel transforming growth factor-beta type I receptor kinase inhibitor, inhibits in vitro invasion and in vivo bone metastasis of a human breast cancer cell line. Cancer Sci 2007; 98(1):127-33; PMID:17129361
- Shinto O, Yashiro M, Kawajiri H, Shimizu K, Shimizu T, Miwa A, Hirakawa K. Inhibitory effect of a TGFbeta receptor type-I inhibitor, Ki26894, on invasiveness of scirrhous gastric cancer cells. Br J Cancer 2010; 102(5):844-51; PMID:20145621
- Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R, Mangadu R, Liu YW, Platten M, Herrlinger U, et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res 2004; 64(21):7954-61; PMID:15520202
- Hayashi T, Hideshima T, Nguyen AN, Munoz O, Podar K, Hamasaki M, Ishitsuka K, Yasui H, Richardson P, Chakravarty S, et al. Transforming growth factor beta receptor I kinase inhibitor down-regulates cytokine secretion and multiple myeloma cell growth in the bone marrow microenvironment. Clin Cancer Res 2004; 10(22):7540-6; PMID:15569984
- Zhou L, Nguyen AN, Sohal D, Ying Ma J, Pahanish P, Gundabolu K, Hayman J, Chubak A, Mo Y, Bhagat TD, et al. Inhibition of the TGF-beta receptor I kinase promotes hematopoiesis in MDS. Blood 2008; 112(8):3434-43; PMID:18474728
- Ge R, Rajeev V, Ray P, Lattime E, Rittling S, Medicherla S, Protter A, Murphy A, Chakravarty J, Dugar S, et al. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clin Cancer Res 2006; 12(14 Pt 1):4315-30; PMID:16857807
- Medicherla S, Li L, Ma JY, Kapoun AM, Gaspar NJ, Liu YW, Mangadu R, O'Young G, Protter AA, Schreiner GF, et al. Antitumor activity of TGF-beta inhibitor is dependent on the microenvironment. Anticancer Res 2007; 27(6B):4149-57; PMID:18229422
- Gaspar NJ, Li L, Kapoun AM, Medicherla S, Reddy M, Li G, O'Young G, Quon D, Henson M, Damm DL, et al. Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Molecular Pharmacol 2007; 72(1):152-61; PMID:17400764
- Mohammad KS, Javelaud D, Fournier PG, Niewolna M, McKenna CR, Peng XH, Duong V, Dunn LK, Mauviel A, Guise TA. TGF-beta-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res 2011; 71(1):175-84; PMID:21084275
- Xu Q, Zhang XM, Duan KZ, Gu XY, Han M, Liu BL, Zhao ZQ, Zhang YQ. Peripheral TGF-beta1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. J Neurosci 2013; 33(49):19099-111; PMID:24305807
- Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, Abbruzzese JL, Chiao PJ. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther 2008; 7(4):829-40; PMID:18413796
- Zhang B, Halder SK, Kashikar ND, Cho YJ, Datta A, Gorden DL, Datta PK. Antimetastatic role of Smad4 signaling in colorectal cancer. Gastroenterology 2010; 138(3):969-80 e1-3
- Zhang B, Halder SK, Zhang S, Datta PK. Targeting transforming growth factor-beta signaling in liver metastasis of colon cancer. Cancer Lett 2009; 277(1):114-20; PMID:19147275
- Mazzocca A, Fransvea E, Lavezzari G, Antonaci S, Giannelli G. Inhibition of transforming growth factor beta receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology 2009; 50(4):1140-51; PMID:19711426
- Fransvea E, Mazzocca A, Santamato A, Azzariti A, Antonaci S, Giannelli G. Kinase activation profile associated with TGF-beta-dependent migration of HCC cells: a preclinical study. Cancer Chemother Pharmacol 2011; 68(1):79-86; PMID:20844878
- Dituri F, Mazzocca A, Fernando J, Papappicco P, Fabregat I, De Santis F, Paradiso A, Sabbà C, Giannelli G. Differential inhibition of the TGF-β signaling pathway in HCC cells using the small molecule inhibitor LY2157299 and the D10 monoclonal antibody against TGF-β receptor type II. PLoS One 2013; 8(6):e67109; PMID:23826206
- Zhang M, Herion TW, Timke C, Han N, Hauser K, Weber KJ, Peschke P, Wirkner U, Lahn M, Huber PE. Trimodal glioblastoma treatment consisting of concurrent radiotherapy, temozolomide, and the novel TGF-beta receptor I kinase inhibitor LY2109761. Neoplasia 2011; 13(6):537-49; PMID:21677877
- Zhang M, Kleber S, Rohrich M, Timke C, Han N, Tuettenberg J, Martin-Villalba A, Debus J, Peschke P, Wirkner U, et al. Blockade of TGF-beta signaling by the TGFbetaR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res 2011; 71(23):7155-67; PMID:22006998
- Bouquet F, Pal A, Pilones KA, Demaria S, Hann B, Akhurst RJ, Babb JS, Lonning SM, DeWyngaert JK, Formenti SC, et al. TGFbeta1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res 2011; 17(21):6754-65; PMID:22028490
- Wan X, Li ZG, Yingling JM, Yang J, Starbuck MW, Ravoori MK, Kundra V, Vazquez E, Navone NM. Effect of transforming growth factor beta (TGF-beta) receptor I kinase inhibitor on prostate cancer bone growth. Bone 2012; 50(3):695-703; PMID:22173053
- Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-beta targeted cancer therapy. Int J Biol Sci 2012; 8(7):964-78; PMID:22811618
- Maier A, Peille AL, Vuaroqueaux V, Lahn M. Anti-tumor activity of the TGF-beta receptor kinase inhibitor galunisertib (LY2157299 monohydrate) in patient-derived tumor xenografts. Cell Oncol (Dordr) 2015; 38(2):131-44; PMID:25573078
- Lee GT, Hong JH, Mueller TJ, Watson JA, Kwak C, Sheen YY, Kim DK, Kim SJ, Kim IY. Effect of IN-1130, a small molecule inhibitor of transforming growth factor-beta type I receptor/activin receptor-like kinase-5, on prostate cancer cells. J Urol 2008; 180(6):2660-7; PMID:18951571
- Park CY, Min KN, Son JY, Park SY, Nam JS, Kim DK, Sheen YY. An novel inhibitor of TGF-beta type I receptor, IN-1130, blocks breast cancer lung metastasis through inhibition of epithelial-mesenchymal transition. Cancer Lett 2014; 351(1):72-80; PMID:24887560
- Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, Trocóniz IF. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer 2008; 44(1):142-50; PMID:18039567
- Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba SC, Benhadji KA, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther 2015; 9: 4479-99; PMID:26309397
- Gueorguieva I, Cleverly AL, Stauber A, Sada Pillay N, Rodon JA, Miles CP, Yingling JM, Lahn MM. Defining a therapeutic window for the novel TGF-beta inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic model. Br J Clin Pharmacol 2014; 77(5):796-807; PMID:24868575
- Fujiwara Y, Nokihara H, Yamada Y, Yamamoto N, Sunami K, Utsumi H, Asou H, TakahashI O, Ogasawara K, Gueorguieva I, et al. Phase 1 study of galunisertib, a TGF-beta receptor I kinase inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 2015; 76(6):1143-52; PMID:26526984
- Rodon J, Carducci M, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Braña I, Sicart E, Gueorguieva I, Cleverly A, et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-beta receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs 2015; 33(2):357-70; PMID:25529192
- Cui Q, Lim SK, Zhao B, Hoffmann FM. Selective inhibition of TGF-beta responsive genes by Smad-interacting peptide aptamers from FoxH1, Lef1 and CBP. Oncogene 2005; 24(24):3864-74; PMID:15750622
- Zhao BM, Hoffmann FM. Inhibition of transforming growth factor-beta1-induced signaling and epithelial-to-mesenchymal transition by the Smad-binding peptide aptamer Trx-SARA. Mol Biol Cell 2006; 17(9):3819-31; PMID:16775010