ABSTRACT
Respiratory syncytial virus (RSV) is a significant respiratory pathogen but no vaccine is available. RSV infections present 2 major, unique problems. First, humans can experience repeated infections caused by the same virus sero-group indicating that protective memory responses to RSV infection are defective. Second, most people have been infected with RSV by age 5. Immune responses to these infections, while poorly protective, could impact the effectiveness of a vaccine. The goal of this study was to assess the generation of protective immune responses in mice previously infected with RSV by virus-like particle (VLP) vaccine candidates containing a stabilized pre-fusion form of the RSV F protein or a stabilized post-fusion F protein. We report that a single immunization of RSV-experienced animals with a stabilized pre-fusion F protein VLP stimulated high titers of neutralizing antibody while a single injection of a post-fusion F protein VLP or a second RSV infection only weakly stimulated neutralizing antibody titers. These results suggest that prior RSV infection can induce neutralizing antibody memory responses, which can be activated by pre-F protein VLPs but not by post-F protein VLPs or a subsequent infection. Thus the F protein conformation has a major impact on enhancing production of neutralizing antibodies in RSV-experienced animals. Furthermore, although both VLPs contained the same RSV G protein, the pre-F VLP stimulated significantly higher titers of total anti-G protein IgG than the post-F VLP in both naïve and RSV-experienced animals. Thus the F protein conformation also influences anti-G protein responses.
Introduction
Respiratory syncytial virus (RSV) is a significant human pathogen most severely affecting infants, young children, and the elderly. The virus is the single most important cause of acute viral respiratory disease in infants and young children frequently resulting in hospitalization in the US and in significant mortality rates in developing countries.Citation1 RSV infection also substantially impacts elderly and immunocompromised populations,Citation2-5 and results in considerable morbidity in normal adult populations.Citation6 Despite the significance of RSV disease, there are no vaccines available although numerous candidates have been characterized in preclinical and clinical studies spanning 5 decades. This failure is due in large part to a lack of understanding of some fundamental issues related to immune responses to RSV.
A significant problem has been a lack of understanding of RSV antigens required for generation of protective, neutralizing anti-RSV antibodies. Many vaccine candidates, while stimulating antibody responses in experimental animals or humans, have failed to induce protection in human trials (reviewed inCitation7-9). One reason for this failure is that many candidates did not contain the appropriate form of the F protein. Like other paramyxovirus F proteins, the RSV F protein is folded into a metastable pre-fusion conformation and upon fusion activation refolds into a structurally very different post-fusion conformation.Citation10-14 The pre-fusion form of F protein is most effective in stimulating optimally neutralizing antibodies.Citation14,15 Furthermore, McLellan, et alCitation15 have shown that a soluble form of pre-fusion F protein, stabilized by mutation (DS-Cav1 mutant F protein), stimulated significantly higher neutralizing antibody titers in mice than those stimulated by post-fusion forms. What has not been appreciated until recently is that the pre-fusion form of the RSV F protein is unusually unstable and that many previous vaccine candidates contained primarily the post-fusion form.
Another significant problem for vaccine development has been a lack of understanding of requirements for the generation of long-lived and memory responses to RSV. One hallmark of RSV infection is that humans can experience repeated infections caused by the same virus sero-group over several years or even within the same seasonCitation6,7,16 indicating that memory responses to RSV infection are defective.Citation16
A further complication for vaccine development is that most of the human population has experienced RSV infection by 2–5 y of age.Citation17 While pre-existing immunity is poorly protective, it could well impact the effectiveness of a vaccine. Thus a successful vaccine candidate must stimulate high titers of neutralizing antibody in the face of any preexisting immunity, a topic that has not been widely addressed. Results in model animal systems using naïve animals may not directly bear on human responses, which will virtually always be in the context of previous infection.
We have developed novel virus-like particle (VLP) vaccine candidates for RSV.Citation18,19 Because of their particulate nature and their presentation of antigens in a repetitive array, VLPs do not need the addition of adjuvant for potent immune responses, in contrast to soluble proteins.Citation20 Because production of VLPs does not require viral replication, different conformational forms of antigens, such as a stabilized pre-fusion F protein or a stabilized post fusion F protein, can be assembled into VLPs, in contrast to attenuated virus, which must remain infectious. VLPs are also safer as vaccines than infectious attenuated or vector viruses for many populations since they do not contain a genome. We have recently reported that a VLP vaccine candidate containing a stabilized pre-fusion F protein induces neutralization titers, in both mice and cotton rats, at levels deemed protective in humans.Citation21,22
To assess the influence of previous RSV infections on the efficacy of our VLP vaccine candidates, we characterized immune responses, in mice previously infected with RSV, to VLPs containing a stabilized pre-fusion F protein or a stabilized post-fusion F protein, contrasting results with a second RSV infection. We report that in these RSV-experienced mice a single injection of a pre-fusion F-containing VLP stimulates extremely high titers of neutralizing antibodies while a single injection of a post-fusion F-containing VLP or a second RSV infection only weakly stimulates neutralizing antibodies. We also found that the conformation of the F protein in VLPs impacts the generation of anti-G protein IgG. The combined results suggest that the conformation of the F protein is an important consideration in RSV vaccine development.
Results
Characterization of protein content of VLP stocks
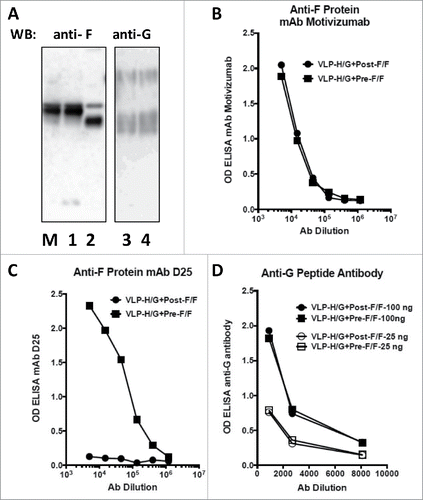
VLPs, based on Newcastle disease virus (NDV) core proteins and containing the RSV G protein and either the pre-fusion or post-fusion forms of the RSV F protein, were generated by transfection of ELL-0 cells with plasmids encoding NDV M protein, NDV NP, the H/G chimera protein,Citation19 and either the Pre-F/F or the Post-F/F chimera proteins to generate stocks of VLP-H/G+Pre-F/F or VLP-H/G+Post-F/FCitation21. The protein content of the 2 purified VLP preparations was quantified by Western blots and antibody binding to the purified VLPs. , panel A, shows a Western blot of proteins in the 2 VLP preparations probed with anti-RSV F (lanes 1 and 2) or anti-RSV G antibodies (lane 3 and 4). The results show that stocks of the 2 VLPs had equivalent levels of Pre-F/F and Post-F/F chimera proteins and equivalent levels of the H/G chimera protein. The 2 F protein chimeras are different sizes since the Pre-F/F contains the inserted foldon sequence and the Post-F/F chimera has a deletion of 9 amino acids. The H/G chimera protein resolves into heterogeneous species due to inefficient glycosylation of the RSV G protein sequences as we have described previously.Citation19,21 To further verify protein concentrations in VLPs, a monoclonal antibody that will bind either form of the RSV F protein, motavizumab,Citation13,23 binds equally to the 2 VLPs (, panel B) verifying that the 2 VLPs have assembled equivalent levels of F protein. However, a monoclonal antibody specific for site φ present only in the pre-fusion form of F protein but not in the post fusion form Citation14 binds only VLP-H/G+Pre-F/F and not VLP-H/G+Post-F/F (, panel C), a result verifying the conformation of the pre-F protein and the post-F protein in the 2 VLPs. A polyclonal antibody raised against a G protein derived peptide bound equivalently to 2 different concentrations of the 2 VLPs (, panel D) verifying that the 2 VLPs have the same amount of H/G chimera protein.
Figure 1. Protein content of VLPs. Panel A shows a Western blot of proteins present in stocks of VLP-H/G+Pre-F/F and VLP-H/G+Post-F/F. Proteins (electrophesed in the presence of reducing agent) in a polyacrylamide gel containing duplicate lanes of the proteins in the 2 VLPs were transferred to a membrane. One half was incubated with anti-F antibody (lanes M, 1, 2). The other half was incubated with anti-G antibody (lanes 3, 4). M: marker Pre-F/F protein. Lanes 1, 3: VLP-H/G+Pre-F/F; Lanes 2, 4: VLP-H/G+Post-F/F. The panel shows results of one of 3 separate blots with identical results. Panels B and C show binding of different concentrations of mAb motivizumab (panel B) or mAb D25 (Panel C) to each VLP in an ELISA as described previously.Citation21 Panel D shows binding of an anti-G protein peptide antibody to 2 different concentrations of VLPs (concentrations in ng of F protein). Results were identical in 3 or 4 separate determinations.
Infection and immunization
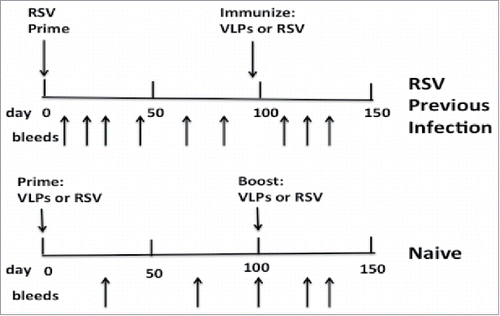
To assess the generation of neutralizing antibody responses in mice previously infected with RSV, 3 groups of 5 mice were prepared by infection with RSV by intranasal inoculation. After 95 days, one group was immunized with VLP-H/G+Pre-F/F, another group immunized with VLP-H/G+Post-F/F, and a third group was infected a second time with RSV (, top). To directly compare responses in previously infected mice with those in naïve mice, in parallel, groups of 5 naïve mice were immunized in a prime (day 0) and a boost (day 100) with the VLP-H/G+Pre-F/F, with the VLP-H/G+Post-F/F, or RSV infection (, bottom). Serum samples were obtained from each mouse at different times starting at day 0.
Figure 2. Immunization/infection timelines. Top panel shows timing of infection of animals with RSV (day 0) and their subsequent immunization with VLPs (day 95) or a second RSV infection (day 95). Sera were harvested from each animal at times indicated by arrows pointing upwards. Bottom panel shows timing of prime immunization with VLPs or RSV infection of naïve animals (day 0) and the subsequent boost with VLPs or a second RSV infection (day 100). Sera were harvested at times indicated by arrows pointing upward.
Neutralization titers in previously infected and naïve animals
To determine the effect of previous RSV infection on generation of neutralizing antibodies (NA), the neutralization titers in pooled sera of mice at different times after an RSV prime and VLP immunization were determined using an in vitro plaque reduction assay (, panel A). A single injection of these RSV-experienced animals with VLP-H/G+Pre-F/Fs stimulated significantly higher NA titers than VLP-H/G+Post-F/Fs or a second RSV infection. VLP-H/G+Pre-F/F immunization resulted in titers of approximately 4000 by day 128 while VLP-H/G+Post-F/Fs stimulated NA titers of approximately 600 at day 128, only slightly higher than a second RSV infection.
Figure 3. Neutralization titers in sera from RSV-experienced or naïve animals. Panel A shows neutralization titers in pooled sera after a single immunization with VLPs of RSV previously infected animals. At day 128, the difference between results of VLP-H/G+Pre-F/F immunization and VLP-H/G+Post-F/F immunization was significant with a p value of 0.0009. The difference between VLP-H/G+Pre-F/F and RSV immunization was significant with a p value of 0.0005. Difference between VLP-H/G+Post-F/F and RSV immunization was not significant. All results are the average of 4 separate determinations with mean and standard deviation shown. Panel B shows neutralization titers in pooled sera after a prime and after a boost of naïve animals with VLPs or RSV. At day 71, p values for the difference between results of immunization with VLP-H/G+Pre-F/F and VLP-H/G+Post-F/F was 0.0005 and for the difference between VLP-H/G+Pre-F/F and RSV was 0.0030. The difference between VLP-H/G+Post-F/F and RSV was not significant. At day 128, the difference between results of VLP-H/G+Pre-F/F immunization and VLP-H/G+Post-F/F immunization was not significant. The p values for difference between VLP-H/G+Pre-F/F and RSV immunization was 0.035 and for the difference between VLP-H/G+Post-F/F and RSV immunization was 0.0012. Results are the average of 3 separate determinations with mean and standard deviation shown.
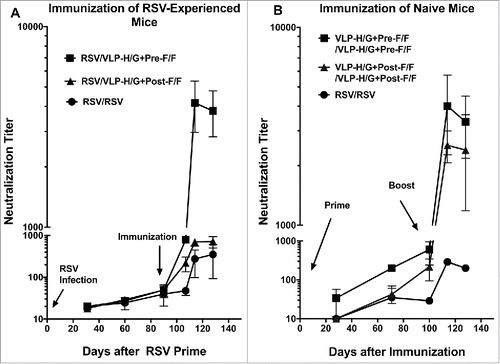
, panel B, illustrates, in parallel groups of naïve mice, the neutralization titers in animals after a prime and after a boost with the either VLP-H/G+Pre-F/Fs, VLP-H/G+Post-F/Fs, or after one or 2 RSV infections. These results are very similar to results previously reported for VLP immunization of naïve animals.Citation21 In a prime immunization, the VLP-H/G+Pre-F/Fs stimulated significantly higher titers than the VLP-H/G+Post-F/Fs or a single RSV infection. A boost with VLP-H/G+Pre-F/Fs increased titers to approximately 4000 while a VLP-H/G+Post-F/Fs boost resulted in titers of approximately 2500. Two consecutive RSV infections produced NA titers of approximately 200.
Total anti-F IgG titers after immunization of RSV-experienced animals
To determine if the differences in the NA titers after a single immunization of RSV-experienced mice with VLP-H/G+Pre-F/Fs or VLP-H/G+Post-F/Fs could be accounted for by differences in total anti-F protein antibody, the amounts of total anti-F protein IgG in the sera of the 2 groups were determined at each time point and compared with IgG levels in RSV infected mice. The titers of anti-F protein IgG that bind to the soluble pre-fusion F protein are shown in , panels A, while the binding of serum IgG to the soluble post-fusion F protein is shown in panel B. The results show that a single immunization with VLP-H/G+Pre-F/Fs or VLP-H/G+Post-F/Fs stimulated virtually equivalent titers of IgG specific for soluble pre-fusion F protein or soluble post-fusion F protein. A second RSV infection did stimulate anti-F protein IgG but the levels were 10-fold lower than those stimulated by both VLPs. Thus different levels of total anti-F protein IgG cannot account for the differences in NA titers after immunization with the VLP-H/G+Pre-F/Fs or VLP-H/G+Post-F/Fs.
Figure 4. Total anti-F protein antibody in animal sera. Total anti-F protein antibody was measured in ELISA using as target purified soluble pre-fusion F (panels A and C) or purified soluble post-fusion F protein (panels B and D). Panels A and B show ng/ml of anti-F protein IgG at different time points in RSV-experienced animals. Results are the average of 2 separate determinations. For the pre-F target as well as post-F target the difference at day 128 between RSV/VLP-H/G+Pre-F/F and RSV/VLP-H/G+Post-F/F groups was not significant. For the pre-F target, p value for difference between RSV/VLP-H/G+Pre-F/F and RSV/RSV was 0.030 while the difference between RSV/RSV and RSV/VLP-H/G+Post-F/F immunization was not significant. For the post F target, the p values for differences between RSV/VLP-H/G+Pre-F/F or RSV/VLP-H/G+Post-F/F VLP immunization and RSV/RSV immunization were 0.034 and 0.0011, respectively. Panels C and D show ng/ml of anti-F protein IgG at different time-points in immunized naïve animals. Figure shows results of one of 2 determinations with identical results and replicates results previously reported.Citation21 For the pre-F target or the post-F target the differences in values at day 128 between all groups were not significant.
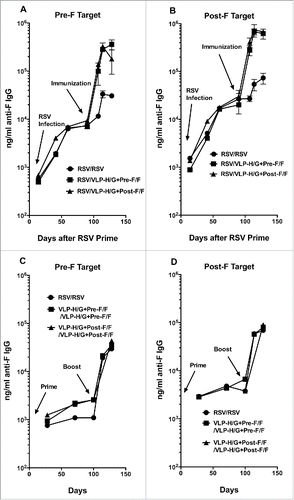
The IgG levels specific for pre-F and post-F targets generated in naïve mice after a prime VLP immunization and after a boost immunization are shown in , panels C and D, respectively. As previously reported,Citation21 levels of IgG specific to the pre-F target are lower than those specific to the post-F target after immunization with either VLP. Interestingly, the levels of IgG specific to both pre-F and post-F targets after a prime and boost are approximately 10-fold lower than levels generated after RSV priming and a single VLP immunization
Total anti-G protein IgG titers after immunization of RSV-experienced or naïve animals
Antibodies specific for the RSV G protein also have a role in protective responses to RSV infection.Citation24-27 Thus it was of interest to determine the influence of previous RSV infection on generation of anti-G protein antibodies. The titers of anti-G protein IgG antibodies in the parallel sets of naïve and RSV-experienced mice were determined using soluble G protein as target in ELISA. , panel A, shows antibody titers in sera after VLP-H/G+Pre-F/F or VLP-H/G+Post-F/F immunization of RSV-experienced mice while panel B shows titers after a prime and a boost of naïve mice with VLP-H/G+Pre-F/Fs or VLP-H/G+Post-F/Fs or RSV. In both naïve and RSV-experienced mice, anti-G protein antibody levels were extremely low after a single RSV infection or after a single VLP immunization. A second RSV infection in both sets of mice only minimally stimulated anti-G protein antibody levels. In contrast, VLP prime and boost immunization of naïve mice substantially increased anti-G protein antibody titers. Importantly, in RSV-experienced animals, a single VLP immunization with either the VLP-H/G+Pre-F/F or the VLP-H/G+Post-F/F considerably increased the anti-G protein antibody titers and this increase was approximately 4-fold over that stimulated by a prime and boost with either VLP in naïve animals.
Figure 5. Total anti-G protein antibody in animal sera. Total anti-G protein IgG was measured in ELISA using as target soluble G protein. Panel A shows ng/ml of anti-G protein IgG at different times in RSV-experienced animals. The results are the average of 4 separate determinations with average and standard deviations shown. At day128, p value for the difference between RSV/VLP-H/G+Pre-F/F and RSV/VLP-H/G+Post-F/F immunization was 0.0057, the p value for the difference between RSV/VLP-H/G+Post-F/F and RSV/RSV was 0.002. The p value for the difference between RSV/VLP-H/G+Pre-F/F and RSV/RSV was 0.0002. Panel B shows ng/ml of anti-G protein IgG in immunized naïve animals. Results are the average of 2 separate determinations with standard deviations shown. At day 128, p values for differences between VLP-H/G+Pre-F/F and VLP-H/G+Post-F/F immunization, for RSV and VLP-H/G+Pre-F/F-VLP immunization, and for RSV and VLP-H/G+Post-F/F immunization were 0.042, 0.019, and 0.055 respectively.
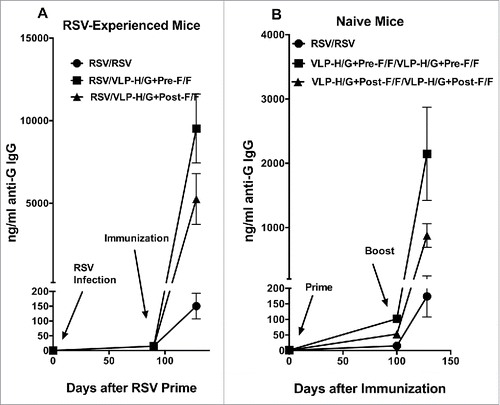
A surprising result was that the levels of anti-G protein antibodies after a single VLP immunization of RSV-experienced animals or after a VLP prime and boost of naïve animals were significantly different depending upon the VLP used although both VLPs contained similar amounts of the same H/G protein (, panels A and D).Citation21 VLPs containing the pre-fusion F protein simulated significantly higher titers of anti-G protein antibody than the VLPs containing the post-F protein.
Protection from RSV challenge
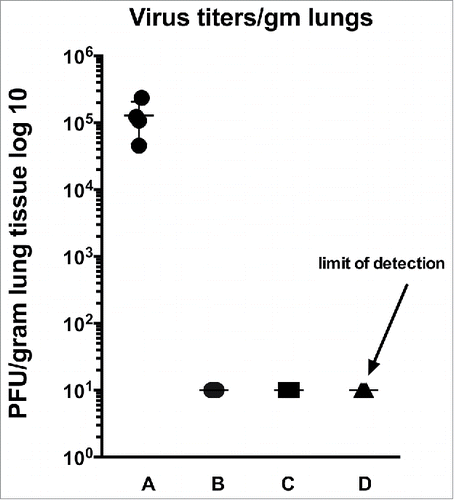
To determine if a single VLP immunization of RSV-experienced animals could protect them from RSV replication in lungs after RSV challenge, mice were challenged with RSV 125 d after VLP immunization. shows titers of virus in lung homogenates. While good titers were obtained in the unprimed, unimmunized controls (lane A), no virus was detected at the limits of detection in lungs of immunized animals. The results demonstrated that immunization with either VLP of RSV primed animals protected them from RSV replication.
Figure 6. Protection from challenge. Shown are lung titers after challenge of RSV-experienced VLP immunized animals. RSV challenge was 125 d after VLP immunization. A: no RSV prime, no immunization; B: RSV primed, RSV immunized; C: RSV primed, VLP-H-G+Pre-F/F immunized; D: RSV primed, VLP-H/G+Post-F/F immunized. Each group contained 5 animals and titers of each animal are shown in the graph. The p value for the differences between group A and the other groups is 0.0182.
Results of the challenge of naïve, immunized mice have been previously published.Citation21
Discussion
Most people have been infected with RSV by 5 y of age,Citation17 but these infections do not generate robust protective immune responses as many individuals experience repeated RSV infections throughout life, infections that have not been attributed to different strains or antigenic variants.Citation7,16,28 However, these infections likely result in some level of pre-existing immunity that could impact the effectiveness of any vaccine. Thus a successful vaccine candidate targeted to adult populations or older children must generate protective responses in the context of any pre-existing immunity.
We have developed VLP vaccine candidates for RSV.Citation18 Our previous studies have demonstrated that VLPs containing the pre-fusion form of the RSV F protein stimulate high titers of NA in both naïve mice and cotton rats, in contrast to RSV infection.Citation21,22 The goal of the studies reported here was to mimic human populations by assessing immune responses to our VLP vaccine candidates in mice previously infected with RSV.
When comparing NA titers, we found that in animals previously infected with RSV, a single immunization with VLP-H/G+Pre-F/Fs stimulated significantly higher NA titers than a single immunization with VLP-H/G+Post-F/Fs or a second RSV infection. The NA titers after a single VLP-H/G+Pre-F/F immunization of previously infected mice were comparable to titers in sera of naïve mice only after both a prime and a boost with VLP-H/G+Pre-F/Fs. This result suggests the hypothesis that RSV infection does induce potent neutralizing antibody memory responses that can be activated by the VLP-H/G+Pre-F/F immunization but not by VLP-H/G+Post-F/Fs or a second RSV infection.
A recent paper from Gilman, et alCitation29 supports the idea that RSV infection induces pre-F memory cells. These investigators report the isolation and characterization of 364 F protein specific monoclonal antibodies (mAbs) from memory B cells in serum of 3 human donors, donors who were infected with RSV in the past. The main conclusions of this comprehensive study are that most of the antibodies target one of 6 defined antibody-binding sites on the F protein. Importantly, approximately 50% of the mAbs were specific to one of the 3 sites present only on the pre-fusion F protein, sites φ, III, and V, and these mAbs were potent virus neutralizers. The vast majority of the rest of the antibodies bound sites common to both the pre-fusion and post-fusion F proteins but, in general, were far less potent neutralizers requiring 10-fold or higher concentrations to neutralize than those mAbs that bound to sites unique to the pre-fusion F protein. These poorly neutralizing antibodies bound primarily to sites I, II, and IV. These findings show that RSV infection does indeed induce significant levels of memory B cells that encode high titer neutralizing antibodies, at least in humans. Our results suggest that RSV infection can induce protective memory in mice but a subsequent infection cannot activate these memory B cells.
In contrast to results with VLP-H/G+Pre-F/Fs, a single immunization with VLP-H/G+Post-F/F in RSV infected mice resulted in NA titers more similar to those observed after a single VLP-H/G+Post-F/F immunization of naïve mice. However, a prime/boost immunization of naïve mice with VLP-H/G+Post-F/Fs stimulated good NA titers, titers that were approximately 50% that simulated by VLP-H/G+Pre-F/Fs. That the VLP-H/G+Post-F/F immunization in RSV-experienced mice did not stimulate these higher NA titers suggests that the RSV infection may not induce memory responses to some determinants present in the VLP-H/G+Post-F/Fs preventing high NA titers with a single VLP-H/G+Post-F/F immunization.
While it was possible that differences in NA titers after VLP-H/G+Pre-F/F or VLP-H/G+Post-F/F immunization of RSV-experienced mice were due to differences in total levels of anti-F protein antibodies in sera of the animals, our data clearly demonstrated that the levels of total anti-F protein antibodies in animals immunized with VLP-H/G+Pre-F/Fs were virtually identical to levels of total antibodies in VLP-H/G+Post-F/F immunized animals. Thus the differences in NA titers in the VLP-H/G+Pre-F/F and VLP-H/G+Post-F/F immunized, RSV-experienced animals must be due to qualitative differences between the populations of anti-F protein antibodies in the 2 groups of animals. Immunization of RSV-experienced mice with either VLP did result in much higher anti-F protein antibody titers than a second RSV infection indicating that a second RSV infection very poorly activates secondary antibody responses in contrast to the VLP immunization. It is noteworthy that naïve mice have total IgG anti-F protein levels after a prime and boost with VLPs similar to that observed after RSV infection. However, total antibody levels by day 128 in these naïve mice were approximately 10-fold lower than total levels in the VLP immunized RSV-experienced mice. The reasons for this difference are a topic of future investigations.
Studies of protective immune responses to RSV have largely focused on the role of the F protein. Indeed, the most broadly neutralizing antibodies are specific to the F protein since the G protein sequence varies with the serotype of RSV, in contrast to the F protein.Citation30 Furthermore, anti-G protein antibody responses are generally poorly neutralizing, a conclusion we confirmed by assessing neutralizing antibody responses in mice immunized with VLPs containing only the G protein.Citation19 However, antibodies to G protein do have a role in protection from RSV induced disease. The G protein central region contains a conserved sequence that is a mimic of the chemokine CX3C (fractalkine).Citation27 The G protein competes for the binding of CX3C to its receptor, CX3CR1, inhibiting immune responses to RSV in several ways that enhance the pathology of the infectionCitation27,31,26 Antibodies to the G protein CX3C sequence block G protein binding to the CX3CR1 moderating RSV disease. Importantly, antibody to this CX3C sequence decrease enhanced respiratory disease that results from RSV challenge of FI-RSV vaccinated animals.Citation32 Treatment with mAb to CX3C sequence decreased symptoms in RSV infected mice.Citation25 For these reasons, we compared levels of total anti-G protein IgG in naïve mice immunized with VLPs or infected with RSV to levels of these antibodies in RSV-experienced mice after VLPs immunization or a second RSV infection. Results show that in naïve mice a single RSV infection or one VLP immunization (prime) both generate anti-G protein antibodies very poorly. However, a single immunization with either VLP in RSV-experienced animals resulted in significant titers of anti-G protein antibodies suggesting that RSV infection does induce memory responses to the G protein. In contrast, a second infection with RSV results in barely detectable levels of anti-G protein antibody suggesting that RSV cannot effectively stimulate this anti-G protein memory. One surprising result of this analysis is that the VLP-H/G+Pre-F/F induced significantly higher titers of anti-G protein IgG than the VLP-H/G+Post-F/F in both naïve and RSV-experienced animals. It is important to point out that the 2 different VLPs, VLP-H/G+Pre-F/Fs and VLP-H/G+Post-F/Fs, contain the same H/G chimera protein and in the same amounts.Citation21 These results suggest that the conformation of the F protein in VLPs influences induction and stimulation of total anti-G IgG.
In summary, results of assessing levels of anti-F or anti-G protein antibodies in RSV-experienced animals vs naïve animals suggests the hypothesis that RSV infection can induce memory responses but infection is defective in stimulating or activating that memory. Further, these results indicate that the conformation of the F protein in a vaccine candidate has significant impact on the nature of anti-RSV immune responses in mice previously infected with RSV.
Materials and methods
Cells, virus, plasmids
ELL-0 (avian fibroblasts) (CLR-12203), Vero cells (CLR-1586), COS-7 cells (CLR-1651), and Hep2 cells (CCL-23) were obtained from the American Type Culture Collection. Expi293F cells were obtained from ThermoFisher/Invitrogen (A14527). ELL-0 cells, Vero cells, COS-7 cells, and Hep2 cells were grown in DMEM (Invitrogen 1195–073) supplemented with penicillin, streptomycin (Invitrogen 15140–122), and 5% (Vero cells) or 10% fetal calf serum (Invitrogen 10437–028). Expi293F cells were grown in Expi293 media (ThermoFisher/Gibco/Invitrogen A1435101). RSV, A2 strain, was obtained from Dr. Robert Finberg.
VLPs containing the RSV F and G proteins are formed with the Newcastle disease virus (NDV) core proteins NP and M.Citation18,33 The cDNAs encoding the NDV NP and M protein have been described previously.Citation34 The RSV F and G proteins are incorporated into these VLPs by constructing chimera protein genes composed of ectodomains of the G or F glycoproteins fused to the transmembrane (TM) and cytoplasmic (CT) domains of the NDV HN protein or NDV F glycoprotein, respectively. These NDV domains specifically interact with the NDV NP and M protein resulting in efficient incorporation of the chimera proteins into VLPs.
The construction, expression, and incorporation of the chimera protein NDVHN/ RSVG (H/G) into VLPs have been described previously.Citation19 The construction, expression, and incorporation into VLPs of the stabilized pre-fusion F protein (Pre-F/F DS-Cav1) to generate VLP-H/G+Pre-F/F, and the stabilized post-fusion F protein (Post-F/F) to create VLP-H/G+Post-F/F have been described previously.Citation21
The construction of genes encoding the soluble pre-F protein, the soluble post-F protein, and the soluble G protein used for target in ELISA was described previously.Citation21
Polyacrylamide gel electrophoresis, silver staining, and western analysis
Proteins were resolved on 8% Bis-Tris gels (NuPage, ThermoFisher/Invitrogen WB1001/WG1002)). Silver staining of proteins in the polyacrylamide gels was accomplished as recommended by the manufacturer (ThermoFisher/Pierce 24600). Quantification of NP, M, different forms of F/F, H/G protein, and soluble pre-F, post-F, and soluble G was accomplished after their separation in polyacrylamide gels followed by silver staining or by Western blots of the proteins as well as protein standards as described previously.Citation35,36 For Western analysis, proteins in the polyacrylamide gels were transferred to PVDF membranes using dry transfer (iblot, ThermoFisher/Invitrogen iB401001). Proteins were detected in the blots using anti-RSV HR2 peptide antibody or anti-RSV antibody.
Antibodies
RSV F monoclonal antibody clone 131–2A (Millipore MAB8599) was used in RSV plaque assays. Monoclonal antibody (mAb) 1112, mAb 1200, mAb 1243, were generous gifts of Dr. J. BeelerCitation37 and used to verify F protein conformations, and mAb D25 and mAb motavizumab, generous gifts of Dr. J. McLellan,,Citation14 were used for ELISA analysis of VLPs and soluble F proteins. Anti-RSV F protein HR2 antibody used for Western Blots is a polyclonal antibody specific to the HR2 domain of the RSV F protein.Citation18 Anti-RSV G protein antibody is a polyclonal antibody raised against a peptide containing G protein amino acids 180–198 (ThermoFisher PA5–22827). Secondary antibodies against goat (A5420), mouse (A5906) and rabbit IgG (A0545) were purchased from Sigma.
VLP preparation, purification, and characterization
For preparations of VLPs to be used as immunogens (VLP-H/G+Pre-F/F, VLP-H/G+Post-F/F), ELL-0 cells growing in T-150 flasks were transfected with cDNAs encoding the NDV M protein, NP, the chimeric proteins H/G, and either Pre-F/F or Post-F/F as described previously.Citation18,19 At 24 hours post-transfection, heparin (Sigma, H4784) was added to the cells at a final concentration of 10 μg/mlCitation19 to inhibit rebinding of released VLPs to cells. At 72, 96, and 120 hours post-transfection, cell supernatants were collected and VLPs purified by sequential pelleting and sucrose gradient fractionation as described previously.Citation18,19,35 Concentrations of proteins in the purified VLPs were determined by silver-stained polyacrylamide gels and by Western analysis using marker proteins for standard curves.Citation18,35 The conformation of F protein in the VLP preparations was verified by reactivity to mAbs.
Preparation of soluble F proteins
Expi293F cells were transfected with pCAGGS vector containing sequences encoding the soluble pre-F protein or the soluble post-F protein. At 5 to 6 d post transfection, total cell supernatants were collected and cell debris removed by centrifugation. Pre-fusion and post-fusion polypeptides were then purified on columns using the His tag and then the strep tag as described previously.Citation15
Quantification of soluble F protein and VLP associated F protein
Determinations of amounts of RSV F protein in VLPs or in soluble F protein preparations were accomplished by Western blots using anti-HR2 antibody for detection and comparing the signals obtained with a standard curve of purified F proteins as described previously.Citation35 Quantification of amounts of soluble G protein was determined on Western blots using anti-RSV G protein antibody for detection.
Preparation of RSV, RSV plaque assays, and antibody neutralization
RSV was grown in Hep2 cells,Citation18,19 and RSV plaque assays were accomplished on Vero cells as described previously.Citation21 Antibody neutralization assays in a plaque reduction assay have been described previously.Citation21,22 Neutralization titer was defined as the reciprocal of the dilution of serum that reduced virus titer by 50%.
Animals, animal immunization, and RSV challenge
Mice, 4-week-old female BALB/c, from Taconic laboratories (BALB-F), were housed (groups of 5) under pathogen-free conditions in microisolator cages at the University of Massachusetts Medical Center animal quarters. Female mice were used to assess the potential of VLPs for maternal immunization. Protocols requiring open cages were accomplished in biosafety cabinets. BALB/c mice were immunized by intramuscular (IM) inoculation of 30 μg total VLP protein (5 μg F protein) in 0.05 ml of TNE (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA) containing 10% sucrose. For infections with RSV, the animals were lightly anesthetized with isoflurane and then infected by intranasal (IN) inoculation of 50 μl of RSV (1 × 107 pfu/ml). All animal procedures and infections were performed in accordance with the University of Massachusetts Medical School IACUC and IBC approved protocols.
ELISA protocols
For determination of anti-F protein or anti-G protein serum antibody titers, blood was obtained from immunized animals by tail vein nicks and centrifuged in BD microtainer serum separator tubes (ThermoFisher 365967) to remove blood cells. For ELISA, wells of microtiter plates (ThermoFisher/Costar 2797) were coated with either purified soluble pre-fusion F protein, soluble post-fusion F protein, or soluble G protein and incubated for 24 hours at 4°C. Wells were then incubated in PBS-2% BSA for 16 hours. Different dilutions of sera, in 0.05% Tween and 2% BSA, were added to each well and incubated for 2 hours at room temperature. After 6 washes in PBS, sheep anti-mouse antibody coupled to HRP (Sigma A5906) was added in 50 μl PBS-2%BSA and incubated for 1.5 hours at room temperature. Bound HRP was detected by adding 50 μl TMB (3,3′5,5′-tetramethylbenzidin, ThermoFisher34028) and incubating for 5–20 minutes at room temperature until blue color developed. The reaction was stopped with 50μl 2N sulfuric acid. Color was read in SpectraMax Plus Plate Reader (Molecular Devices) using SoftMax Pro software. Amounts of IgG bound to the wells was calculated using a standard curve generated using defined amounts of purified IgG.Citation35
Statistical analysis
Statistical analyses (student T test) of data were accomplished using Graph Pad Prism 6 software.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank J. McLellan and J. Beeler for generous donation of antibodies.
Funding
This work was supported, in part, by The Hood Foundation, and by the National Institutes of Health, AI 114809.
References
- Karron RA. Respiratory syncytial virus and parainfluenza virus vaccines. In: Plotkin SA, Orenstein WA, Offit P, eds. Vaccines. 5th ed: Saunders-Elsevier; 2008:1146
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749-59; PMID:15858184; https://doi.org/10.1056/NEJMoa043951
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev 2000; 13:371-84; PMID:10885982; https://doi.org/10.1128/CMR.13.3.371-384.2000
- Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis 1999; 179:25-30; PMID:9841818; https://doi.org/10.1086/314567
- Raboni SM, Nogueira MB, Tsuchiya LR, Takahashi GA, Pereira LA, Pasquini R. Respiratory tract viral infections in bone marrow transplant patients. Transplant 2003; 76:142-6; https://doi.org/10.1097/01.TP.0000072012.26176.58
- Hall CB, Long CE, Schnabel KD. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis 2001; 33:792-6; PMID:11512084; https://doi.org/10.1086/322657
- Power UF. Respiratory syncytial virus (RSV) vaccines–Two steps back for one leap forward. J Clin Virol 2008; 41:38-44; PMID: 18340669; https://doi.org/10.1016/j.jcv.2007.10.024
- Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev 2012;239:149-66; https://doi.org/10.1111/j.1600-065X.2010.00972.x
- Morrison TG, Walsh EE. Subunit and Virus-like Particle Vaccine Approached for Respiratory Syncytial Virus. In: Anderson LJ, Graham BS, eds. Challenges and opportunities for respiratory syncytial virus vaccines. Heidelberg, Berlin: Springer; 2013
- Jardetsky TS, Lamb RA. A class act. Nature 2004; 427:307-8; https://doi.10.1038/427307a
- Lamb RA, Parks GD. Paramyxoviridae: The Viruses and Their Replication. In: Knipe DM, Howley PM, Griffin DE, et al., eds. Fields Virology. Fifth Edition ed. Philadelphia: LippincottWilliams & Wilkins; 2007:1450-96
- Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci USA 2011; 108:9619-24; PMID:21586636; https://doi.org/10.1073/pnas.1106536108
- McLellan JS, Yang Y, Graham BS, Kwong PD. Structure of Respiratory Syncytial Virus Fusion Glycoprotein in the Postfusion Conformation Reveals Preservation of Neutralizing Epitopes. J Virol 2011; 85:7788-96; PMID:21613394; https://doi.org/10.1128/JVI.00555-11
- McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, et al. Structure of RSV Fusion Glycoprotein Trimer Bound to a Prefusion-Specific Neutralizing Antibody. Science 2013; 340:1113-7; PMID:23618766; https://doi.org/10.1126/science.1234914
- McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GBE, Yang Y. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013;342:592-8; PMID:24179220; https://doi.org/10.1126/science.1243283
- Hall CB, Simoes EAF, Anderson LJ. Clinical and Epidemiologic Features of Respiratory Syncytial Virus. In: Anderson LJ, Graham BS, eds. Challenges and Opportunities for Respiratory Syncytial Virus Vaccines. Heidelberg, New York, Dordrecht, Londaon: Springer; 2013: 39-58
- Glezen W, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J of Dis of Child 1986; 140:543-6
- McGinnes LW, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J Virol 2011; 85:366-77; PMID:20980510; https://doi.org/10.1128/JVI.01861-10
- Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare M, Smith G. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice with no evidence of immunopathology. J Virol 2010; 84:1110-23; PMID:19889768; https://doi.org/10.1128/JVI.01709-09
- Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 2010; 10:787-96; PMID:20948547; https://doi.org/10.1038/nri2868
- McGinnes-Cullen L, Schmidt MR, Kenward SA, Woodland RT, Morrison TG. Murine Immune Responses to Virus-Like Particle-Associated Pre- and Postfusion Forms of the Respiratory Syncytial Virus F Protein. J of Virol 2015; 89:6835-47; https://doi.org/10.1128/JVI.00384-15
- Cullen LM, Blanco JCG, Morrison TG. Cotton rat immune responses to virus-like particles containing the pre-fusion form of respiratory syncytial virus fusion protein. J Transl Med 2015; 13:1-13; PMID:25591711; https://doi.org/10.1186/s12967-015-0705-8
- McLellan JS, Chen M, Kim A, Yang Y, Graham BS, Kwong PD. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nat Struct Mol Biol 2011; 17:248-50; https://doi.org/10.1038/nsmb.1723
- Boyoglu-Barnum S, Todd SO, Chirkova T, Barnum TR, Gaston KA, Haynes LM. An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice. Virology 2015; 483:117-25
- Boyoglu-Barnum S, Todd SO, Chirkova T, Barnum TR, Gaston KA, Haynes LM, Tripp RA, Moore ML, Anderson LJ. An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice. Virology 2015; 483:117-25; PMID:25965801; https://doi.org/10.1016/j.virol.2015.02.035
- Tripp RA. Pathogenesis of respiratory syncytial virus infection. Viral Immunol 2004; 17:165-81; PMID:15279697; https://doi.org/10.1089/0882824041310513
- Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2001; 2:732-8; PMID:11477410; https://doi.org/10.1038/90675
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med 2001; 344:1917-28; PMID:11419430; https://doi.org/10.1056/NEJM200106213442507
- Gilman MSA, Castellanos CA, Chen M, Ngwuta JO, Goodwin E, Moin SM, Mas V, Melero JA, Wright PF, Graham BS, et al. Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci Immunol 2016; 1:1879; https://doi.org/10.1126/sciimmunol.aaj1879
- Collins PL, Crowe JE. Respiratory syncytial virus and metapneumovirus. 5 ed. Philadelphia: LippincottWilliams and Wilkins; 2007
- Chirkova T, Boyoglu-Barnum S, Gaston KA, Malik FM, Trau SP, Oomens AG, Anderson LJ.. Respiratory Syncytial Virus G Protein CX3C Motif Impairs Human Airway Epithelial and Immune Cell Responses. J of Virol 2013; 87:13466-79; https://doi.org/10.1128/JVI.01741-13
- Rey GU, Miao C, Caidi H, Trivedi SU, Harcourt JL, Tripp RA, Anderson LJ, Haynes LM. Decrease in Formalin-Inactivated Respiratory Syncytial Virus (FI-RSV) Enhanced Disease with RSV G Glycoprotein Peptide Immunization in BALB/c Mice. PLoS ONE 2013; 8:e83075; PMID:24376637; https://doi.org/10.1371/journal.pone.0083075
- McGinnes LW, Pantua H, Laliberte JP, Gravel KA, Jain S, Morrison TG. Assembly and biological and immunological properties of Newcastle disease virus-like particles. J Virol 2010; 84:4513-23; PMID:20181713; https://doi.org/10.1128/JVI.01931-09
- McGinnes LW, Reitter J, Pantua HD, Morrison TG. Newcastle disease virus: propagation, quantification, and storage: John Wiley and sons, Inc; 2006
- McGinnes LW, Morrison TG. Newcastle Disease Virus-Like Particles: Preparation, Purification, Quantification, and Incorporation of Foreign Glycoproteins. Current Protocols in Microbiology: John Wiley & Sons, Inc; 2013
- Gravel KA, McGinnes LW, Reitter J, Morrison TG. The transmembrane domain sequence affects the structure and function of the Newcastle disease virus fusion protein. J Virol 2011; 85:3486-97; PMID:21270151; https://doi.org/10.1128/JVI.02308-10
- Beeler JA, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol 1989; 63:2941-50; PMID:2470922
