ABSTRACT
Japanese Red Cedar (JRC) pollen induced allergy affects one third of Japanese and the development of effective therapies remains an unachieved challenge. We designed a DNA vaccine encoding CryJ2 allergen from the JRC pollen and Lysosomal Associated Membrane Protein 1 (LAMP-1) to treat JRC allergy. These Phase IA and IB trials assessed safety and immunological effects of the investigational CryJ2-LAMP DNA vaccine in both non-sensitive and sensitive Japanese expatriates living in Honolulu, Hawaii. In the Phase IA trial, 6 JRC non-sensitive subjects and 9 JRC and/or Mountain Cedar (MC) sensitive subjects were given 4 vaccine doses (each 4mg/1ml) intramuscularly (IM) at 14-day intervals. Nine JRC and/or MC sensitive subjects were given 4 doses (2 mg/0.5 ml) IM at 14-day intervals. The safety and functional biomarkers were followed for 132 d. Following this, 17 of 24 subjects were recruited into the IB trial and received one booster dose (2 mg/0.5 ml) IM approximately 300 d after the first vaccination dose to which they were randomized in the first phase of the trial. All safety endpoints were met and all subjects tolerated CryJ2-LAMP vaccinations well. At the end of the IA trial, 10 out of 12 JRC sensitive and 6 out of 11 MC sensitive subjects experienced skin test negative conversion, possibly related to the CryJ2-LAMP vaccinations. Collectively, these data suggested that the CryJ2-LAMP DNA vaccine is safe and may be immunologically effective in treating JRC induced allergy.
KEYWORDS:
Introduction
As a major source of environmental allergens in Japan, Japanese red cedar (JRC) pollen causes pollinosis (JCP) in 30–35% of the Japanese population during early spring.Citation1 Cry j 1 and Cry j 2 proteins are the 2 major allergenic components in JRC pollen.Citation2-4 T cell responses and IgE antibodies specific for these 2 proteins have been found in most JCP patients.Citation5,6 Because of the high sequence identity and cross-reactivity between JRC pollen and Japanese cypress pollen, which is dispersed after the season of JRC pollen, the pollinosis symptoms might last as long as 4 months in some patients.Citation7-9 The quality of life of such patients is greatly affected. Meanwhile, identifying an effective therapy for JRC allergy remains an unmet need.
JRC is not native to North America, but is found as an ornamental tree across the Southeastern and Southwestern United States. Furthermore, pollen from Mountain Cedar (MC), a close relative native of JRC in North America, cross-reacts to a high degree with JRC pollen. It has been found that Jun a 1 and Jun a 2 proteins from MC share 80% and 71% identity with Cry j 1 and Cry j 2, respectively.Citation10-13 MC pollen causes a severe respiratory tract allergy in Texas during winter months.Citation14 Similarly to JCP, there is no effective immunotherapy available for MC allergy.
The concept of a DNA vaccine was described in early 1990s.Citation15,16 One unique feature of DNA vaccination is its ability to rapidly induce strong CD4+ and CD8+ T cell and antibody responses. Therefore, DNA vaccination has been substantially studied in a wide range of diseases including allergy, cancer, infectious diseases, and autoimmune diseases.Citation17 Type-I allergic diseases, including JRC and MC pollens induced allergy, are mediated by CD4+ Th2 cells, which help B cells produce IgE antibodies.Citation18 In several animal models for allergic diseases, it has been demonstrated that DNA vaccination can induce a Th1 type immune response, which could counterbalance the Th2 response.Citation19-23 Thus, DNA immunization represents a potential intervention for preventing or treating JRC/MC induced allergy.
Immunomic Therapeutics, Inc.'s research group developed a novel allergy immunotherapy, based on LAMP technology, to treat pollen induced allergies. Lysosomal Associated Membrane Protein 1 (LAMP-1 or LAMP) is a lysosomal residential protein. Its lysosomal targeting property has been initially used in the DNA vaccine fields in animal models for infectious diseases as well as in a variety of cell therapies for human oncology indications.Citation24-29 It has been shown that inclusion of LAMP in the DNA plasmids significantly enhanced both cellular and humoral responses in vaccinated animals. In a recent study, DNA plasmids encoding LAMP fused with Cry j 1 and Cry j 2 protein elicited a strong Th1 response in mice. After repeated allergen exposure, vaccinated mice were well protected, as indicated by a minimal level of allergen-specific IgE production. In contrast, the control mice exhibited a typical Th2 response. Based upon these data we believed that the LAMP based DNA vaccination skewed the allergic reaction from a Th2 toward a Th1 dominant response.Citation30
In the current Phase IA and IB clinical trials, we evaluated the safety and immunological effects of an investigational DNA vaccine encoding CryJ2-LAMP protein in human subjects. Cry j 2 was chosen as our first investigational product because it has been found that immunogenicity of Cry j 2 is stronger than that of Cry j 1.Citation31 Both JRC and/or MC atopic subjects were vaccinated with CryJ2-LAMP plasmid 4 times in the Phase IA trial and some subjects were boosted once in the Phase IB stage. The safety and immunologic biomarkers were assessed in these subjects for an accumulated time from Day 0 of the Phase IA to the end of the Phase IB, which ranged between 331–416 d. The results indicated that CryJ2-LAMP DNA vaccine is safe and has a potential as a therapeutic for JRC and/or MC sensitive subjects.
Methods
This protocol was reviewed and approved by the Sterling Institutional Review Board (Atlanta, Georgia), an independent review broad. This trial was conducted under an Investigational New Drug Application (IND) and registered on clinicaltrials.gov as NCT01707069 and NCT01966224.
Subject recruitment
Subjects were recruited from the Japanese community in Honolulu, Hawaii, favoring those who had more recently arrived from Japan within the last 5 y. The inclusion and exclusion criteria are given in detail in the Supplementary Documents. Subjects were identified as either non-atopic or had atopic reactivity to JRC or MC allergens. It should be noted that we were unable to identify in Honolulu and on the island any mature JRC trees; thus, any Japanese subjects exhibiting skin test reactivity were presumed to have naturally become sensitive to JRC in Japan. Subjects were also screened for atopic sensitivity to a variety of JRC unrelated allergens, including southern grasses, southern California tree, ragweed, and dust mites. Laboratory evaluations were performed by LabCorp for Phase IA, a local reference laboratory in Honolulu for Phase IB, and physical examinations were performed at the clinical site, East-West Medical Research Institute (Honolulu, HI).
41 subjects were screened, of which 11 subjects failed the inclusion/exclusion criteria and were not included in the trial. 30 subjects underwent the informed consent process, however, 6 of the 30 subjects withdrew their Informed Consent before entering into the first vaccination of this trial because of family issues or scheduling conflicts. One subject returned to Japan after the 4th vaccination and was therefore considered lost to follow up. All remaining 23 subjects completed the 0–72 day initial trial protocol. An amendment to the protocol for a 60 day extension was requested but not authorized in time; hence, 9 subjects were not able to complete visit 7 (day 102). However, all of these subjects did return for visit 8 on day 132.
During the Phase IB trial, 4 subjects from Cohort 1, 7 from the cohort 2, and 6 from Cohort 3 of the Phase IA trial were re-recruited. Subject #102 (Cohort 2) and subject #122 (Cohort 3) completed the trial without receiving a booster dose due to either anemia at screening or subject choice, respectively. Subject #120 from Cohort 2 did not finish the Phase IB trial because she moved to Japan.
Trial design and treatment
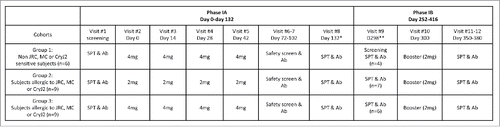
This study included a Phase IA and a Phase IB trials (). The primary end point of the study was to assess the safety as determined by self-reported AE's, vital signs clinical laboratory evaluations and changes in physical examination in non-atopic (no allergic sensitivities to CryJ2 allergen) subjects and atopic subjects with known allergy to JRC or MC allergen as identified by positive skin test reactivity. The secondary endpoints of this study were to examine whether there are increases in beneficial immunoglobulins - classes of CryJ2 specific IgG, as well as changes in the IgE antibody levels in the serum of non-atopic (no allergic sensitivities to CryJ2 allergen) subjects and in atopic subjects with known allergy to JRC CryJ2 allergen as identified by positive skin test reactivity and/or IgG specific antibody titers above baseline. The Phase IA was designed to assess acute and chronic toxicity up to 132 d after the first of 4 intramuscular (IM) immunizations. 24 subjects were divided into 3 cohorts. Subjects in Cohort 1 (JRC non atopic, n = 6) received a full 4 (4 mg dose) dosing regimen. Subjects (JRC and/or MC atopic) in Cohort 2 (n = 9) and Cohort 3 (n = 9) received a total of 4 half (2 mg dose) or 4 full (4 mg dose) dosing regimen, respectively. All subjects were treated with plasmid DNA by IM injection at 14 day intervals. The Phase IB trial was designed to continue evaluating the safety and immunological responses of subjects from the Phase IA trial. In the Phase IB trial, 15 out of 17 subjects were boosted with 2 mg plasmid DNA approximately 300 d after the 1st vaccination in the Phase IA trial.
Safety, skin prick test (SPT), and antibody measurement
Methods for safety assessment, SPT, and antibody measurement are provided in the Supplementary Documents (Methods).
Results
Subject demographics
24 subjects were enrolled in the Phase IA trial. Their demographics and characteristics are described in . There were more females (n = 6), than males (n = 3) in Cohort 2 and 3, but this did not appear to influence the safety or immunologic data. Seventeen subjects from the Phase IA trial were recruited to the Phase IB trial. Their demographics and characteristics are described in the Supplementary Documents (Table S1). Again, more females than males were enrolled (Cohort 2, 5 females and 2 males; Cohort 3, 5 females and 1 male). There were no other differences among groups.
Table 1. Demographics and Baseline Characteristics.
Adverse events
During the Phase IA trial (0 day-132 days), a total 88 treatment-emergent adverse events (TEAEs) were reported () over 8 trial visits. Twenty subjects reported at least one TEAE, 5 from Cohort 1, 6 from Cohort 2, and 9 from Cohort 3 (Supplementary Documents, Table S2). There was no reported early or late phase anaphylaxis or other systemic illness. The most frequently reported TEAE was injection site erythema which was considered definitely related. The majority of TEAEs were mild in severity. There were a total of 41 “definitely related” events of local injection site reactions, commonly expected with vaccinations. These local reactions did not change with time nor increase in severity after each vaccination. During the Phase IB trial a total of 9 TEAEs in 3 subjects were reported (). Only one definitely related TEAE was reported, which was itchiness at the injection site. In addition, fatigue was reported by one subject and was considered possibly related to the vaccine. The event was transient (4 hours duration) and there was no reoccurrence on further follow-up. None of these reported TEAEs required medical intervention.
Table 2. Summary of all TEAEs by Cohort (day 0 - day 132).
Table 3. Summary of all AEs during the Phase IB Trial.
One subject who participated in both IA and IB trials experienced 3 raised systolic blood pressure results on Day 28. The subject had no prior or subsequent raised systolic blood pressure reading. All other subject's vital signs remained in the normal range throughout the trial period (data not shown). No subjects experienced significant changes in the clinical laboratory parameters (hematology, blood chemistry, urinalysis, and serology), which required medical intervention.
Anti-LAMP antibodies were monitored before each vaccination and thereafter in total 8 time points in the Phase IA and 3 time points in the Phase IB study. Anti-LAMP IgG antibody in serum samples were measured by ELISA and spontaneous competitive inhibition ELISA. We did not find any anti-LAMP IgG positive samples throughout the study in any subjects (data not shown).
Skin prick test (SPT) for JRC/MC allergens
The SPT for JRC/MC results for both Phase IA and IB trial are summarized in . Seventeen subjects in Cohort 2 and 3 completed the Phase IA trial. Of the 17 subjects, 12 were JRC atopic by SPT and 11 were MC atopic. At the end of the Phase IA trial, of these 12 JRC atopic subjects, 10 experienced SPT reaction conversion from positive to negative for JRC extract. Of the 11 MC atopic subjects, 6 experienced SPT negative conversion for MC extract. All 3 subjects (#105, #112, and #136), who were found skin test positive for Cry j 2 allergen at screening, showed conversion to negative for Cry j 2 on day 132 (data not shown).
Table 4. Skin Prick Test Results for JRC and MC.
The SPT negative conversion for JRC/MC was either maintained or achieved for all subjects who enrolled in the Phase IB trial. The only exception was one subject that experienced MC SPT negative conversion but did not maintain the conversion at the end of trial. Also, this atopic subject at visit #12 had a positive SPT reaction for JRC for the first time. It is worth noting that 2 subjects #102 and #122, who did not receive the single booster injection, remained negative by SPT for JRC and/or MC.
Skin prick test for allergens unrelated to JRC
The SPT results for the unrelated allergens from all subjects who completed the Phase IA and/or Phase IB trials are summarized in . At day 132, 10 of the 17 subjects in Cohorts 2 and 3, who completed the Phase IA trial, experienced SPT negative conversion for at least one of the 4 unrelated skin test allergens. Eight out of these 10 subjects also experienced a shift from positive skin tests for JRC, MC, or CryJ2 at screening to negative skin tests at day 132. The majority of the subjects who enrolled in the Phase IB maintained the negative SPT conversion to the end of trial.
Table 5. Skin Prick Test Results for Allergens Unrelated to JRC and MC.
At day 132, 3 subjects converted from SPT reaction negative to positive for southern grass and one was positive converted for western ragweed.
Antibody detection
Serological IgG and IgE antibodies for JRC, MC, and other allergens and total IgG, IgG1, IgG2, IgG3, and IgG4 antibodies for Cry j 2 were tested by the ImmunoCAP Allergen Specific IgG/E method. Cry j 2 specific IgE levels were examined by the Conventional RAST method. At the end of the Phase IA trial, we did not observe any significant changes in anti-JRC, -Cry j 2, and -MC IgG (total IgG and/or subclasses) antibodies (data not shown) and IgE antibodies (Supplementary Documents, Tables S3–S5) in any cohorts. At the end of the Phase IB trial, most subjects showed a trend of increasing of anti-JRC IgG production (Supplementary Documents, Table S6). No significant change was found in serological levels of IgG and IgE against unrelated allergens including southern grass, ragweed, dust mite and southern California tree (data not shown) and there were no concomitant changes in subjects' allergen-specific IgG and IgE antibody levels with the SPT results for these unrelated allergens.
Discussion
The primary objective of these Phase IA and IB trials was to determine the safety of the investigational CryJ2-LAMP DNA vaccine. The 4 initial and a boosting dose regimen of the CryJ2-LAMP vaccination was well tolerated by all subjects and the safety endpoints were met. A majority of the atopic subjects experienced conversions of skin test from positive to negative for JRC and/or MC at the end of the trial, possibly due to the CryJ2-LAMP vaccination.
Accumulated evidence from clinical trials for infectious diseases and cancer indicate that DNA vaccines are safe in humans.Citation32-35 However, one concern with the administration of DNA products in allergic patients is that the plasmid encoded allergens might trigger or worsen allergic responses. The investigational CryJ2-LAMP DNA plasmid contains 3 major segments: the luminal domain of LAMP, the CryJ2 sequence, and the transmembrane/cytoplasmic signaling domain of LAMP. Theoretically, this strategy eliminates the release of free allergen into circulation; thus, allowing patient exposure to DNA vaccines without the fear of atopic reactions. The intracellular accumulation of LAMP fused target antigens in antigen presenting cells has been confirmed by using several viral proteins.Citation27,28 Our safety data support the concept as no anaphylactic/allergic response or other systemic illness was found during the trials. 42 out of 97 observed TEAEs were mild skin injection site reactions, which were expected. The total number and frequency of TEAEs from Cohort 2, which is the 2mg-dose group, was lower than those from the 4mg-dose groups, indicating a correlation between TEAEs and the amount of administrated DNA plasmid. However, there was no difference between groups in term of the severity of adverse events. Eighty six out of 88 of observed TEAEs occurred during the period from day 0 to day 72. The majority of these incidences were transitory and none of them required medication or medical attention. However, one limitation is that this study was conducted at Honolulu where we were unable to identify any JRC trees. Thus, we were unable to evaluate if natural exposure to JRC pollen simultaneously with CryJ2-LAMP vaccination. Because of a lack of mock control (empty LAMP plasmid), we were unable to determine whether the observed TEAEs were results of the expression of CryJ2 or of the LAMP technology. Nevertheless, no LAMP IgG antibody positive sample was detected at any of the up to elven time points in these trials. This correlates with the lack of clinical symptoms or data for induction of an adverse immune response to the LAMP vaccine. The physical and clinical laboratory results also support the conclusion that the CryJ2-LAMP vaccine is safe as no adverse events requiring medical intervention were found.
Another advantage of LAMP technology is the induction of robust CD4 T cell responses. LAMP protein mediates the trafficking of its cargo antigens to the lysosomal/endosomal compartment and enhances the subsequent MHC class II presentation.Citation24-26 We demonstrated in an animal model that a robust antigen specific Th1 type CD4+ T cell response was induced upon CryJ-LAMP vaccination.Citation30 As a result, high levels of Cry j 1 or Cry j 2 specific IgG2a (Th1 type) antibody and low IgE antibody were found. In this Phase I clinical trial, we evaluated the immunological effects of CryJ2-LAMP vaccine by using skin prick test and antibody detection. SPT for JRC negative conversion was achieved in the JRC sensitive subjects (10/12 in Phase IA and 8/8 in Phase IB), with either 2mg- or 4mg- dose of plasmid DNA. The JRC SPT negative reaction in these JRC sensitive subjects had been maintained until the end of IB trial. One subject (#140, Cohort 3) who was initially negative for JRC, transiently became to SPT positive on day 132, but returned back to SPT negative for JRC. It is surprising that 2 subjects had maintained SPT negative reaction until the end of the Phase IB trial even without receiving the single booster dose, suggesting a long-term effect of the initial CryJ2-LAMP vaccination. However, because of a lack of placebo control and natural exposition, we could not exclude the possibility that the negative skin test conversion is a result of subjects naturally outgrowing their JRC/MC sensitivity. It should be noted that these results were achieved by using only one allergen-encoding vaccine, indicating the potency of the LAMP-based DNA technique in inducing the beneficial effects in allergic patients. Because the primary goal of this study is safety evaluation and Cry j 2 has a strong immunogenicity, CryJ2-LAMP, but not the combination of CryJ2 and CryJ1 LAMP, was chosen as our first generation investigational product. Based on results from the current study, we predict that a combination of CryJ1-LAMP and CryJ2-LAMP vaccines, our next investigational products, could elicit a stronger immunological effect than the CryJ2-LAMP alone.
Five subjects who were skin test negative for JRC but positive for MC were included in these trial. Because Jun a 2 is a high homologous protein of the Cry j 2, it is reasonable that these allergens share T cell and IgE epitopes. Indeed, CryJ2-LAMP vaccination resulted in SPT negative conversion in 6 out of total 11 MC sensitive subjects on day 132. For the remaining 5 subjects, 3 of them were enrolled in the Phase IB trial and all 3 subjects were found SPT test negative for MC during the Phase IB trial screening and before receiving the booster dose. These results suggest that in some subjects, the immunological effects might not be induced rapidly after vaccination, but can be delayed. This delay is consistent with the purported mechanism of action of LAMP vaccines since they require “re-education” of the immune system. Another possibility of this delayed conversion is that some of these MC positive SPT reaction (at screening) may be reactive to several of the MC pollen allergens, such as Jun a 1 and Jun a 2, and the single allergen encoding vaccine needs a long period to exert its effect. Only one subject (#118, Cohort 2), who experienced a MC negative conversion, was unable to maintain the negative SPT reaction at the end of trial. This subject at the last visit also had a positive skin test to JRC for the first time. Because this subject showed SPT reaction negative conversion in the previous visits, it is unlikely that this phenomenon was caused by the boosting vaccination. No other atopic and non-atopic subjects experienced this phenomenon.
It has been found that immunotherapies can induce a bystander suppression to unrelated allergens.Citation36-38 In line with these findings, we did observed a substantial number of SPT conversion from positive to negative for tested unrelated allergens. Although we do not know the exact mechanism, it is possibly due to the bystander T cell help. In future studies, including a placebo control group or a control group which has subjects atopic to non-JRC/MC allergens, for example, ragweed, will help define whether the conversion of skin test to that unrelated allergens is related to the bystander effects caused by the CryJ2-LAMP vaccination. Though a few subjects experienced positive conversion for any of these unrelated allergens, there is no correlation between the SPT results for these allergens and for JRC/MC. For example, subject #125, who became SPT reaction positive for southern California tree and dust mite during the Phase IB trial, converted SPT reaction to negative for MC and then remained the negative reaction until the end of trial. Thus, it is possible that these subjects exposed to such unrelated allergens became sensitized during the trials.
CryJ2-LAMP vaccinations did not induce any significant changes in the production of JRC and/or MC specific IgE antibodies, indicating that the CryJ2-LAMP is safe. However, it is worth noting that the JRC-specific IgE binding titers in this study are much lower (about 5-fold) than the typical titers in JRC allergic patients.Citation39 We speculate that the decrease in JRC-specific IgE titers was a result of lack of JRC pollen exposure in Honolulu. In addition, the binding titers of Cry j 2-specific IgE were lower than expected, indicating the subjects were sensitized to other allergens in the JRC pollen. Unlike the results from preclinical studies in which CryJ-LAMP plasmid DNA vaccinated mice exhibited a robust JRC specific IgG antibody production, herein we only observed a marginal increase of anti-JRC IgG antibody at the end of the Phase IB trial. Human subjects usually produce little or no antibodies by only DNA vaccination.Citation32,40,41 For example, even combined with an immunoregulatory cytokine granulocyte macrophage-colony stimulating factor encoding plasmid, DNA vaccines for malaria still failed to induce antibody response, although specific CD8+ T cell response was induced.Citation42 Nevertheless, recent studies indicate that DNA vaccines are excellent in priming the immune system, both cellular and humoral, if followed with a protein boost.Citation43,44 Thus, the DNA priming/protein boost immunization regimen has been used to improve the levels of neutralizing antibody responses, particularly in the infectious disease studies.Citation45,46 Therefore, we propose that in future clinical studies, vaccinated subjects might produce higher JRC specific IgG antibodies once they are exposed naturally to the JRC pollen. Considering the highly homology and cross-activities among the major allergens of JRC (Cry j 1), MC (Jun a 1), Japanese cypress (Cha o 1) and Cupressus arizonica (Cup a 1)and among Cry j 2, Jun a 2, and Cha o 2,Citation10,12,47,48 the CryJ-LAMP vaccines might have a potential as a therapeutic to individual allergic to such pollens, particularly to Japanese cypress, which follows the season of JRC pollen.
Conclusions
In summary, the investigational CryJ2-LAMP DNA vaccine was safe as all safety endpoints were met at the end of the 2 trials. The 4 dose vaccination regimen was well tolerated by all subjects and no serious safety issue was identified. Meanwhile, the investigational product showed immunological effects in these JRC/MC sensitive subjects, suggesting its potential as a therapeutic for JRC/MC allergic patients. However, these were open trials with a small number of subjects without placebo control group. A double-blind placebo-controlled study with more subjects, more control groups, and more biomarker measurement is needed to confirm the true efficacy.
Abbreviations
| JRC | = | Japanese Red Cedar |
| LAMP | = | Lysosomal Associated Membrane Protein |
| MC | = | Mountain Cedar |
| SPT | = | Skin Prick Test |
Disclosure of conflicts of interest
This trial was supported by Immunomic Therapeutics, Inc. WH and TH are shareholders of Immunomic Therapeutics, Inc. YS, ER, and AA are employees of Immunomic Therapeutics, Inc. DF. has declared that he has no relevant conflicts of interest.
Author contributions
YS wrote the manuscript and participated in the data analysis. ER and AA reviewed the manuscript and participated in the data extraction and analysis. DF supervised the clinical aspects of the trials. WH and TH conceived and designed the study and supervised data extraction and analysis. All authors approved the final version of the manuscript as submitted.
Supplemental_Material.zip
Download Zip (71.9 KB)Acknowledgments
We thank the subjects for their participation in these trials. We thank the staff of the Clinical Site at the of the East-West Medical Research Institute (Honolulu, HI) without whose help this trial could not have been completed. We thank the staff of the Viracor-IBT Laboratories and KCAS Laboratory for antibody detection. We also thank our Medical Monitor, Dr. Larry Weiner, as well as our Regulatory Advisor, Dr. Bruce Mackler, for their involvement in conducting this study.
References
- Yamada T, Saito H, Fujieda S. Present state of Japanese cedar pollinosis: The national affliction. J Allergy Clin Immunol 2014; 133:632-9 e5; PMID: 24361081; https://doi.org/10.1016/j.jaci.2013.11.002
- Sone T, Komiyama N, Shimizu K, Kusakabe T, Morikubo K, Kino K. Cloning and sequencing of cDNA coding for Cry j I, a major allergen of Japanese cedar pollen. Biochem Biophys Res Commun 1994; 199:619-25; PMID: 8135802; https://doi.org/10.1006/bbrc.1994.1273
- Yasueda H, Yui Y, Shimizu T, Shida T. Isolation and partial characterization of the major allergen from Japanese cedar (Cryptomeria japonica) pollen. J Allergy Clin Immunol 1983; 71:77-86; PMID: 6822692; https://doi.org/10.1016/0091-6749(83)90550-X
- Sakaguchi M, Inouye S, Taniai M, Ando S, Usui M, Matuhasi T. Identification of the second major allergen of Japanese cedar pollen. Allergy 1990; 45:309-12; PMID: 2382797; https://doi.org/10.1111/j.1398-9995.1990.tb00501.x
- Hashimoto M, Nigi H, Sakaguchi M, Inouye S, Imaoka K, Miyazawa H, Taniguchi Y, Kurimoto M, Yasueda H, Ogawa T. Sensitivity to two major allergens (Cry j I and Cry j II) in patients with Japanese cedar (Cryptomeria japonica) pollinosis. Clin Exp Allergy 1995; 25:848-52; PMID: 8564723; https://doi.org/10.1111/j.1365-2222.1995.tb00027.x
- Sugimura K, Hashiguchi S, Takahashi Y, Hino K, Taniguchi Y, Kurimoto M, Fukuda K, Ohyama M, Yamada G. Th1/Th2 response profiles to the major allergens Cry j 1 and Cry j 2 of Japanese cedar pollen. Allergy 1996; 51:732-40; PMID: 8905002
- Sone T, Dairiki K, Morikubo K, Shimizu K, Tsunoo H, Mori T, Kino K. Identification of human T cell epitopes in Japanese cypress pollen allergen, Cha o 1, elucidates the intrinsic mechanism of cross-allergenicity between Cha o 1 and Cry j 1, the major allergen of Japanese cedar pollen, at the T cell level. Clin Exp Allergy 2005; 35:664-71; PMID: 15898991; https://doi.org/10.1111/j.1365-2222.2005.02221.x
- Sone T, Dairiki K, Morikubo K, Shimizu K, Tsunoo H, Mori T, Kino K. Recognition of T cell epitopes unique to Cha o 2, the major allergen in Japanese cypress pollen, in allergic patients cross-reactive to Japanese cedar and Japanese cypress pollen. Allergol Int 2009; 58:237-45; PMID: 19307778; https://doi.org/10.2332/allergolint.08-OA-0027
- Okamoto Y, Horiguchi S, Yamamoto H, Yonekura S, Hanazawa T. Present situation of cedar pollinosis in Japan and its immune responses. Allergol Int 2009; 58:155-62; PMID: 19307773; https://doi.org/10.2332/allergolint.08-RAI-0074
- Aceituno E, Del Pozo V, Minguez A, Arrieta I, Cortegano I, Cardaba B, Gallardo S, Rojo M, Palomino P, Lahoz C. Molecular cloning of major allergen from Cupressus arizonica pollen: Cup a 1. Clin Exp Allergy 2000; 30:1750-8; PMID: 11122214; https://doi.org/10.1046/j.1365-2222.2000.00949.x
- Midoro-Horiuti T, Goldblum RM, Kurosky A, Wood TG, Schein CH, Brooks EG. Molecular cloning of the mountain cedar (Juniperus ashei) pollen major allergen, Jun a 1. J Allergy Clin Immunol 1999; 104:613-7; PMID: 10482836; https://doi.org/10.1016/S0091-6749(99)70332-5
- Ito H, Nishimura J, Suzuki M, Mamiya S, Sato K, Takagi I, Baba S. Specific IgE to Japanese cypress (Chamaecyparis obtusa) in patients with nasal allergy. Ann Allergy Asthma Immunol 1995; 74:299-303; PMID: 7719888
- Yokoyama M, Miyahara M, Shimizu K, Kino K, Tsunoo H. Purification, identification, and cDNA cloning of Jun a 2, the second major allergen of mountain cedar pollen. Biochem Biophys Res Commun 2000; 275:195-202; PMID: 10944464; https://doi.org/10.1006/bbrc.2000.3273
- Ramirez DA. The natural history of mountain cedar pollinosis. J Allergy Clin Immunol 1984; 73:88-93; PMID: 6537956; https://doi.org/10.1016/0091-6749(84)90489-5
- Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature 1992; 356:152-4; PMID: 1545867; https://doi.org/10.1038/356152a0
- Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993; 259:1745-9; PMID: 8456302; https://doi.org/10.1126/science.8456302
- Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev 2011; 239:62-84; PMID: 21198665; https://doi.org/10.1111/j.1600-065X.2010.00980.x
- Kapsenberg ML, Jansen HM, Bos JD, Wierenga EA. Role of type 1 and type 2 T helper cells in allergic diseases. Curr Opin Immunol 1992; 4:788-93; PMID: 1466803; https://doi.org/10.1016/0952-7915(92)90063-K
- Hsu CH, Chua KY, Tao MH, Lai YL, Wu HD, Huang SK, Hsieh KH. Immunoprophylaxis of allergen-induced immunoglobulin E synthesis and airway hyperresponsiveness in vivo by genetic immunization. Nat Med 1996; 2:540-4; PMID: 8616712; https://doi.org/10.1038/nm0596-540
- Tan LK, Huang CH, Kuo IC, Liew LM, Chua KY. Intramuscular immunization with DNA construct containing Der p 2 and signal peptide sequences primed strong IgE production. Vaccine 2006; 24:5762-71; PMID: 16740347; https://doi.org/10.1016/j.vaccine.2006.04.064
- Peng HJ, Su SN, Chang ZN, Chao PL, Kuo SW, Tsai LC. Induction of specific Th1 responses and suppression of IgE antibody formation by vaccination with plasmid DNA encoding Der f 11. Vaccine 2002; 20:1761-8; PMID: 11906763; https://doi.org/10.1016/S0264-410X(02)00029-4
- Toda M, Sato H, Takebe Y, Taniguchi Y, Saito S, Inouye S, Takemori T, Sakaguchi M. Inhibition of immunoglobulin E response to Japanese cedar pollen allergen (Cry j 1) in mice by DNA immunization: different outcomes dependent on the plasmid DNA inoculation method. Immunology 2000; 99:179-86; PMID: 10692034; https://doi.org/10.1046/j.1365-2567.2000.00935.x
- Toda M, Kasai M, Hosokawa H, Nakano N, Taniguchi Y, Inouye S, Kaminogawa S, Takemori T, Sakaguchi M. DNA vaccine using invariant chain gene for delivery of CD4+ T cell epitope peptide derived from Japanese cedar pollen allergen inhibits allergen-specific IgE response. Eur J Immunol 2002; 32:1631-9; PMID: 12115646; https://doi.org/10.1002/1521-4141(200206)32:6<1631::AID-IMMU1631>3.0.CO;2-O
- Rowell JF, Ruff AL, Guarnieri FG, Staveley-O'Carroll K, Lin X, Tang J, August JT, Siliciano RF. Lysosome-associated membrane protein-1-mediated targeting of the HIV-1 envelope protein to an endosomal/lysosomal compartment enhances its presentation to MHC class II-restricted T cells. J Immunol 1995; 155:1818-28; PMID: 7636236
- Ruff AL, Guarnieri FG, Staveley-O'Carroll K, Siliciano RF, August JT. The enhanced immune response to the HIV gp160/LAMP chimeric gene product targeted to the lysosome membrane protein trafficking pathway. J Biol Chem 1997; 272:8671-8; PMID: 9079699; https://doi.org/10.1074/jbc.272.13.8671
- Wu TC, Guarnieri FG, Staveley-O'Carroll KF, Viscidi RP, Levitsky HI, Hedrick L, Cho KR, August JT, Pardoll DM. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci U S A 1995; 92:11671-5; PMID: 8524826; https://doi.org/10.1073/pnas.92.25.11671
- Marques ET, Jr., Chikhlikar P, de Arruda LB, Leao IC, Lu Y, Wong J, Chen JS, Byrne B, August JT. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J Biol Chem 2003; 278:37926-36; PMID: 12824194; https://doi.org/10.1074/jbc.M303336200
- Anwar A, Chandrasekaran A, Ng ML, Marques E, August JT. West Nile premembrane-envelope genetic vaccine encoded as a chimera containing the transmembrane and cytoplasmic domains of a lysosome-associated membrane protein: increased cellular concentration of the transgene product, targeting to the MHC II compartment, and enhanced neutralizing antibody response. Virology 2005; 332:66-77; PMID: 15661141
- Bonehill A, Heirman C, Tuyaerts S, Michiels A, Breckpot K, Brasseur F, Zhang Y, Van Der Bruggen P, Thielemans K. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J Immunol 2004; 172:6649-57; PMID: 15153480
- Su Y, Connolly M, Marketon A, Heiland T. CryJ-LAMP DNA Vaccines for Japanese Red Cedar Allergy Induce Robust Th1-Type Immune Responses in Murine Model. J Immunol Res 2016; 2016:4857869; PMID: 27239481
- Hirahara K, Saito S, Serizawa N, Sasaki R, Sakaguchi M, Inouye S, Taniguchi Y, Kaminogawa S, Shiraishi A. Oral administration of a dominant T-cell determinant peptide inhibits allergen-specific TH1 and TH2 cell responses in Cry j 2-primed mice. J Allergy Clin Immunol 1998; 102:961-7; PMID: 9847437
- Wang R, Doolan DL, Le TP, Hedstrom RC, Coonan KM, Charoenvit Y, Jones TR, Hobart P, Margalith M, Ng J, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 1998; 282:476-80; PMID: 9774275
- Chudley L, McCann K, Mander A, Tjelle T, Campos-Perez J, Godeseth R, Creak A, Dobbyn J, Johnson B, Bass P, et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8(+) T-cell responses and increases PSA doubling time. Cancer Immunol Immunother 2012; 61:2161-70; PMID: 22729556
- Vardas E, Stanescu I, Leinonen M, Ellefsen K, Pantaleo G, Valtavaara M, Ustav M, Reijonen K. Indicators of therapeutic effect in FIT-06, a Phase II trial of a DNA vaccine, GTU((R))-Multi-HIVB, in untreated HIV-1 infected subjects. Vaccine 2012; 30:4046-54; PMID: 22549090
- Kibuuka H, Berkowitz NM, Millard M, Enama ME, Tindikahwa A, Sekiziyivu AB, Costner P, Sitar S, Glover D, Hu Z, et al. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet 2015; 385:1545-54; PMID: 25540891
- Prado N, Canamero M, Villalba M, Rodriguez R, Batanero E. Bystander suppression to unrelated allergen sensitization through intranasal administration of tolerogenic exosomes in mouse. Mol Immunol 2010; 47:2148-51; PMID: 20478618
- Scholl I, Wiedermann U, Forster-Waldl E, Ganglberger E, Baier K, Boltz-Nitulescu G, Scheiner O, Ebner C, Jensen-Jarolim E. Phage-displayed Bet mim 1, a mimotope of the major birch pollen allergen Bet v 1, induces B cell responses to the natural antigen using bystander T cell help. Clin Exp Allergy 2002; 32:1583-8; PMID: 12569978; https://doi.org/10.1046/j.1365-2222.2002.01527.x
- Schabussova I, Ul-Haq O, Hoflehner E, Akgun J, Wagner A, Loupal G, Joachim A, Ruttkowski B, Maizels RM, Wiedermann U. Oesophagostomum dentatum extract modulates T cell-dependent immune responses to bystander antigens and prevents the development of allergy in mice. PLoS One 2013; 8:e67544; PMID: 23844022; https://doi.org/10.1371/journal.pone.0067544
- Uekusa Y, Inamine A, Yonekura S, Horiguchi S, Fujimura T, Sakurai D, Yamamoto H, Suzuki H, Hanazawa T, Okamoto Y. Immunological parameters associated with the development of allergic rhinitis: a preliminary prospective study. Am J Rhinol Allergy 2012; 26:92-6; PMID: 22487284; https://doi.org/10.2500/ajra.2012.26.3706
- MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, Chattergoon MA, Baine Y, Higgins TJ, Ciccarelli RB, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis 1998; 178:92-100; PMID: 9652427; https://doi.org/10.1086/515613
- Epstein JE, Gorak EJ, Charoenvit Y, Wang R, Freydberg N, Osinowo O, Richie TL, Stoltz EL, Trespalacios F, Nerges J, et al. Safety, tolerability, and lack of antibody responses after administration of a PfCSP DNA malaria vaccine via needle or needle-free jet injection, and comparison of intramuscular and combination intramuscular/intradermal routes. Hum Gene Ther 2002; 13:1551-60; PMID: 12228010; https://doi.org/10.1089/10430340260201644
- Richie TL, Charoenvit Y, Wang R, Epstein JE, Hedstrom RC, Kumar S, Luke TC, Freilich DA, Aguiar JC, Sacci JB Jr, et al. Clinical trial in healthy malaria-naive adults to evaluate the safety, tolerability, immunogenicity and efficacy of MuStDO5, a five-gene, sporozoite/hepatic stage Plasmodium falciparum DNA vaccine combined with escalating dose human GM-CSF DNA. Hum Vaccin Immunother 2012; 8:1564-84; PMID: 23151451; https://doi.org/10.4161/hv.22129
- Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, Shen S, Green S, Rothman AL, Ennis FA, et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine 2008; 26:3947-57; PMID: 18724414; https://doi.org/10.1016/j.vaccine.2007.12.060
- Spearman P, Lally MA, Elizaga M, Montefiori D, Tomaras GD, McElrath MJ, Hural J, De Rosa SC, Sato A, Huang Y, et al. A trimeric, V2-deleted HIV-1 envelope glycoprotein vaccine elicits potent neutralizing antibodies but limited breadth of neutralization in human volunteers. J Infect Dis 2011; 203:1165-73; PMID: 21451004; https://doi.org/10.1093/infdis/jiq175
- Villarreal DO, Talbott KT, Choo DK, Shedlock DJ, Weiner DB. Synthetic DNA vaccine strategies against persistent viral infections. Expert Rev Vaccines 2013; 12:537-54; PMID: 23659301; https://doi.org/10.1586/erv.13.33
- Lu S. Immunogenicity of DNA vaccines in humans: it takes two to tango. Hum Vaccin 2008; 4:449-52; PMID: 18443427; https://doi.org/10.4161/hv.4.6.6179
- Mori T, Yokoyama M, Komiyama N, Okano M, Kino K. Purification, identification, and cDNA cloning of Cha o 2, the second major allergen of Japanese cypress pollen. Biochem Biophys Res Commun 1999; 263:166-71; PMID: 10486272; https://doi.org/10.1006/bbrc.1999.1261
- Yasueda H, Saito A, Sakaguchi M, Ide T, Saito S, Taniguchi Y, Akiyama K, Inouye S. Identification and characterization of a group 2 conifer pollen allergen from Chamaecyparis obtusa, a homologue of Cry j 2 from Cryptomeria japonica. Clin Exp Allergy 2000; 30:546-50; PMID: 10718852; https://doi.org/10.1046/j.1365-2222.2000.00747.x