ABSTRACT
Two large pivotal phase III studies demonstrated the efficacy of the tetravalent dengue vaccine (CYD-TDV; Dengvaxia®, Sanofi Pasteur) against all dengue serotypes. Here we present an unprecedented integrated summary of the immunogenicity of CYD-TDV to identify the parameters driving the neutralizing humoral immune response and evolution over time. We summarized the immunogenicity profiles of a 3-dose schedule of CYD-TDV administered 6 months apart across 10 phase II and 6 phase III trials undertaken in dengue endemic and non-endemic countries. Dengue neutralizing antibody titers in sera were determined at centralized laboratories using the 50% plaque reduction neutralization test (PRNT50) at baseline, 28 d after the third dose, and annually thereafter for up to 4 y after the third dose in some studies. CYD-TDV elicits neutralizing antibody responses against all 4 dengue serotypes; geometric mean titers (GMTs) increased from baseline to post-dose 3. GMTs were influenced by several parameters including age, baseline dengue seropositivity and region. In the 2 pivotal studies, GMTs decreased initially during the first 2 y post-dose 3 but appear to stabilize or slightly increase again in the third year. GMTs persisted 1.2–3.2-fold higher than baseline levels for up to 4 y post-dose 3 in other studies undertaken in dengue endemic countries. Our integrated analysis captures the fullness of the CYD-TDV immunogenicity profile across studies, age groups and regions; by presenting the available data in this way general trends and substantial outliers within each grouping can be easily identified. CYD-TDV elicits neutralizing antibody responses against all dengue serotypes, with differences by age and endemicity, which persist above baseline levels in endemic countries.
Introduction
A recombinant yellow fever-17D–dengue virus, live, attenuated, tetravalent dengue vaccine (CYD-TDV) has recently been approved in several endemic countries for the prevention of dengue in those aged ≥ 9 y. The World Health Organization (WHO) has also recommended the introduction of CYD-TDV in regions with high dengue endemicity.Citation1 These decisions were based on efficacy, safety and immunogenicity data from 2 pivotal phase III studies involving ≥ 31,000 children and adolescents in Asia and Latin America,Citation2,3 14 other supportive studies as well as modeling analyses which suggested that the benefit-risk profile of the vaccine may depend on the endemicity level of the region considered.Citation4,5 The approved CYD-TDV schedule, 3 doses administered 6 months apart, was shown in the 2 pivotal phase III studies to have pooled efficacy rates of 60.3% against virologically-confirmed dengue for all participants and 72.7% against hospitalizations during the first 25 months.Citation6 However, vaccine efficacy differed by serotype, was higher in individuals with pre-existing dengue immunity (seropositive), and appeared to increase directly with age in the Asian phase III study. Whether age simply reflects accumulated exposure to dengue or to other factors related to host physiology such as maturity of the immune system and/or microvascular are unknown.Citation7
The CYD-TDV immunogenicity profile has been documented in reports from regions with varying epidemiological settings, age groups and baseline dengue status.Citation2,3,8-21 Here we present an unprecedented integrated summary of dengue neutralizing antibody geometric mean titers (GMTs) as measured at centralized laboratories using the same standardized 50% plaque reduction neutralization test (PRNT50) after 3 CYD-TDV doses in infants, toddlers, children, adolescents and adults aged up to 60 y from studies undertaken in endemic or non-endemic areas. These results further elucidate vaccine-induced dengue neutralizing antibody responses and persistence, and complement critical analyses identifying factors that may influence vaccine protection. We also describe the neutralizing antibody response in participants aged <9 y and those living in non-endemic regions, populations which are not indicated in the approved CYD-TDV label.
Results
Studies and participants
A total of 5,780 participants aged ≥ 9 months received at least one dose of CYD-TDV and 5,499 (95.1%) received the third CYD-TDV dose. The numbers of participants in studies undertaken in Asia Pacific (including Australia) and Latin America were 3,233 (55.9%) and 2,329 (40.3%), respectively, and there were 218 (3.8%) in the 2 US studies (CYD12 and CYD51). Participants included in the immunogenicity subset for CYD14, CYD15, CYD23 and CYD28 had similar baseline demographic data to the overall study population. The majority of participants were from endemic regions (4,400; 76.1%) and most were children 6 to 11 y (1,833; 31.7%) and adolescents 12 to 17 y (1,510; 26.1%). Overall, there were 3,104 (53.7%) participants aged 9–45 y (2,810 [48.6%] aged 9 to 17 years) and 241 [4.2%] aged 46–60 y included in this integrated analysis. Studies undertaken in non-endemic regions recruited only adult participants.
Dengue and Japanese encephalitis (JE)/Yellow fever (YF) seropositivity rates at baseline in CYD-TDV recipients are summarized in . In studies undertaken in dengue non-endemic regions, the proportion of participants who were dengue seropositive at baseline ranged from 6.6% (43/653; CYD17) to 16.8% (17/101; CYD12). The most common dengue serotypes detected were serotypes 3 and 4. Some participants in CYD51 had previously been vaccinated against YF and as such, the proportion of flavivirus seropositive participants corresponded mainly to those who were YF seropositive (66.7%; 78/117).
Table 1. Studies included in the analysis.
A high proportion of participants were dengue seropositive in studies undertaken in dengue endemic Asia-Pacific countries; seropositive rates ranged from 44.1% (78/177; CYD08) to 86.5% (109/126; CYD47), except for one study undertaken in Singapore, CYD28 (26.7%; 114/427), which had a much lower seropositivity rate (). Baseline dengue seropositivity generally increased with age: dengue seropositivity ranged from 47.1% (66/140; CYD28) to 95.0% (19/20; CYD22) in adults; 13.6% (19/140; CYD28) to 80.0% (320/400; CYD14) in adolescents; 19.8% (20/101; CYD28) to 72.5% (124/171; CYD23) in children aged 6–11 years; 19.6% (9/46; CYD28) to 60.0% (24/40; CDY22) in children aged 2–5 years; and it was 44.1% in the one study with infants and toddlers. The most common dengue serotypes detected were serotypes 1, 2 and 3 in adults, serotypes 2 and 3 in adolescents, children aged 6 to 11 y and 2 to 5 y, and serotype 3 in infants and toddlers.
Studies undertaken in dengue endemic Latin American countries had similar trends in baseline dengue seropositivity rates as those undertaken in dengue endemic Asia-Pacific countries. Dengue seropositivity rates ranged from 37.8% (74/196; CYD24) to 80.7% (1048/1299; CYD15), except in CYD29 (3.9%; 4/103) and CYD33 (6.5%; 14/216) where dengue seropositive rates were much lower than in other studies which may be explained by the lower age group recruited into these 2 studies (infants and toddlers only) (). In addition, dengue seropositivity rates generally increased with age: these ranged from 80.2% (186/232; CYD13) to 84.3% (554/657; CYD15) in adolescents; 36.1% (35/97; CYD24) to 76.9% (494/642; CYD15) in children aged 6–11 years; and 39.4% (39/99; CYD24) in the one study that included children aged 2–5 y. The most and equally represented serotypes were serotypes 2 and 3 in all age groups.
The proportion of participants with neutralizing dengue antibody responses to at least 2 serotypes at baseline is summarized in Supplementary Table S1 by age group, study and region. In general, the proportion of participants with neutralizing dengue antibody responses to at least 2 serotypes increased with age in the 2 endemic regions.
CYD-TDV immunogenicity
Baseline and post-dose 3 dengue neutralizing antibody GMTs varied across studies depending on region and age group () — in general, GMTs were usually higher in adults and adolescents compared with children aged 6 to 11 years, 2 to 5 y and infants and toddlers in trials conducted in endemic regions (studies in non-endemic regions were in adults only). However, the reverse tended to occur in those who were seronegative at baseline, with higher post-dose 3 GMTs in younger children than adolescents and adults. Nonetheless, GMTs against each of the 4 dengue serotypes increased from baseline to 28 d post-dose 3 in all studies, regardless of region and age. There was a trend toward lower GMTs against serotype 1 in most studies compared with serotypes 2, 3 and 4: the GMT ratios (GMTRs) post-dose 3 to baseline ranged from 1.42 (CYD51) to 9.99 (CYD08) for serotype 1; from 2.03 (CYD22) to 18.9 (CYD33) for serotype 2; from 2.46 (CYD22) to 19.8 (CYD33) for serotype 3; and 3.35 (CYD14) to 22.9 (CYD30) for serotype 4. No specific gender effect was observed on neutralizing antibody GMTs (data not shown).
Table 2. Dengue neutralizing antibody geometric mean titers at baseline (pre-dose 1) and post-dose 3 for each serotype, by region and age group (full analysis set).
In adults, both baseline and post-dose 3 GMTs were consistently higher in endemic regions than non-endemic regions (Fig. S1). No data are available for adolescents and children in non-endemic region with CYD-TDV. There was a trend toward higher GMTs post-dose 3 for all serotypes in endemic Latin American countries compared with Asia Pacific endemic countries in adolescents and children aged 6–11 y (Figs. S2–S3), despite limited differences in baseline titers between the 2 regions. No differences were observed between endemic regions in infants and toddlers.
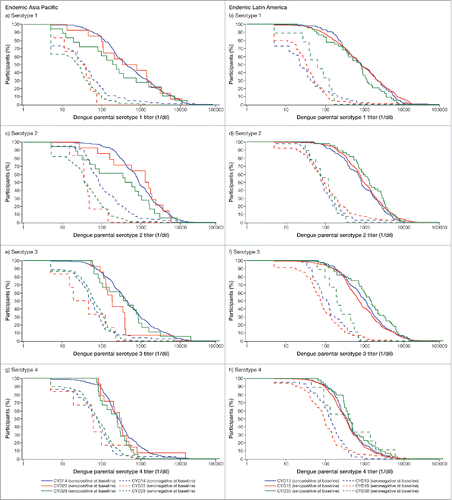
Participants who were seropositive to at least one dengue serotype at baseline in general achieved higher post-dose 3 GMTs for all serotypes than those who were seronegative regardless of the region. The impact of the dengue immune status at baseline on the immune response against each serotype is illustrated by the reverse cumulative distribution curves by region for adolescents (). In children, the same trend was observed for all 4 serotypes but to a lesser extent than in adolescents (Fig. S4). In infants and toddlers, there were insufficient data to draw any conclusions.
Figure 1. Serotype-specific reverse cumulative distribution curves of post-dose 3 titers by baseline dengue status in adolescents (12 to 17 y): Data are summarized by region (Full Analysis Set).
Although dengue seropositive participants achieved higher post-dose 3 GMTs than those who were seronegative, the magnitude of the increase in immune response was higher in the latter group of participants. The post-dose 3/baseline GMTRs for each serotype ranged from 3.53 to 13.7 for baseline dengue seronegative participants compared with 2.37 to 3.75 for baseline dengue seropositive participants in the 2 pivotal efficacy studies. These observations were consistent across all studies and age groups, except in infants and toddlers where insufficient data are available to draw any conclusions. In addition, GMTRs generally decreased with age; higher GMTRs were observed in infants and toddlers, than in older children, adolescents and adults. In adults, GMTRs tended to be higher in studies undertaken in non-endemic areas than in endemic areas for serotypes 2, 3 and 4 (range across the 3 serotypes 4.38–13.0 vs 2.03–7.83, respectively), but were similar for serotype 1 (1.42–2.44 vs 2.10–2.33).
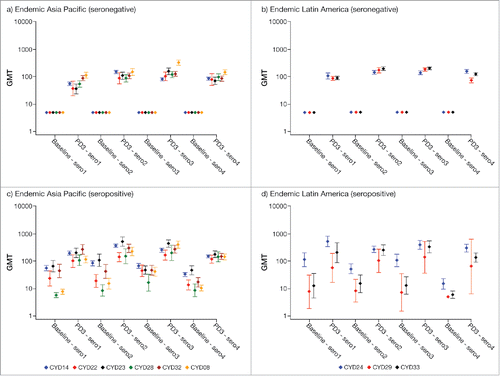
Dengue neutralizing antibody GMTs in participants aged <9 y in endemic countries at baseline and post-dose 3 are summarized by study and region in . Although the increase in GMTs post-dose 3 in dengue seronegative participants aged <9 y varied by study and serotype, the increase observed was similar across the 2 endemic regions. In dengue seropositive participants, both baseline and the observed increase in post-dose 3 GMTs also varied by study and serotype but were also similar across the 2 endemic regions. There was a trend toward higher post-dose 3 GMTs for each serotype in seropositive participants compared with those who were dengue seronegative.
Figure 2. Dengue neutralizing antibody GMTs (95% CI) in participants aged <9 y by baseline dengue status, region and study. PD3, post-dose 3; sero1–4, serotype 1–4.
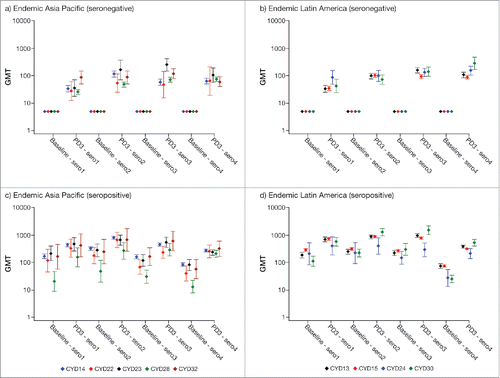
Dengue neutralizing antibody GMTs in participants aged 9–17 y in endemic countries at baseline and post-dose 3 are summarized by study and region in ; corresponding data for participants aged 18–45 y in endemic Asia-Pacific countries are presented in Figure S5 (similar data for adults in endemic Latin American countries are lacking). In dengue seronegative participants, the increase in GMTs post-dose 3 varied by study and serotype. There was a trend toward higher GMTs against serotype 4 in Latin America compared with Asia Pacific. In dengue seropositive participants, both baseline and the observed increase in post-dose 3 GMTs also varied by study and serotype but were generally similar across the 2 endemic regions. Of note, baseline GMTs for all serotypes except for serotype 4 in seropositive participants aged 9–17 y in Latin American were similar to those achieved post-dose 3 in seropositive participants aged <9 y ( and ).
Figure 3. Dengue neutralizing antibody GMTs (95% CI) in participants aged 9–17 y by baseline dengue status, region and study. PD3, post-dose 3; sero1–4, serotype 1–4.
The effect of seropositivity to other flaviviruses at baseline was investigated despite the low number of participants who were dengue seronegative but seropositive for either JE or YF. No effect on dengue immune response could be clearly established or ruled out in those with prior exposure to these other flaviviruses.
Antibody persistence
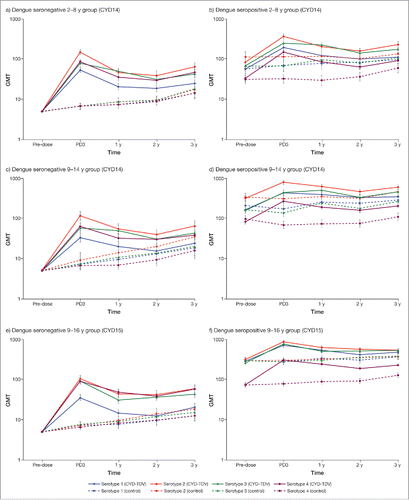
Dengue neutralizing antibody persistence data post-dose 3 were available from the following studies: CYD14, CYD15, CYD22, CYD23 and CYD28. In this section, we focus on data from CYD14 and CYD15 as these were pivotal studies and included the most number of participants accessed for immunogenicity and have long-term follow-up up to 3 y post-dose 3 (follow-up is still ongoing through to 5 y post-dose 3). During the first 2 y of follow-up, GMTs for all serotypes decreased relative to post-dose 3 in both studies, regardless of participant's age or dengue immune status at baseline (). The decrease in GMTs against all 4 serotypes from Year 1 to Year 2 of follow-up tended to be lower than between post-dose 3 and Year 1 of follow-up in all age groups. However, GMTs at Year 3 of follow-up remain stable or slightly increased relative to those at Year 2 for all serotypes in both studies. Of note, GMTs for placebo recipients seronegative at baseline gradually increased throughout the duration of follow-up. For placebo recipients seropositive at baseline GMTs tended to decrease between pre-dose and post-dose 3 in those aged ≥ 9 y before gradually increasing throughout the duration of follow-up; in those aged <9 years, GMTs tended to gradually increase from pre-dose levels. In general, GMTs for each serotype in the CYD-TDV group remained at higher levels than those observed at baseline or in the control group for all age groups across both studies. In addition, the GMTs remained higher in seropositive participants aged ≥ 9 y than those aged <9 y throughout follow-up. Dengue neutralizing antibody persistence data in 2 studies (CYD22 and CYD28) with longer follow-up to 4 y post-dose 3 (Year 4 of follow-up) also show that GMTs remain 1.2–3.2-fold higher than baseline (Fig. S6).
Discussion
Three doses of CYD-TDV 6 months apart consistently induces an increase in dengue neutralizing antibody GMTs, as measured by PRNT50, in all studied populations encompassing a wide age range and different regions with various dengue endemicity. In addition, several factors including age, previous exposure to dengue and region are known to impact on the level of CYD-TDV induced immune response. Therefore, to capture the fullness of the available immunogenicity profiles we did not pool the immunogenicity data across studies; all GMTs are reported by age, study, and region in this article. By presenting all the available data in this way, the reader should be able to identify general trends and any substantial outliers within each grouping.
One of the strengths of our analysis is that we present laboratory data that were determined at centralized laboratories using the same validated PRNT50 across all studies rather than compare results obtained from multiple different neutralization assays. Although it is recognized to be a valuable assay in dengue research, intra- and inter-laboratory variation may impact comparability and interpretation of results between laboratories or studies.Citation22,23 As such, while CYD-TDV was consistently shown to induce increased GMTs, there are examples of minimal increases (e.g. against serotype 1 in adults living in non-endemic areas) which considering the variability of the assay, are arguably negligible increases. In addition, its relevance is uncertain with regard to thresholds of protection as it lacks qualitative aspects — for example, it does not differentiate between homotypic (expected to provide life-long protection) and heterotypic antibodies (cross-protective during a certain window of time).Citation24,25
A multifaceted approach may be required where the PRNT50 assay is supplemented with more physiologically relevant neutralization tests capable of identification of homotypic and heterotypic antibodies, and their relative contributions to the immune response and protection.Citation25,26 Moreover, protective PRNT50 titers have not been established for any dengue serotype and it is possible that these may vary by serotype and/or be vaccine-specific, which has to be taken into account when interpreting immunogenicity results.Citation25 Analyses have been undertaken to better define correlates of CYD-TDV protection using efficacy and PRNT50 immunogenicity obtained in the 2 large-scale pivotal studies (CYD14 and CYD15) (submitted for publication). Although the data suggest that high post-dose 3 titers are predictive of high vaccine efficacy across all serotypes and age groups, other factors may play a role in protection particularly among those with low titers.
The current analysis confirms that there is a link between the level of endemicity (and therefore, between baseline dengue immune status) and the immune response, with higher GMTs after 3 vaccinations observed in endemic regions than in non-endemic regions. In addition, GMTs also increased with age in endemic countries: the older the participant, the higher the baseline and post-dose 3 GMTs. This observation is consistent with the increased likelihood of prior dengue exposure with age in endemic countries boosting memory responses.
A modeling study that included PRNT50 data from 5 phase II trials (also included in the current analysis) in South East Asia and Latin America reported that CYD-TDV immunogenicity in endemic countries was principally influenced by the baseline dengue immunological status.Citation27 However, we did not examine the effect of previous homotypic vs. heterotypic dengue infection on CYD-TDV induced GMTs in our analysis. Additional studies are required to better define the immune response as it evolves sequentially with exposure or repeat exposure to the varying dengue serotypes.
JE or YF seropositivity was expected to increase the immune response to dengue vaccination based on previous studies.Citation27-29 Participants who were dengue seropositive at baseline, as well as JE seropositive, were shown to have higher GMTs following CYD-TDV administration for all 4 serotypes than dengue seropositive but JE seronegative participants.Citation27 Similarly, prior exposure to the YF vaccine increases antibody responses to CYD-TDV,Citation28 and vaccination of YF seropositive participants with a monovalent live-attenuated dengue candidate vaccine was shown to boost antibody responses to both flaviviruses compared with those who were YF seronegative.Citation29 However, JE and YF seropositivity in our studies was determined using the PRNT, and as such cross-reactivity with dengue (due to antigenic epitopes common to flaviviruses) in regions where the viruses co-circulate would result in false positives.Citation30,31 Consequently, after ruling out all dengue seropositive participants, the number who were dengue seronegative but seropositive for either JE or YF at baseline was limited in our analysis and therefore, a potential increase in dengue immune response because of prior exposure to these flaviviruses could not be clearly established nor ruled out. Further research would need to be undertaken to address the question of cross-reactivity before a more definitive effect of baseline JE or YF seropositivity on CYD-TDV immune response can be established.
We showed that GMTs post-dose 3 decline annually across all 4 serotypes but this decline appears to stabilize from Y2 of follow-up and remains above baseline against all 4 serotypes up to 4 y post-dose 3 regardless of age and baseline dengue immunological status. However, it is unclear to what extent exposure to wild-type dengue contributed to antibody persistence in these analyses in the absence of an appropriate comparator (e.g., long-term follow-up from vaccinated participants living in a non-endemic area). There was a rise in GMTs observed in the placebo groups of the 2 efficacy studies. Indeed, the annual incidence of symptomatic and asymptomatic dengue infections in the immunogenicity subsets of the 2 pivotal phase III CYD-TDV studies was 8.1–14.8% and 0.9–3.5%, respectively, depending on age or allocated group.Citation32 Whether symptomatic and asymptomatic infections contribute differentially to the boosting/persistence of the GMTs remains to be established. In addition, it is not known whether the decay in vaccine-induced antibody titers is quantitatively similar to the decay of naturally-acquired dengue antibodies. Additional studies would be required to identify factors that modulate antibody persistence.
In conclusion, a 3-dose schedule of CYD-TDV 6 months apart elicits neutralizing antibody responses against all 4 dengue serotypes, with raised neutralizing antibody GMTs persisting above baseline levels over the longer term in dengue endemic countries. Our conclusions are based on an unprecedented integrated summary of the immunogenicity of CYD-TDV attained across studies using the same validated PRNT50 undertaken at centralized laboratories.
Methods
This summary includes immunogenicity data from 16 pivotal and supportive clinical studies, conducted by Sanofi Pasteur from 2008 to the present, in which participants aged 9 months to 60 y received CYD-TDV (∼5 log10 cell-culture infectious dose 50% [CCID50]/serotype) as a 3-dose schedule 6 months apart. The 16 studies included in the current report consisted of 10 phase II and 6 phase III studies (); these were conducted in non-endemic (USA and Australia) and in endemic regions (Asia Pacific and Latin America countries) to assess the safety and immunogenicity of CYD-TDV. Few studies investigated long-term safety and immunogenicity. Three studies also assessed the safety and immunogenicity of CYD-TDV co-administration with other childhood vaccines in infants and toddlers (< 2 y of age). Although there were differences in vaccine lots used across the studies, a formal analysis of lot-to-lot consistency was performed in the CYD17 study, which established biological and clinical equivalence between phase II and phase III vaccine lots (based on post-dose 3 GMTs).Citation10
The study designs, enrollment details, and inclusion/exclusion criteria are described in detail in the original publications. Key characteristics of the trials are summarized in . All studies were performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization-Good Clinical Practice. Study protocols and amendments were approved by the individual review board/independent ethics committee for each participating site. All participants or their parents/guardians provided informed written consent. A pooled analysis of safety data from most of the studies included in the current analysis and others in which the dengue vaccine was administered has recently been published.Citation33
Immunogenicity
In the current analysis, we focus on dengue neutralizing antibody titers obtained before the first CYD-TDV dose and 28 d after the third dose (in all participants except in CYD14,Citation3 CYD15,Citation2 CYD2316 and CYD2813 where subsets were assessed) as these were evaluated in all studies. Studies where CYD-TDV was co-administered with other childhood vaccines were also included in the current analysis as the co-administered products did not impact the humoral response to CYD-TDV; in these studies the immunogenicity data were combined across the groups that received 3 CYD-TDV doses.Citation11,19,21 In addition, we present persistence data where available. Pre-vaccination baseline blood samples were also assessed for antibodies against Japanese encephalitis (JE) for studies undertaken in Asia Pacific (except for CYD1710 and CYD2813) and for antibodies against Yellow Fever (YF) in those undertaken in the Americas (except for CYD128 and CYD3319). Baseline JE and YF immune status were assessed using the PRNT50 for JE (CYD08,Citation11 CYD143 and CYD22,Citation12 CYD23,Citation16 CYD32,Citation14 CYD4715), and PRNT50 (CYD 13,Citation17 CYD15,Citation2 CYD29,Citation21 CYD30,Citation18 CYD51 (however, a derived PRNT80 value was reported in the publicationCitation9)) or PRNT80 (CYD2420) for YF. The lower limit of quantitation (LLOQ) of the JE and YF PRNT50 was 10 (1/dil) and of the YF PRNT80 was 5; seropositivity for JE and YF was defined as antibody titers ≥ 10 1/dil.
Neutralizing antibodies serum levels against each of the 4 CYD-TDV's dengue parental strains (dengue 1 strain PUO-359, dengue 2 strain PUO-218, dengue 3 strain PaH881/88, and dengue 4 strain 1228) were determined using a validated PRNT50 at a centralized Sanofi Pasteur laboratory (GCI, Swiftwater, USA) or outsourced to another laboratory after demonstration of successful method transfer and concordance across a panel of samples (CYD29, CYD33 and CYD47).Citation22 The validated PRNT50 was continuously monitored at both laboratories using a panel of samples with known values and compared with historical results to ensure consistency of results over time. This harmonized approach in dengue PRNT50 undertaken during the CYD-TVD clinical development was intended to minimize inter-laboratory variation, which can be considerable if different methods are used to perform and analyze the PRNT50 data,Citation23 and would ultimately impact comparability and interpretation of results between laboratories. Nonetheless, variations in precision exist even within the same laboratory, and assay variation may be as much as 3-fold.Citation22 The LLOQ for the PRNT50 was 10 (1/dil), and dengue seropositivity was defined by dengue neutralization antibodies titer ≥ 10 for at least one serotype. Titers below the LLOQ were assigned a value of 5; for the calculation of GMT ratios (GMTRs), baseline titers < LLOQ (i.e. for the denominator) were assigned a value of 10. Dengue neutralizing antibody persistence in samples collected annually for at least 2 y after the last CYD-TDV dose were also summarized.
Statistical analyses
There was no hypothesis testing undertaken in our current analysis. Statistical analyses were performed with SAS® software. GMTs and 95% CIs were calculated for antibodies against each of the 4 dengue serotypes for the full analysis set assuming normal distribution of the log10 transformed titers. The definitions of the study populations used in this integrated analysis were the same as those used in the individual studies; the full analysis set in most studies consisted of all participants who received at least one dose of CYD-TDV and who had at least one blood sample drawn and one valid post-injection serology result.
Disclosure of potential conflicts of interest
All authors are employees or former employees of Sanofi Pasteur.
Supplemental_Material.pdf
Download PDF (930.4 KB)Acknowledgments
This integrated analysis was performed by Sanofi Pasteur.
Editorial assistance with the preparation of the manuscript was provided by Richard Glover, inScience Communications, Springer Healthcare, Chester, UK. Funding for this assistance was also provided by Sanofi Pasteur. The authors also thank Jean-Sébastien Persico for editorial assistance and manuscript coordination on behalf of Sanofi Pasteur.
We thank all the parents and participants who agreed to participate in these trials; the trial investigators and staff in the countries; and to the clinical research organization staff who contributed to the successful completion of the trials. The authors also wish to thank Stephen W. Hildreth (Sanofi Pasteur) for his contribution to the dossier writing, and the Common Technical Document task force.
References
- World Health Organization. Dengue vaccine: WHO position paper – July 2016. Wkly Epidemiol Rec 2016; 91:349-64; PMID: 27476189
- Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372:113-23; PMID: 25365753; https://doi.org/10.1056/NEJMoa1411037
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384:1358-65; PMID: 25018116; https://doi.org/10.1016/S0140-6736(14)61060-6
- Flasche S, Jit M, Rodriguez-Barraquer I, Coudeville L, Recker M, Koelle K, Milne G, Hladish TJ, Perkins TA, Cummings DA, et al. The Long-Term safety, public health impact, and Cost-Effectiveness of routine vaccination with a recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A model comparison study. PLoS Med 2016; 13:e1002181; PMID: 27898668; https://doi.org/10.1371/journal.pmed.1002181
- Ferguson NM, Rodriguez-Barraquer I, Dorigatti I, Mier YT-RL, Laydon DJ, Cummings DA. Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science 2016; 353:1033-6; PMID: 27701113; https://doi.org/10.1126/science.aaf9590
- Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. Efficacy and Long-Term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195-206; PMID: 26214039; https://doi.org/10.1056/NEJMoa1506223
- Guy B, Jackson N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat Rev Microbiol 2016; 14:45-54; PMID: 26639777; https://doi.org/10.1038/nrmicro.2015.2
- Dayan GH, Thakur M, Boaz M, Johnson C. Safety and immunogenicity of three tetravalent dengue vaccine formulations in healthy adults in the USA. Vaccine 2013; 31:5047-54; PMID: 24021313; https://doi.org/10.1016/j.vaccine.2013.08.088
- Kirstein J, Douglas W, Thakur M, Boaz M, Skipatrova A, Plennevaux E. Immunogenicity of a compressed schedule of a tetravalent dengue vaccine in adults: A phase II randomized study. Submitted.
- Torresi J, Heron LG, Qiao M, Marjason J, Chambonneau L, Bouckenooghe A, Boaz M, van der Vliet D, Wallace D, Hutagalung Y, et al. Lot-to-lot consistency of a tetravalent dengue vaccine in healthy adults in Australia: A randomised study. Vaccine 2015; 33:5127-34; PMID: 26279339; https://doi.org/10.1016/j.vaccine.2015.08.008
- Crevat D, Brion JD, Gailhardou S, Laot TM, Capeding MR. First experience of concomitant vaccination against dengue and MMR in toddlers. Pediatr Infect Dis J 2015; 34:884-92; PMID: 25966916; https://doi.org/10.1097/INF.0000000000000752
- Tran NH, Luong CQ, Vu TQH, Lang J, Vu QD, Bouckenooghe A, et al. Safety and immunogenicity of recombinant, live attenuated tetravalent dengue vaccine (CYD- TDV) in healthy Vietnamese adults and children. J Vaccin Vaccinol 2012; 3:1000162
- Leo YS, Wilder-Smith A, Archuleta S, Shek LP, Chong CY, Leong HN, Low CY, Oh ML, Bouckenooghe A, Wartel TA, et al. Immunogenicity and safety of recombinant tetravalent dengue vaccine (CYD-TDV) in individuals aged 2–45 y: Phase II randomized controlled trial in Singapore. Hum Vaccin Immunother 2012; 8:1259-71; PMID: 22894958; https://doi.org/10.4161/hv.21224
- Hss AS, Koh MT, Tan KK, Chan LG, Zhou L, Bouckenooghe A, Crevat D, Hutagalung Y. Safety and immunogenicity of a tetravalent dengue vaccine in healthy children aged 2–11 years in Malaysia: A randomized, placebo-controlled, Phase III study. Vaccine 2013; 31:5814-21; PMID: 24135573; https://doi.org/10.1016/j.vaccine.2013.10.013
- Dubey AP, Agarkhedkar S, Chhatwal J, Narayan A, Ganguly S, Wartel TA, Bouckenooghe A, Menezes J. Immunogenicity and safety of a tetravalent dengue vaccine in healthy adults in India: A randomized, observer-blind, placebo-controlled phase II trial. Hum Vaccin Immunother 2016; 12:512-8; PMID: 26291554; https://doi.org/10.1080/21645515.2015.1076598
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet 2012; 380:1559-67; PMID: 22975340; https://doi.org/10.1016/S0140-6736(12)61428-7
- Villar LA, Rivera-Medina DM, Arredondo-Garcia JL, Boaz M, Starr-Spires L, Thakur M, Zambrano B, Miranda MC, Rivas E, Dayan GH. Safety and immunogenicity of a recombinant tetravalent dengue vaccine in 9–16 year olds: A randomized, controlled, phase II trial in Latin America. Pediatr Infect Dis J 2013; 32:1102-9; PMID: 24067553; https://doi.org/10.1097/INF.0b013e31829b8022
- Dayan GH, Garbes P, Noriega F, Izoton de Sadovsky AD, Rodrigues PM, Giuberti C, Dietze R. Immunogenicity and safety of a recombinant tetravalent dengue vaccine in children and adolescents ages 9–16 years in Brazil. Am J Trop Med Hyg 2013; 89:1058-65; PMID: 24189367; https://doi.org/10.4269/ajtmh.13-0304
- Rodriguez FI, Morales JJR, De Los Santos AHM, Rivas E, Vigne C, Noriega F. Immunogenicity and safety of a booster injection of DTaP-IPV//Hib (Pentaxim®) administered concomitantly with tetravalent dengue vaccine in healthy toddlers aged 15 to 18 months in Mexico: A randomized trial. Pediatr Infect Dis J 2017; 36:602-608; PMID:28067718; https://doi.org/10.1097/INF.0000000000001542
- Lanata CF, Andrade T, Gil AI, Terrones C, Valladolid O, Zambrano B, Saville M, Crevat D. Immunogenicity and safety of tetravalent dengue vaccine in 2–11 year-olds previously vaccinated against yellow fever: Randomized, controlled, phase II study in Piura, Peru. Vaccine 2012; 30:5935-41; PMID: 22863660; https://doi.org/10.1016/j.vaccine.2012.07.043
- Lopez P, Lanata CF, Zambrano B, Cortes M, Andrade T, Amemiya I, Terrones C, Gil AI, Verastegui H, Marquez V, et al. Immunogenicity and safety of yellow fever vaccine (Stamaril®) when administered concomitantly with a tetravalent dengue vaccine candidate in healthy toddlers at 12–13 months of age in Colombia and Peru: A randomized trial. Pediatr Infect Dis J 2016; 35:1140-7; PMID: 27254034; https://doi.org/10.1097/INF.0000000000001250
- Timiryasova TM, Bonaparte MI, Luo P, Zedar R, Hu BT, Hildreth SW. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am J Trop Med Hyg 2013; 88:962-70; PMID: 23458954; https://doi.org/10.4269/ajtmh.12-0461
- World Health Organization. Guidelines for plaque reduction neutralization testing of human antibodies to dengue viruses. Available from: http://apps.who.int/iris/bitstream/10665/69687/1/who_ivb_0707_eng.pdf (Accessed 19 July 2016) 2007.
- The SAGE working group on dengue vaccines and WHO Secretariat. Background paper on dengue vaccines. Available from: http://www.who.int/immunization/sage/meetings/2016/april/1_Background_Paper_Dengue_Vaccines_2016_03_17.pdf (Accessed 19 July 2016) 2016.
- Guy B, Briand O, Lang J, Saville M, Jackson N. Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine 2015; 33:7100-11; PMID: 26475445; https://doi.org/10.1016/j.vaccine.2015.09.108
- Henein S, Swanstrom J, Byers AM, Moser J, Shaik F, Bonaparte M, et al. Dissecting antibody response induced by a chimeric yellow fever-dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in naïve and dengue exposed individuals. J Infect Dis (in press) 2017; 215:351-8
- Dorigatti I, Aguas R, Donnelly CA, Guy B, Coudeville L, Jackson N, Saville M, Ferguson NM. Modelling the immunological response to a tetravalent dengue vaccine from multiple phase-2 trials in Latin America and South East Asia. Vaccine 2015; 33:3746-51; PMID: 26051515; https://doi.org/10.1016/j.vaccine.2015.05.059
- Qiao M, Shaw D, Forrat R, Wartel-Tram A, Lang J. Priming effect of dengue and yellow fever vaccination on the immunogenicity, infectivity, and safety of a tetravalent dengue vaccine in humans. Am J Trop Med Hyg 2011; 85:724-31; PMID: 21976579; https://doi.org/10.4269/ajtmh.2011.10-0436
- Kanesa-Thasan N, Sun W, Ludwig GV, Rossi C, Putnak JR, Mangiafico JA, Innis BL, Edelman R. Atypical antibody responses in dengue vaccine recipients. Am J Trop Med Hyg 2003; 69:32-8; PMID: 14740953; https://doi.org/10.4269/ajtmh.2003.69.32
- Johnson BW, Kosoy O, Hunsperger E, Beltran M, Delorey M, Guirakhoo F, Monath T. Evaluation of chimeric Japanese encephalitis and dengue viruses for use in diagnostic plaque reduction neutralization tests. Clin Vaccine Immunol 2009; 16:1052-9; PMID: 19458204; https://doi.org/10.1128/CVI.00095-09
- Houghton-Trivino N, Montana D, Castellanos J. Dengue-yellow fever sera cross-reactivity; challenges for diagnosis. Rev Salud Publica (Bogota) 2008; 10:299-307; PMID: 19039426; https://doi.org/10.1590/S0124-00642008000200010
- Olivera-Botello G, Coudeville L, Fanouillere K, Guy B, Chambonneau L, Noriega F, Jackson N, CYD-TDV Vaccine Trial Group. Tetravalent dengue vaccine reduces symptomatic and asymptomatic dengue infections in healthy children and adolescents aged 2–16 years in Asia and Latin America. J Infect Dis 2016; 214:994-1000; https://doi.org/10.1093/infdis/jiw297
- Gailhardou S, Skipetrova A, Dayan GH, Jezorwski J, Saville M, Van der Vliet D, Wartel TA. Safety overview of a recombinant Live-Attenuated tetravalent dengue vaccine: Pooled analysis of data from 18 clinical trials. PLoS Negl Trop Dis 2016; 10:e0004821; PMID: 27414655; https://doi.org/10.1371/journal.pntd.0004821