ABSTRACT
A Typhoid Conjugate Vaccine (TCV) is expected to acquire WHO prequalification soon, which will pave the way for its use in many low- and middle-income countries where typhoid fever is endemic. Thus it is critical to forecast future vaccine demand to ensure supply meets demand, and to facilitate vaccine policy and introduction planning. We forecasted introduction dates for countries based on specific criteria and estimated vaccine demand by year for defined vaccination strategies in 2 scenarios: rapid vaccine introduction and slow vaccine introduction. In the rapid introduction scenario, we forecasted 17 countries and India introducing TCV in the first 5 y of the vaccine's availability while in the slow introduction scenario we forecasted 4 countries and India introducing TCV in the same time period. If the vaccine is targeting infants in high-risk populations as a routine single dose, the vaccine demand peaks around 40 million doses per year under the rapid introduction scenario. Similarly, if the vaccine is targeting infants in the general population as a routine single dose, the vaccine demand increases to 160 million doses per year under the rapid introduction scenario. The demand forecast projected here is an upper bound estimate of vaccine demand, where actual demand depends on various factors such as country priorities, actual vaccine introduction, vaccination strategies, Gavi financing, costs, and overall product profile. Considering the potential role of TCV in typhoid control globally; manufacturers, policymakers, donors and financing bodies should work together to ensure vaccine access through sufficient production capacity, early WHO prequalification of the vaccine, continued Gavi financing and supportive policy.
Introduction
Typhoid fever is a major public health concern in many low- and middle-income countries (LMICs).Citation1 The disease is transmitted fecal-orally and is common in settings with limited sanitation, hygiene and unclean water. Until the infrastructure for provision of clean water and sanitation is improved, vaccination against typhoid fever is considered a short-term to medium-term control measure.Citation2 Currently, there are 3 types of vaccines available: oral Ty21a, injectable Vi polysaccharide vaccine, and new-generation injectable typhoid conjugate vaccines (TCV). Only the first 2 vaccine types are commercially available; they have been prequalified by the World Health Organization (WHO) and are recommended by WHO for programmatic use in typhoid-endemic countries.Citation2 However, these vaccines have limitations, including short duration of protection and inability to confer protection in children under the age of 2,Citation3 an age group that has a considerably higher disease burden in typhoid-endemic countries.Citation1
Many of the limitations of the 2 vaccines mentioned above can be resolved with TCV. The vaccine is considered to be safe in infants and generates a good immune response. TCV is also expected to provide a longer duration of protection and is co-administrable with other vaccines used under the routine childhood Expanded Programme on Immunization (EPI). Because of these advantages, TCV is likely to play a major role in typhoid prevention and control in the near future.Citation4 Although there are high expectations of the vaccine and more than 10 TCV candidates are under development, only one vaccine is currently licensed in India and it is not available for wider use in LMICs.Citation3 However, the manufacturer of the Indian-licensed vaccine submitted a dossier to the WHO Prequalification Program for approval of the vaccine; if successful, the prequalified vaccine may be available to LMICs as early as 2017–2018.
Considering the future availability of TCVs, several global activities are ongoing to support vaccine introduction in LMICs. Gavi, the Vaccine Alliance committed to financing TCV for routine use by including it in their investment portfolio in 2008, which was reiterated in 2013.Citation5 The “Expert Consultation to Review Evidence in Support of the Use of TCV” held by WHO in Geneva in July, 2014 stated that while the clinical data are promising, overall evidence is inadequate.Citation6 The Immunization and Vaccine Related Implementation Research (IVIR) Advisory Committee of WHO reviewed available evidence on typhoid fever and TCV in 2014 to explore the possibility of developing new recommendations through its Strategic Advisory Group of Experts (SAGE) on Immunization.Citation7 More recently, WHO has set-up a SAGE working group to review scientific evidence and programmatic considerations to formulate updated recommendations on the use of typhoid vaccines, with a particular focus on TCVs.Citation8
Understanding and anticipating vaccine demand can help in planning activities that shape the vaccine market. For example, in the case of the oral cholera vaccine, which was stockpiled by WHO and financed by Gavi, deployment of the vaccine was limited due to supply constraints despite multiple requests to WHO for the vaccine from countries.Citation9 The supply constraint was due to the fact there was only one manufacturer of the vaccine. The issue was alleviated when a second manufacturer came on to the market with its oral cholera vaccine whose development and WHO prequalification was supported through a public-private product development partnership.Citation9
Although vaccine deployment depends on several factors, it is imperative to compare vaccination strategies, a major demand driver; and to select the most suitable options for typhoid-endemic countries to control the disease effectively.Citation10 To understand vaccine demand and to make policy decisions, one needs to know which country will introduce the TCV, when, and what the vaccine demand will be based on proposed vaccination strategies.Citation11-13
There is no perfect method to predict a new vaccine introduction and to determine vaccine demand. In such situations, the vaccine demand forecast approach is widely used to represent potential future demand as close to reality as possible.Citation11-13 In this paper, we use forecasting techniques for vaccine introduction among countries included in the recent global disease burden study.Citation1 Here, we present the underlying evidence and assumptions used in developing a vaccine demand forecast and results from our analyses.
Results
Rapid introduction scenario
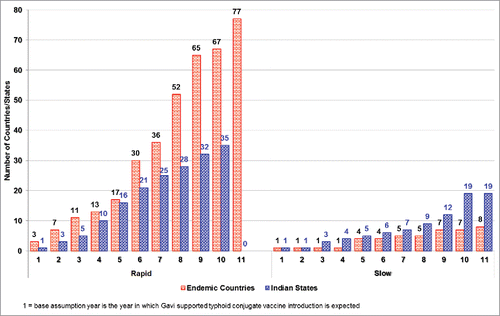
The 4 indicator-based score was applied to 92 typhoid-endemic countries to forecast vaccine adoption starting from the base assumption year and continuing thereafter. In the qualitative adjustment, the typhoid experts' responses suggested that Bangladesh, Cuba and Indonesia would introduce the vaccine earlier than quantitatively forecasted. It is believed that Bangladesh would introduce the vaccine as early as 2020 since the vaccine is being developed by a domestic manufacturer, and may attain in-country licensure in 2018. Though its TCV is currently in the preclinical research phase, Cuba may introduce the vaccine by 2020 based on its past experience with the typhoid vaccine, which may allow it to attain national licensure earlier. Indonesia may introduce the vaccine in 2021; the vaccine is in early-stage development and may be licensed by 2020. These changes were incorporated into the introduction forecast. With the availability of an already-licensed vaccine in India, the states' forecasts showed early introductions in selected states ahead of many other countries.
After the quantitative forecast and qualitative adjustment, 17 countries were forecasted to introduce TCV in the first 5 y (). This included 10 Gavi-eligible countries, 4 Gavi-graduating countries, and 3 Gavi non-eligible countries. More details on vaccine adoption classified by the countries' Gavi eligibility status (for year 2015) is available in Annex 1. For the Indian states, we found the earliest adoption will be in Delhi, India by the base assumption year, followed by 15 more states introducing the vaccine in the next 5 y.
High-risk population versus general population
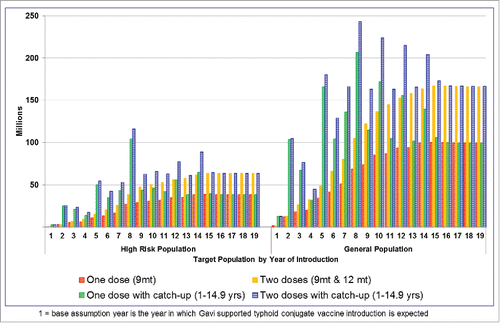
In the rapid introduction scenario, if the vaccine is targeted to high-risk populations only, the routine vaccine demand peaks around 40 million doses per year to 60 million doses per year for single-dose and 2-dose strategies respectively (). In addition, there would be a need for a large number of doses in intermittent years for catch-up vaccinations, if added. If the general population is targeted, the vaccine demand peaks at around 100 million doses per year to 160 million per year for single-dose and 2-dose strategies respectively. The demand in certain years will sharply increase as the catch-up campaigns are added in certain countries.
Slow introduction scenario
The slow introduction scenario forecasted 4 typhoid-endemic countries introducing the vaccine in the first 5 y and 7 countries by the end of 10 y (). For the Indian States, this method forecasted 5 states introducing the vaccine in the first 5 y and 19 by the end of 10 y.
Discussion
This paper describes a method of forecasting vaccine introduction and estimating vaccine demand in the future. Such a forecast is often used in vaccine investment cases and is useful in estimating vaccine quantity to meet demand per year once a vaccine is WHO-prequalified. We presented rapid and slow TCV introduction scenarios, which show stark differences in the number of countries introducing the vaccine, signifying high uncertainty in the forecast. Based on the rapid introduction scenario, we showed that annual demand ranges from 40 million doses to 160 million doses depending on the vaccination strategy proposed. Vaccine manufacturers and product development partners should consider vaccine demand when deciding their production capacities. Besides being used for market shaping activities by Gavi and other global agencies, this forecast would be useful for budgeting and economic analyses that advise policy decisions at global and country levels.
This demand forecast is likely to be affected by many factors. It is not certain whether TCV will have strong advocacy groups to empower and support countries to introduce the vaccine at a faster pace such as those for the Hib Initiative, the Pneumococcal vaccines Accelerated Development and Introduction Plan (Pneumo ADIP), and the Rotavirus Vaccine Program (RVP).Citation14 If a similar initiative is started and is successful for TCV, introduction may happen at a faster pace. An explicit recommendation from WHO for the global use of TCV may further influence vaccine introduction decisions. Many countries will only start implementation of a TCV program after WHO recommendations. A delay in the WHO recommendations may delay introduction.
There are other potential interventions that may affect the TCV introduction forecast, which are not accounted here. Rapid economic growth in typhoid-endemic countries may improve the water and sanitation infrastructure, changing country priorities and strategies for typhoid control. Similarly, other health interventions including new vaccines for other diseases may compete with the TCV, which may affect introduction decisions. Moreover, decision makers need to consider the impact of TCV on their country's cold chain and logistics system, programmatic feasibility, and so on, which can influence introduction decisions.
Besides program-specific aspects, ongoing changes such as political instability and other macroeconomic events in any of the forecasted countries may jeopardize the results of our forecast. These uncertainties cannot be foreseen and integrated into our analysis. Particularly, long-term predictions for the future are affected by a lot of uncertainty, which cannot be accounted for now. Finally, this forecast is based on a modeling exercise, which may not represent the true interests of individual countries in vaccine introduction. Updating this forecast based on results from country-specific interviews with experts, policymakers and decision makers will be a valuable exercise in the future.
It is important to note that forecasts are only as good as their underlying assumptions. Some of the key underlying assumptions used at the time of analysis need not be true for long. The optimal number of doses, requirement of booster, targeting strategies, WHO prequalification timeline and status of continued Gavi financing for TCV in Gavi-eligible countries may change in the future. Gavi will be revisiting its financing strategy for TCV in 2018, which is applicable for the next 5 y. Changes in Gavi's financing position may adversely influence vaccine introduction decisions. Similarly, a country's Gavi-eligibility status may have a large influence on a country's vaccine introduction decision. Some high-burden countries such as India will be graduating from Gavi soon, and financing TCV introduction is more difficult in Gavi-ineligible countries. This may also influence the TCV introduction timeline by possibly adding a delaying effect, which remains unaccounted for in the forecast. We have not accounted for what would happen when countries become 100% self-financing in our analysis. Thus, any change that may affect the vaccine demand influences the forecast. Also, longer forecasts are more difficult and uncertain as there will be many influencing factors accumulating over time. These limitations need to be accounted for before interpreting the results.
Conclusion
In conclusion, our demand forecast provides an estimate of vaccine demand with a clearly defined rationale, although the actual demand depends on country priorities, vaccine introduction, vaccination strategies, Gavi financing, vaccine price, cost of vaccination, operational feasibility, and overall product profile. Considering the importance of TCV in disease control, policymakers, donors, manufacturers and financing bodies at the global level should consider working toward strengthening capacity to ensure sufficient quantities of vaccine are produced, accelerate early WHO prequalification, continue Gavi financing support, define vaccination strategies, and facilitate support for policy and access.
Methods
We developed the TCV demand forecast in 3 logical steps. The first step was to estimate which country would introduce the vaccine and when, based on systematically organized evidence and rational assumptions as described below. The next step was to define what would be the potential vaccination strategies for vaccine deployment in LMICs. The third and the last step was to calculate the number of vaccine doses required for deployment estimated by vaccine introduction year. This forecast covered 92 LMICs for which typhoid disease burden estimations were available and were adjusted for water-related risk-factors and blood culture sensitivity.Citation1,15,16 Indian states and union territories, totaling 35 in number, were considered as independent country-like entities because of their large size and history of state vaccine introductions. Each of these steps are described in detail below.
Vaccine introduction forecast
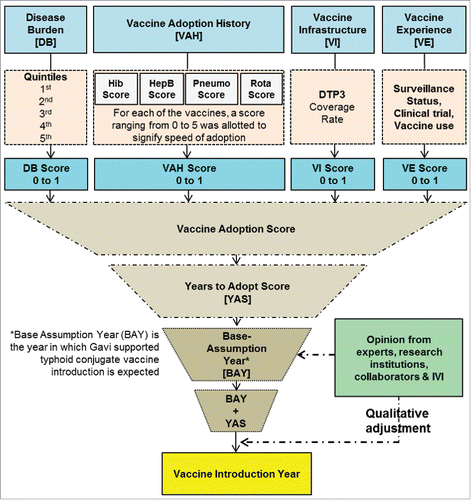
We deployed both quantitative and qualitative methods for the vaccine introduction forecast. Four quantitative indicators were used to formulate a composite measure score for each typhoid- endemic country to forecast TCV introduction. The indicators included were: (1) typhoid disease burden based on recent publicationsCitation1; (2) previous vaccine adoption history based on the Vaccine Information Management System (VIMS) databank hosted by the Johns Hopkins Bloomberg School of Public Health and on their updated March, 2014 reportCitation17 that presents introduction information for Haemophilus influenzae type b (Hib), Hepatitis B (HepB), Pneumococcal (Pneumo), and Rotavirus (Rota) vaccines; (3) immunization system capacity as represented by 3 doses of diphtheria-tetanus-pertussis (DTP3) coverage rates reported by WHO's immunization surveillance, assessment and monitoring siteCitation18; and (4) typhoid fever surveillance, typhoid vaccine clinical trial experience and Vi-polysaccharide vaccination experience at the country level from published literature.Citation19-41 The DTP3 coverage for various Indian States was retrieved from the 2009 Coverage Evaluation Survey Report.Citation42 Data on Indian States' past vaccine introduction experience was represented by the pentavalent vaccine introduction as accrued from Gavi, (personal communication: Melissa Ko). Data for typhoid research experience in India were retrieved from 3 publications.Citation27,28,41
We assumed that a country would introduce a vaccine early if its disease burden is high, has earlier vaccine adoption history, has high DTP3 coverage, and has experience in typhoid surveillance or typhoid vaccine use. For each of the above 4 factors, a score from 0 to 1 was allocated where the lower the score, the earlier would be vaccine introduction (). The sum of the scores for each of the 4 variables constituted a TCV introduction forecast score, which predicted the number of years required for vaccine introduction from the base assumption year, 2020. The notion of base assumption year was developed based on consensus between research teams at the International Vaccine Institute and Gavi in August, 2014 with the assumptions that the first TCV will be WHO-prequalified in 2018 and that Gavi-supported introduction will take 2 y of preparation thereafter.
Figure 3. Vaccine introduction forecast methodology (DTP3 = 3 doses of Diphtheria-tetanus-pertussis vaccine) showing how 4 quantitative indicators were used to formulate a composite measure score. In the last step a qualitative adjustment was done.
After the quantitative forecast, a qualitative adjustment was done through a consultation with typhoid fever experts. The experts consulted included program leaders, epidemiologists, clinicians, health economists, and representatives of global decision-makers. An inquiry email was sent to 30 typhoid experts from various organizations, and responses from 6 of the experts were deemed relevant for TCV introduction. These 6 inputs were used to semi-qualitatively adjust the already-analyzed introduction years.
We also looked at the scenario of typhoid-endemic countries and Indian States introducing TCV in a slow manner. In this method, the vaccine adoption score was stretched to mirror the history of hepatitis B vaccine introduction. It took on average 20 y to introduce the hepatitis B vaccine,Citation43 with some countries introducing it earlier while others introduced it later. Therefore, we applied an assumption that half of the countries will introduce the TCV earlier than 20 y and the other half later than 20 y. The Indian States were forecasted to introduce at a faster pace in 22 y. This is because India already has a licensed TCV, which may enable the country to introduce the vaccine rapidly. However, we need to remember that the first hepatitis B vaccine introduction in India took place 17 y after WHO prequalification.Citation44 Unlike the interval scale used for typhoid incidence in rapid introduction scenario, we applied a continuous scale for typhoid incidence in the slow introduction scenario, which reduced crowding of countries and states in the same year.
Vaccination strategies
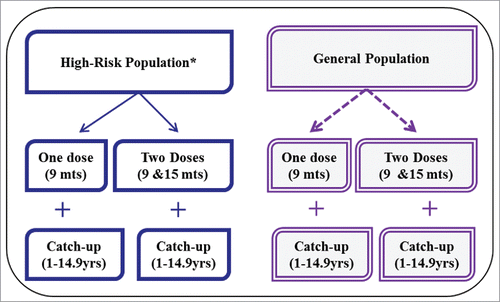
In the absence of robust evidence on vaccine efficacy, doses required, and duration of protection offered by TCV, the following 2 vaccination strategies were proposed for the vaccine introduction scenario analysis (). In a 2-dose strategy, the vaccine is routinely given at 9 months and at 15–18 months. An optional one-time catch-up dose is considered for children aged 2 to 14.9 y. In a one-dose strategy, the vaccine is given at 9 months with an optional one-time catch-up dose for 1 to 14.9 y olds. Both strategies were risk-targeted based on WHO guidanceCitation2 where the vaccine is given to urban slum populations plus rural populations without access to improved water in high- and medium-typhoid burden countries.Citation1,45 The fraction of populations residing in urban slums and populations with poor access to improved water sources were extracted from the United Nations (UN) Millennium Development Goals Indicators database.Citation46 For countries without this information, we used the regional population-weighted average estimate. As a comparator, both strategies were also applied to the same age groups in the general population in high- and medium-typhoid burden countries. We extracted population estimates for the year 2010 for LMICs from a UN database.Citation47
Vaccine demand forecast
The vaccine demand forecast is a mathematical calculation that estimates the number of vaccine doses required based on target population size and vaccination strategies. As the vaccine is given at the same age as the measles vaccine, we assumed the coverage rate would be similar to that of dose one of the measles vaccine (MCV1). Based on data from the WHO-UNICEF immunization database,Citation48 we applied different MCV1 vaccination coverage rates for each country over a 30-year period based on the forecasted population. For vaccination at 9 months of age, we assumed TCV coverage will be 100% of MCV1 coverage. For vaccination at 15–18 months and for catch-up, we assumed TCV coverage will be 75% of MCV1 coverage. This means that each country has different vaccination coverage for different years depending on the vaccination strategy. We also assumed a wastage factor of 1.33 for routine vaccination and a wastage factor of 1.11 for campaign-based vaccination. Although we forecasted vaccine introduction and demand for 30 years, considering the significant uncertainty in long-term predictions, we presented results only for 10 y.
Abbreviations
| DTP | = | Diphtheria-tetanus-pertussis vaccine |
| EPI | = | Expanded Programme on Immunization |
| Gavi | = | Gavi, the vaccine alliance |
| HepB | = | Hepatitis B |
| Hib | = | Haemophilus Influenzae b vaccine |
| IVI | = | International Vaccine Institute |
| IVIR | = | Vaccine Related Implementation Research |
| LMIC | = | low and middle income countries |
| MCV | = | measles containing vaccine |
| Pneumo | = | Pneumococcal vaccine |
| Rota | = | Rota virus vaccines |
| SAGE | = | Strategic Advisory Group of Experts |
| TCV | = | typhoid conjugate vaccine |
| UN | = | United Nations |
| UNICEF | = | United Nations Children's Fund |
| VIVA | = | Vi-based Vaccines for Asia Initiative |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all typhoid experts who contributed to this work. We thank Deborah Hong for English Language editing.
Funding
This work was supported through the Vi-based Vaccines for Asia (VIVA) Initiative, which was funded by the Bill & Melinda Gates Foundation (Grant no. 417.01). The International Vaccine Institute also receives core funding from the Governments of the Republic of Korea and Sweden.
References
- Mogasale V, Maskery B, Ochiai RL, Lee JS, Mogasale VV, Ramani E, Kim YE, Park JK, Wierzba TF. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health 2014; 2(10):e570-80; PMID:25304633; https://doi.org/10.1016/S2214-109X(14)70301-8
- WHO. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec 2008; 83(6):49-59; PMID:18260212
- Szu SC. Development of Vi conjugate - a new generation of typhoid vaccine. Expert Rev Vaccines 2013; 12(11):1273-86; PMID:24156285; https://doi.org/10.1586/14760584.2013.845529
- Steele AD, Hay Burgess DC, Diaz Z, Carey ME, Zaidi AK. Challenges and opportunities for typhoid fever control: a call for coordinated action. Clin Infect Dis 2016; 62 Suppl 1:S4-8; PMID:26933019; https://doi.org/10.1093/cid/civ976
- Kallenberg J, Nguyen A, Schwalbe N. Final report on Vaccine Investment Strategy(Phase II)- November board papet. 2013, Gavi, the vaccine alliance Geneva. p. 7
- WHO. Expert Consultation to Review Evidence in Support of the Use of Typhoid Conjugate Vaccines 2–3 July, 2014, Geneva, Switzerland. 2014, WHO
- WER. Immunization and Vaccine related Implementation Research Advisory Committee (IVIR-AC): summary of conclusions and recommendations 17–19 September 2014 meeting. Wkly Epidemiol Rec 2015; 90(1–2):1-8
- WHO SAGE Working Group on Typhoid Vaccines (established March 2016). 2016
- Desai SN, Pezzoli L, Martin S, Costa A, Rodriguez C, Legros D, Perea W. A second affordable oral cholera vaccine: implications for the global vaccine stockpile. Lancet Glob Health 2016; 4(4):e223-4; PMID:27013303; https://doi.org/10.1016/S2214-109X(16)00037-1
- Date KA, Bentsi-Enchill A, Marks F, Fox K. Typhoid fever vaccination strategies. Vaccine 2015; 33 Suppl 3:C55-61; PMID:25902360; https://doi.org/10.1016/j.vaccine.2015.04.028
- Amarasinghe A, Wichmann O, Margolis HS, Mahoney RT. Forecasting dengue vaccine demand in disease endemic and non-endemic countries. Hum Vaccin 2010; 6(9); pii: 12587; PMID:20930501; https://doi.org/10.4161/hv.6.9.12587
- Smith G, Michelson J, Singh R, Dabbagh A, Hoekstra E, van den Ent M, Mallya A. Is there enough vaccine to eradicate measles? An integrated analysis of measles-containing vaccine supply and demand. J Infect Dis 2011; 204 Suppl 1:S62-70; PMID:21666215; https://doi.org/10.1093/infdis/jir130
- Gilchrist SA, Nanni A. Lessons learned in shaping vaccine markets in low-income countries: a review of the vaccine market segment supported by the GAVI Alliance. Health Policy Plan 2013; 28(8):838-46; PMID:23174880; https://doi.org/10.1093/heapol/czs123
- Gavi Getting vaccines on the agenda. 2014
- Mogasale V, Ramani E, Mogasale VV, Park J. What proportion of Salmonella Typhi cases are detected by blood culture? A systematic literature review. Ann Clin Microbiol Antimicrob 2016; 15(1):32; PMID:27188991; https://doi.org/10.1186/s12941-016-0147-z
- Mogasale V, Mogasale VV, Ramani E, Lee JS, Park JY, Lee KS, Wierzba TF. Revisiting typhoid fever surveillance in low and middle income countries: lessons from systematic literature review of population-based longitudinal studies. BMC Infect Dis 2016; 16:35; PMID:26822522; https://doi.org/10.1186/s12879-016-1351-3
- JHSPH. Vaccine Information Management System (VIMS) - IVAC - International Vaccine Access Center - Johns Hopkins Bloomberg School of Public Health [Internet]. VIMS - Vaccine Information Management System. Report on Global Introduction. 2014
- WHO, 5. Immunisation system indicators. 2014
- Naheed A, Ram PK, Brooks WA, Hossain MA, Parsons MB, Talukder KA, Mintz E, Luby S, Breiman RF. Burden of typhoid and paratyphoid fever in a densely populated urban community, Dhaka, Bangladesh. Int J Infect Dis 2010; 14 Suppl 3:e93-9; PMID:20236850; https://doi.org/10.1016/j.ijid.2009.11.023
- Srikantiah P, Girgis FY, Luby SP, Jennings G, Wasfy MO, Crump JA, Hoekstra RM, Anwer M, Mahoney FJ. Population-based surveillance of typhoid fever in Egypt. Am J Trop Med Hyg 2006; 74(1):114-9; PMID:16407354
- Crump JA, Youssef FG, Luby SP, Wasfy MO, Rangel JM, Taalat M, Oun SA, Mahoney FJ. Estimating the incidence of typhoid fever and other febrile illnesses in developing countries. Emerg Infect Dis 2003; 9(5):539-44; PMID:12737736; https://doi.org/10.3201/eid0905.020428
- Marks F, Adu-Sarkodie Y, Hünger F, Sarpong N, Ekuban S, Agyekum A, Nkrumah B, Schwarz NG, Favorov MO, Meyer CG, et al. Typhoid fever among children, Ghana. Emerg Infect Dis 2010; 16(11):1796-7; PMID:21029549; https://doi.org/10.3201/eid1611.100388
- Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB, Wamola N, Bigogo GM, Awiti G, Tabu CW, et al. Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One 2012; 7(1):e29119; PMID:22276105; https://doi.org/10.1371/journal.pone.0029119
- Thriemer K, Ley B, Ame S, von Seidlein L, Pak GD, Chang NY, Hashim R, Schmied WH, Busch CJ, Nixon S, et al. The burden of invasive bacterial infections in Pemba, Zanzibar. PLoS One 2012; 7(2):e30350; PMID:22363426; https://doi.org/10.1371/journal.pone.0030350
- Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN. Immunogenicity, efficacy and serological correlate of protection of Salmonella typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine 1996; 14(5):435-8; PMID:8735556; https://doi.org/10.1016/0264-410X(95)00186-5
- Yang HH, Wu CG, Xie GZ, Gu QW, Wang BR, Wang LY, Wang HF, Ding ZS, Yang Y, Tan WS, et al. Efficacy trial of Vi polysaccharide vaccine against typhoid fever in south-western China. Bull World Health Organ 2001; 79(7):625-31; PMID:11477965
- Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, Singh B, Rao M, Naficy A, Clemens JD, Bhan MK. Typhoid fever in children aged less than 5 years. Lancet 1999; 354(9180):734-7; PMID:10475185; https://doi.org/10.1016/S0140-6736(98)09001-1
- Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, Agtini MD, Bhutta ZA, Canh DG, Ali M, Shin S, et al. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ 2008; 86(4):260-8; PMID:18438514; https://doi.org/10.2471/BLT.06.039818
- Brooks WA, Hossain A, Goswami D, Nahar K, Alam K, Ahmed N, Naheed A, Nair GB, Luby S, Breiman RF. Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerg Infect Dis 2005; 11(2):326-9; PMID:15752457; https://doi.org/10.3201/eid1102.040422
- Owais A, Sultana S, Zaman U, Rizvi A, Zaidi AK. Incidence of typhoid bacteremia in infants and young children in southern coastal Pakistan. Pediatr Infect Dis J 2010; 29(11):1035-9; PMID:21046701
- Khan MI, Soofi SB, Ochiai RL, Habib MA, Sahito SM, Nizami SQ, Acosta CJ, Clemens JD, Bhutta ZA; DOMI Typhoid Karachi Vi Effectiveness Study Group. Effectiveness of Vi capsular polysaccharide typhoid vaccine among children: a cluster randomized trial in Karachi, Pakistan. Vaccine 2012; 30(36):5389-95; PMID:22721899; https://doi.org/10.1016/j.vaccine.2012.06.015
- Simanjuntak CH, Paleologo FP, Punjabi NH, Darmowigoto R, Soeprawoto , Totosudirjo H, Haryanto P, Suprijanto E, Witham ND, Hoffman SL. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet 1991; 338(8774):1055-9; PMID:1681365; https://doi.org/10.1016/0140-6736(91)91910-M
- Lin FY, Vo AH, Phan VB, Nguyen TT, Bryla D, Tran CT, Ha BK, Dang DT, Robbins JB. The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am J Trop Med Hyg 2000; 62(5):644-8; PMID:11289678; https://doi.org/10.4269/ajtmh.2000.62.644
- Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Eng J Med 2001; 344(17):1263-9; PMID:11320385; https://doi.org/10.1056/NEJM200104263441701
- Levine MM, Ferreccio C, Cryz S, Ortiz E. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet 1990; 336(8720):891-4; PMID:1976928; https://doi.org/10.1016/0140-6736(90)92266-K
- Black RE, et al. Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Chilean Typhoid Committee. Vaccine 1990; 8(1):81-4; PMID:2180234; https://doi.org/10.1016/0264-410X(90)90183-M
- Siddiqui FJ, Rabbani F, Hasan R, Nizami SQ, Bhutta ZA. Typhoid fever in children: some epidemiological considerations from Karachi, Pakistan. Int J Infect Dis 2006; 10(3):215-22; PMID:16431148; https://doi.org/10.1016/j.ijid.2005.03.010
- Feasey NA, Archer BN, Heyderman RS, Sooka A, Dennis B, Gordon MA, Keddy KH. Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg Infect Dis 2010; 16(9):1448-51; PMID:20735930; https://doi.org/10.3201/eid1609.100125
- Acharya IL, Lowe CU, Thapa R, Gurubacharya VL, Shrestha MB, Cadoz M, Schulz D, Armand J, Bryla DA, Trollfors B, et al. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med 1987; 317(18):1101-4; https://doi.org/10.1056/NEJM198710293171801
- Date KA, Bentsi-Enchill AD, Fox KK, Abeysinghe N, Mintz ED, Khan MI, Sahastrabuddhe S, Hyde TB; Centers for Disease Control and Prevention (CDC). Typhoid Fever surveillance and vaccine use - South-East Asia and Western Pacific regions, 2009–2013. MMWR Morb Mortal Wkly Rep 2014; 63(39):855-60; PMID:25275329
- Acosta CJ, Galindo CM, Ali M, Elyazeed RA, Ochiai RL, Danovaro-Holliday MC, Page AL, Thiem VD, Jin Y, Park JK, et al. A multi-country cluster randomized controlled effectiveness evaluation to accelerate the introduction of Vi polysaccharide typhoid vaccine in developing countries in Asia: rationale and design. Trop Med Int Health 2005; 10(12):1219-28; PMID:16359401; https://doi.org/10.1111/j.1365-3156.2005.01517.x
- UNICEF, UNICEF India - Health - Coverage Evaluation Survey Report 2009- State Fact Sheets 2009
- Brooks A, et al. Implementing new health interventions in developing countries: why do we lose a decade or more? BMC Public Health 2012; 12:683
- Lahariya C. A brief history of vaccines & vaccination in India. Indian J Med Res 2014; 139(4):491-511; PMID:24927336
- WHO-UNICEF. Types of drinking-water sources and sanitation, WHO / UNICEF Joint Monitoring Programme (JMP) for Water Supply and Sanitation. [cited 2014 March 18th]; Available from: http://www.wssinfo.org/definitions-methods/watsan-categories
- UN. Millenium Development Goals Indicators, UN Statistics Division, U.S. Division, Editor. 2010, UN Statistics Division
- UN. World Population Prospects:The 2010 Revision. 2011, Population Division, Department of Economic and Social Affairs: United Nations
- UNICEF-WHO. Immunization Summary. 2009, United Nations Children's Fund
Appendix Annex 1: Typhoid conjugate vaccine introduction forecast by year