ABSTRACT
Stings of hymenoptera can induce IgE-mediated hypersensitivity reactions in venom-allergic patients, ranging from local up to severe systemic reactions and even fatal anaphylaxis. Allergic patients' quality of life can be mainly improved by altering their immune response to tolerate the venoms by injecting increasing venom doses over years. This venom-specific immunotherapy is highly effective and well tolerated. However, component-resolved information about the venoms has increased in the last years. This knowledge is not only able to improve diagnostics as basis for an accurate therapy, but was additionally used to create tools which enable the analysis of therapeutic venom extracts on a molecular level. Therefore, during the last decade the detailed knowledge of the allergen composition of hymenoptera venoms has substantially improved diagnosis and therapy of venom allergy. This review focuses on state of the art diagnostic and therapeutic options as well as on novel directions trying to improve therapy.
Hymenoptera venom hypersensitivity
There are 24 described orders of insects out of which hymenoptera are the main inducers of severe allergies in humans. Hymenoptera belonging to the genus bee (Apis), bumblebee (Bombus), wasp (Family: Vespidae, Genus: Dolichovespula, Polistes, Vespula), hornet (Vespa) and stinging ant (Family: Formicidae, Genus: Myrmecia, Solenopsis), sting humans with high frequency, and thus are the most studied in terms of allergy and allergen-specific immunotherapy Citation1 (). Honeybees (Apis mellifera) and yellow jackets (Vespula vulgaris, in Europe also called wasp) are observed as the most frequent inducers of allergic reactions to hymenoptera venoms in humans, and therefore this review will mainly focus on specific immunotherapy for allergy to stings of these species (also in comparison to Polistes species (paper wasps), relevant in the US and Mediterranean areas of Europe). For the review of allergy to other insects, the reader is referred to recent publications in the field. Citation2,Citation3
Figure 1. Relevant species and allergens in hymenoptera venom allergy. A, Taxonomy of hymenoptera, Citation154,Citation155 with examples of prominent species, which are relevant elicitors of venom allergy. B, Identified allergens of the allergy-relevant hymenoptera species Polistes dominula, Vespula vulgaris and Apis mellifera. Allergens which are marked with an asterisk are available for routine diagnosis. Indicated in red are commercially available marker allergens, used to discriminate between allergies against Polistes/Vespula and honeybee venom allergy. Cross-reactive allergens and their sequence identity (in percent) are shown in gray boxes.
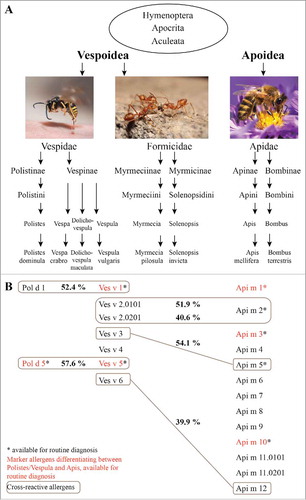
The frequency of stings, and thus of subsequent allergic reactions, is dependent on geographic, environmental and ecological factors. Citation1 These factors can change very fast, which is reflected by the fact that species like Polistes dominula, known to be domestic in southern Europe, are invading the US (1970s) from the north-east area to the west coast (1990s), Citation4 South Africa (2008) Citation5 and also central Europe (1956), Citation6 which is most probably due to climate change. Therefore, allergy to Polistes will gain importance in these areas within the next years.
Hymenoptera venoms are complex mixtures of various substances including numerous relevant allergens. The amount of venom that is injected during a sting is species specific. Honeybees inject up to 140 µg of venom, Citation7 with a protein content of around 59 µg. Citation8 In comparison, wasps inject venom with a protein content ranging from 1.7 to 3.1 µg (yellow jacket), up to 17 µg (Polistes species). Citation7 Nevertheless, 70.6% of anaphylactic reactions reported in europe are caused by stings of wasps and only 23.4% and 4.1% are caused by bees and hornets, respectively. Citation9
Clinical manifestations of hymenoptera venom hypersensitivity
In contrast to airborne allergens that have to cross mucosal barriers, venom allergens are injected into the skin and reach the blood easy and fast. Transient pain, itching and swelling are part of the normal response to stings of hymenoptera due to irritative and toxic venom components. In contrast, large local reactions (LLRs) that peak at one to 2 d after the sting and resolve 3 to 10 d later are thought to be part of an allergic reaction to the venom. LLRs are defined by edema, erythema and pruritus and have diameters greater than 10 cm. Citation10 LLRs are supposed to be IgE-dependent Citation11 or cell-mediated, Citation10 although previous work described them to be independent of detectable IgE (with the detection limit at that time). Citation12 It is believed that only very few patients that suffer from LLRs develop more severe reactions when they are re-stung by the same insect, hence, they are not a predictor of the severity of allergy. Citation12-Citation17 Due to the low risk of systemic reactions, immunotherapy is not recommended for patients experiencing only LLRs. Citation18,Citation19
Systemic or generalized reactions (SR) or anaphylaxis include cutaneous urticaria, angioedema, pruritus, flush, unusual nephropathy, central and peripheral neurologic syndromes, idiopathic thrombocytopenic purpura, rhabdomyolysis, vascular or respiratory symptoms, bradycardia, arrhythmia, angina, myocardial infarction, abdominal cramps, gastrointestinal tract and/or uterine smooth muscle contraction. Citation1 SRs usually begin 10 to 30 minutes after the sting, but can also arise faster (i.e. in patients with mast cell disorders) or slower (1–4 h) although being less life threatening in the latter case. Citation1 It is assumed, that 0.4–0.8% of children and 3% of adults show potentially life threating systemic reactions after an insect sting. Citation20,Citation21 Anaphylactic reactions due to stings of hymenoptera can cause a rapid death, since cardiorespiratory arrests can be observed in a median time of 15 min after the sting, a fact that leaves people at risk of severe allergic reaction in great anxiety. Citation22 The only therapeutic options for venom allergy are the prescription of emergency medication (adrenaline/epinephrine auto-injector, anti-histamines, corticosteroids) or, as the only curative treatment, venom-specific immunotherapy (VIT, also named allergen-specific immunotherapy).
Prevalence of venom allergy
Epidemiologic studies by Biló et al. Citation23 showed that most of the venom-allergic patients suffer from LLRs (ranging from 2.4 to 26.4% in the general population) and that this number can be as high as 38% in beekeepers. Between 0.3 and 7.5% of the population studied, have experienced systemic anaphylaxis (self-reports), whereas the number of severe systemic reactions is as high as 14–43% among beekeepers. The prevalence of venom-allergic reactions in children is only 0.15–0.3%. The estimated number of annual mortalities ranges from 0.03 to 0.45 fatalities per one million inhabitants. But this number could be underestimated as many deaths due to anaphylactic reactions to insect stings probably remain undetected. Citation23 A survey of the European network of severe allergic reactions (NORA) found that 48.2% (> 18 years) and 20.2% (in children) of severe anaphylactic reactions occur due to insect stings. Citation9
Risk factors for severe allergic reactions
Main risk factors for severe allergic reactions to insect venoms seem to be an elevated serum tryptase concentration Citation24 (independent of mast cell disorders) and mastocytosis. Citation25 Nevertheless, it could be shown that if tryptase concentration is above a certain threshold, the risk of severe insect venom-allergic reactions declines. Citation25 The prevalence of severe allergic reactions was found to be 50% in patients with a tryptase level of 20.4 to 29.9 µg/mL and this prevalence is lower than 10% in patients with a tryptase level below 6.1 µg/L and above 191 µg/L. However, this prevalence is still higher than in the general population. Citation25 Further risk factors include older age, male sex, medication of hypertension (ACE, β blockers), diagnosed vespid allergy and preceding stings with systemic reactions. Citation26 Of note, there is no significant difference in the frequency of hymenoptera venom allergy in the non-atopic and atopic population. Citation27,Citation28
Diagnosis and selection of patients for venom-specific immunotherapy
The correct identification of the allergy-relevant insect is of major importance for accurate therapy of venom-allergic patients, as de novo sensitizations to the wrong therapeutic extract are possible. Citation29 Besides the thorough and important clinical history, several additional tools exist to analyze sensitization and/or allergy against insect venoms in vivo and in vitro. According to the guidelines of the allergy academies of Europe and the USA, there is a unique diagnostic algorithm for each patient which has to be gone through in different ways, depending on the diagnostic results. Citation19 During diagnosis and the decision process for venom-specific immunotherapy, clinicians are advised to discuss the risks and benefits of therapy for each individual case. Citation19
History
Standard diagnosis begins with the detailed survey of the medical history of the patient, including previous sting reactions (time course and severity of the reaction, number of stings, all associated symptoms and treatments), the assessment of potential risk factors (such as medication, cardiovascular risks and other diseases) and the identification of the insect causing the allergic reaction. Citation1 Unfortunately, most of the patients have difficulties to correctly identify the insect that has stung and caused the allergic reaction. Citation30,Citation31 Additionally, the severity of stings is often under- or overestimated because of fear, panic, exercise, heat, alcohol or underlying cardiorespiratory disease. Furthermore, if the sting reaction was experienced already a long time ago, sometimes it is only poorly remembered. Citation1 Therefore, in most of the cases further diagnostic tests are needed to correctly identify the allergy-relevant insect.
Skin testing
If a patient has had previous systemic reactions or severe dermal reactions (systemic cutaneous or LLRs) after insect stings, further diagnostic tests are recommended. Additionally, under special circumstances such as frequent exposure or a certain lifestyle, further diagnosis and therapy is considerable. Citation19,Citation32 Despite improvements in molecular in vitro diagnosis, skin testing is the gold standard to diagnose insect venom allergy. There are 2 options for skin testing, skin prick testing and intradermal testing, whereas the intradermal testing is the more sensitive procedure. Skin testing is safe Citation33 and should be performed at least 2 weeks after the sting reaction to avoid possible false-negative results during the refractory period, in which sIgE can be exausted. Ideally, skin tests should be repeated one to 2 months later. Citation34
Baseline serum tryptase
It is recommended to determine the basal serum tryptase concentration in all patients with a history of a severe reaction after a hymenoptera sting. Adult patients with mastocytosis and/or elevated baseline serum tryptase are at risk to experience more severe reactions following stings, as well as for severe side effects during venom immunotherapy. Citation25,Citation26 In addition, proper diagnosis of venom allergy in mastocytosis patients can be affected since results of sIgE-testing might be more often negative compared with venom allergic patients without mastocytosis. Citation35
Specific IgE
Candidates for VIT can be considered for complementary in vitro testing. Citation19 This can supplement the information of the clinical history and skin testing, even though testing for specific IgE (sIgE) to the hymenoptera venoms in serum is a little loewer than (intradermal) skin testing. Citation34 Before the late 1970s testing for sIgE was done with whole insect body extracts, Citation36 but sensitivity could be increased by using purified venoms. The sensitivity could even be increased further by the use of natural or recombinant single allergens. Citation37 Limitations of in vitro testing are demonstrated by the fact, that 15% of a population studied by Mosbech et al. Citation38 showed sensitization to wasp and/or bee venom, but only 31% of those had reactions to stings. In a smaller study group of sensitized patients (n = 94), which were additionally diagnosed by sting challenge, only 5.3% showed systemic reactions. Citation39 Nevertheless, the increasing knowledge of single allergen components in the venom of hymenoptera is able to increase the diagnostic sensitivity and specificity in many (unclear) cases by component-resolved diagnosis (see below). Citation40,Citation41
Allergens of hymenoptera venoms
The increasing knowledge of the exact composition of hymenoptera venoms created added value for accurate diagnosis of venom allergy. Moreover, it turns out that the understanding of all allergen components, not only in the venom, but also in therapeutic extracts used for specific immunotherapy, might influence the outcome of the therapy. Citation42,Citation43
According to the WHO/IUIS Allergen Nomenclature Sub-committee, Citation44 protein components of one allergen source (in this case venom) are considered as allergen if specific IgE binding from at least 2 out of 10 subjects with allergy to the source and no IgE binding from subjects without allergy to the source can be shown. This IgE binding should preferably be demonstrated to the purified (natural or recombinant) protein as well as to the extract of the source. In general, hymenoptera venoms are composed of low molecular weight substances like biogenic amines, basic peptides and proteins of higher molecular weight, of which most have an enzymatic activity. One of the best characterized venoms is that of the honeybee Apis mellifera, for which detailed analyses of venom proteins are available. Citation45 More recently 113 proteins and peptides were identified in honeybee venom. Citation46 These detailed analyses were also able to reveal seasonal changes of venom composition. Citation47 Together with the proteomic data, the unraveling of the genomic information of the honeybee Citation48 simplified the production of single recombinant allergens, advancing the field of component-resolved diagnosis. Recently, also the genome of the invasive species Polistes dominula was sequenced, Citation49 a fact that will probably simplify the discovery of novel allergens of Polistes species in the future. Unfortunately, detailed proteomic venom analyses comparable to those for the honeybee are still missing for Vespula species. An overview of identified honeybee, yellow jacket and Polistes allergens is given in .
Table 1. Identified venom allergens from honeybee (Apis mellifera), yellow jacket (Vespula vulgaris) and paper wasp (Polistes dominula). MW, molecular weight; CRP, carbohydrate-rich protein; DPP IV, Dipeptidyl peptidase IV; DW, dry weight; MRJP, major royal jelly protein.
The best characterized allergen of honeybee venom is the phospholipase A2 or Api m 1. Citation50,Citation44 In contrast, the venoms of yellow jackets contain phospholipase A1 (Ves v 1, Pol d 1), which differs in sequence and substrate specificity. Citation50 Honeybee and yellow jacket venoms contain hyaluronidases (Api m 2 and Ves v 2). Citation50 Recently, for the yellow jacket it was shown, that the hyaluronidase exists in 2 isoforms, Ves v 2.0101 and Ves v 2.0201, whereby the latter is an inactive, but surprisingly the predominant isoform. Citation51 Not described in other species so far, are the acid phosphatase (Api m 3) Citation52,Citation53 and the small peptide melittin (Api m 4) of honeybee venom. However, melittin is an allergen of minor importance. Citation54 In contrast, Api m 3 was recently identified as a major allergen. Citation41
Shared between almost all Vespoidea venoms (except Myrmecia), is an allergen named “antigen 5” (i.e., Ves v 5, Pol d 5). Moreover, it has been shown, that there exists an antigen 5-like protein also in honeybees, which is only expressed in the winter and probably shows no IgE reactivity with allergic-patients' sera due to missing sensitizing stings in winter. Citation47 The antigen 5 protein is of high abundance in wasp venoms, however, its function is still unknown (antigen 5 of Vespa mandarinia was shown to be neurotoxic at the neuro-muscular junctions of lobster legs). Citation55 Since antigens 5 share a high degree of sequence similarity, and thus of protein epitopes, they are highly cross-reactive and recognized by many Vespoidea-allergic patients, independent of the sensitizing species. Citation56
Other major allergens of honeybee and yellow jacket venom are the dipeptidyl peptidases IV (DPP IV, Api m 5 and Ves v 3), which also exhibit cross-reactivity due to sequence identity. Citation57 Additional minor allergens include the putative protease inhibitor Api m 6, Citation58,Citation59 the protease Api m 7, Citation60 the esterase Api m 8, the peptidase Api m 9, the major royal jelly proteins (MRJP) 8 and 9 (Api m 11.0101, Api m 11.0201) Citation61 and the cross-reactive vitellogenins Api m 12 and Ves v 6. Citation62
Another clinically relevant protein of honeybee venom is Api m 10 (named icarapin). Citation63 This carbohydrate-rich and unstable allergen of unknown function with at least 9 known transcript isoforms Citation64 was recently identified as major allergen with important implications for diagnostics an therapy. Citation41,Citation43,Citation65 Api m 10 is not only a marker allergen for honeybee venom allergy but also underrepresented in some therapeutic extracts that are commonly used for honeybee venom immunotherapy. Citation43,Citation65
Component-resolved diagnosis (CRD)
In the last years it has become more and more evident that testing for specific IgE reactivity against several single allergens (molecular allergology Citation66 or component-resolved diagnosis, CRD) is evolving as a superior tool to support classical allergy testing. For a deep review of developments in the field of CRD in insect venom allergy we refer the reader to a recent review by Ollert and Blank. Citation42
Natural venom extracts used for diagnosis can lead to “false positive” results due to cross-reactivity of IgE directed against antigenic carbohydrate determinants (cross-reactive carbohydrate determinants, CCDs). Up to 75% of double-positive in vitro test results with honeybee and yellow jacket venom are caused by IgE to CCDs Citation67 and only a minor portion by true allergy to both venoms. IgE antibodies directed against glycostructures of insect and plant proteins were shown to be of high affinity Citation68 but their clinical relevance seems to be low, meaning that to an unknown reason they are causing no clinical symptoms. Citation69 Measuring sIgE to CCD markers (MUXF3, horseradish peroxidase, bromelain, ascorbate oxidase) is able to confirm the presence of CCD-specific IgE antibodies as reason of multiple positive test results. However, since specific IgE directed against both (CCD and protein epitopes might be present), the detection of CCD-specific IgE alone does not allow the exclusion of sensitization to protein epitopes of multiple venoms. Citation66,Citation67
To circumvent the problem of CCDs, allergens that cannot be recombinantly produced in E.coli (without glycosylation) due to complex 3-dimensional folding, can be expressed in Sf9 (Spodoptera frugiperda) insect cells without the naturally occurring insect glycosylation (CCDs). Citation70 In contrast to other hymenoptera venoms, venom allergens of Polistes species show no immunologically detectable CCD-reactivity. Citation71
Currently, commercially available hymenoptera allergens for component-resolved allergy testing include rApi m 1, rApi m 2, rApi m 3, rApi m 5 and rApi m 10 for honeybee venom as well as rPol d 5, rVes v 1 and rVes v 5 for yellow jacket venom for the ImmunoCAP platform (Phadia/Thermo Fisher Scientific) and rApi m 1, rApi m 2 and rVes v 5 for the Immulite platform (Siemens Healthcare Diagnostics). EUROIMMUN Medizinische Labordiagnostika AG offers a different test system for rVes v 1, rVes v 5, rApi m 1, rApi m 2, rApi m 10 and in the near future rPol d 1 and rPol d 4. All allergens available for routine diagnosis are produced in a CCD-free form.
By using all of the commercially available allergens for the diagnosis of honeybee venom allergy, it is possible to detect sIgE reactivity in 94.4% of the patients. Citation41 Köhler et al Citation41 additionally calculated the contribution of sIgE to single honeybee venom allergens to sIgE reactivity to whole honeybee venom. It was shown, that there is high contribution of Api m 1 (19.6%) and Api m 10 (14.4%), medium contribution of Api m 2 (7.6%), Api m 3 (7.2%) and Api m 5 (8.9%) and low contribution of Api m 4 (2%) and CCDs (2.5%). However, until now there is no clinically meaningful interpretation of such data. The diagnostic sensitivity for yellow jacket (Vespula vulgaris) venom allergy was increased to a sensitivity of almost 100% by the use of the 2 recombinant marker allergens Ves v 1 and Ves v 5. Citation35,Citation72 Therefore, in most cases where the venom extract-based diagnostics do not allow the differentiation between honeybee and yellow jacket venom allergy due to clinically irrelevant cross-reactivity, the newly available component-resolved diagnostics, using CCD-free allergens, enable the detailed characterization of sensitization profiles and the identification of the venom causing clinical symptoms. Moreover, for patients who are difficult to diagnose due to very low levels of sIgE, component-resolved sIgE testing shows a higher sensitivity compared with venom extract-based testing. Citation72-Citation76
The situation for the diagnostic differentiation between allergies to the different wasp species is more difficult, as cross-reactivity, independent from CCD-reactivity, Citation35,Citation71 is frequently observed between Vespula and Polistes species, when sIgE to venom extracts Citation77 or single allergens Citation78 is assessed. This cross-reactivity is not limited to Polistes and Vespula species and shows the need for novel marker allergens. Citation56
In the last years component-resolved approaches were also used to analyze products used for venom immunotherapy. These analyses revealed that particular allergens are underrepresented in some therapeutic extracts Citation65 and this might be a reason for therapeutic failures. Citation43 An overview of clinically relevant allergens and allergens which are relevant for molecular diagnostics is given in .
Basophil activation test (BAT)
In line with component-resolved diagnostics is the testing for the activation of patient-derived basophils by allergens (basophil activation test, BAT). In this test, whole blood is stimulated with venoms (or single allergens) and the subsequent activation of basophils is measured by the detection of CD63 upregulation (as a consequence of cell degranulation) on the surface of basophils by flow cytometry.
Diagnosis with the BAT is only recommended if all other diagnostic methods fail to detect an allergy against venom, despite a convincing history of an allergic reaction. Citation19,Citation23 A study could demonstrate that 81% out of 21 patients without detectable sIgE could be diagnosed using BAT. Citation79 This method can even be useful to gain more detailed information about the insect, the patient is allergic to. Citation80 Unfortunately, detailed clinical information whether if BAT can predict the severity of a reaction to an insect sting is missing due to ethical limitations on diagnostic sting challenges. Citation81 Moreover, there is some evidence, that BAT can support the diagnosis of venom allergy in mastocytosis patients with negative sIgE and low total IgE, Citation82 whereas other studies are not able to show clear results for the diagnosis of this patient group using BAT. Citation83,Citation84 Nevertheless, it was demonstrated that BAT can be useful for monitoring the effectiveness of VIT. Citation19
Allergen-specific immunotherapy and monitoring of venom hypersensitivity
History of VIT
The first described case of specific immunotherapy of venom allergy was published by Braun in 1925, reporting the desensitization of one patient sensitive to bee stings. Citation85 This therapeutic approach was based on the administration of a body extract of the abdomen. Whole insect body extract-based therapy was continued for many years, until a randomized trial proved its ineffectiveness. In 1978, the first randomized controlled study using whole venom, extracted from venom sacks, was published and demonstrated high effectiveness. Citation86
Treatment protocols for VIT
Modern therapeutic products for specific immunotherapy of venom allergy are venom extracts which are purified by a standardized procedure. The production process of these products is regulated by country-specific guidelines. Moreover, due to confidential manufacturer-specific processing, the venom extracts produced might differ in terms of composition and allergen activity. Products for VIT are available as aqueous (or lyophilized) extracts or as depot preparations adsorbed to aluminum hydroxide.
Current guidelines for VIT of the American Academy of Allergy and Clinical Immunology (AAAAI) recommend the treatment of patients who had a history of a systemic sting reaction. Citation19 Guidelines of the European Academy of Allergy and Clinical Immunology (EAACI) further specify the indication for VIT and include children and adults who had systemic reactions including respiratory and cardiovascular symptoms upon stings of hymenoptera. Citation23 Additionally, both guidelines demand the definite diagnosis either by skin tests and/or specific IgE tests. Patients only suffering from LLRs should not be treated.
The injection for specific immunotherapy represents a medical task and should thus be performed by the physician and done with a 1 ml syringe with fine graduation down to 0.01 ml with an injection needle (size 14–18, short bevel, sufficient length). The injection is made strictly subcutaneously into a lifted skin fold a hand's width above the olecranon on the extensor side of the upper arms. Citation87 The guidelines for VIT Citation19,Citation23 recommend a starting dose of 1 µg which is contradictory to package inserts of therapy products (starting dose of 0.1 µg), as it was shown, that this dose is well tolerated. Citation88 The starting dose is then gradually increased up to a maintenance dose of 100 µg, chosen out of historical reasons, as it was assumed that this is the equivalent venom amount of 2 honeybee stings. To reach the suggested maintenance dose, several protocols exist. The conventional protocol gradually increases the amount of injected venom during 8 to 16 weeks treatment, by injecting increasing volumes and/or concentrations of therapy product every week. The detailed protocol can be found on package inserts or in the guideline for venom immunotherapy. Citation19 Faster protocols reach the maintenance dose within one week, 2 to 3 d or in up to 4 to 8 hours in rush or ultrarush treatment protocols, respectively. Citation89 In clustered or modified rush up-dosing protocols, 2 or 3 injections are given in 30 minutes intervals every 3 to 7 d. Citation34 All up-dosing protocols are safe in most of the patients, with the only exception of ultrarush protocols, where the probability of systemic reactions is somewhat increased. Citation90 Fast up-dosing protocols are used in cases, when patients do not have access to specialists for treatment (maintenance treatment can be given by general practitioners) or when there is need for fast protection. Citation19,Citation23
Several studies could show by sting challenge tests, that clinical protection is reached as soon as the maintenance dose is reached Citation91,Citation92 and that the treatment interval can be gradually increased from monthly injections to a treatment course of every 6 to 8 weeks without loss of clinical protection. Citation93 In some cases it was shown, that the interval can even be increased to treatment every 3 months. Citation19,Citation23 After its introduction, VIT was thought to be a lifelong treatment or a treatment that should be performed until serum IgE and skin tests turn negative. As the majority of patients will stay positive in diagnostic screenings, studies could show that 80–90% of patients are protected from having SR to sting challenge tests or field stings when VIT is discontinued after 3–5 y. Citation19,Citation23 Of note, stopping VIT after 3 y might only be feasible for patients with good prognosis (patients with mild reactions and a reduction of sensitivity to venom in response to VIT, either measured by skin tests or sIgE serum tests) and should not be performed when sting challenge during therapy cannot be performed. Citation94 Extending the treatment to 5 y protects the majority of unselected patients. Citation95 Several specific risk factors such as older age, very severe reactions to previous stings or during VIT (injection or sting), treatment of less than 5 years, elevated basal serum tryptase and/or mastocytosis, honeybee venom allergy, cardiovascular disease, concomitant treatment with angiotensin-converting enzyme inhibitors or β-blockers, exposure to repeated stings and high skin sensitivity at the time point of stopping VIT are associated with the loss of clinical protection after discontinuation of VIT. Citation96 However, all patients continue to have a 10% chance of having a reaction to a future sting, even if venom skin tests become negative. Citation97 Hence, it should be kept in mind that that longer treatment periods are associated with a lower risk of relapse Citation98 and that the only way to keep the risk down to 2% is to remain on maintenance immunotherapy. Citation94 Therefore, to keep the risk of relapse after stopping VIT as low as possible, thought must be given to prolonging treatment or even maintaining it lifelong, especially for high-risk patients. Considering life-long treatment regimens it surely would be worth to investigate if treatment intervals can be extended to longer than 3 month. Citation19,Citation23
Immunological mechanisms of VIT
Venom-specific immunotherapy aims to induce a shift from pro-allergic and pro-inflammatory Th2 conditions, present in an allergic individual, toward a tolerating state of the immune system (). The induction of this tolerogenic reaction to venom allergens during VIT is characterized by several changes within cellular and humoral parameters.
Figure 2. Mechanisms of venom-specific immunotherapy (VIT) compared with the allergic immune response. Venom allergens are injected into the skin, either by the allergy-causing insect (natural exposure) or by subcutaneous injection during VIT. Skin-resident dendritic cells take up the allergens, process them and present derived peptides in a complex with MHC class II molecules to allergen-specific naive CD4+ T cells. In a venom-allergic individual this leads to their differentiation into Th2 cells (key transcription factor: GATA-3), which secrete the cytokines IL-3, IL-5 and IL-9, contributing to the activation and degranulation of mast cells, eosinophils and basophils, as well as IL-4 and IL-13 which induce the production of IgE by B cells. These inflammatory processes elicit the allergic reaction and suppress a tolerogenic phenotype of the immune response, which can be observed in individuals not allergic to insect venom. A shift of this Th2-directed reaction toward a tolerogenic reaction is observed during VIT, characterized by the differentiation of allergen-specific naive T cells to Tregs (key transcription factor: Foxp3) and Th1 cells (key transcription factor: T-bet). The effector cytokines of Tregs and Th1 cells then lead to the suppression of Th2 cells and their inflammation-promoting functions, therefore causing desensitization of mast cells and basophils, as well as the induction of IgA-producing B cells and Bregs. These Bregs then produce protective blocking IgG4 antibodies, further enhance the differentiation of Tregs via TGF-β and can inhibit the differentiation of Th2 cells via IL-10 and TGF-β. Citation99,Citation100,Citation102-Citation104 Breg: regulatory B cell; Foxp3: Forkhead Box P3; GATA-3: GATA binding protein 3; Ig: Immunoglobulin; IL: interleukin; MHC: major histocompatibility complex; T-bet: T-Box 21; TCR: T cell receptor; TGF-β: Transforming growth factor β; Th: T helper cell; Treg: regulatory T cell; VIT: venom-specific immunotherapy.
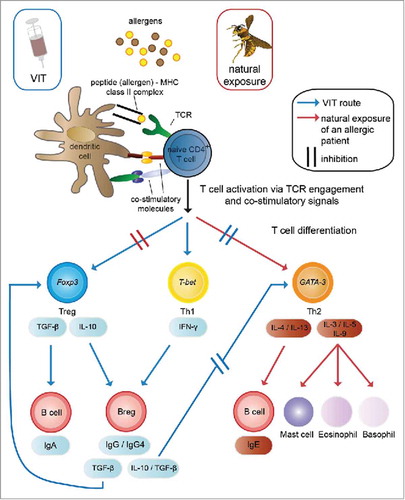
Whereas in an allergic individual upon a sting, venom-specific Th2 cells are thought to induce and initiate the allergic reaction, venom-specific regulatory T cells (Treg) are thought to be induced during VIT. Citation99 These Tregs are able to suppress the pro-allergic Th2 cells. Further, the Th2 suppression leads to the reduction of the secretion of particular cytokines such as IL-3, IL-4, IL-5, IL-9 and IL-13, reduces the activation and degranulation of mast cells, eosinophils and basophils and, therefore, dampens the inflammation. Citation99
During the last years, the beneficial role of changes within the B cell compartment during VIT, became more and more obvious. Citation100 A switch from IgE-producing pro-allergic B cells to IgG4-producing B cells with a regulatory phenotype (secretion of IL-10 and TGF-β) was shown to be important, as blocking IgG4 antibodies are supposed to have a protective anti-inflammatory role. Citation101 These regulatory B cells are also able to suppress venom-specific T cell proliferation Citation102 and to induce additional Tregs, Citation103,Citation104 and thus amplify the shift toward a tolerogenic phenotype of the immune system (). During the course of immunotherapy, specific IgE increases at the beginning and gradually decreases starting some month after the initiation of immunotherapy. Nevertheless, this time course of sIgE does not correlate with the clinical improvements of allergy. Citation105
These protective shifts in the T and B cell compartment together with the resulting changes in cytokine profiles and antibody classes are useful characteristics which should be monitored during VIT studies, to be able to define predictive markers for clinical outcome of the therapy in the future.
Putative options for therapy monitoring
Until now there is no laboratory test available to predict the safe end point of VIT. In most cases, this is examined by sting challenge tests in a clinic situation. Citation106 This procedure can be quite stressful for patients because of the risk to develop severe systemic reactions. Hence, there is ongoing research effort to find predictive biomarkers to monitor VIT to decide if the therapy has been successful. One big drawback of most of the studies is the lack of sting challenges during or after VIT, to be able to correlate any of the biomarkers to the success of therapy.
Starting in 1983, studies of IgG (and IgE) antibody concentrations in serum against venoms were conducted, but they could not correlate the amount of IgG to the protection against allergy after stings. Citation107,Citation108 Nevertheless, monitoring the thresholds of skin reactivity, total serum IgE, specific IgE, specific IgG and specific IgG4 in patients that completed VIT showed that no severe reactions upon field stings were observed when VIT induced changes in at least 3 of these parameters. Citation109
Studying restimulated PBMCs of patients before, during and after VIT showed a shift from Th2 to Th1 cells Citation110 or a reduction of ICOS upregulation Citation111 and an increase in IL-10 producing T-cells. Citation112 None of the mentioned studies correlated these findings to the clinical outcome of VIT. In line with these results, an induction of regulatory T cells during VIT could be observed. This finding also correlated with a shift of venom-specific IgG4 and IgE, but no correlation to the clinical outcome was made. Citation113
B cell responses of patients on VIT showed clear similarity to non-allergic beekeepers. This includes the expansion of IL-10 producing BR1 cells, plasmablasts and as Api m 1-specific class-switched memory B cells within the IgG4 producing cells. Citation114 However, there are no conclusive data sets that show whether these parameters could be useful markers of clinical successful VIT.
Studies of basophil activation before, during and after VIT show that basophil activation sensitivity at low allergen concentrations decreases during VIT. Citation115,Citation116 Moreover, recent studies demonstrate that there is an increase of basophil sensitivity during therapy, but this increase declines again after 18 months of therapy. Citation117 The lower sensitivity of basophil activation correlates with the increase of serum specific IgG4. Citation118 Correlations to the clinical outcome of therapy were not made in studies with basophil activation. Nevertheless, it could be shown that a higher sensitivity before VIT was associated with more severe side effects during the up-dosing phase of VIT. There is evidence, that no changes in basophil sensitivity can be correlated with a positive sting challenge after therapy. Citation116
Other changes during VIT include the increased expression of osteopontin on mRNA Citation119 and protein level in serum, Citation120 changes of miRNA expression (lower expression of miRNAs involved in allergic inflammation and higher expression of those involved in tolerance induction) during VIT up-dosing Citation121 and the decreased spontaneous release of prostaglandin E2 and an increase of lipoxin A4 at the beginning and a decrease after 6 months of therapy. Citation122 Non of the studied biomarkers were linked to clinical outcomes. These studies are needed, to find predictive markers, so the use of potentially dangerous sting challenge tests are not needed anymore.
Studies of effectiveness and safety
Side effects
Comparable to allergen-specific immunotherapy for other allergens, there is a significant risk of systemic reactions (14.2% honeybee VIT and 2.8% wasp VIT). Citation123 Nevertheless, a study by Mosbech et al. Citation124 showed that most of these systemic reactions do not need medical treatment, proving that the side effects are not severe. The development of LLRs at the site of injection is experienced by 12.7%, 15.2% and 11.5% of patients undergoing bee, wasp and bee and wasp VIT, respectively. Citation123 Such reactions can easily be managed by premedication with anti-histamines or glucocorticoids. Citation125-Citation127 There is even evidence, that premedication with anti-histamines can improve the efficacy of VIT. Citation128 If systemic reactions occur repeatedly, especially before reaching the maintenance dose, the change from standard treatment protocols to rush up-dosing protocols, optionally together with premedication, can be very helpful. Citation129
Premedication may include the use of Omalizumab, a recombinant humanized monoclonal anti-IgE antibody approved as add-on therapy for severe allergic asthma and chronic spontaneous urticaria. Omalizumab binds free IgE and, hence, prevents IgE binding to FcϵRI on mast cells, basophils and eosinophils, consequently leading to its downregulations. Citation130 In patients with repeated systemic reactions to VIT it is difficult to reach the maintenance dose. In several published case reports Omalizumab has been successfully used for the pre-treatment of patients who experienced systemic reactions to VIT, including patients with indolent systemic mastocytosis. Citation131-Citation135 Most of these patients were able to tolerate VIT after Omalizumab pre-treatment. However, the duration of therapy and optimal dosing schedule in this clinical setting is not clearly established and a wide variety of different approaches were used. The dosing schedule in most cases was obtained from the approved table for asthma therapy, which is based on body weight and total IgE. While in some cases a single injection before initiation of VIT was used Citation131 others used 3 to 5 injections. Citation132,Citation136 In other cases Omalizumab therapy and VIT were combined for several months Citation132,Citation133,Citation135 or even an unlimited pre-treatment before every maintenance dose was administered. Citation134 This suggests, that the optimal treatment schedule with Omalizumab depends on the individual response to VIT administration.
Risk factors for severe side effects during VIT include honeybee venom allergy and elevated basal serum tryptase or mastocytosis. Citation137 The use of antihypertensive medication is controversially discussed as a risk factor, as it is a risk factor for anaphylaxis to stings. Citation19,Citation26 Nevertheless, a prospective study of patients taking antihypertensive medication during VIT could show that there is no adverse effect on safety and efficacy. Citation138 The current guidelines recommend, that the start of VIT as well as the up-dosing phase should be avoided during pregnancy, however, maintenance dose treatments should and can be continued. Citation19
Efficacy
The first controlled trial on the efficacy of venom-specific immunotherapy in 197886 was already able to show, that the risk of subsequent systemic reactions caused by re-stings can be reduced to less than 5%. The same study observed that sting reactions that happened during VIT are usually milder than those before treatment. In general, venom immunotherapy is 75–98% effective in preventing sting anaphylaxis, Citation139 whereby efficacy of yellow jacket venom-specific immunotherapy is higher compared with honeybee venom-specific immunotherapy. Citation140 To date it is not known why VIT is not successful in a minor population of venom-allergic patients. So far, the only identified risk factor for treatment failure in honeybee venom allergy is a predominant sensitization (> 50% of sIgE to HBV) to the major allergen Api m 10, Citation43 which is present in the venom as well as in therapeutic extracts only in minimal amounts. Citation63,Citation65 If patients still react to the venom of hymenoptera stings in a sting challenge setting, increasing the dose used for therapy can protect these patients from further reactions. Citation141 The analysis of 7 controlled clinical trials of venom immunotherapy could not show a statistically significant reduction of fatalities with or without treatment due to their rarity, but a significant reduced risk of systemic reactions to a future sting after VIT could be demonstrated. Citation123 Nevertheless, the analysis of the studies mentioned before, suggested that venom immunotherapy is efficient concerning the prevention of allergic reactions of different severities. Furthermore, the authors analyzed 2 non-blinded studies in this context. Here, they could show an significant improvement of quality of life. Citation123 Even after short treatment times of one year, patients had a positive view on VIT, reduced anxiety and less limitations to activities that cause fear of insects. Citation123
Novel approaches to improve immunotherapy
Allergen-specific immunotherapy in general
Allergen-specific immunotherapy is the only curative treatment of allergies of all kinds and despite all efforts to improve patient's quality of life, treatment protocols have not changed in the last years. The patient is treated with increasing dosages of allergen up to a certain maintenance concentration and the treatment continued for several years. The treatment is supposed to re-educate the immune system toward a tolerogenic response to the allergen source. Despite of studies showing significant reduction of symptom scores after allergen-specific immunotherapy, Citation142 this therapy is still facing challenges. The probability of side effects is high and the long duration of therapy (3–5 years) leads to high costs and low patient adherence. Hence, there is a need for novel adjuvant systems to improve the efficacy of therapy and further attempts to increase the safety of allergen-specific immunotherapy.
The most common adjuvant for allergen-specific immunotherapy is aluminum hydroxide (75%) which is in use since 80 y for this therapy. Citation143,Citation144 In products for VIT an excess of aluminum hydroxide is mixed with the venom extract and the mixture injected subcutaneously. Unlike for prophylactic vaccines, the manufacturers of products for allergen-specific immunotherapy are not required to specify the amount of aluminum in their summary of product characteristics or package leaflets. Citation144 In Europe, 1.25 mg aluminum per injection is considered as the maximum value permitted. Citation145 The efficacy of aluminum-based adjuvants is not subject to discussion, but it was shown that they are able to stimulate the immune system into the unwanted Th2 direction and to induce the production of IgE. Citation146,Citation147 In allergen-specific immunotherapy the cumulative aluminum dose can be more than 50 times higher, compared with the vaccination against hepatitis B. Citation144
Alternative adjuvants, which are approved for immunotherapy, include L-tyrosine (depot effect) and the Toll-like receptor agonist monophosphoryl lipid A (MPL). Further adjuvants like LPS, CpG-oligodeoxynucleotides and imiquimod/resiquimod (R837, R848) are currently investigated. Novel delivery systems are liposomes, virus-like particles or biodegradable polymeric carriers. Citation148
For the reduction of side effects through IgE-mediated adverse reactions to injected allergens and to increase the safety of immunotherapy, allergens can be chemically modified. This includes the use of recombinant hypoallergenic molecules, dimers, trimers, fusion proteins or peptides. For an overview of novel approaches in the field of allergen-specific immunotherapy the reader is referred to a recent review. Citation148
Venom-specific immunotherapy
Even though novel treatment approaches were tested already in 1987149 (passive and active immunization to overcome severe side effects), other new methods to improve venom immunotherapy are scarcely investigated and were only tested in animal models. This includes treatment with T-cell epitopes, already published in 1998, Citation150 and the mucosal pretreatment with a single yellow jacket allergen, a procedure that reduced sensitization of mice to yellow jacket venom. Citation151 Moreover, it was shown that novel delivery systems such as PLGA microspheres and microbubbles could be efficiently loaded with venom or venom allergens. Citation152,Citation153 The microbubbles were even used to prevent sensitization in a honeybee venom allergy model in mice. Issues of study design, efficacy and safety for allergen products being developed for specific immunotherapy of allergic diseases are addressed by guidelines of national and international medical agencies. Citation87,Citation145
Conclusion
Venom-specific immunotherapy is proven to be highly effective in improving the patient's quality of life and to significantly reduce the risk for systemic sting reactions during and after therapy. The increasing knowledge about the molecular composition of hymenoptera venoms has created added clinical value over the last decade. Component-resolved diagnostics using recombinant CCD-free allergens enables the differentiation between cross-reactivity and true allergy, and thus in many patients improves the selection of the appropriate immunotherapeutic intervention. Nevertheless, there is still a lack of information about the relevant allergens present in the venoms of different hymenoptera species. This knowledge would help to further increase the sensitivity and specificity of diagnostics and to improve therapeutic extracts used in the clinics. The aim of current developments is to increase efficacy of therapy, and therefore to reduce treatment times and to increase patient compliance. In line, there is a need to identify biomarkers that can efficiently predict the end point of therapy.
As a consequence of the development of component-resolved diagnostic approaches, enabling the identification of various different sensitization profiles to particular venom allergens, a patient-tailored recombinant immunotherapy would be highly desirable. Such a therapy would allow treating the patient only with adequate amounts of the allergens he is sensitized to. Considering the complex sensitization profiles of venom allergic-patients and the unequal distribution of relevant allergens in therapeutic extracts, such a therapy might have a high potential for superior efficacy and, moreover, would avoid de novo sensitizations to additional allergens. However, due to current regulatory requirements for the approval of novel products for allergen-specific immunotherapy and associated costs, such developments might be in the distant future.
Abbreviations
| AAAAI | = |
American Academy of Allergy, Asthma and Immunology |
| ACE | = |
angiotensin-converting enzyme |
| Api m | = |
Apis mellifera |
| BAT | = |
basophil activation test |
| Breg | = |
regulatory B cell |
| CCD | = |
cross-reactive carbohydrate determinant |
| CD | = |
cluster of differentiation |
| CRD | = |
component-resolved diagnosis |
| DPP | = |
dipeptidyl peptidase |
| EAACI | = |
European Academy of Allergy and Clinical Immunology |
| E. coli | = |
Escherichia coli |
| Foxp3 | = |
Forkhead Box P3 |
| GATA-3 | = |
GATA binding protein 3 |
| ICOS | = |
inducible T cell costimulator |
| Ig | = |
Immunoglobulin |
| IL | = |
interleukin |
| LLR | = |
large local reaction |
| LPS | = |
lipopolysaccharide |
| MHC | = |
major histocompatibility complex |
| MPL | = |
monophosphoryl lipid A |
| MRJP | = |
major royal jelly protein |
| PLGA | = |
poly lactic-co-glacolic acid |
| Pol d | = |
Polistes dominula |
| r | = |
recombinant |
| Sf9 | = |
Spodoptera frugiperda |
| sIgE | = |
specific Immunoglobulin E |
| SR | = |
systemic (generalized) reaction |
| T-bet | = |
T-Box 21 |
| TGF- β | = |
transforming growth factor β |
| Th | = |
T helper |
| Treg | = |
regulatory T cell |
| Ves v | = |
Vespula vulgaris |
| VIT | = |
venom-specific immunotherapy |
Disclosure of potential conflicts of interest
MS has received travel support from ALK-Abelló and Bencard. MO has received consultancy fees from Siemens Healthcare, Hitachi Chemical Diagnostics and Bencard; has received lecture fees from Thermo Fisher Scientific, Bencard and Siemens Healthcare; is co-founder of PLS-Design GmbH. CBS-W has received grants from Allergopharma, Leti, PLS-Design and Regeneron; is member of the scientific advisory board of Leti and Bencard; has received consultancy fees from Leti, GLG Consultancy, Allergopharma and Bencard. SB has received speaker's honorarium and/or travel support from ALK-Abelló, Bencard and Thermo Fisher Scientific; has received consultancy fees as an advisory board member and research support from Bencard. AG declares to have no conflict of interest.
References
- Golden DB. Anaphylaxis to insect stings. Immunol Allergy Clin North Am 2015; 35:287-302; PMID:25841552; https://doi.org/10.1016/j.iac.2015.01.007
- Tankersley MS , Ledford DK . Stinging Insect Allergy: State of the Art 2015. J Allergy Clin Immunol Pract 2015; 3:315-22; PMID:25956310; https://doi.org/10.1016/j.jaip.2015.03.012
- Potiwat R , Sitcharungsi R . Ant allergens and hypersensitivity reactions in response to ant stings. Asian Pacific J allergy Immunol 2015; 33:267-75
- Cervo R , Zacchi F , Turillazzi S . Polistes dominulus (Hymenoptera, Vespidae) invading North America: some hypotheses for its rapid spread. Insectes Soc 2000; 47:155-7; https://doi.org/10.1007/PL00001694
- Eardley C , Koch F , Wood AR . Polistes dominulus (Christ, 1791) (Hymenoptera: Polistinae: Vespidae) newly recorded from South Africa. African Entomol 2009; 17:226-7; https://doi.org/10.4001/003.017.0214
- Höcherl N , Tautz J . Nesting behavior of the paper wasp Polistes dominula in Central Europe—a flexible system for expanding into new areas. Ecosphere 2015; 6:1-11; https://doi.org/10.1890/ES15-00254.1
- Schumacher M , Tveten M , Egen N . Rate and quantity of delivery of venom from honeybee stings. J Allergy Clin Immunol 1994; 93:831-5; PMID:8182223; https://doi.org/10.1016/0091-6749(94)90373-5
- Hoffman DR , Jacobson RS . Allergens in hymenoptera venom XII: How much protein is in a sting? Ann Allergy 1984; 52:276-8; PMID:6711914
- Worm M , Moneret-Vautrin A , Scherer K , Lang R , Fernandez-Rivas M , Cardona V , Kowalski ML , Jutel M , Poziomkowska-Gesicka I , Papadopoulos NG , et al. First European data from the network of severe allergic reactions (NORA). Allergy 2014; 69:1397-404; PMID:24989080; https://doi.org/10.1111/all.12475
- Severino M , Bonadonna P , Passalacqua G . Large local reactions from stinging insects: from epidemiology to management. Curr Opin Allergy Clin Immunol 2009; 9:334-7; PMID:19458526; https://doi.org/10.1097/ACI.0b013e32832d0668
- Wright DN , Lockey RF . Local Reactions to Stinging Insects (Hymenoptera). Allergy Asthma Proc 1990; 11:23-8; https://doi.org/10.2500/108854190778999474
- Mauriello P , Barde S , Georgitis J , Reisman R . Natural history of large local reactions from stinging insects. J Allergy Clin Immunol 1984; 74:494-8; PMID:6491095; https://doi.org/10.1016/0091-6749(84)90384-1
- Schuberth KC , Lichtenstein LM , Kagey-Sobotka A , Szklo M , Kwiterovich KA , Valentine MD . An epidemiologic study of insect allergy in children. I. Characteristics of the disease. J Pediatr 1982; 100:546-51; PMID:7062201; https://doi.org/10.1016/S0022-3476(82)80750-6
- Graft DF , Schuberth KC , Kagey-Sobotka A , Kwiterovich KA , Niv Y , Lichtenstein LM , Valentine MD . A prospective study of the natural history of large local reactions after Hymenoptera stings in children. J Pediatr 1984; 104:664-8; PMID:6716215; https://doi.org/10.1016/S0022-3476(84)80940-3
- Abrecht I , Eichler G , Müller U , Hoigné R . On the significance of severe local reactions to Hymenoptera stings. Clin Allergy 1980; 10:675-82; PMID:7460262; https://doi.org/10.1111/j.1365-2222.1980.tb02151.x
- Fernandez J , Soriano V , Mayorga L , Mayor M . Natural history of Hymenoptera venom allergy in Eastern Spain. Clin Exp Allergy 2005; 35:179-85; PMID:15725189; https://doi.org/10.1111/j.1365-2222.2005.02169.x
- Pucci S , D'Alò S , De Pasquale T , Illuminati I , Makri E , Incorvaia C . Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy 2015; 13:21; PMID:26557045; https://doi.org/10.1186/s12948-015-0030-z
- Valentine MD , Schuberth KC , Kagey-Sobotka A , Graft DF , Kwiterovich KA , Szklo M , Lichtenstein LM . The Value of Immunotherapy with Venom in Children with Allergy to Insect Stings. N Engl J Med 1990; 323:1601-3; PMID:2098016; https://doi.org/10.1056/NEJM199012063232305
- Golden DBK , Demain J , Freeman T , Graft D , Tankersley M , Tracy J , Blessing-Moore J , Bernstein D , Dinakar C , Greenhawt M , et al. Stinging insect hypersensitivity. Ann Allergy Asthma Immunol 2017; 118:28-54; https://doi.org/10.1016/j.anai.2016.10.031
- Bilò BM , Bonifazi F . Epidemiology of insect-venom anaphylaxis. Curr Opin Allergy Clin Immunol 2008; 8:330-7; PMID:18596590; https://doi.org/10.1097/ACI.0b013e32830638c5
- Golden DB . Epidemiology of insect venom sensitivity. JAMA J Am Med Assoc 1989; 262:240-4; https://doi.org/10.1001/jama.1989.03430020082033
- Pumphrey RSH . Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy 2000; 30:1144-50; PMID:10931122; https://doi.org/10.1046/j.1365-2222.2000.00864.x
- Biló BM , Rueff F , Mosbech H , Bonifazi F , Oude-Elberink JNG , Birnbaum J , Bucher C , Forster J , Hemmer W , Incorvaia C , et al. Diagnosis of Hymenoptera venom allergy. Allergy 2005; 60:1339-49; PMID:16197464; https://doi.org/10.1111/j.1398-9995.2005.00963.x
- Haeberli G , Brönnimann M , Hunziker T , Müller U . Elevated basal serum tryptase and hymenoptera venom allergy: Relation to severity of sting reactions and to safety and efficacy of venom immunotherapy. Clin Exp Allergy 2003; 33:1216-20; PMID:12956741; https://doi.org/10.1046/j.1365-2222.2003.01755.x
- Niedoszytko M , Bonadonna P , Elberink JNGO , Golden DBK . Epidemiology, diagnosis, and treatment of Hymenoptera venom allergy in mastocytosis patients. Immunol Allergy Clin North Am 2014; 34:365-81; PMID:24745680; https://doi.org/10.1016/j.iac.2014.02.004
- Ruëff F , Przybilla B , Biló MB , Müller U , Scheipl F , Aberer W , Birnbaum J , Bodzenta-Lukaszyk A , Bonifazi F , Bucher C , et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: Importance of baseline serum tryptase—a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. J Allergy Clin Immunol 2009; 124:1047-54; PMID:19895993; https://doi.org/10.1016/j.jaci.2009.08.027
- Pastorello EA , Incorvaia C , Sarassi A , Qualizza R , Bigi A , Farioli L . Epidemiological and clinical study on bee venom allergy among beekeepers. Boll Ist Sieroter Milan 1988; 67:386-92; PMID:3154984
- Settipane GA , Newstead GJ , Boyd GK . Frequency of Hymenoptera allergy in an atopic and normal population. J Allergy Clin Immunol 1972; 50:146-50; PMID:5050325; https://doi.org/10.1016/0091-6749(72)90045-0
- Juarez C , Blanca M , Miranda A , Sanchez F , Carmona MJ , Avila MJ , Fernandez S , Fernandez J , Terrados S . Specific IgE antibodies to vespids in the course of immunotherapy with Vespula germanica administered to patients sensitized to Polistes dominulus. Allergy 1992; 47:299-302; PMID:1443448; https://doi.org/10.1111/j.1398-9995.1992.tb02057.x
- Baker TW , Forester JP , Johnson ML , Stolfi A , Stahl MC . The HIT study: Hymenoptera Identification Test - How accurate are people at identifying stinging insects? Ann Allergy Asthma Immunol 2014; 113:267-70; PMID:24969241; https://doi.org/10.1016/j.anai.2014.05.029
- Baker TW , Forester JP , Johnson ML , Sikora JM , Stolfi A , Stahl MC . Stinging insect identification: Are the allergy specialists any better than their patients? Ann Allergy Asthma Immunol 2016; 116:431-4; PMID:26993171; https://doi.org/10.1016/j.anai.2016.01.025
- Przybilla B , Ruëff F , Walker A , Räwer H-C , Aberer W , Bauer CP , Berdel D , Biedermann T , Brockow K , Forster J , et al. Diagnose und Therapie der Bienen- und Wespengiftallergie. Allergo J 2011; 20:318-39; https://doi.org/10.1007/BF03362543
- Bilò BM , Bonifazi F . Hymenoptera venom immunotherapy. Immunotherapy 2011; 3:229-46; PMID:21322761; https://doi.org/10.2217/imt.10.88
- Ludman SW , Boyle RJ . Stinging insect allergy: current perspectives on venom immunotherapy. J Asthma Allergy 2015; 8:75-86; PMID:26229493
- Michel J , Brockow K , Darsow U , Ring J , Schmidt-Weber CB , Grunwald T , Blank S , Ollert M . Added sensitivity of component-resolved diagnosis in hymenoptera venom-allergic patients with elevated serum tryptase and/or mastocytosis. Allergy 2016; 71:651-60; PMID:26836051; https://doi.org/10.1111/all.12850
- Müller UR . Recombinant Hymenoptera venom allergens. Allergy 2002; 57:570-6; PMID:12100296; https://doi.org/10.1034/j.1398-9995.2002.02157.x
- Vachová M , Panzner P , Malkusová I , Hanzlíková J , Vlas T . Utility of laboratory testing for the diagnosis of Hymenoptera venom allergy. Allergy Asthma Proc 2016; 37:248-55; PMID:27178893; https://doi.org/10.2500/aap.2016.37.3934
- Mosbech H , Tang L , Linneberg A . Insect Sting Reactions and Specific IgE to Venom and Major Allergens in a General Population. Int Arch Allergy Immunol 2016; 170:194-200; PMID:27591992; https://doi.org/10.1159/000448399
- Sturm GJ , Kranzelbinder B , Schuster C , Sturm EM , Bokanovic D , Vollmann J , Crailsheim K , Hemmer W , Aberer W . Sensitization to Hymenoptera venoms is common, but systemic sting reactions are rare. J Allergy Clin Immunol 2014; 133:1635-43.e1; PMID:24365141; https://doi.org/10.1016/j.jaci.2013.10.046
- Blank S , Michel Y , Seismann H , Plum M , Greunke K , Grunwald T , Bredehorst R , Ollert M , Braren I , Spillner E . Evaluation of Different Glycoforms of Honeybee Venom Major Allergen Phospholipase A2 (Api m 1) Produced in Insect Cells. Protein Pept Lett 2011; 18:415-22; PMID:21171948; https://doi.org/10.2174/092986611794653923
- Köhler J , Blank S , Müller S , Bantleon F , Frick M , Huss-Marp J , Lidholm J , Spillner E , Jakob T . Component resolution reveals additional major allergens in patients with honeybee venom allergy. J Allergy Clin Immunol 2014; 133:1383-9.e6; PMID:24440283; https://doi.org/10.1016/j.jaci.2013.10.060
- Ollert M , Blank S . Anaphylaxis to Insect Venom Allergens: Role of Molecular Diagnostics. Curr Allergy Asthma Rep 2015; 15:26; PMID:26139335; https://doi.org/10.1007/s11882-015-0527-z
- Frick M , Fischer J , Helbling A , Ruëff F , Wieczorek D , Ollert M , Pfützner W , Müller S , Huss-Marp J , Dorn B , et al. Predominant Api m 10 sensitization as risk factor for treatment failure in honey bee venom immunotherapy. J Allergy Clin Immunol 2016; 138:1663-71.e9; PMID:27372568; https://doi.org/10.1016/j.jaci.2016.04.024
- Radauer C , Nandy A , Ferreira F , Goodman RE , Larsen JN , Lidholm J , Pomés A , Raulf-Heimsoth M , Rozynek P , Thomas WR , et al. Update of the WHO/IUIS Allergen Nomenclature Database based on analysis of allergen sequences. Allergy 2014; 69:413-9; PMID:24738154; https://doi.org/10.1111/all.12348
- Peiren N , Vanrobaeys F , de Graaf DC , Devreese B , Van Beeumen J , Jacobs FJ . The protein composition of honeybee venom reconsidered by a proteomic approach. Biochim Biophys Acta 2005; 1752:1-5; PMID:16112630; https://doi.org/10.1016/j.bbapap.2005.07.017
- Van Vaerenbergh M , Debyser G , Devreese B , de Graaf DC . Exploring the hidden honeybee (Apis mellifera) venom proteome by integrating a combinatorial peptide ligand library approach with FTMS. J Proteomics 2014; 99:169-78; PMID:24606962; https://doi.org/10.1016/j.jprot.2013.04.039
- Van Vaerenbergh M , Cardoen D , Formesyn EM , Brunain M , Van Driessche G , Blank S , Spillner E , Verleyen P , Wenseleers T , Schoofs L , et al. Extending the honey bee venome with the antimicrobial peptide apidaecin and a protein resembling wasp antigen 5. Insect Mol Biol 2013; 22:199-210; PMID:23350689; https://doi.org/10.1111/imb.12013
- Weinstock GM , Robinson GE , Gibbs RA , Weinstock GM , Weinstock GM , Robinson GE , Worley KC , Evans JD , Maleszka R , Robertson HM , et al. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 2006; 443:931-49; PMID:17073008; https://doi.org/10.1038/nature05260
- Standage DS , Berens AJ , Glastad KM , Severin AJ , Brendel VP , Toth AL . Genome, transcriptome and methylome sequencing of a primitively eusocial wasp reveal a greatly reduced DNA methylation system in a social insect. Mol Ecol 2016; 25:1769-84; PMID:26859767; https://doi.org/10.1111/mec.13578
- Binder M , Fierlbeck G , King TP , Valent P , Bühring HJ . Individual Hymenoptera venom compounds induce upregulation of the basophil activation marker ectonucleotide pyrophosphatase/phosphodiesterase 3 (CD203c) in sensitized patients. Int Arch Allergy Immunol 2002; 129:160-8; PMID:12403934; https://doi.org/10.1159/000065875
- Kolarich D , Leonard R , Hemmer W , Altmann F . The N-glycans of yellow jacket venom hyaluronidases and the protein sequence of its major isoform in Vespula vulgaris. FEBS J 2005; 272:5182-90; PMID:16218950; https://doi.org/10.1111/j.1742-4658.2005.04841.x
- Hoffman D , Shipman W , Babin D . Allergens in bee venom II. Two new high molecular weight allergenic specificities. J Allergy Clin Immunol 1977; 59:147-53; PMID:401840; https://doi.org/10.1016/0091-6749(77)90217-2
- Hoffman DR . Allergens in bee venom. III. Identification of allergen B of bee venom as an acid phosphatase. J Allergy Clin Immunol 1977; 59:364-6; PMID:853176; https://doi.org/10.1016/0091-6749(77)90019-7
- Arbesman CE , Reisman RE , Wypych JI . Allergenic potency of bee antigens measured by RAST inhibition. Clin Allergy 1976; 6:587-95; PMID:1016291; https://doi.org/10.1111/j.1365-2222.1976.tb01945.x
- King TP , Spangfort MD . Structure and Biology of Stinging Insect Venom Allergens. Int Arch Allergy Immunol 2000; 123:99-106; PMID:11060481; https://doi.org/10.1159/000024440
- Schiener M , Eberlein B , Moreno-Aguilar C , Pietsch G , Serrano P , Mcintyre M , Schwarze L , Russkamp D , Biedermann T , Spillner E , et al. Application of recombinant antigen 5 allergens from seven allergy-relevant Hymenoptera species in diagnostics. Allergy 2017; 72:98-108; PMID:27496543; https://doi.org/10.1111/all.13000
- Blank S , Seismann H , Bockisch B , Braren I , Cifuentes L , McIntyre M , Ruhl D , Ring J , Bredehorst R , Ollert MW , et al. Identification, Recombinant Expression, and Characterization of the 100 kDa High Molecular Weight Hymenoptera Venom Allergens Api m 5 and Ves v 3. J Immunol 2010; 184:5403-13; PMID:20348419; https://doi.org/10.4049/jimmunol.0803709
- Kettner A , Hughes GJ , Frutiger S , Astori M , Roggero M , Spertini F , Corradin G . Api m 6: A new bee venom allergen. J Allergy Clin Immunol 2001; 107:914-20; PMID:11344362; https://doi.org/10.1067/mai.2001.113867
- Michel Y , McIntyre M , Ginglinger H , Ollert M , Cifuentes L , Blank S , Spillner E . The putative serine protease inhibitor Api m 6 from Apis Mellifera venom: Recombinant and structural evaluation. J Investig Allergol Clin Immunol 2012; 22:476-84; PMID:23397669
- Winningham KM , Fitch CD , Schmidt M , Hoffman DR . Hymenoptera venom protease allergens. J Allergy Clin Immunol 2004; 114:928-33; PMID:15480337; https://doi.org/10.1016/j.jaci.2004.07.043
- Blank S , Bantleon FI , McIntyre M , Ollert M , Spillner E . The major royal jelly proteins 8 and 9 (Api m 11) are glycosylated components of Apis mellifera venom with allergenic potential beyond carbohydrate-based reactivity. Clin Exp Allergy 2012; 42:976-85; PMID:22909169; https://doi.org/10.1111/j.1365-2222.2012.03966.x
- Blank S , Seismann H , McIntyre M , Ollert M , Wolf S , Bantleon FI , Spillner E . Vitellogenins Are New High Molecular Weight Components and Allergens (Api m 12 and Ves v 6) of Apis mellifera and Vespula vulgaris Venom. PLoS One 2013; 8:e62009; PMID:23626765; https://doi.org/10.1371/journal.pone.0062009
- Peiren N , de Graaf DC , Brunain M , Bridts CH , Ebo DG , Stevens WJ , Jacobs FJ . Molecular cloning and expression of icarapin, a novel IgE-binding bee venom protein. FEBS Lett 2006; 580:4895-9; PMID:16914147; https://doi.org/10.1016/j.febslet.2006.08.005
- Van Vaerenbergh M , De Smet L , Rafei-Shamsabadi D , Blank S , Spillner E , Ebo DG , Devreese B , Jakob T , de Graaf DC . IgE recognition of chimeric isoforms of the honeybee (Apis mellifera) venom allergen Api m 10 evaluated by protein array technology. Mol Immunol 2015; 63:449-55; PMID:25451974; https://doi.org/10.1016/j.molimm.2014.09.018
- Blank S , Seismann H , Michel Y , McIntyre M , Cifuentes L , Braren I , Grunwald T , Darsow U , Ring J , Bredehorst R , et al. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Allergy 2011; 66:1322-9; PMID:21658068; https://doi.org/10.1111/j.1398-9995.2011.02667.x
- Matricardi PM , Kleine-Tebbe J , Hoffmann HJ , Valenta R , Hilger C , Hofmaier S , Aalberse RC , Agache I , Asero R , Ballmer-Weber B , et al. EAACI Molecular Allergology User's Guide. Pediatr Allergy Immunol 2016; 27:1-250; PMID:27288833; https://doi.org/10.1111/pai.12563
- Jappe U , Raulf-Heimsoth M , Hoffmann M , Burow G , Hubsch-Muller C , Enk A . In vitro hymenoptera venom allergy diagnosis: improved by screening for cross-reactive carbohydrate determinants and reciprocal inhibition. Allergy 2006; 61:1220-9; PMID:16942573; https://doi.org/10.1111/j.1398-9995.2006.01232.x
- Jin C , Hantusch B , Hemmer W , Stadlmann J , Altmann F . Affinity of IgE and IgG against cross-reactive carbohydrate determinants on plant and insect glycoproteins. J Allergy Clin Immunol 2008; 121:185-190.e2; PMID:17881041; https://doi.org/10.1016/j.jaci.2007.07.047
- Altmann F . Coping with cross-reactive carbohydrate determinants in allergy diagnosis. Allergo J Int 2016; 25:98-105; PMID:27656353; https://doi.org/10.1007/s40629-016-0115-3
- Seismann H , Blank S , Braren I , Greunke K , Cifuentes L , Grunwald T , Bredehorst R , Ollert M , Spillner E . Dissecting cross-reactivity in hymenoptera venom allergy by circumvention of α-1,3-core fucosylation. Mol Immunol 2010; 47:799-808; PMID:19896717; https://doi.org/10.1016/j.molimm.2009.10.005
- Blank S , Neu C , Hasche D , Bantleon FI , Jakob T , Spillner E . Polistes species venom is devoid of carbohydrate-based cross-reactivity and allows interference-free diagnostics. J Allergy Clin Immunol 2013; 131:1239-42; PMID:23228245; https://doi.org/10.1016/j.jaci.2012.10.047
- Hofmann SC , Pfender N , Weckesser S , Huss-Marp J , Jakob T . Added value of IgE detection to rApi m 1 and rVes v 5 in patients with Hymenoptera venom allergy. J Allergy Clin Immunol 2011; 127:265-7; PMID:20719373; https://doi.org/10.1016/j.jaci.2010.06.042
- Korošec P , Valenta R , Mittermann I , Čelesnik N , Šilar M , Zidarn M , Košnik M . High sensitivity of CAP-FEIA rVes v 5 and rVes v 1 for diagnosis of Vespula venom allergy. J Allergy Clin Immunol 2012; 129:1406-8; PMID:22277201; https://doi.org/10.1016/j.jaci.2011.12.975
- Sturm GJ , Hemmer W , Hawranek T , Lang R , Ollert M , Spillner E , Blank S , Bokanovic D , Aberer W . Detection of IgE to recombinant Api m 1 and rVes v 5 is valuable but not sufficient to distinguish bee from wasp venom allergy. J Allergy Clin Immunol 2011; 128:247-8; PMID:21439627; https://doi.org/10.1016/j.jaci.2011.02.021
- Müller UR , Johansen N , Petersen AB , Fromberg-Nielsen J , Haeberli G . Hymenoptera venom allergy: analysis of double positivity to honey bee and Vespula venom by estimation of IgE antibodies to species-specific major allergens Api m1 and Ves v5. Allergy 2009; 64:543-8; PMID:19120073; https://doi.org/10.1111/j.1398-9995.2008.01794.x
- Müller U , Schmid-Grendelmeier P , Hausmann O , Helbling A . IgE to recombinant allergens Api m 1, Ves v 1, and Ves v 5 distinguish double sensitization from crossreaction in venom allergy. Allergy 2012; 67:1069-73; PMID:22676144; https://doi.org/10.1111/j.1398-9995.2012.02847.x
- Caruso B , Bonadonna P , Severino MG , Manfredi M , Dama A , Schiappoli M , Rizzotti P , Senna G , Passalacqua G . Evaluation of the IgE cross-reactions among vespid venoms. A possible approach for the choice of immunotherapy. Allergy 2007; 62:561-4; PMID:17441797; https://doi.org/10.1111/j.1398-9995.2007.01353.x
- Monsalve RI , Vega A , Marqués L , Miranda A , Fernández J , Soriano V , Cruz S , Domínguez-Noche C , Sánchez-Morillas L , Armisen-Gil M , et al. Component-resolved diagnosis of vespid venom-allergic individuals: phospholipases and antigen 5s are necessary to identify Vespula or Polistes sensitization. Allergy 2012; 67:528-36; PMID:22229815; https://doi.org/10.1111/j.1398-9995.2011.02781.x
- Korošec P , Šilar M , Eržen R , Čelesnik N , Bajrović N , Zidarn M , Košnik M . Clinical routine utility of Basophil activation testing for diagnosis of hymenoptera-allergic patients with emphasis on individuals with negative venom-specific IgE antibodies. Int Arch Allergy Immunol 2013; 161:363-8; PMID:23689117; https://doi.org/10.1159/000348500
- Eberlein-König B , Rakoski J , Behrendt H , Ring J . Use of CD63 expression as marker of in vitro basophil activation in identifying the culprit in insect venom allergy. J Investig Allergol Clin Immunol 2004; 14:10-6; PMID:15160437
- Eberlein B , Krischan L , Darsow U , Ollert M , Ring J . Double positivity to bee and wasp venom: Improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J Allergy Clin Immunol 2012; 130:155-61; PMID:22421265; https://doi.org/10.1016/j.jaci.2012.02.008
- González-De-Olano D , Álvarez-Twose I , Morgado JM , Esteban López MI , Castro AV , Díaz De Durana MD , Sánchez-Muñoz L , Matito A , De La Hoz Caballer B , Sanz ML , et al. Evaluation of basophil activation in mastocytosis with hymenoptera venom anaphylaxis. Cytom Part B Clin Cytom 2011; 80 B:167-75; https://doi.org/10.1002/cyto.b.20577
- Bonadonna P , Zanotti R , Melioli G , Antonini F , Romano I , Lenzi L , Caruso B , Passalacqua G . The role of basophil activation test in special populations with mastocytosis and reactions to hymenoptera sting. Allergy 2012; 67:962-5; PMID:22676063; https://doi.org/10.1111/j.1398-9995.2012.02849.x
- Rietveld MJ , Schreurs MW , Gerth Van Wijk R , Van Daele PL , Hermans MA . The basophil activation test is not a useful screening tool for hymenoptera venom-related anaphylaxis in patients with systemic mastocytosis. Int Arch Allergy Immunol 2016; 169:125-9; PMID:27055231; https://doi.org/10.1159/000444996
- Braun D . Notes on Desensitisation of a Patient Hypersensitive to Bee Stings. South African Med J 1925; 18:408-9
- Hunt KJ , Valentine MD , Sobotka AK , Benton AW , Amodio FJ , Lichtenstein LM . A Controlled Trial of Immunotherapy in Insect Hypersensitivity. N Engl J Med 1978; 299:157-61; PMID:78446; https://doi.org/10.1056/NEJM197807272990401
- Pfaar O , Bachert C , Bufe A , Buhl R , Ebner C , Eng P , Friedrichs F , Fuchs T , Hamelmann E , Hartwig-Bade D , et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases. Allergo J Int 2014; 23:282-319; PMID:26120539; https://doi.org/10.1007/s40629-014-0032-2
- Roumana A , Pitsios C , Vartholomaios S , Kompoti E , Kontou-Fili K . The safety of initiating Hymenoptera immunotherapy at 1 μg of venom extract. J Allergy Clin Immunol 2009; 124:379-81; PMID:19560804; https://doi.org/10.1016/j.jaci.2009.05.026
- Sturm G , Kränke B , Rudolph C , Aberer W . Rush Hymenoptera venom immunotherapy: A safe and practical protocol for high-risk patients. J Allergy Clin Immunol 2002; 110:928-33; PMID:12464961; https://doi.org/10.1067/mai.2002.129124
- Brown SGA , Wiese MD , van Eeden P , Stone SF , Chuter CL , Gunner J , Wanandy T , Phillips M , Heddle RJ . Ultrarush versus semirush initiation of insect venom immunotherapy: A randomized controlled trial. J Allergy Clin Immunol 2012; 130:162-8; PMID:22460067; https://doi.org/10.1016/j.jaci.2012.02.022
- Goldberg A , Confino-Cohen R . Bee venom immunotherapy - how early is it effective? Allergy 2010; 65:391-5; PMID:19839973; https://doi.org/10.1111/j.1398-9995.2009.02198.x
- Goldberg A , Yogev A , Confino-Cohen R . Three Days Rush Venom Immunotherapy in Bee Allergy: Safe, Inexpensive and Instantaneously Effective. Int Arch Allergy Immunol 2011; 156:90-8; PMID:21447964; https://doi.org/10.1159/000322258
- Cavallucci E , Ramondo S , Renzetti A , Turi MC , Di Claudio F , Braga M , Incorvaia C , Schiavone C , Ballone E , Di Gioacchino M . Maintenance venom immunotherapy administered at a 3-month interval preserves safety and efficacy and improves adherence. J Investig Allergol Clin Immunol 2010; 20:63-8; PMID:20232775
- Golden DBK . Discontinuing venom immunotherapy. Curr Opin Allergy Clin Immunol 2001; 1:353-6; PMID:11964712; https://doi.org/10.1097/00130832-200108000-00012
- Golden DB , Kwiterovich KA , Kagey-Sobotka A , Valentine MD , Lichtenstein LM . Discontinuing venom immunotherapy: outcome after five years. J Allergy Clin Immunol 1996; 97:579-87; PMID:8621842; https://doi.org/10.1016/S0091-6749(96)70302-0
- Bonifazi F , Jutel M , Bilo BM , Birnbaum J , Muller U . Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice. Allergy 2005; 60:1459-70; PMID:16266376; https://doi.org/10.1111/j.1398-9995.2005.00960.x
- Golden DBK , Kwiterovich KA , Kagey-Sobotka A , Lichtenstein LM . Discontinuing venom immunotherapy: Extended observations. J Allergy Clin Immunol 1998; 101:298-305; PMID:9525443; https://doi.org/10.1016/S0091-6749(98)70239-8
- Golden DB . Long-term outcome after venom immunotherapy. Curr Opin Allergy Clin Immunol 2010; 10:337-41; PMID:20610978; https://doi.org/10.1097/ACI.0b013e32833bc0ba
- Akdis CA , Akdis M . Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J 2015; 8:17; PMID:26023323; https://doi.org/10.1186/s40413-015-0063-2
- van de Veen W , Stanic B , Wirz OF , Jansen K , Globinska A , Akdis M . Role of regulatory B cells in immune tolerance to allergens and beyond. J Allergy Clin Immunol 2016; 138:654-65; PMID:27596706; https://doi.org/10.1016/j.jaci.2016.07.006
- Möbs C , Ipsen H , Mayer L , Slotosch C , Petersen A , Würtzen PA , Hertl M , Pfützner W . Birch pollen immunotherapy results in long-term loss of Bet v 1–specific TH2 responses, transient TR1 activation, and synthesis of IgE-blocking antibodies. J Allergy Clin Immunol 2012; 130:1108-16.e6; PMID:23021882; https://doi.org/10.1016/j.jaci.2012.07.056
- van de Veen W , Stanic B , Yaman G , Wawrzyniak M , Söllner S , Akdis DG , Rückert B , Akdis CA , Akdis M . IgG4 production is confined to human IL-10–producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol 2013; 131:1204-12; PMID:23453135; https://doi.org/10.1016/j.jaci.2013.01.014
- Kessel A , Haj T , Peri R , Snir A , Melamed D , Sabo E , Toubi E . Human CD19+CD25high B regulatory cells suppress proliferation of CD4+ T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev 2012; 11:670-7; PMID:22155204; https://doi.org/10.1016/j.autrev.2011.11.018
- Lee KM , Stott RT , Zhao G , SooHoo J , Xiong W , Lian MM , Fitzgerald L , Shi S , Akrawi E , Lei J , et al. TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol 2014; 44:1728-36; PMID:24700192; https://doi.org/10.1002/eji.201344062
- Akdis CA , Akdis M . Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol 2011; 127:18-27; PMID:21211639; https://doi.org/10.1016/j.jaci.2010.11.030
- Ruëff F , Przybilla B , Müller U , Mosbech H . The sting challenge test in Hymenoptera venom allergy Position paper of the subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy 1996; 51:216-25; PMID:8792917
- Urbanek R , Krauss U , Ziupa J , Smedegard G . Venom-specific IgE and IgG antibodies as a measure of the degree of protection in insect-sting-sensitive patients. Clin Allergy 1983; 13:229-34; PMID:6602010; https://doi.org/10.1111/j.1365-2222.1983.tb02592.x
- Ewan PW , Deighton J , Wilson AB , Lachmann PJ . Venom-specific IgG antibodies in bee and wasp allergy: lack of correlation with protection from stings. Clin Exp Allergy 1993; 23:647-60; PMID:8221268; https://doi.org/10.1111/j.1365-2222.1993.tb01791.x
- Brasch J , Maidusch T . Immunotherapy with wasp venom is accompanied by wide-ranging immune responses that need further exploration. Acta Derm Venereol 2009; 89:466-9; PMID:19734970; https://doi.org/10.2340/00015555-0690
- Kosnik M , Wraber B . Shift from Th2 to Th1 response in immunotherapy with venoms. Pflugers Arch 2000; 440:R70-1; PMID:11005617; https://doi.org/10.1007/s004240000010
- Bellinghausen I , Klostermann B , Bottcher I , Knop J , Saloga J . Importance of the inducible costimulator molecule for the induction of allergic immune responses and its decreased expression on T helper cells after venom immunotherapy. Immunology 2004; 112:80-6; PMID:15096187; https://doi.org/10.1111/j.1365-2567.2004.01845.x
- Mamessier E , Birnbaum J , Dupuy P , Vervloet D , Magnan A . Ultra-rush venom immunotherapy induces differential T cell activation and regulatory patterns according to the severity of allergy. Clin Exp Allergy 2006; 36:704-13; PMID:16776670; https://doi.org/10.1111/j.1365-2222.2006.02487.x
- Pereira-Santos MC , Baptista AP , Melo A , Alves RR , Soares RS , Pedro E , Pereira-Barbosa M , Victorino RMM , Sousa AE . Expansion of circulating Foxp3+CD25bright CD4 + T cells during specific venom immunotherapy. Clin Exp Allergy 2008; 38:291-7; PMID:18070166; https://doi.org/10.1111/j.1365-2222.2007.02887.x
- Boonpiyathad T , Meyer N , Moniuszko M , Sokolowska M , Eljaszewicz A , Wirz OF , Tomasiak-Lozowska MM , Bodzenta-Lukaszyk A , Ruxrungtham K , van de Veen W . High-dose bee venom exposure induces similar tolerogenic B-cell responses in allergic patients and healthy beekeepers. Allergy 2017; 72:407-15; PMID:27341567; https://doi.org/10.1111/all.12966
- Žitnik SEK , Vesel T , Avčin T , Šilar M , Košnik M , Korošec P . Monitoring honeybee venom immunotherapy in children with the basophil activation test. Pediatr Allergy Immunol 2012; 23:167-72; https://doi.org/10.1111/j.1399-3038.2011.01233.x
- Eržen R , Košnik M , Šilar M , Korošec P . Basophil response and the induction of a tolerance in venom immunotherapy: A long-term sting challenge study. Allergy 2012; 67:822-30; PMID:22469017; https://doi.org/10.1111/j.1398-9995.2012.02817.x
- Trabado AR , Hijón CC , Cantariño AR , Romero-Chala S , García-Trujillo JA , Fernández Pereira LM . Short-, intermediate-, and long-term changes in basophil reactivity induced by venom immunotherapy. Allergy Asthma Immunol Res 2016; 8:412-20; PMID:27334779; https://doi.org/10.4168/aair.2016.8.5.412
- Bidad K , Nawijn MC , Van Oosterhout AJM , Van Der Heide S , Oude Elberink JNG . Basophil activation test in the diagnosis and monitoring of mastocytosis patients with wasp venom allergy on immunotherapy. Cytom Part B Clin Cytom 2014; 86:183-90; https://doi.org/10.1002/cyto.b.21148
- KONNO S , GOLDEN D , SCHROEDER J , HAMILTON R , LICHTENSTEIN L , HUANG S . Increased expression of osteopontin is associated with long-term bee venom immunotherapy. J Allergy Clin Immunol 2005; 115:1063-7; PMID:15867867; https://doi.org/10.1016/j.jaci.2005.01.055
- Yavuz ST , Soyer OU , Sekerel BE , Buyuktiryaki B , Cavkaytar O , Sahiner UM , Sackesen C , Tuncer A . Increased osteopontin levels in children undergoing venom immunotherapy may serve as a marker of clinical efficacy. Int Arch Allergy Immunol 2014; 165:206-13; PMID:25531371; https://doi.org/10.1159/000368925
- Specjalski K , Maciejewska A , Pawłowski R , Chełmińska M , Jassem E . Changes in the Expression of MicroRNA in the Buildup Phase of Wasp Venom Immunotherapy: A Pilot Study. Int Arch Allergy Immunol 2016; 170:97-100; PMID:27441833; https://doi.org/10.1159/000447637
- Lech Z , Chalubinski M , Kosiński S , Smorawska E , Bassin C , Akdis CA , Grzegorczyk J , Kowalski ML . Prostaglandin E2 and lipoxin A4 in PBMCs are associated with immune tolerance during venom immunotherapy. J Allergy Clin Immunol 2016; 138:1199-202.e2; PMID:27221137; https://doi.org/10.1016/j.jaci.2016.03.024
- Boyle RJ , Elremeli M , Hockenhull J , Cherry MG , Bulsara MK , Daniels M , Oude Elberink JN . Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev 2012; 10:CD008838; PMID:23076950
- Mosbech H , Müller U . Side-effects of insect venom immunotherapy: results from an EAACI multicenter study. European Academy of Allergology and Clinical Immunology. Allergy 2000; 55:1005-10; PMID:11097308; https://doi.org/10.1034/j.1398-9995.2000.00587.x
- Brockow K , Kiehn M , Riethmüller C , Vieluf D , Berger J , Ring J . Efficacy of antihistamine pretreatment in the prevention of adverse reactions to Hymenoptera immunotherapy: A prospective, randomized, placebo-controlled trial. J Allergy Clin Immunol 1997; 100:458-63; PMID:9338537; https://doi.org/10.1016/S0091-6749(97)70135-0
- Müller UR , Jutel M , Reimers A , Zumkehr J , Huber C , Kriegel C , Steiner U , Haeberli G , Akdis M , Helbling A , et al. Clinical and immunologic effects of H1 antihistamine preventive medication during honeybee venom immunotherapy. J Allergy Clin Immunol 2008; 122:1001-7.e4; PMID:18845330; https://doi.org/10.1016/j.jaci.2008.08.007
- Wöhrl S , Gamper S , Hemmer W , Heinze G , Stingl G , Kinaciyan T . Premedication with montelukast reduces local reactions of allergen immunotherapy. Int Arch Allergy Immunol 2007; 144:137-42; PMID:17536222; https://doi.org/10.1159/000103225
- Müller U , Hari Y , Berchtold E . Premedication with antihistamines may enhance efficacy of specific-allergen immunotherapy. J Allergy Clin Immunol 2001; 107:81-6; PMID:11149995; https://doi.org/10.1067/mai.2001.111852
- Goldberg A , Confino-Cohen R . Rush venom immunotherapy in patients experiencing recurrent systemic reactions to conventional venom immunotherapy. Ann Allergy Asthma Immunol 2003; 91:405-10; PMID:14582821; https://doi.org/10.1016/S1081-1206(10)61689-4
- Shankar T , Petrov AA . Omalizumab and hypersensitivity reactions. Curr Opin Allergy Clin Immunol 2013; 13:19-24; PMID:23242113; https://doi.org/10.1097/ACI.0b013e32835bf3f5
- Kontou-Fili K . High omalizumab dose controls recurrent reactions to venom immunotherapy in indolent systemic mastocytosis. Allergy 2008; 63:376-8; PMID:18269681; https://doi.org/10.1111/j.1398-9995.2007.01604.x
- Galera C , Soohun N , Zankar N , Caimmi S , Gallen C , Demoly P . Severe anaphylaxis to bee venom immunotherapy: Efficacy of pretreatment and concurrent treatment with omalizumab. J Investig Allergol Clin Immunol 2009; 19:225-9; PMID:19610266
- da Silva EN , Randall KL . Omalizumab mitigates anaphylaxis during ultrarush honey bee venom immunotherapy in monoclonal mast cell activation syndrome. J Allergy Clin Immunol Pract 2013; 1:687-8; PMID:24565720; https://doi.org/10.1016/j.jaip.2013.07.004
- Pałgan K , Bartuzi Z , Gotz-Żbikowska M . Treatment with a Combination of Omalizumab and Specific Immunotherapy for Severe Anaphylaxis after a Wasp Sting. Int J Immunopathol Pharmacol 2014; 27:109-12; PMID:24674685; https://doi.org/10.1177/039463201402700114
- Ricciardi L . Omalizumab: A useful tool for inducing tolerance to bee venom immunotherapy. Int J Immunopathol Pharmacol 2016; 29:726-8; PMID:27679679; https://doi.org/10.1177/0394632016670920
- Wedi B , Ruëff F . Pharmakoprophylaxe und Begleitmedikation bei spezifischer Immuntherapie. Der Hautarzt 2011; 62:663-70; https://doi.org/10.1007/s00105-011-2157-2
- Ruëff F , Przybilla B , Biló MB , Müller U , Scheipl F , Aberer W , Birnbaum J , Bodzenta-Lukaszyk A , Bonifazi F , Bucher C , et al. Predictors of side effects during the buildup phase of venom immunotherapy for Hymenoptera venom allergy: The importance of baseline serum tryptase†. J Allergy Clin Immunol 2010; 126:105-11.e5; PMID:20542320; https://doi.org/10.1016/j.jaci.2010.04.025
- Stoevesandt J , Hosp C , Kerstan A , Trautmann A . Hymenoptera venom immunotherapy while maintaining cardiovascular medication: safe and effective. Ann Allergy Asthma Immunol 2015; 114:411-6; PMID:25952636; https://doi.org/10.1016/j.anai.2015.03.001
- Golden DBK . Insect Sting Anaphylaxis. Immunol Allergy Clin North Am 2007; 27:261-72; PMID:17493502; https://doi.org/10.1016/j.iac.2007.03.008
- Muller U , Helbling A , Berchtold E . Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J Allergy Clin Immunol 1992; 89:529-35; PMID:1740583; https://doi.org/10.1016/0091-6749(92)90319-W
- Ruëff F , Wenderoth A , Przybilla B . Patients still reacting to a sting challenge while receiving conventional Hymenoptera venom immunotherapy are protected by increased venom doses. J Allergy Clin Immunol 2001; 108:1027-32; PMID:11742283; https://doi.org/10.1067/mai.2001.119154
- Calderon MA , Alves B , Jacobson M , Hurwitz B , Sheikh A , Durham S . Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev 2007; 5:CD001936
- Exley C . Aluminium adjuvants and adverse events in sub-cutaneous allergy immunotherapy. Allergy Asthma Clin Immunol 2014; 10:4; https://doi.org/10.1186/1710-1492-10-4
- Kramer MF , Heath MD . Aluminium in allergen-specific subcutaneous immunotherapy – A German perspective. Vaccine 2014; 32:4140-8; PMID:24892252; https://doi.org/10.1016/j.vaccine.2014.05.063
- Council of Europe. European pharmacopoeia . Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases. Strasbourg: Council of Europe, 2013; 3971
- Lindblad EB . Aluminium compounds for use in vaccines. Immunol Cell Biol 2004; 82:497-505; PMID:15479435; https://doi.org/10.1111/j.0818-9641.2004.01286.x
- Brewer JM , Conacher M , Satoskar A , Bluethmann H , Alexander J . In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to Freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol 1996; 26:2062-6; PMID:8814247; https://doi.org/10.1002/eji.1830260915
- Chesné J , Schmidt-Weber CB , Esser von-Bieren J . The Use of Adjuvants for Enhancing Allergen Immunotherapy Efficacy. Immunol Allergy Clin North Am 2016; 36:125-45; PMID:26617231; https://doi.org/10.1016/j.iac.2015.08.009
- Bousquet J , Fontez A , Aznar R , Robinet-Levy M , Michel FB . Combination of passive and active immunization in honeybee venom immunotherapy. J Allergy Clin Immunol 1987; 79:947-54; PMID:3584749; https://doi.org/10.1016/0091-6749(87)90245-4
- King TP , Lu G , Agosto H . Antibody responses to bee melittin (Api m 4) and hornet antigen 5 (Dol m 5) in mice treated with the dominant T-cell epitope peptides. J Allergy Clin Immunol 1998; 101:397-403; PMID:9525458; https://doi.org/10.1016/S0091-6749(98)70254-4
- Winkler B , Bolwig C , Seppala U , Spangfort MD , Ebner C , Wiedermann U . Allergen-specific immunosuppression by mucosal treatment with recombinant Ves v 5, a major allergen of Vespula vulgaris venom, in a murine model of wasp venom allergy. Immunology 2003; 110:376-85; PMID:14632666; https://doi.org/10.1046/j.1365-2567.2003.01751.x
- Trindade RA , Kiyohara PK , de Araujo PS , Bueno da Costa MH . PLGA microspheres containing bee venom proteins for preventive immunotherapy. Int J Pharm 2012; 423:124-33; PMID:21356289; https://doi.org/10.1016/j.ijpharm.2011.02.027
- Bioley G , Lassus A , Terrettaz J , Tranquart F , Corthésy B . Prophylactic immunization of mice with phospholipase A2-loaded gas-filled microbubbles is protective against Th2-mediated honeybee venom allergy. Clin Exp Allergy 2016; 46:153-62; PMID:25900397; https://doi.org/10.1111/cea.12555
- Benson DA , Karsch-Mizrachi I , Lipman DJ , Ostell J , Sayers EW . .GenBank. Nucleic Acids Res 2009; 37:D26-31; PMID:18940867; https://doi.org/10.1093/nar/gkn723
- Sayers EW , Barrett T , Benson DA , Bryant SH , Canese K , Chetvernin V , Church DM , DiCuccio M , Edgar R , Federhen S , et al. Database resources of the national center for biotechnology information. Nucleic Acids Res 2009; 37:D5-15; PMID:18940862; https://doi.org/10.1093/nar/gkn741
