ABSTRACT
It is becoming increasingly important for clinicians to identify a safer intramuscular (IM) injection site in the deltoid muscle because of possible complications following the vaccine administration of IM injections. We herein examined 4 original IM sites located on the perpendicular line through the mid-acromion to establish a safer IM injection site. Thirty healthy volunteers participated in this study and the distances from our 4 IM sites to some anatomical landmarks on their left arms were measured. Ultrasonography (US) was also performed to measure the thickness of the deltoid muscle and identify the posterior circumflex humeral artery (PCHA) along the course of the axillary nerve. Subcutaneous thickness was measured using 2 methods: measuring the skin thickness with caliper after pinching the skin, and with US. The results obtained revealed that the intersection between the anteroposterior axillary line (the line between the upper end of the anterior axillary line and the upper end of the posterior axillary line) and the perpendicular line from the mid-acromion was the most appropriate site for IM injections because it was distant from the axillary nerve, PCHA, and subdeltoid/subacromial brusa. At this site, depth of needle insertions was 5 mm greater than the subcutaneous thickness at a 90° angle, which was sufficient to penetrate subcutaneous tissue in both sexes. Subcutaneous thickness can be assessed with almost the same accuracy by US or measuring with calipers after pinching the skin. The results of the present study support the improved vaccine practice for safer IM injections.
Introduction
Determining a safer site for intramuscular (IM) injections is very important since these injections are a general practice for clinicians when patients are administered vaccines or other medications. The dorsogluteal, ventrogluteal, vastus lateralis, rectus femoris, and deltoid muscles are currently advocated as IM injection sites.Citation1,2 The deltoid muscle has been used in clinical settings because it is easy for clinicians to administer injections at this site and for patients to expose it,Citation3 and it is the most commonly used site for vaccines worldwide.Citation4 Four injection sites have been recommended as safer and appropriate IM injection sites in the deltoid muscle: the first site is 1 to 3 finger breadths (5 cm) below the mid-acromion,Citation5,6 the second is a triangular injection site,Citation1,7,8 the third is the middle third of the deltoid muscle,Citation9,10 and the fourth is a mid-deltoid site.Citation11,12 The first injection site is easy to identify, and this site is frequently used in clinical settings in Japan.Citation13 The second injection site is formed by an apex based on a line drawn laterally from the upper end of the anterior axilla line and a line base on 1 or 3 finger breadths (5 cm) below the acromion.Citation1,7,8 The third and fourth injection sites are defined by the acromion as the origin of the deltoid muscle and the deltoid tuberosity as the insertion of the deltoid muscle. The third site is the densest part of the deltoid muscle.Citation11 However, CookCitation14 reported that these 4 injection sites have the potential to cause injury to the subdeltoid/subacromial brusa and/or anterior branch of the axillary nerve with the arm in the anatomical position. Additionally, we showed that the axillary nerve often runs near the site 5 cm below the mid-acromion lateral border, and concluded that this site is unsuitable for IM injection in terms of the high risk for the complications related to this nerve.Citation15-17 The following complications have been reported after the administration of IM injections: injection site reactions such as pain, erythema, and swelling due to over- or underpenetration by the needle, axillary or radial nerve palsies, musculoskeletal injuries, local sepsis, and vascular complications.Citation10 Therefore, it is becoming increasingly important to establish a safer site for IM injections.
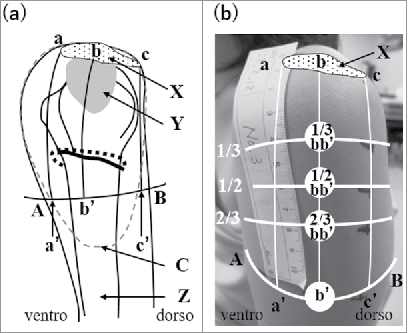
We previously proposed safer IM injection sites in the deltoid muscle.Citation15,18,19 A perpendicular line was drawn from the mid-acromion lateral border to the line between the upper end of the anterior axillary line and the upper end of the posterior axillary line (anteroposterior axillary line). We identified the upper one second to upper one third area of this segment or the intersection of this line with the anteroposterior line as appropriate sites for IM injections (see ). In living bodies, we showed that visualizing the course of the posterior circumflex humeral artery (PCHA) using ultrasonography (US) was useful for assessing the course of the axillary nerve.Citation20,21 Hence, by using US in living bodies, it is now possible to establish whether our injection sites are suitable as IM injection sites.
Figure 1. Anatomical structure of the left shoulder (a) and locations of 4 examined sites in a living body (b). A: The upper end of the anterior axillary line, B: the upper end of the posterior axillary line, a: the anterior edge of the mid-acromion lateral border, C: Deltoid tuberosity for attachment of the deltoid muscle, b: the midportion of the mid-acromion lateral border, c: the posterior edge of the mid-acromion lateral border, a’: the intersection between the perpendicular line drawn from the anterior edge of the mid-acromion lateral border and line AB, b’: the intersection between a perpendicular line drawn from the mid-acromion lateral border and line AB, c’: the intersection between the perpendicular line drawn from the posterior edge of the mid-acromion lateral border and line AB, X: acromion, Y: subdeltoid/subacromial brusa, Z: humerus, dotted circle: the deltoid muscle, dotted line: the posterior circumflex humeral artery (PCHA), black line below the PCHA: the axillary nerve. (b) One third, half, and two thirds of bb’ are marked on the skin. 1/3 bb’, 1/2 bb’, 2/3 bb’, and b’ are the sites examined in the present study.
For the selection of safer sites for IM injections, the appropriate depth of needle insertion into the muscle needs to be assessed. An inappropriate IM injection deposits medication into the muscle fascia or subcutaneous tissue, resulting in severe complications or reduced efficacy. Hence, the assessment of subcutaneous thickness is also important when attempting to safely administer IM injections. Two methods are currently used to assess subcutaneous thickness in clinical settings: measuring the skin thickness with caliper after pinching the skin, and with US. Limited information is available on the accuracy of pinching the skin and measuring its thickness using calipers relative to US. In the present study, we aim to establish a safer IM injection site and identify the appropriate depth of needle insertion in this site in living bodies. Additionally, we compare subcutaneous thickness measured by calipers with that by US.
Results
Locations at each examined site and the main trunk of the PCHA
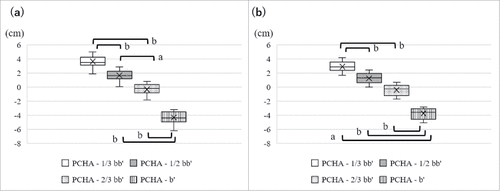
The distances from the mid-acromion lateral border to each examined site were measured to compare with distances from the mid-acromion to currently recommended IM injection sites in ref.15. The distances from the mid-acromion lateral border to each examined site in the present study are shown in . The distance from the mid-acromion lateral border to b’ was significantly longer than that to 1/3 bb’ in males and females (p = 0.0193, p = 0.0088, respectively).
Table 1. Mean distances from the mid-acromion lateral border to each examined site.
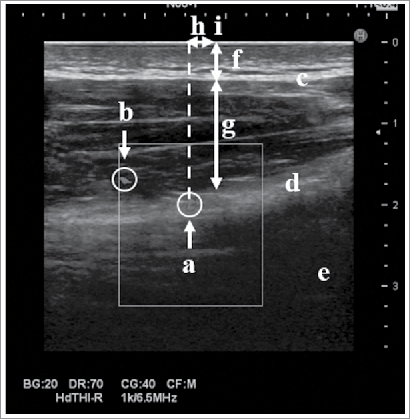
The safer site which were distant from the axillary nerve and the PCHA were examined in our original IM sites by using US. shows an Ultrasound image at 2/3 bb’. Although the PCHA was detected in most subjects, it was not identified in 2 male subjects. The axillary nerve was not identified in any subjects because it is thin and resembles collagen. shows the distances from each examined site to the PCHA when the PCHA was expressed as 0 cm. In both sexes, most PCHAs were located around 2/3 bb’, while some were detected around 1/2 bb’. 1/3bb’ was far from the PCHA as was b’ in males, while b’ was farther from the PCHA than 1/3 bb’ in females (p = 0.0103). 1/2 bb’ was farther from the PCHA than 2/3 bb’ in males (p = 0.0160), while 1/2 bb’ was near the PCHA as was 2/3 bb’ in females. The average distances from the PCHA to 1/3 bb’, 1/2 bb’, 2/3 bb’, and b’ in males were 3.7 ± 0.8 (1.9 – 5.0) cm, 1.7 ± 0.8 (0.03 – 2.9) cm, 0.4 ± 0.8 (0.8 – 1.8) cm, and 4.3 ± 0.9 (3.2 – 6.2) cm, respectively, while those in females were 2.9 ± 0.7 (1.7 – 4.2) cm, 1.2 ± 0.7 (0.0 – 2.5) cm, 0.4 ± 0.7 (0.7 – 1.7) cm, and 3.7 ± 0.8 (2.8 – 5.1) cm, respectively. The PCHA was located 7.6 ± 1.0 (5.5 – 9.2) cm below the mid-acromion lateral border in males and 6.2 ± 0.8 (5.1 – 7.7) cm below the mid-acromion lateral border in females.
Thickness of subcutaneous tissue and the deltoid muscle
The subcutaneous thickness measured by calipers or US was used to determine the appropriate depth of needle insertion for IM injection. In males, subcutaneous thickness measured by calipers or US was significantly greater at b’ than at 1/3 bb’ (p = 0.0193, p = 0.0088, respectively) ( and ). In females, subcutaneous thickness measured by calipers and US was the thinnest at 1/3 bb’ and gradually increased distal to the examined site ( and ). Each mean value of subcutaneous thickness is shown in .
Figure 4. Thickness of subcutaneous tissue and the deltoid muscle. (a) Subcutaneous thickness measured by calipers in males, (b) subcutaneous thickness measured by ultrasonography (US) in males, (c) subcutaneous thickness measured by calipers in females, (d) subcutaneous thickness measured by US in females, (e) The thickness of the deltoid muscle in males, (f) the thickness of the deltoid muscle in females. a: p < 0.05, b: p < 0.01, (males; n = 15, females; n = 15, subcutaneous thickness: Kruskal-Wallis and Steel-Dwass, thickness of the deltoid muscle: ANOVA, Tukey-Kramer HSD).
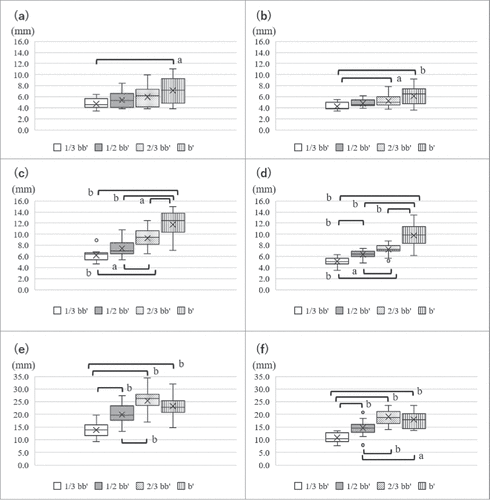
Table 2. Mean values of subcutaneous thickness measured by calipers and ultrasonography US.
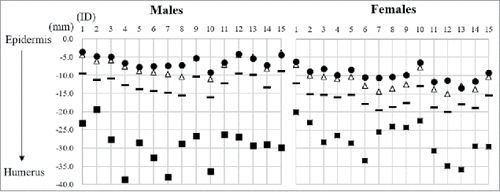
The thickness of the deltoid muscle was measured to identify whether each examined site is enough thick to insert the needle for IM injection. The thickness of the deltoid muscle was shown in and . In both sexes, the deltoid muscle at 1/3 bb’ was the thinnest among all examined sites, and while similar thicknesses were observed at b’ and 2/3 bb’. The thickness of the deltoid muscle gradually increased distal to the examined site. shows the subcutaneous thickness from the skin surface and the thickness of the deltoid muscle from the skin surface and appropriate depths at b’. This figure lets us easily understand the depth of needle insertions at b’.
Figure 5. Appropriate depth of the intramuscular (IM) injection at b’. The skin surface is expressed as 0 cm. The X axis shows each subject ID and the Y axis the depth from the skin surface. The black short lines are values that are 5 mm greater than the subcutaneous thickness measured by calipers, and represent the appropriate depth of needle insertion to administer IM injections. Black circles: subcutaneous thickness measured by ultrasonography (US), white triangles; subcutaneous thickness measured by calipers, black squares: the thickness of the deltoid muscle from the skin surface measured by US.
We also compared the differences of subcutaneous thickness between calipers or US because limited information is available on the accuracy between these 2 methods. also shows differences of subcutaneous thickness between the 2 methods. Subcutaneous thickness measured by calipers was greater than that by US at all sites. However, differences in the mean values obtained for subcutaneous thickness between the 2 methods were only within 1 mm in males and 1 to 2 mm in females. The greatest difference in subcutaneous thickness at b’ between 2 methods was 3.2 mm in males and 3.9 mm in females.
Discussion
The safest site among those examined in the present study
IM injections for vaccination practice in the deltoid muscle can be safely performed without risking injury to the axillary nerve, PCHA, subacromial/subdeltoid bursa, musculocutaneous nerve, or radial nerve. The musculocutaneous nerve arises from the lateral cord of the brachial plexus and descends laterally between the biceps and brachialis to the lateral side of the arm.Citation22 The radial nerve appeared in the deltoid muscle at the site of c’ in our previous study.Citation16 Hence, safer injection sites have been located on the mid-acromion line. The axillary nerve arises from the posterior cord of the brachial plexus and passes through the quadrilateral space under the deltoid muscle. It divides into 2 branches: the anterior branch mainly gives off branches to the anterior and middle aspects of the deltoid muscle, while the posterior branches mainly give off branches to the teres minor and posterior aspects of the deltoid muscle.Citation23-25 The anterior axillary nerve anatomically follows the PCHA. Additionally, in the bottom of the superior area of the deltoid muscle, the subacromial/subdeltoid bursa covers the acromioclavicular joint. Therefore, the sites examined herein need to avoid these structures.
In the present study, the safest site for IM injections was identified as b’ for the following reasons. Most PCHAs were located around 2/3 bb’, while some were around 1/2 bb’. These results show that the course of the PCHA and axillary nerve was the transverse lines situated at 2/3 bb’ or sometimes 1/2 bb’, and, thus, it was not considered safer to administer IM injections at the 1/2 bb’ and 2/3 bb’ sites. These results support our previous findings.Citation15-17 CookCitation14 recommended that a safer IM injection site was 7.4 cm below the mid-acromion in both sexes due to the course of the axillary nerve and position of the subacromial/subdeltoid bursa. The distances from the mid-acromion lateral border to the PCHA were 5.5 to 9.2 cm in males and 5.1 to 7.7 cm in females in this study. The distances from the mid-acromion lateral border to 1/3 bb’ were 3.5 to 4.6 cm in males and 2.8 to 3.7 cm in females in this study. In contrast, the distances from the mid-acromion lateral border to b’ were 10.5 to 13.8 cm in males and 8.5 to 11.0 cm in females. Consequently, the location of b’ was distant from the axillary nerve, PCHA, and subacromial/subdeltoid bursa. Therefore, b’ was identified as the safest site for IM injections in this study.
shows comparisons between the distance from the PCHA to the mid-acromion lateral border in the present study as well as the distances from the mid-acromion to each injection site currently recommended in ref.15. Although the subjects who participated in this study were young, the distances from the mid-acromion lateral border to the PCHA were almost the same in our previous study using elderly cadavars;Citation17 therefore, it was possible to compare the data obtained between these 2 age groups. As shown in , some injection sites in previous studies were close to the location of the PCHA in the present study. Although the site at 1 to 3 finger breadths/5 cm from the mid-acromion was distant from the location of the PCHA in this study, the axillary nerve was less than 5 cm from the acromion in some cadavers,Citation3,15,26 and the mean distances from the mid-acromion to subacromial/subdeltoid bursa were 4.1 cm in males and 4.0 cm in females.Citation27,28 Therefore, we consider the injection sites recommended by previous studies to potentially cause injuries to the PCHA and axillary nerve. Additionally, there is currently no evidence for aspiration of the syringe plunger except for the dorsogluteal procedure because no large blood vessels exist at injection sites.Citation2,4 It currently remains unclear whether these injection sites recommended by previous studies in the deltoid muscle reduce the risk of vascular injury. We recommend aspiration to avoid vascular injury or the administration of medications into blood vessels.
Table 3. Distance from the mid-acromion lateral border to the PHCA in this study and injection sites in a previous study.
Appropriate depth and angle of needle insertion
Inappropriate depth of needle insertions can result in complications associated with IM injections or inadequate protection by vaccine. For instance, in adults, the immunogenicity of hepatitis B is lower substantially when IM site is used for administration.Citation4 Medication, especially vaccine, are required to be administrated by appropriate route and depth, and thus deltoid IM injections generally require needle penetration into the deltoid muscle layer by 5 mm or more to ensure that medication is administered into the muscle mass.Citation29 In the present study, we defined the appropriate depths of needle insertion at b’ to be 5 mm greater than the subcutaneous thickness. As shown in , the needle insertions of these depth can completely penetrate subcutaneous tissue and delivery medications into the muscle in all subjects if clinicians adopt subcutaneous thickness measured by pinching the skin and measuring with calipers. Based on these findings, the needle depth of 5 mm greater than the subcutaneous thickness prevents under- or overpenetration and reduce the risk of complications associated with IM injections or inadequate protection by vaccine.
Needle angles are also important to determine the appropriate depth in IM injections. Some previous studies reported that few studies proposed varying needle angles from 45 – 60° or 72°.Citation2,30 The nearest distance from the PCHA to b’ was 3.2 cm in males and 2.8 cm in females. If clinicians insert a needle at a slanting angle, the needle may be close to the PCHA or axillary nerve. In addition to the increased risk of nerve or vascular injuries, a slanting angle may lead to the deposition of medications into fascia or subcutaneous tissue because assessments of subcutaneous thickness become more difficult at a slanting angle. Accordingly, we recommend a needle angle of 90° for IM injections.
Assessment of subcutaneous thickness
In clinical settings, we generally assess subcutaneous thickness by pinching the skin, measuring it, and then halving the value obtained. Subcutaneous thickness with calipers was found to be greater than with US in the present study. However, only slight differences were observed in the mean values obtained using the 2 methods: only within 1 mm in males and 1 to 2 mm in females. In regards to the maximum differences obtained using the 2 methods, their values were only 3.2 mm in males and 3.9 mm in females. We think that these differences are very little and ignored when clinicians administer IM injections to patients. Furthermore, the adoption of subcutaneous thickness measured by calipers lead to the penetration of subcutaneous tissue in all subjects, and this adoption lead to decrease complications. Therefore, the method of pinching the skin and using calipers is reliable for determining appropriate depth for IM injections.
Limitation of this study
This study was conducted on a limited number of subjects in terms of body mass index as well as the small sample size and limited age. Concerning obese subjects, we guess that our new IM injection site in the deltoid muscle is also applicable to obese subjects because we relatively decide our IM site by using anatomical landmarks. However, as we do not have any data in the location of axillary nerve, PCHA and subcutaneous thickness, further studies are required to identify safer sites for IM injection in obese subjects. Moreover, our established site for IM injections is for a single dose; the effects of multiple doses at this site have not yet been investigated. Further studies are needed to support the results obtained in the present study.
Conclusion
We herein established a new site for IM injections in the deltoid muscle that is located in the intersection between the anteroposterior axillary line and perpendicular line from the mid-acromion. At this site, the appropriate depths of needle insertion for IM injections are 5 mm greater than the subcutaneous thickness with a needle angle of 90°. We can determine the appropriate depth for IM injections by pinching the skin and measuring its thickness using calipers in clinical settings. We believe that our established site for IM injections is beneficial for clinicians in clinical settings.
Methods and materials
Subjects and setting
Thirty healthy volunteers participated in this study and met the following inclusion criteria: age >17 y old, no history of upper limb injury/surgery or neurological or muscular disease of the upper limb, and no allergies to implements used in this study. They provided informed consent before their inclusion in the study. Fifteen subjects were males and 15 were females. The average values for age, height, weight, and BMI in males were 21.0 ± 1.3, 172.5 ± 5.1, 60.3 ± 6.4, and 20.2 ± 1.4, respectively, while those in females were 21.8 ± 0.6, 158.5 ± 6.3, 48.5 ± 4.9, and 19.3 ± 1.1, respectively. This study was conducted at a laboratory in Kanazawa University and was approved by the Kanazawa University Ethical Committee (Approval No. HS28–6–1).
Definition of examined sites
All examinations were conducted by 2 nursing students trained by a medical doctor specializing in anatomy and researchers acquiring basic nursing skills between August and October 2016. shows the anatomy of the shoulder and the sites examined in the living body. All subjects were seated with the arms in the anatomical position. The acromion on the left arm was identified and the anterior edge (a), midportion (b), and posterior edge (c) of the mid-acromion lateral border were marked. The line between the upper end of the anterior axillary line (A) and upper end of the posterior axillary line (B) was drawn laterally. Three perpendicular lines were drawn from a, b, and c on the acromion and the sites of their intersection with line AB were defined as a’, b’, and c’. The distance of bb’ was measured with a tape measure and one third, half, and two thirds of this distance were marked on the skin. These sites were named 1/3 bb’, 1/2 bb’, and 2/3 bb’. The 4 sites, which were 1/3 bb’, 1/2 bb’, 2/3 bb’, and b’, were examined as IM sites in the present study.
Data collection
The distances from the mid-acromion lateral border to each examined site were measured by a tape measure. As shown in , Ultrasound diagnostic equipment (18 – 5 MHz probe, Hitachi Aloka Medical Inc., Tokyo, Japan) was used to identify the PCHA, deltoid muscle, humerus, subcutaneous tissue, and fascia. Ultrasound images using the B-mode were obtained 3 times to evaluate the thickness of subcutaneous tissue and the deltoid muscle. Subcutaneous thickness at each examined site was measured 3 times using 2 methods: pinching the skin, measuring its thickness by calipers, and halving the value obtained, and measuring its thickness using US. The thickness of the deltoid muscle at each examined site was also measured 3 times. Compression by the probe against the skin was avoided and the probe was held at a 90° angle to the plane of the examined sites. The PCHA was identified with Doppler mode US. To identify the main trunk of the PCHA, the probe was put on the cc’ line with a vertical angle to the humerus and moved vertically. After identifying the main duct of the PCHA on deep fascia on the cc’ line, the probe was anterolaterally moved to the bb’ line. The main trunk of the PCHA on the bb’ line was marked on the skin surface and the distance from each examined site to the PCHA marked on the skin surface was measured by a tape measure.
Statistical analysis
All data were expressed as the mean ± SD (maximum – minimum), and were analyzed by the following methods. Distances from the mid-acromion lateral border to the 4 examined sites and subcutaneous thickness were analyzed by the Kruskal-Wallis and Steel-Dwass tests. Distances from the PCHA to the 4 examined sites and the thickness of the deltoid muscle were analyzed by ANOVA and the Tukey-Kramer HSD test. Differences in subcutaneous thickness at each examined site measured by calipers or US were analyzed by the paired t-test. Differences were considered significant at p < 0.05.
Disclosure of potential conflicts of interest
The authors report no conflicts of interest related to this research. All authors were involved in the study design, data collection, data analysis, and editorial process and approved the final version of the manuscript.
Acknowledgments
The authors are indebted to all study participants.
Funding
This work was supported by JSPS KAKENHI under Grant Number 25293430.
References
- Treas LS, Wilkinson JM. Basic nursing: concepts, skills & reasoning. Philadelphia PA: F.A.Davis Company; 2014
- Malkin B. Are techniques used for intramuscular injection based on research evidence? Nurs Times 2008; 104:48-51; PMID:19165987
- Fujimoto E. The problem of using deltoid muscle for intramuscular injection. Aino J 2007; 6:49-53
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization— recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 2011; 60(2):1-64
- Beyea SC, Nicoll LH. Administration of medications via the intramuscular route: an integrative review of the literature and research-based protocol for the procedure. Appl Nurs Res 1995; 8(1):23-33; PMID:7695353; https://doi.org/10.1016/S0897-1897(95)80279-7
- Davidson LT, Carter GT, Kilmer DD, Han JJ. Latrogenic axillary neuropathy after intramuscular injection of the deltoid muscle. Am J Phys Med Rehabil 2007; 86(6):507-11; PMID:17515691; https://doi.org/10.1097/PHM.0b013e31805b7bcf
- Gray R, Spilling R, Burgess D, Newey T. Antipsychotic long-acting injections in clinical practice: medication management and patient choice. Br J Psychiatry Suppl 2009; 52:S51-6; PMID:19880918; https://doi.org/10.1192/bjp.195.52.s51
- Rodger MA, King L. Drawing up and administering intramuscular injections: a review of the literature. J Adv Nurs 2000; 31(3):574-82; PMID:10718876; https://doi.org/10.1046/j.1365-2648.2000.01312.x
- Funnell R, Koutoukidis G, Lawrence K. Tabbner's nursing care 4th Ed. Marrickville: Elsevier; 2005
- Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother 2015; 11(5):1184-91; PMID:25868476; https://doi.org/10.1080/21645515.2015.1017694
- Cocoman A, Murray J. Intramuscular injections: a review of best practice for mental health nurses. J Psychiatr Ment Health Nurs 2008; 15(5):424-34; PMID:18454829; https://doi.org/10.1111/j.1365-2850.2007.01236.x
- National Health and Medical Research Council. The Australian Immunization Handbook: 10th Edition. Australia: Commonwealth of Australia; 2015; 2015 June [accessed 2017 Mar 8]. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/7B28E87511E08905CA257D4D001DB1F8/$File/Aus-Imm-Handbook.pdf
- Kikuchi K, Oyama N, Takahashi Y, Ishida Y. Analysis of Practical Knowledge of Deltoid Intramuscular Injections by Nurses in Hospitals. J Fac Nurs lwate Pref Univ 2009; 11:79-85. ( in Japanese, abstract in English)
- Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin 2011; 7(8):845-8; PMID:21832883; https://doi.org/10.4161/hv.7.8.16271
- Nakatani T, Sanada H, Sugama J, Nagakawa T, Konya C, Ohkuwa M. Appropriate site for intramuscular injection in the deltoid muscle evaluated in 35 cadaverous arms. Memoirs Health Sci Med Kanazawa Univ 2000; 24:27-31
- Nakatani T, Kitagawa A, Kitayama Y, Tanaka A, Yamazaki M, Konya C, Tanaka S. The course of the axillary nerve projected on the skin covering the deltoid muscle of a cadaver for safety administering intramuscular injection in the deltoid muscle. J Tsuruma Health Sci Soc Kanazawa Univ 2003; 27:33-7
- Nakatani T, Tanaka A, Tanaka S, Sugama J, Ohkuwa M, Matsui Y, Murata M, Futamura M, Kinoshita S, Morita H, et al. Appropriate site for intramural injection in the deltoid comparing cadaverous with living arms. J Tsuruma Health Sci Soc Kanazawa Univ 2004; 28:121-6
- Nakatani T, Inagaki M, Sugama J, Sanada H, Nagakawa T, Takeda Y, Tawara T, Hiramatsu T, Kawamura K, Ohkuwa M. Intramuscular injection into the deltoid muscle: Where is the suitable site for the injection? Memoirs Health Sci Med Kanazawa Univ 1999; 23(1):83-6. ( in Japanese)
- Komatsu E, Mukai K, Nakajima Y, Ozaki N, Nakatani T. Examination of the utility of a new tool for intramuscular injection points into the deltoid muscle and the safety of the points decided by a method using it. Struct Function 2014; 13(1):17-24. ( in Japanese, abstract in English)
- Hara Y, Kurokawa K, Urai T, Okuwa M, Nakatani T. To determine the course of the axillary nerve, it is useful to identify the course of the posterior humeral circumflex artery that runs along the nerve using a handheld ultrasound blood flowmeter and ultrasound diagnostic equipment. Struct Function 2010; 8(2):59-65. ( in Japanese, abstract in English)
- Shimamura K, Kimoto H, Kuroda C, Nakazawa E, Hirose S, Miyajima M, Watanabe N, Yamamoto H, Okuwa M, Nakatani T. A study to determine the usefulness of measuring the position of the posterior humeral circumflex artery which runs along with the axillary nerve using a handhold ultrasound blood flowmeter in order to estimate the course of the axillary nerve. Struct Function 2008; 7(1):3-6. ( in Japanese, abstract in English)
- Macchi V, Tiengo C, Porzionato A, Parenti A, Stecco C, Bassetto F, Scapinelli R, Taglialavoro G, De Caro R. Musculocutaneous nerve: histotopographic study and clinical implications. Clin Anat 2007; 20(4):400-6; PMID:17022027; https://doi.org/10.1002/ca.20402
- Kontakis GM, Steriopoulos K, Damilakis J, Michalodimitrakis E. The position of the axillary nerve in the deltoid muscle. A cadaveric study. Acta Orthop Scand 1999; 70(1):9-11; https://doi.org/10.3109/17453679909000948
- Uz A, Apaydin N, Bozkurt M, Elhan A. The anatomic branch pattern of the axillary nerve. J Shoulder Elbow Surg 2007; 16(2):240-4; PMID:17097311; https://doi.org/10.1016/j.jse.2006.05.003
- Loukas M, Grabska J, Tubbs RS, Apaydin N, Jordan R. Mapping the axillary nerve within the deltoid muscle. Surg Radiol Anat 2009; 31(1):43-7; PMID:18766295; https://doi.org/10.1007/s00276-008-0409-3
- Burkhead WZ Jr, Scheinberg RR, Box G. Surgical anatomy of the axillary nerve. J Shoulder Elbow Surg 1992; 1(1):31-6; https://doi.org/10.1016/S1058-2746(09)80014-1
- Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine 2007; 25:585-7; https://doi.org/10.1016/j.vaccine.2006.08.034
- Beals TC, Harryman DT, Lazarus MD. Useful boundaries of the subacromial bursa. Arthroscopy J Arthro 1998; 14:465-70; https://doi.org/10.1016/S0749-8063(98)70073-8
- Poland GA, Borrud A, Jacobson RM, McDermott K, Wollan PC, Brakke D, Charboneau JW. Determination of deltoid fat pad thickness. Implications for needle length in adult immunization. JAMA 1997; 277(21):1709-11; https://doi.org/10.1001/jama.1997.03540450065037
- Wynaden D, Landsborough I, Chapman R, McGowan S, Lapsley J, Finn M. Establishing best practice guidelines for administration of intra muscular injections in the adult: a systematic review of the literature. Contemp Nurse 2005; 20(2):267-77; https://doi.org/10.5172/conu.20.2.267