ABSTRACT
An HBsAg-HBIG therapeutic vaccine (Yeast-derived Immune Complexes, YIC) for chronic hepatitis B (CHB) patients has undergone a series of clinical trials. The HBeAg sero-conversion rate of YIC varied from 21.9% to 14% depending on the immunization protocols from 6 to 12 injections. To analyze the immunological mechanisms exerted by 6 injections of YIC, 44 CHB patients were separately immunized with YIC, alum as adjuvant control or normal saline as blank control, with add on of antiviral drug Adefovir in all groups. Kinetic increase in Th1 and Th2 cells CD4+ T cell sub-populations with association in decrease in Treg cells and increase of Tc1 and Tc17 cells in CD8+ T cells were observed in YIC immunized group. No such changes were found in the other groups. By multifunctional analysis of cytokine profiles, significant increase of IL-2 levels was observed, both in CD4+ and CD8+ T cells in the YIC immunized group, accompanied by increase in IFN-gamma and decrease of inhibitory factors (IL-10, TGF-β and Foxp3) in CD4+ T cells. In the alum immunized group, slight increase of IL-10, TGF-β and Foxp3 in CD4+ T cells was found after the second injection, but decreased after more injections, suggesting that alum induced early inflammatory responses to a certain extent. Similar patterns of responses of IL-17A and TNF-α in CD8+T cells were shown between YIC and the saline group. Results indicate that add on of Adefovir, did not affect host specific immune responses.
Introduction
There are approximately 300 million people infected with Hepatitis B virus (HBV), making it one of the leading health threats worldwide. Some infected patients will progress to chronic liver diseases, liver cirrhosis, and hepatocellular carcinoma. In the past 20 years, antiviral treatment of chronic hepatitis B has progressed tremendously with 5 oral nucleoside analogs (NAs: lamivudine, telbivudine, adefovir, entecavir, tenofovir) and PEGylated interferon currently available in the market. However, due to side effects and emergence of drug-resistant mutants, alternative immunotherapies aiming to develop effective host immune responses against the infection have been tested, with some advanced to clinical trials.Citation1-4
The immune system plays a pivotal role in controlling HBV and can be harnessed to trigger viral clearance in CHB patients. Reports showed that CD4+ and CD8+ T cell responses against HBV surface antigen (HBsAg) were markedly impaired in long-term chronic hepatitis B patients, while HBV-specific CD8+ T cell activation or re-activation could lead to a successful clearance of HBV.Citation5 Different approaches have been developed to reshape immune responses against HBV, and immunotherapy, either alone or in combination with antiviral drugs has been actively pursued.Citation6,7
Although activation of host immune response against HBV infection is the key factor to cure, the mechanisms by which HBV establishes and maintains chronic infection are still elusive. A number of experimental results from animal studies and in CHB patients have been reported. Among these, naive reactive CD8+ T cells were shown to ignore antigens expressed in the liver.Citation8 The immune-tolerance state of liver was partly attributable to arginase, TGF-β and IL-10 produced by resident myeloid-derived suppressor cells (MDSC) or Kupffer cells.Citation9 Furthermore, HBV infection regulated expression of TLRs and related receptors. Pretreatment of cells with HBV virions, HBsAg or HBeAg which inhibited the expression of IFN-stimulated genes (ISGs) almost fully abrogated TLR antiviral activity.Citation10 T-cell exhaustion has been contributed by several co-inhibitory molecules including PD-1, BTLA, TIM-3, LAG-3 and CTLA-4 for such T-cell exhaustion.Citation11 During HBV infection, CD4+ T-cells, particularly Treg cells play dominant roles in regulating CD8+ T cells-mediated HBV specific response. However, the rule of Treg cells in HBV infection is still in question.Citation11 In addition, some immunosuppressive cytokines may contribute to the regulation of T-cell exhaustion, such as TGF-β and IL-10, which limit the proliferative and effector abilities of HBV-specific T cells have been shown.Citation11 Recently, it was suggested that γδT cells promoted MDSC infiltrating into the liver and resulted in MDSC- mediated CD8+ T cell exhaustion.Citation12 Inhibition of both CD4+ and CD8+ T cell responses leading to arginase, TGF-β and IL-10 production in CHB have been shownCitation13 and natural killer (NK) cells which could negatively regulate HBV-specific CD8+ T responses via upregulation of TRAIL-R214 have been observed to increase several folds in the liver and have the capacity to produce IFN-γ to control of HBV infection in animal models, and in CHB patients.Citation15 TNF-α, IFN-γ are traditionally recognized as playing key roles in the clearance of HBV infection. In recent years, IL-17, IL-9, IL-22, IL-33 produced by CD4+ T-cell were shown contribute to HBV infection and progression of HBV related diseases.Citation16,17
To overcome these immune defects, currently, several clinical trials based on reshaping host immune responses have been conducted.Citation18,19 In 1995, we conducted a pilot study on the use of HBsAg-HBIG (immune complex, IC of hepatitis B surface antigen complexed with high-titer immunoglobulin) to treat CHB patients.Citation20 Our hypothesis was, by complexing HBsAg with anti-HBs at an appropriate ratio, HBsAg can be more effectively delivered into host antigen presenting cells (APCs) via their Fc receptors, as being amply demonstrated in animal models.Citation21 Once delivered into APCs, effective processing and presentation of HBsAg are predicted to initiate specific adaptive immunity. This hypothesis has been proven true in animal models, in vitro cell-cell interactions, and by HBeAg seroconversion in CHB patients by several laboratories including ours.Citation21-23 Though this IC-based immunotherapy has undergone sequential phase I, II and III clinical trials,Citation24-26 and efficacy as well as safety of YIC have been well studied in around 700 CHB patients, detailed analysis on the immune mechanisms, especially cell-mediated mechanisms exerted by YIC have not been fully explored. This pilot study was aimed to kinetically analyze the antigen specific T cell responses and cytokine profiles in CHB patients, separately treated with YIC, alum or saline. Results provided will benefit future applications of immune therapies for CHB patients
Results
The percentage of patients seroconverting to HBeAg was higher in the YIC-treated group
The clinical and virological characteristics of the 44 patients who participated in this study are shown in . Among the 44 patients, complete immunological assay data were only available from 39 patients (YIC, 10; Alum 15; saline 14). Patients were given 6 intramuscular injections of either YIC, alum or saline at 4-week intervals. Virological responsive results are shown in . Although the small number of recruited patients in this study, the number of patients seroconverting to HBeAg was higher in the YIC-treated group. At the end of follow-up, 2 out of 11 patients treated with YIC had HBeAg seroconverted. As a result of treatments with Adefovir, serum HBV DNA levels declined to less than 1000 copies in 64.7%, 50% and 58.8% of patients in the YIC, alum and saline-treated groups respectively (p > 0.05).
Table 1. Characteristics of the participants at baseline (Per Protocol Set, PPS).
Table 2. HBeAg seroconversion after treatment (Per Protocol Set, PPS).
Increased proportions of Th cell subtypes and decreased Treg cell subtypes were observed in the YIC group
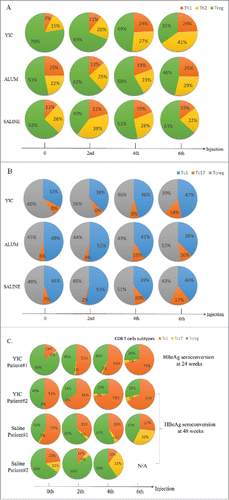
The percentages of T cell subtypes from the YIC group, alum group and the saline group after 2, 4, and 6 injections are shown in and . When the baseline status was compared with that at the end of treatment, the percentage of Treg cells among the total CD4+ T cells in the YIC-treated group decreased from 78% to 35%, and the Th1 and Th2 cells increased from 7% to 24% and 15% to 41%, respectively. Neither the alum group nor the saline group showed such significant change. The kinetic changes of CD8+ T cells were not as marked as in CD4+ T cells. There were slight increases in the percentages of Tc1 cells and Tc17 cells in the YIC-treated group and in the alum group only the latter was found increased at the end of treatment. Interestingly, there were 2 patients seroconverted after all 6 injections of the YIC and 2 with the saline. One (patient 1) was seroconverted after 6 injections of YIC at weeks 24 and had significant level of Tc1 (in which the Anti-HBsAg CD8+ T cell expressing IFN-γ) increased dynamically along with the YIC injections from 15% at zero time point to 51% after the 2nd injection, to 56% after its 4th injection and to 71% after the 6th injection; The second patient was seroconverted at weeks 48 and significant Tc1 proportion changes dynamically along with the injections from 51% at zero time point to 66% after the 2nd injection, to 73% after its 4th injection and to 67% after the 6th injection (). In contrast, 2 patients from the saline group with seroconversions did not show increase in Tc1, but rather decreased (). Although the number of seroconverted patients were small, the consistency of antigen specific responses against HBsAg miantained. Results from the 2 YIC treated patients suggest that increase of Tc1 against HBsAg in CHB patients has important impact on HBeAg seroconversion.
Figure 1. The percentages of T cell subtypes from the YIC, alum and saline groups after 0, 2, 4 and 6 injections. (A) Percentage changes of the HBsAg specific CD4+ T cells responded to corresponding treatments were represented by the 10 individual cytokine populations. Multifunctional Th1 cells are depicted in orange, Th2 in yellow and Treg in green. (B) Percentage changes of the HBsAg specific CD8+ T cells responded to corresponding treatments were represented by the 11 individual cytokine populations. Multifunctional Tc1 cells are depicted in blue, Tc17 in orange and Tcreg in gray. Data are presented as means from 10, 15 and 14 patients in the YIC, alum and saline groups, respectively. (C) Percent changes of the HBsAg specific CD8+ T cells of the YIC treated 2 patients with HBeAg seroconversions at weeks 24 and 48, and 2 patients in the saline control with HBeAg seroconversions at week 48.
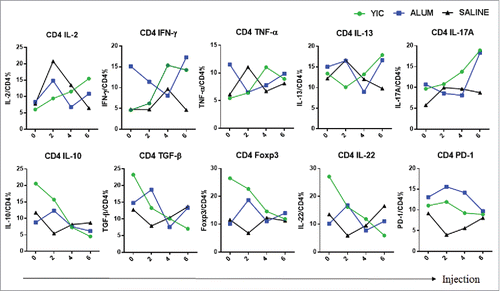
Increase of IL-2, IFN-γ, decrease of IL-10, TGF-β and Foxp3 expression levels in CD4+ T cells from the YIC-treated group
CD4+ T cells from YIC-treated group were stimulated with specific peptides, and the kinetic changes in intracellular cytokines, chemokines and expression of transcriptional regulatory factor Foxp3 were analyzed and shown in . Consistent decreases in anti-inflammatory factors IL-10 and TGF-β, as well as expression of Foxp3, and increases in IL-2, TNF-α and IFN-γ were observed in the YIC group. However, in the alum-immunized group, at first, levels of anti-inflammatory cytokines and expression levels of Foxp3 were increased, but decreased later. Inconsistent changes in levels of IL-2, TNF-α and IFN-γ were observed. Complete individual data sets on CD4+ T cell cytokines/chemokines/transcriptional factors in the 3 groups after 2, 4, and 6 injections are shown in Fig. S2.
Figure 2. CD4+ T cell cytokine profiles from the YIC, alum and saline groups after 2, 4 and 6 injections. HBsAg specific cytokine production by PBMC fractionated CD4+ T cells after the injections. The mean frequency of each cytokine producing CD4+ T cells was analyzed by ICS from 10, 15 and 14 patients in the YIC, alum and saline groups, respectively. Cytokine producing CD4+ T cells of YIC as indicated in green, Alum in blue, Saline in black.
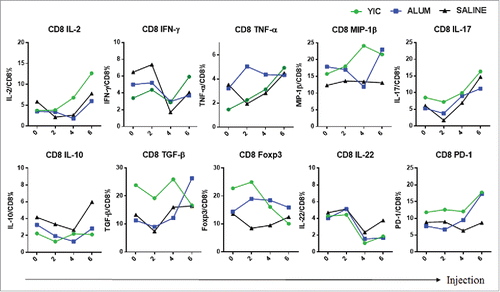
Increase of IFN-γ, and decrease of Foxp3 expression in CD8+ T cells from the YIC-treated group
The kinetics of cytokine and Foxp3 expression in CD8+ T cells stimulated with the pooled HBsAg peptides are shown in . Overall, differences in expression levels between the YIC-treated and other groups were only observed for IFN-γ and Foxp3. Complete data sets on CD8+ T cell cytokines/chemokines/transcriptional factors of each patient in the 3 treated groups after 2, 4, and 6 injections are shown in Fig. S3.
Figure 3. CD8+ T cell cytokine profiles from the YIC group, alum group and the saline group after 2, 4 and 6 injections. HBsAg specific cytokine production by PBMC fractionated CD8+ T cells after the injections. The mean frequency of each cytokine producing CD8+ T cells was analyzed by ICS from 10, 15 and 14 patients in the YIC, alum and saline groups, respectively. Cytokine producing CD8+ T cells of YIC as indicated in green, Alum in blue, Saline in black.
Discussion
CHB induces a systemic immune tolerance or immune exhaustion with 2 possible mechanisms.Citation27,28 One is by down-regulating the frequency and functions of innate cells such as NK, NKT, and DCs and the other is by inducing deficiency of HBV specific CTL and low levels of antiviral cytokine production.Citation28-30 In addition to surgery, radiation and drugs, immunotherapies, including active, passive and modulatory immunotherapies have evolved as a fast progressing therapy.Citation3,31-33 For the past 2 decades, we have focused on using active immunotherapy to enhance or reshape the immune responses in CHB patients with YIC immunization. YIC was shown to reduce serum HBsAg and induce anti-HBs in HBsAg-positive transgenic mice,Citation21 and to trigger DCs from CHB patients for higher production of IL-12. This was corroborated by higher IFN-γ and IL-2 responses in the co-culture of DC-T cell cells from CHB patients in vitro.Citation22 All those encouraging outcomes led to the approval for our Phase I, II and III trials.
Compared to drug treatment, the history of immune therapy in clinical trial is short and relatively immature. Not only is it necessary to optimize the protocol for effective therapeutic immunization, but also it is crucial to collect immunological data in clinical trials to reveal immune mechanisms uniquely associated with this new intervention. In a previous phase IIA clinical study, we kinetically compared cytokines between 5 responders and 5 non-responders to YIC. At the end of treatment, 4/5 responders showed increased IFN-γ and TNF-α responses in cells stimulated with HBsAg, and 3/5 showed increased levels of IL-10 and IL-2, but these changes were not sustained in the 24-week follow-up.Citation34 To gain further understanding of the immune mechanisms, in this study we expanded coverage to monitor the kinetic changes in CD4+ and CD8+ T cells by intracellular staining of a range of cytokine/chemokine/regulatory factors in samples from YIC, alum, and saline treated CHB patients. To quantify different cytokines produced by T cells in individual patients, T-cell functions were analyzed after in vitro culture to yield a comprehensive representation of the global T-cell response to HBV. CD4+ T cells were classified as Th1 cells (IL-2, IFN-γ, TNF-α), Th17 (IL-17A), Th2 cells (IL-13) and Treg cells (TGF-β, IL-22, IL-10, Foxp3, PD-1). CD8+ cells were classified as Tc1 (MIP-1β, IL-2, TNF-α, IFN-γ), Tc17 (IL-17A) and Tcreg (TGF-β, IL-22, IL-10, Foxp3, PD-1). Due to ethical concerns, all patients were given antiviral drug Adefovir at the beginning and throughout the trial. The most notable impact of the use of Adefovir was the significant reduce of HBV loads in all the patients within the first month. Compared to alum and saline groups, the YIC immunized group showed increase in percentages of Th1 and Th2 CD4+ T cells, which was associated with a decrease in Treg cells. Increases in Tc1 and Tc17 cells and decrease in Tcreg cells were also observed in CD8+ T cells in the YIC immunized group. Though slight increase in Th17 was observed in the alum and saline groups, there was no decrease in Treg cells. These results support that alum could induce inflammatory changes that were unrelated to antigen specific T cell-mediated responses ().
The most striking change in cytokine/chemokine/transcriptional factor staining in CD4+ T cells and CD8+ T cells was the increase in IL-2 in the YIC immunized group. Consistent with the T cell subtyping, while IFN-γ and IL-17A increased steadily in the YIC immunized group, there were decreased in expression of anti-inflammatory cytokines IL-10, TGF-β and the Foxp3 expression. In the alum group, IFN-γ and IL-17A at first decreased but then increased with the last injections, suggesting that with more injections, these cytokines might continue to increase, but no decrease in anti-inflammatory cytokines and expression of Foxp3 gene was observed.
Looking at the overall kinetic changes in the YIC immunized group, the changes of cytokines/regulatory factors in CD4+ T cells occurred earlier and were more intense than those in CD8+ T cells. These findings suggest that specific immune responses of CD4+ T cells were stimulated first, with the subsequent activation of CD8+ T cells giving rise to the increases in IFN-γ, TNF-α and IL-17A, accompanied by decreases in regulatory factors. This contrasted with the alum group, where there was a marked increase in IL-17A which coincided with induction of inflammatory changes, and while there was an increasing rather than decreasing effect on regulatory factors. Interestingly, there were similar kinetic changes in the pattern of IL-17A and IL-2 responses between patients immunized with YIC and only immunized with saline, suggesting that Adefovir may have some immunoregulatory effects, which needs further studies.
The small number of patients recruited in this clinical trial was aimed to analyze the HBsAg specific T cell responses and cytokine profiles to YIC treatment versus alum and saline. Therefore, we did not expect to see the efficacy and safety of YIC in this small group of patients. Another aim was to have a pilot study on whether Adefovir would affect immune responses in HBeAg positive CHB patients. In 2011, GlobeImmune has in collaboration with Gilead Sciences developed a therapeutic vaccine GS-4774. GS-4774 has been designed to generate T cell immune responses against cells containing HBV antigens in combination with antiviral therapy with the goal of increasing the cure rate in patients with CHB. This randomized, open-label phase II study with the GS-4774 failed to meet the primary end point recently.Citation35 Another therapeutic vaccine to treat of CHB was ABX203, which was formulated as a nasal spray solution and as a solution for sub-cutaneous injection. ABX203 has been designed to induce neutralizing serum antibodies to HBsAg as well as strong cellular responses, which were weak or undetectable in patients with CHB. The therapeutic vaccine was composed of the surface antigen (HBsAg) and the core antigen (HBcAg). The immune responses in CHB patients receiving ABX203 showed that this approach overcome the immune paralysis which is the major problem for the chronicity of CHB.Citation36 With a novel combination of anti-viral drugs and pegylated interferon 2a to treat naïve patients with chronic HBeAg negative HBV infection has been reported recently from Replicor, in which the REP 401 combinations were intended to achieve functional cure in patients with chronic HBV.Citation37 Most recently, Transgene presented preclinical results of TG1050, based on a viral vector expressing 3 HBV antigens.Citation38 Transgene has initiated a randomized, multi-center, double-blind, placebo-controlled safety and dose-finding first-in-man Phase 1 study evaluating the safety and tolerability of TG1050 in patients who are currently being treated for chronic HBV infection with standard-of-care antiviral therapy.
Clinical trials are golden standards for evaluation of therapeutic vaccination. In our previous phase IIB clinical trial with 6 injections of YIC, the HBeAg seroconversion rate in CHB patients was 21.8%. While in the phase III clinical trial with 12 injections of YIC, due to over stimulation of host immune responses, the HBeAg seroconversion rate dropped to 14.0%, which may be due to an immune fatigue. In this pilot study, though the number of patient was small, based on 6 injections of YIC in Adefovir add-on-treated CHB patients, the HBeAg seroconversion rate reached 20%, which was in accordance with the results of phase IIB clinical trial. Furthermore, immunological mechanisms exerted by YIC were shown as upregulation of Th1 cell type cytokines from CD4+ T cells and CD8+ T cells and downregulation of anti-inflammatory and regulatory factors; whereas alum immunization triggered inflammatory cytokines, with upregulation of anti-inflammatory and regulatory factors. The add-on of Adefovir to YIC immunization did not show inhibitory therapeutic effects, which is encouraging for future combination therapy.
Our study is the first attempt to define the immunological mechanisms initiated by YIC. This study provides valuable information on antigen specific cellular and cytokine immune responses, with saline and alum as controls. In addition, this study further provides information that the add-on Adefovir with YIC did not interfere with long-term host specific immune responses, which may guide us in future combination therapies.
Materials and methods
Patients
This study was approved by the ethical committees of participating hospitals entitled “Safety and efficacy of antigen-antibody complexed therapeutic hepatitis B vaccine combined with Adefovir Dipivoxil” with the clinical registration number: ChiCTR-TRC-11003189.
Fifty-two patients were screened and 44 met the criteria to be included in this study. Patients were: aged 20–50 y old; HBsAg and HBeAg positive for at least 6 months; anti-HBe negative with an HBV viral load more than 100,000 copies/mL; serum ALT of 2 to 5 times over the upper limit of normal value within the 4 weeks before randomized enrollment. The patients were recruited to Huashan Hospital (Shanghai, China), Fifth Hospital of Wuxi (Jiangsu Province, China), Xiangya Hospital (Hunan Province, China) and 2nd Hospital of Chongqing (Chongqing, China). All were naive patients without previous antiviral or immuno-therapies. Exclusion criteria were: co-infection with hepatitis A, C, D and E virus, or HIV; taking antiviral, hepatotoxic or immunosuppressive drugs or products within the preceding 6 months; other causes of liver disease; serious medical or psychiatric illness; hepatic cirrhosis or AFP 100 ng/mL; abnormal serum creatinine, thrombocyte count, hemoglobin or serum total bilirubin; pregnancy. All patients were treated with adefovir dipivoxil (10 mg/day) throughout the 48 weeks of this study.
Immune complexes and controls
Yeast-derived immune complexes (YIC) composed of HBsAg complexed to HBIG and alum were manufactured by Beijing Institute of Vaccine and Biological Products (Beijing, China), and in compliance with the Chinese Good Manufacture Practice (GMP) regulation. Each dose of 1 mL YIC consisted of 60 µg of HBsAg complexed to human anti-HBs immunoglobulin (HBIG) at an appropriate ratio (described in US patent 6,221,664 B1 and European patent 913157), using alum as the adjuvant, which was a mixture of KAl(SO4)2 and NaOH. One mL of 0.1% alum, identical to that being used in YIC as the adjuvant, was used for alum immunization, and 1 mL of normal saline (0,9% NaCl, Beijing Biological Product Institute) certified for medical use was used for immunization as the blank control.
Study design
Patients were divided into 3 groups randomly, and all patients were given adefovir dipivoxil together with the following injections: 6 intramuscular injections of either YIC, alum or normal saline (NS, as blank control) at 4-week intervals. Patients were followed-up for a further 24 weeks (Fig. S1).
Synthetic peptides and antibodies
Two pools of HBV peptides were used to test HBV-specific CD4+ or CD8+ T cell responses. The synthetic peptides representing the HLA class I restricted epitopes on the envelope peptides were those spanning amino acids S14 to 22 (VLQAGFFLL), S20 to 28 (FLLTRILTI), S41 to 49 (FLGGTPVCL), S88 to 96 (LLCLIFLLV), S95 to 104 (LVLLDYQGML), S97 to 106 (LLDYQGMLPV), S172 to 180 (WLSLLVPFV), S185 to 194 (GLSPTVWLSV), S207 to 216 (SIVSPFIPLL), and S208 to 216 (ILSPFLPLL); The HLA class II restricted epitopes on the envelope peptides were those spanning amino acids S19 to 28 (FFLLTRILTI), S19 to 33 (FFLLTRILTIPQSLD), S37 to 51 (TSLNFLGGTTVCLGQ), S54 to 69 (QSPTSNHSPTSCPPIC), S124 to 137 (CTTPAQGNSMFPSC), S139 to 146 (CTKPTDGN), S165 to 172 (WASVRFSW), S215 to 223 (LLPIFFCLW). All were purchased from Science Peptide Biological Technology co., Ltd. (Shanghai, China). Anti-CD4-PerCP-Cy5.5, anti-CD8-PerCP-Cy5.5, anti-IFN-γ-APC-Cy7, anti-TNF-α- APC, anti-IL-2-PE-CY7, anti-IL-17A-FITC, anti-IL-13-FITC, anti-IL-10-PE-Cy7, anti-PD-1-APC-Cy7, anti-IL-22-PE, anti-Foxp3-APC and anti-TGF-β-FITC monoclonal antibodies were purchased from eBioscience (San Diego, USA). Anti-MIP-1β-FITC was purchased from BD Biosciences (USA).
Immunological assays
Blood samples were collected at one week before immunization, and one week after the 2nd, 4th and 6th injection. Standardized assays for T cell subtyping and multifunctional analysis via stimulation of pooled HBsAg peptides are summarized as follows.
Isolation of PBMC and in vitro expansion of T cells
Peripheral blood mononuclear cells (PBMC) were isolated from fresh blood by Lymphoprep™ (Axis-Shield, Norway) and re-suspended in complete medium R10 (RPMI 1640 supplemented with 25 mM HEPES, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL of streptomycin, and 10% fetal calf serum, all from Gibco (Life technology, USA)). For T cell expansion in vitro, PBMC were suspended in R10 in the presence of anti-CD3 (0.1 µg/mL; Miltenyi Biotec, USA) and anti-CD28 (0.05 µg/mL; Miltenyi Biotec) at a concentration of 5 × 106 cells/mL and seeded at 1 mL/well in a 12-well plate. The immunological assays were performed on day 3 of the expansion.
Cell surface and intracellular cytokine staining
For CD4/CD8+ T cell functional assays, multi-color antibody panels were set up with appropriate fluorescent-labeled anti-human monoclonal antibodies. After 3 days, in vitro-expanded PBMC were seeded at 100 μl/well in a 96-well plate. Cells were washed once with R10 and then stimulated with HBV peptide pools (each peptide at a 10 µg/mL final concentration) or medium alone (control) for 8 hours in the presence of anti-CD28 (0.1 μg/mL; Miltenyi Biotec) and brefeldin A (BD Biosciences). For surface staining, the cells after washing were stained with anti-CD4 and anti-CD8 monoclonal antibodies for 30 min at 4°C, then fixed and permeabilized using 4% paraformaldehyde (PFA, Sinopharm Chemical Reagent Co., Ltd, China) and 0.2% Triton X-100 (Genview, China). For intracellular cytokine staining, the cells were further stained with the selected fluorescent-labeled anti-human monoclonal antibodies as described (anti-IFN-γ, anti-TNF-α, anti-IL-2, anti-MIP-1β, anti-IL-17A, anti-IL-13, anti-IL-10, anti-IL-22, anti-PD-1, anti-Foxp3 and anti-TGF-β) for 2 hours at 4°C and washed. Finally, cell data were acquired using a LSR Fortessa flow cytometer (BD Biosciences, USA) and data were analyzed by FlowJo (TreeStar, Ashland, OR, USA). In summary, CD4+ T cells were characterized as Th1 cells (IL-2, IFN-γ, TNF-α), Th17 (IL-17A), Th2 cells (IL-13) and Treg cells (TGF-β, IL-22, IL-10, Foxp3, PD-1). CD8+ cells were characterized as Tc1 (MIP-1β, IL-2, TNF-α, IFN-γ), Tc17 (IL-17A) and Tcreg (TGF-β, IL-22, IL-10, Foxp3, PD-1).
Virological studies
Serum HBV DNA, HBeAg seroconversion, and the levels of serum HBsAg and ALT were monitored by an independent third party laboratory (Clinical Laboratory, Ruijin Hospital, Shanghai, China), using the same lots of reagents. Sequential samples from one patient were tested on the same day. Abbott EIA AxSYM (Abbott, Abbott Park, IL, USA) was used for detection of HBsAg, HBeAg, and anti-HBe. HBV DNA was quantified by fluorescent PCR assay (PiJi, Shenzhen Co, China with a detection limit of 500 copies/mL). Architect HBsAg QT assay (Abbott, Abbott Park, IL, USA) was used for serum HBsAg quantification. Routine biochemical and hematological tests were performed at each evaluation center using automated assay systems.
Statistical analysis
An unpaired Student's t test analysis was used for all data analysis. A p-value < 0.05 (2-tailed) was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
2016HV0604R1-s02.docx
Download MS Word (9 MB)Acknowledgments
We would like to thank the patients enrolled in this study for their participation. We wish to thank the YIC clinical trial study team for their assistance in this work. We are grateful for Dr. Yan Shi for his critical review and proof readings of the manuscript.
Funding
This work was supported by the National Science and Technology Major Projects (2008ZX10002003001, 2012ZX10002002004, 2012ZX10004701 and 2013ZX10002001).
References
- Wen Y, Wang X, Wang B, Yuan Z. Vaccine therapies for chronic hepatitis B: can we go further? Frontiers Med 2014; 8:17-23; https://doi.org/10.1007/s11684-014-0313-7
- Michel ML, Deng Q, Mancini-Bourgine M. Therapeutic vaccines and immune-based therapies for the treatment of chronic hepatitis B: perspectives and challenges. J Hepatol 2011; 54:1286-96; https://doi.org/10.1016/j.jhep.2010.12.031
- Akbar SM, Furukawa S, Horiike N, Abe M, Hiasa Y, Onji M. Safety and immunogenicity of hepatitis B surface antigen-pulsed dendritic cells in patients with chronic hepatitis B. J Viral Hepatitis 2011; 18:408-14; PMID:20487261; https://doi.org/10.1111/j.1365-2893.2010.01320.x
- Luo J, Li J, Chen RL, Nie L, Huang J, Liu ZW, Luo L, Yan XJ. Autologus dendritic cell vaccine for chronic hepatitis B carriers: a pilot, open label, clinical trial in human volunteers. Vaccine 2010; 28:2497-504; PMID:20117267; https://doi.org/10.1016/j.vaccine.2010.01.038
- Chisari FV. Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am J Pathol 2000; 156:1117-32; PMID:10751335; https://doi.org/10.1016/S0002-9440(10)64980-2
- Michel ML, Pol S, Brechot C, Tiollais P. Immunotherapy of chronic hepatitis B by anti HBV vaccine: from present to future. Vaccine 2001; 19:2395-9; PMID:11257367; https://doi.org/10.1016/S0264-410X(00)00461-8
- Michel ML, Loirat D. DNA vaccines for prophylactic or therapeutic immunization against hepatitis B. Intervirology 2001; 44:78-87; PMID:11509869; https://doi.org/10.1159/000050035
- Voehringer D, Blaser C, Grawitz AB, Chisari FV, Buerki K, Pircher H. Break of T cell ignorance to a viral antigen in the liver induces hepatitis. J Immunol 2000; 165:2415-22; https://doi.org/10.4049/jimmunol.165.5.2415
- Chen S, Akbar SM, Abe M, Hiasa Y, Onji M. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol 2011; 166:134-42; PMID:21762128; https://doi.org/10.1111/j.1365-2249.2011.04445.x
- Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D, et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 2009; 49:1132-40; PMID:19140219; https://doi.org/10.1002/hep.22751
- Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis 2015; 6:e1694; https://doi.org/10.1038/cddis.2015.42
- Kong X, Sun R, Chen Y, Wei H, Tian Z. gammadeltaT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J Immunol 2014; 193:1645-53; https://doi.org/10.4049/jimmunol.1303432
- Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol 2016; 64:S60-70; PMID:27084038; https://doi.org/10.1016/j.jhep.2016.01.028
- Peppa D, Gill US, Reynolds G, Easom NJ, Pallett LJ, Schurich A, Micco L, Nebbia G, Singh HD, Adams DH, et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med 2013; 210:99-114; PMID:23254287; https://doi.org/10.1084/jem.20121172
- Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C, Pallant C, Ellis G, Khanna P, Dusheiko G, et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathogens 2010; 6:e1001227; PMID:21187913; https://doi.org/10.1371/journal.ppat.1001227
- Huan SL, Zhao JG, Wang ZL, Gao S, Wang K. Relevance of serum interleukin-33 and ST2 levels and the natural course of chronic hepatitis B virus infection. BMC Infectious Dis 2016; 16:200; PMID:27180842; https://doi.org/10.1186/s12879-016-1543-x
- Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, Robek MD. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology 2011; 141:1897-906; PMID:21708106; https://doi.org/10.1053/j.gastro.2011.06.051
- Moreno-Cubero E, Larrubia JR. Specific CD8(+) T cell response immunotherapy for hepatocellular carcinoma and viral hepatitis. World J Gastroenterol 2016; 22:6469-83; PMID:27605882; https://doi.org/10.3748/wjg.v22.i28.6469
- Akbar SM, Al-Mahtab M, Khan MS, Raihan R, Shrestha A. Immune therapy for hepatitis B. Annals Translational Med 2016; 4:335; PMID:27761439; https://doi.org/10.21037/atm.2016.08.48
- Wen YM, Wu XH, Hu DC, Zhang QP, Guo SQ. Hepatitis B vaccine and anti-HBs complex as approach for vaccine therapy. Lancet 1995; 345:1575-6; PMID:7791465; https://doi.org/10.1016/S0140-6736(95)91126-X
- Zheng BJ, Ng MH, He LF, Yao X, Chan KW, Yuen KY, Wen YM. Therapeutic efficacy of hepatitis B surface antigen-antibodies-recombinant DNA composite in HBsAg transgenic mice. Vaccine 2001; 19:4219-25; PMID:11457548; https://doi.org/10.1016/S0264-410X(01)00158-X
- Zheng BJ, Zhou J, Qu D, Siu KL, Lam TW, Lo HY, Lee SS, Wen YM. Selective functional deficit in dendritic cell–T cell interaction is a crucial mechanism in chronic hepatitis B virus infection. J Viral Hepatitis 2004; 11:217-24; PMID:15117323; https://doi.org/10.1111/j.1365-2893.2004.00497.x
- Liu H, Geng S, Wang B, Wu B, Xie X, Wang S, Zhong Y, Wang X, Qu D, Wen Y, et al. Immuno-potentiating pathway of HBsAg-HBIG immunogenic complex visualized. Hum Vaccines Immunotherapeutics 2016; 12:77-84; https://doi.org/10.1080/21645515.2015.1072660
- Xu DZ, Huang KL, Zhao K, Xu LF, Shi N, Yuan ZH, Wen YM. Vaccination with recombinant HBsAg-HBIG complex in healthy adults. Vaccine 2005; 23:2658-64; PMID:15780449; https://doi.org/10.1016/j.vaccine.2004.10.040
- Xu DZ, Zhao K, Guo LM, Li LJ, Xie Q, Ren H, Zhang JM, Xu M, Wang HF, Huang WX, et al. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PloS One 2008; 3:e2565; PMID:18596958; https://doi.org/10.1371/journal.pone.0002565
- Xu DZ, Wang XY, Shen XL, Gong GZ, Ren H, Guo LM, Sun AM, Xu M, Li LJ, Guo XH, et al. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol 2013; 59:450-6; PMID:23669281; https://doi.org/10.1016/j.jhep.2013.05.003
- Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005; 41:771-8; PMID:15791617; https://doi.org/10.1002/hep.20649
- Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol 2002; 76:8609-20; PMID:12163580; https://doi.org/10.1128/JVI.76.17.8609-8620.2002
- Han Q, Lan P, Zhang J, Zhang C, Tian Z. Reversal of hepatitis B virus-induced systemic immune tolerance by intrinsic innate immune stimulation. J Gastroenterol Hepatol 2013; 28 Suppl 1:132-7; PMID:23855309; https://doi.org/10.1111/jgh.12034
- Carey I, D'Antiga L, Bansal S, Longhi MS, Ma Y, Mesa IR, Mieli-Vergani G, Vergani D. Immune and viral profile from tolerance to hepatitis B surface antigen clearance: a longitudinal study of vertically hepatitis B virus-infected children on combined therapy. J Virol 2011; 85:2416-28; PMID:21147914; https://doi.org/10.1128/JVI.01449-10
- Sapra P, Shor B. Monoclonal antibody-based therapies in cancer: advances and challenges. Pharmacol Therapeutics 2013; 138:452-69; https://doi.org/10.1016/j.pharmthera.2013.03.004
- Rini B. Future approaches in immunotherapy. Seminars Oncol 2014; 41 Suppl 5:S30-40; PMID:25438998; https://doi.org/10.1053/j.seminoncol.2014.09.005
- Yang FQ, Yu YY, Wang GQ, Chen J, Li JH, Li YQ, Rao GR, Mo GY, Luo XR, Chen GM. A pilot randomized controlled trial of dual-plasmid HBV DNA vaccine mediated by in vivo electroporation in chronic hepatitis B patients under lamivudine chemotherapy. J Viral Hepatitis 2012; 19:581-93; PMID:22762143; https://doi.org/10.1111/j.1365-2893.2012.01589.x
- Yao X, Zheng B, Zhou J, Xu DZ, Zhao K, Sun SH, Yuan ZH, Wen YM. Therapeutic effect of hepatitis B surface antigen-antibody complex is associated with cytolytic and non-cytolytic immune responses in hepatitis B patients. Vaccine 2007; 25:1771-9; PMID:17224217; https://doi.org/10.1016/j.vaccine.2006.11.019
- Lok AS, Pan CQ, Han SH, Trinh HN, Fessel WJ, Rodell T, Massetto B, Lin L, Gaggar A, Subramanian GM, et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol 2016; 65:509-16; PMID:27210427; https://doi.org/10.1016/j.jhep.2016.05.016
- Lobaina Y, Aguiar J, Penton E, Guillen G, Mahtab M, Uddin H, Raihan R, Akbar F, Pujol F, Loureiro CL, Aguilar JC. Demonstration of safety, immunogenicity and evidences of efficacy of the therapeutic vaccine candidate HeberNasvac and characterization of chronic hepatitis B patient populations. Biotecnologia Aplicada 2015; 32:3511-13.
- Al-Mahtab M, Bazinet M, Vaillant A. Safety and Efficacy of Nucleic Acid Polymers in Monotherapy and Combined with Immunotherapy in Treatment-Naive Bangladeshi Patients with HBeAg+ Chronic Hepatitis B Infection. PloS One 2016; 11:e0156667; PMID:27257978; https://doi.org/10.1371/journal.pone.0156667
- Godon O, Evlachev A, Bourgine M, Meritet JF, Martin P, Inchauspe G, Michel ML. Recognition of core-derived epitopes from a novel HBV-targeted immunotherapeutic by T-cells from patients infected by different viral genotypes. Vaccine 2015; 33:4548-53; PMID:26209840; https://doi.org/10.1016/j.vaccine.2015.07.020
