ABSTRACT
Respiratory syncytial virus (RSV) accounts for about 20% of all respiratory infections in children below the age of 5 y. It is associated with up to 63% of all acute respiratory infections and up to 81% of all viral lower respiratory tract infections causing hospitalization in infants and young children. RSV leads to seasonal epidemics between November and April in the northern hemisphere. Most severe infections (RSV accounts for 50 to 80% of all cause bronchiolitis) affect infants younger than 6 months of age and high-risk infants including those born preterm with or without bronchopulmonary dysplasia and those with hemodynamically significant congenital heart disease up to an age of 24 months. Palivizumab, a highly potent RSV-neutralizing monoclonal antibody (Mab), has been licensed in 1998 for prophylactic use to prevent RSV associated hospitalizations in high-risk infants. This Mab is given by monthly intramuscular injection at a dose of 15 mg/kg over the RSV season (up to 5 times). Palivizumab proved to be safe and well-tolerated in this population. Concerns have been raised regarding cost-effectiveness of palivizumab and thus, palivizumab prophylaxis is mainly limited to selected high-risk infants for the first RSV season. Long-lasting Mabs will be the next future approach in the prophylaxis of RSV hospitalization until a vaccine is developed.
Respiratory syncytial virus
In 1955 a new virus was isolated from chimpanzees with symptoms of an upper respiratory tract infection including coughing and sneezing with mucopurulent nasal discharge called chimpanzee coryza agent (CCA). Chanock and colleagues confirmed its human origin when they isolated the virus from an infant with bronchopneumonia and another with laryngotracheobronchitis. Due to its ability to form syncytia in human liver epithelial cell lines the virus was renamed respiratory syncytial virus (RSV), and it was first isolated during a bronchiolitis epidemic in 1960.Citation1,2
RSV is a member of the Paramyxoviridae family. It is a medium-sized (120–200 nm) enveloped virus containing a lipoprotein coat and a linear negative-sense RNA genome of 10 genes encoding 11 proteins (completely sequenced since 1997).Citation3 Two serotypes are known – group A and B – and type A is considered to be the more virulent strain. RSV has 2 major proteins: the F-glycoprotein for fusion and G-glycoprotein for attachment to the host cells. These are the major targets for neutralizing antibodies. The F-glycoprotein is more conserved among strains and by 95% identical between serotypes A and B.Citation4 There is a 40- to 90-fold increase in F-specific compared with a 5- to 20-fold increase in G-specific antibody titer after primary infection.Citation5 Interestingly, severity of disease seems to be unrelated to RSV-specific IgG antibody titers, avidity of RSV-IgG or virus neutralization capacity.Citation6
RSV infects the bronchial, bronchiolar and alveolar epithelium, and also the airway dendritic cells. The virus is recognized by different pattern recognition receptors (PRRs) which trigger the innate immune response. T cell immunity is mandatory for virus clearance. This T helper (Th)-2 and Th-17 T cell response results in the recruitment of T cells, neutrophils and eosinophils with subsequent inflammation and tissue damage of the lung.Citation7,8 Both CD4+ and CD8+ T cells have been demonstrated to be essential for the establishment of an efficient RSV immunity, and these immune reactions are both beneficial and detrimental for the host. Approximately one third of the children can exhibit reinfection during one winter.Citation8 These reinfections are supposed to be the result of deficiencies of the humoral and cellular immune response after the first RSV infection. Disease severity has been associated with high viral loads, but in contrast low viral loads have been observed in severe disease in case of prematurity.Citation9
Respiratory syncytial virus and burden of disease
Most children are infected during the first 2 y of life, and nearly all have been infected after the second RSV season. Despite limited antigenic variation RSV immunity is short and recurrent infections occur life-long with the first episode during the first season being the most severe one.Citation4,9 One to 3% of all healthy term infants exhibit hospitalization due to RSV associated lower respiratory tract infection (LRTI) due to primary RSV infection mainly during the first 6 months of life. This rate increases up to about 10% in high risk populations including preterm infants with and without bronchopulmonary dysplasia, infants with hemodynamically significant congenital heart disease, Down syndrome, neuromuscular disease and severe immune deficiency syndrome or immunosuppression.Citation9,10 RSV is a seasonal virus with infection rates peaking during the cold season in temperate s and rainy seasons in tropical climates. Typically, RSV related hospitalizations occur between November and April in the northern hemisphere and most often peak in January and February.Citation11,12 Sometimes a severe RSV season is followed by a less severe season, and an early peaking season is followed by a late peaking one, but RSV activity has also been (rarely) observed through the whole year – as data from Austria have shown.Citation12
RSV is mainly transmitted by large particle aerosols or direct contact. Incubation time is 4–5 d with initial viral replication in the nasopharynx; thereafter the virus can spread and cause lower LRTI. Thus, RSV infection ranges from that of a mild common cold to bronchiolitis with airway obstruction, hypoxia, and wheezing, or pneumonia.Citation13 Upper respiratory tract infections (URTI) characterized by rhinitis, cough, and mostly mild fever reaction are the most common manifestations of RSV. Acute otitis media – up to 20–30% - and less often croup have been reported to occur in children with RSV illness, and bronchiolitis and pneumonia are the more common features of RSV disease in infants and young children – mostly as the first episode of an RSV infection.Citation4 RSV is responsible for 50 to 80% of all hospitalizations for bronchiolitis during seasonal epidemics.Citation14 RSV was found to be associated with 12 to 63% of all acute respiratory infections (resulting in 1 of 334 hospitalizations, 1 of 38 admissions to the emergency department, and 1 of 13 admissions to the primary care office per year in the USA) and 19 to 81% of all viral LRTIs causing hospitalization in children.Citation15 RSV hospitalization rates increase with decreasing age and vary by a factor of 2 to 3 dependent on severity of the RSV season. The length of hospital stay ranges from median 2 to 11 d in high-risk populations, with 2 to 12% of cases requiring intensive care unit (ICU) admission throughout the studies.Citation15 Risk factors associated with RSV hospitalization include male sex, young age below 6 months, birth during the first half of the RSV season, crowding and/or presence of siblings, and day-care exposure.Citation4,10,15 Bronchiolitis-associated deaths have been reported to be 2.0 per 100 000 livebirths from the late 1990s;Citation16 and case-fatality rates were reported to be below 0.5% - retrieved from studies published between 1995 and 2015.Citation15
About 34 million new cases of RSV associated LRTI globally occur in children younger than 5 years; with 3.4 million hospital admissions and about 199,000 deaths per year(predominantly in developing countries). In developed countries such as the USA, bronchiolitis is the most common reason for hospital admission up to the age of 12 months accounting for about 100,000 infant admissions annually.Citation16,17 In a prospective surveillance study including children up to the age of 5 y with acute respiratory infections from the USA 18% (919 of 5067 infants) had diagnosis of RSV U/LRTI.Citation18 RSV was found in 20% of all hospitalizations due to acute respiratory infection, in 18% of the emergency department admissions, and in 15% of the primary care office admissions between the months November and April. These findings resulted in annual hospitalization rates of 17 per 1000 infants up to the age of 6 months and 3 per 1000 children up to the age of 5 y.Citation18
The incidence of RSV associated apneas is reported to range from 1.2 to 23.8% with higher rates in preterm infants.Citation19 Encephalitis/encephalopathy is a rare but well described complication of RSV infection, predominantly manifesting as ataxia.Citation20 Severe RSV bronchiolitis during early infancy is often associated with subsequent episodes of wheezing which can persist until adolescent age.Citation21
Treatment and prevention of RSV bronchiolitis
Symptomatic treatment
The treatment of RSV bronchiolitis is primarily symptomatic and includes oral or parenteral fluid replacement to maintain adequate hydration, supplemental oxygen in case of desaturation (SpO2 below 92%), and decongestant nose drops.Citation22 In case of acute respiratory failure transfer to the ICU and non-invasive ventilator support via CPAP or invasive mechanical ventilation has to be considered. Extracorporeal membrane oxygenation (ECMO) is a lifesaving option for a minority of cases, mostly with diagnosis of prematurity and bronchopulmonary dysplasia.Citation23
Bronchodilators including salbutamol, albuterol, epinephrine or heliox might have at least short-term benefits for the patients, as it is also the case for aerosolized hypertonic saline. The use of inhaled or systemic corticosteroids and leukotriene antagonists, both as anti-inflammatory agents, showed lack of benefits.Citation22,24 The only FDA-approved antiviral drug against RSV is ribavirin. There are a lot of controversies and concerns around its efficacy, tolerability, and the route of administration. Thus, ribavirin is not recommended for routine use in the treatment of RSV disease but might be considered in life-threatening infections and in immunocompromised hosts with severe disease.Citation22,25
Preventive strategies
In the early 1960s, vaccination of infants with a formalin-inactivated RSV vaccine resulted in a significant increase of neutralizing antibodies but led to the death of 2 infants resulting from a vaccine-enhanced disease during the following RSV season.Citation26 Reasons for the difficulties developing a vaccine include the necessity of vaccination in the presence of maternal antibodies, the phenomenon of recurrent reinfections following wild virus infection, and the lack of adequate animal models completely reproducing human RSV infection.
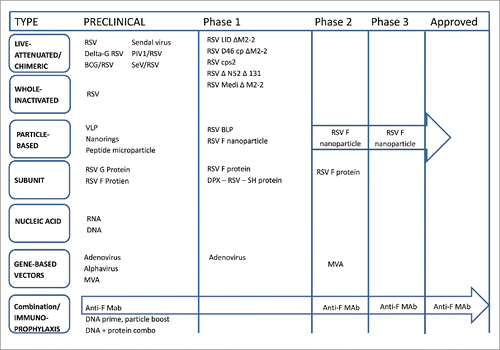
Vaccine development is ongoing based on different approaches as shown in . Details are beyond this product review.
Figure 1. Actual proceedings in the RSV vaccine and monoclonal antibody development (Adapted from Ref. Citation115.)
Standard polyclonal immunoglobulin preparations were neither successful in the prevention of RSV hospitalisation nor in the treatment of RSV disease.Citation27,28 In contrast, an RSV enriched hyperimmmune globulin (RSV-IGIV, RespiGam®) that was given parenteral at doses of 750 mg/kg monthly (5 times) during the RSV season was safe and effectively reduced RSV hospitalization rates in preterm infants with and without bronchopulmonary dysplasia and infants with cyanotic congenital heart disease.Citation29,30 Unfortunately the latter more often experienced serious adverse events including sudden unexplained death following cardiac surgery.Citation31 Thus, the American Academy of Pediatrics (AAP) recommended RSV-IGIV only for preterm infants with or without a history of bronchopulmonary dysplasia and excluded those with congenital heart disease.Citation32 RSV-IGIV had the disadvantage of monthly time-consuming infusions and the minimal risk of blood-borne pathogen transmission.Citation33 Some years following license of palivizumab it was taken from the market.
Origin and research basis for the design of palivizumab
The development of a monoclonal antibody (Mab) that neutralizes RSV was the next step in the development of a simpler administrable and maybe more effective RSV immune prophylaxis tool. Shortly, 18 neutralizing Mabs specific for the F-glycoprotein were used to construct a detailed map of epitopes that were involved in virus neutralization and fusion.Citation34 Competitive binding assays identified further antigenic and bridge sites. Following selection of Mab-resistant mutants to identify additional epitopes cross-neutralization assays were used for examination of antigenic variation in the F epitopes. This resulted in the identification of constant, variable, and hypervariable regions.Citation34
Palivizumab (Synagis®), a monoclonal antibody to the RSV F-protein, was developed over a 10-year period by MedImmune Inc. (Gaithersburg, MD) for prophylactic use against RSV disease.Citation35 The F-protein was selected as the antibody target to enable both the A and B subtype strains to be neutralized. There are 2 effects by antibody binding to the F-protein. The one is that it hampers the viral to fuse with the cell membrane; the second is that it avoids the formation of syncytia in the lung by preventing cell-to-cell spread of the virus.Citation35
In detail, antibody production was initiated following immunization of a mouse with RSV. Thereafter the antibody producing B cells were isolated from the mouse spleen and fused with mouse myeloma cell lines - with the advantage of indefinite living (hybridoma cell line) - that produced the antibody accordingly. The mouse monoclonal antibody was humanized by cloning and sequencing the DNA from both the heavy and light chains of the Mab. Following identification of the complementarity-determining-region-sequences they were molecularly “transplanted” into human immunoglobulin genes by use of computer modeling of the required 3-dimensional structures of both variable regions. Thus, the Mab is by 95% comparable to any other human antibody, and only 5% of its DNA origins from the mouse. Thereafter the genes were inserted into a plasmid expressed from Escherichia coli bacteria and then inserted into the hybridoma cell line by electroporation. This method uses an electric shock application of about 1500 V to opens up holes in the membrane, which allows entrance of the plasmid DNA and integration into the chromosome. This procedure initiated the production of the humanized antibody by the cells. Palivizumab was the first humanized monoclonal antibody ever shown to be effective against an infectious disease.Citation35
Pharmacokinetics and –dynamics
The affinity of palivizumab was slightly better than a chimeric derivative of the parent antibody, and palivizumab was found to be more potent than RSV-IGIV.Citation36 Palivizumab neutralized a broad panel of 57 RSV isolates including both subtypes A and B. At a dose of 2.5 mg/kg prophylactic palivizumab treatment of cotton rats resulted in a 99% reduction of RSV titers in their lungs. Corresponding serum concentrations were at 25 to 30 µg/mL. Furthermore, palivizumab demonstrated no induction of increased RSV pathology by primary or secondary challenge.Citation36
To answer the question for a possible presence of palivizumab-resistant mutants, an immunofluorescence binding assay was investigated to test neutralization of RSV isolates from 458 infants hospitalized for RSV LRTI between 1998 and 2002.Citation37 Palivizumab effectively bound to all 371 evaluated RSV isolates; 25 derived from palivizumab recipients. In comparison to another RSV Mab called felvizumab and to RSV-IGIV palivizumab was better neutralizing RSV by 5 and 20 times, respectively.Citation38 The concentrations necessary for a 50% inhibition of RSV were 0.23 µg/mL for palivizumab, 0.95 µg/mL for felvizumab, and 9.26 µg/mL for RSV-IGIV.Citation38 Interestingly, an 8.7% prevalence of resistance mutations at the F-glycoprotein´s 23 amino acid sequences has been observed in a small cohort of palivizumab recipients that might have an impact on palivizumab efficacy over the years.Citation39
Palivizumab was twice as potent as RSV-IGIV in reducing capsaicin-induced neurogenic extravasation in the airways following RSV infection when given before intranasal inoculation, and, in contrast to RSV-IVIG, also caused significant RSV inhibition following endotracheal inoculation in Fisher rats.Citation40 Furthermore, palivizumab inhibited neurogenic inflammation even when given 3 d following RSV inoculation. These findings suggested efficacy of palivizumab in the protection of inflammation of the respiratory tract by RSV.
Efficacy of palivizumab on reducing RSV loads has been proven in either nasalCitation41 or tracheal aspiratesCitation42 of preterm infants below 2 y of age. Nasal aspirates from 27 hospitalized preterm infants with RSV infection who had not received palivizumab were prospectively compared with 10 infants having received palivizumab regarding RSV loads.Citation41 Mean nasal RSV loads in both groups were 3.36 and 4.89 logPFU/ml, respectively, demonstrating a significant reduction of nasal RSV loads (p = 0.01). The effect of palivizumab on tracheal RSV concentrations was shown in 35 children mechanically ventilated for RSV infection (below 2 y of age).Citation42 RSV concentrations, measured before treatment and at daily intervals after treatment, were significantly reduced from day 0 to day 1 and from day 0 to day 2 (p = 0.004 and 0.012, respectively). Nasal RSV concentrations were not different between groups.
Transient, low level anti-palivizumab binding antibodies with titers ranging from 1:10 to 1:40 were detected in 10 of 65 preterm infants (15%) in a multicenter dose finding trial.Citation43 There was one child showing a transient anti-idiotypic antibody response (1:10) and another with asymptomatic elevation of AST and non-measurable palivizumab concentrations 30 d following the last palivizumab dosage.
In summary these preclinical studies revealed broad neutralizing activity of palivizumab against RSV including both A and B subtypes that exceeded that of RSV-IGIV by 50 to 100 times.
The pharmacokinetic properties of palivizumab were studied in high-risk infants up to the age of 2 y. Following intramuscular injection palivizumab is slowly absorbed and maximum serum concentrations are reached at 3 to 5 d.Citation44 Two days after the first and second intramuscular injection of palivizumab of 15 mg/kg Mean serum concentrations were 91.1 and 150.3 µg/mL, respectively, following the first and second palivizumab administration.Citation43 Cardiopulmonary bypass reduced mean serum palivizumab concentrations by 58%.Citation45 The mean distribution volume of palivizumab was found to be 57 mL/kg.Citation44 Trough serum palivizumab concentrations were below a Cmin ≤ 40 µg/ml at dose intervals of 28 to 30 d following dose 1 in 29 – 68%, dose 2 in 0 – 14%, dose 3 in 0 – 9%, dose 4 in 0 – 4%, and following dose 5 in 0 – 5%.Citation46 Following intravenous as intramuscular palivizumab injection mean serum elimination half-life was reported to differ between19.3 and 26.8 d.Citation47
Clinical studies
Phase I/II
Between 1 and 5 monthly injections were given at doses of 5 mg/kg (n = 11), 10 mg/kg (n = 6) and 15 mg/kg (n = 48), respectively, in 65 high-risk children.Citation43 Mean serum palivizumab concentrations (enzyme-linked immunosorbent assay) were 91.1 (range 52.3 - 174.0) µg/mL 2 d after the initial dose of 15 mg/kg and 49.2 (range 13.5 - 132.0) µg/mL at 30 d. Monthly injections of 15 mg/kg revealed mean trough serum concentrations of 70 µg/mL. Injections were generally well-tolerated. Adverse effects possibly being related to palivizumab were observed in 3 children (3.4%). Two patients in the 5-mg/kg dose group were hospitalized for RSV; and no RSV hospitalizations were found in the higher dose groups.Citation43
In another trial high-risk infants were randomized to receive 3, 10 or 15 mg/kg palivizumab intravenously compared with placebo (0.9% saline) each month for up to 5 administrations.Citation47 Palivizumab was safe and well-tolerated and did not induce a specific anti-palivizumab response. The mean half-life of 20 d compared well to other IgG preparations. Mean trough serum concentrations were 6.8, 36.1 and 60.6 µg/ml for the 3-, 10- and 15-mg/kg dose groups, respectively, 30 d following the first infusion and 11.9, 45.2 and 70.7 µg/mL, respectively, following the second Infusion. Thereafter, mean trough serum concentrations ranged from 14 to 18 µg/mL in the infants given 3 mg/kg palivizumab, from 46 to 72 µg/mL in those given 10 mg/kg, and from 88 to 96 mg/mL for those given 15 mg/kg.Citation47
Phase III/IV
Over the RSV season 1996/97, 1502 preterm infants with or without bronchopulmonary dysplasia received 5 injections of either palivizumab (15 mg/kg) or placebo by intramuscular injection monthly for up to 5 administrations.Citation48 Infants were recruited from 139 centers in the USA, UK and Canada. The placebo and palivizumab groups did not differ regarding demographic data and presence of typical RSV risk factors, and compliance was high (99% completed the study). Palivizumab prophylaxis resulted in an overall 55% reduction in RSV related hospitalizations (10.6% vs 4.8%). Infants without bronchopulmonary dysplasia showed a 78% reduction (8.1% vs 1.8%), those with bronchopulmonary dysplasia a 39% reduction (12.8% vs 7.9%), infants below 32 weeks a 47% and infants between 32 and 35 weeks of gestational age an 80% reduction. Thus, the infants having the most severe underlying morbidity only showed a modest reduction in RSV hospitalization. Further analyses exhibited fewer total RSV hospital days (36.4 vs. 62.6/100 children), fewer days with supplemental oxygen (30.3 vs. 50.6/100 children), fewer days with a moderate/severe LRTI (29.6 vs. 47.4/100 children), and a lower incidence of PICU admission rate (1.3 vs, 3.0%). Mean trough serum concentrations 30 d following injection number 1, 2, 3 and 4 were 37, 57, 68 and 72 µg/mL, respectively. There were no differences regarding adverse events between groups, and only 0.3% discontinued before completion. Injection site reactions occurred in 1.8% in the placebo compared with 2.7% in the palivizumab group. Frequent observations included erythemas and elevations of AST and ALT.Citation48
In 1287 children with congenital heart disease palivizumab prophylaxis given over 4 seasons (1998 – 2002) resulted in a 45% relative reduction of RSV hospitalizations.Citation49 Additionally significant findings included a 56% reduction in hospitalization days per 100 children (9.7% placebo vs 5.3%), and a 73% reduction in days with need for supplemental oxygen per 100 children (p = 0.014). There were similar rates of adverse events observed and no serious adverse events found being related to palivizumab. Deaths occurred in 3.3% in the palivizumab and 4.2% in the placebo group and none was related to palivizumab. No adverse events were recorded in regard to cardiac surgery, thus, compared with RSV-IGIV monthly palivizumab injections were safe, well-tolerated, and effective in preventing RSV disease requiring hospitalization in this population.Citation49
The Expanded Access Study included 565 infants from 80 centers in 15 countries of the Northern Hemisphere over the 1998/99 RSV season.Citation50 The rate of adverse events was low (6.9%) including injection site reaction, fever, diarrhea, and irritability. Serious adverse events included hospitalization and one case of RSV bronchiolitis not requiring hospitalization. The RSV hospitalization rate was calculated with 2.1% (12/565) – not all cases were tested for RSV.Citation50
A total of 285 preterm infants of 29 to 32 weeks of gestational age without bronchopulmonary disease and age below 6 months was enrolled from 35 centers out of 18 countries over the 2000/2001 RSV season (PROTECT study).Citation51 More than half of the infants (56%) were below 12 weeks of age at study entry, and more than 80% of infants received minimum 4 palivizumab injections. Adverse events (> 5%) were observed including rhinitis, cough, fever, pharyngitis, bronchiolitis, and diarrhea, and a minority was possibly attributed to palivizumab. The overall hospitalization rate was 7%, the RSV hospitalization rate 2.1%; and no deaths occurred during the study period.Citation51
The “Second Season Safety Study” was designed to exclude concerns that palivizumab might cause an adverse immune response during a second RSV season.Citation52 Children up to 2 y of age were selected as either first season study participants with no previous palivizumab exposure or second season participants, who had received palivizumab throughout the previous RSV season. Palivizumab was administered for up to 5 months as usual. No first (n = 71) or second (n = 63) season participants experienced a significant anti-palivizumab antibody response defined as a titer ≥ 1:80. Serum palivizumab concentrations were similar for the 2 groups. Serious adverse events were observed in 12.7% of each group; most were respiratory symptoms and all were considered to be not related to palivizumab. No deaths occurred during the study.Citation52
Metaanalyses regarding clinical and economic efficacy
A Cochrane review on palivizumab published in 2013 found palivizumab prophylaxis being effective in reducing RSV hospitalization rates in high-risk infants.Citation53 The cumulative relative reduction was 0.49 (95% confidence interval 0.37 to 0.64) when compared with placebo from 3 randomized controlled trials including 2831 infants. Economic analyses of palivizumab prophylaxis in this review revealed conflicting results with broad variations of incremental cost-effectiveness and/or cost-utility ratio values suggesting that palivizumab either was cost-effective or not cost-effective depending on many variables included in the respective pharmacoeconomic calculation model.Citation53
Another systematic review including 13 studies revealed palivizumab to be cost-effective for particular subgroups at a threshold of £30,000 per quality-adjusted life-year (QALY).Citation54 For preterm infants without bronchopulmonary dysplasia or congenital heart disease cost-effectiveness was given for those being 6 weeks of age or less at the onset of the RSV season and having 2 other RSV risk factors and a gestational age of 24 weeks or less. Palivizumab was cost-effective for children with bronchopulmonary dysplasia if they were 6 months of age or less at the onset of the season and had a gestational age of 28 weeks or less. For children with acyanotic or cyanotic congenital heart disease cost-effectiveness was given when they were younger than 6 months of age and had a gestational age of 24 weeks or less. Limitations of this economic analysis included the poor quality of the studies included. Differences existed across studies regarding true and estimated numerical results that were taken into account for analyses (poor-quality inputs) and finally led to considerable variations.Citation54
A systematic literature review and meta-analysis on RSV associated morbidity and mortality including 10 comparative studies of palivizumab prophylaxis and evaluating more than 15,000 infants reported on an all-cause mortality associated with palivizumab of 0.19% (12 of 6380) compared with 0.53% (33 of 8182) for infants not having received palivizumab prophylaxis over the RSV season (odds ratio, 0.30; 95% confidence interval, 0.17–0.55).Citation55 Only 5 RSV related deaths were reported. The RSV hospitalization rate was significantly reduced in palivizumab recipients (4.1% vs. 10.4%; odds ratio 0.35; 95% confidence interval 0.25–0.47).Citation55
Regulatory issues
In 1998, palivizumab was approved by the Food and Drug Administration (FDA) for RSV prophylaxis of high-risk children in the USA; approval of the European Agency for the Evaluation of Medicinal Products (EMEA) followed in 1999 for Europe.Citation56 Palivizumab received approval in over 45 countries worldwide in the early 2000s. The main goal of immunoprophylaxis with palivizumab was achieved by significantly reducing RSV hospitalization rates in high-risk infants in North America and Europe.Citation56
AAP guidelines over the years
The first guidelines for the use of palivizumab were published by the AAP Citation57 as a result of the phase III study published in 1998.Citation48 Palivizumab was mainly recommended for infants and children up to the age of 2 y with bronchopulmonary dysplasia (requiring medical therapy or respiratory support for their disease within 6 months before the second RSV season), and for preterm infants (those born at 28 weeks of gestational age or less up to 12 months and those born at 29 to 32 weeks up to 6 months of age). For those infants of 33 to 35 weeks of gestational age additional risk factors had to be evident including neurologic disease, presence of young siblings, child care attendance, passive tobacco smoke exposure, planned cardiac surgery or difficulties regarding medical support for severe respiratory disease). In 2003 the AAP provided additional guidelines for administering RSV prophylaxis to infants and children with hemodynamically significant congenital heart disease.Citation58 Palivizumab recommendations were changed for preterm infants of 32 to 35 weeks of gestational age by risk factor stratification (presence of 2 or more risk factors including child care attendance, school-aged siblings, exposure to environmental air pollutants, congenital abnormalities of the airways, or severe neuromuscular disease). In 2009 again guidelines were changed, now reducing the moderate and late preterm infant population to 32 to 34 weeks of gestational age. Additional risk factors required were the presence of either child care attendance or one or more siblings less than 5 y of age.Citation59
A bulk of largely divergent cost-benefit analyses of palivizumab prophylaxis was published over the last decade.Citation53-55,60 Results varied enormously, as stated shortly above, and the majority of studies did not reveal cost-effectiveness except for subgroups of high-risk infants. Some authors concluded that reducing the costs of palivizumab would lead to broader use of the Mab in high-risk infants.
In 2014 the AAP revised its recommendations completely.Citation61 The authors stated that palivizumab might be given to preterm infants at 28 weeks of gestational age or less at a maximum of 5 doses during one season. No recommendations for otherwise healthy preterm infants of 29 to 35 weeks of gestational age were provided and unchanged ones for infants with bronchopulmonary dysplasia or congenital heart disease. The committee further could not found clear evidence for the use of palivizumab in infants with anatomic pulmonary abnormalities or neuromuscular disease, Down syndrome, cystic fibrosis, or immunocompromised status.Citation61
Ongoing debate regarding palivizumab recommendations
The 2014 recommendations are in contrast to a bulk of literature describing the increased risk of RSV related hospitalizations in these populations;Citation62-67 and in disregard to the evidence of prospective studies.Citation68,69 The AAP published an additional technical report on their updated guidance for palivizumab prophylaxis.Citation70 Shortly, the committee provides different and/or alternate data regarding palivizumab pharmacokinetics, regarding the seasonality of RSV circulation, regarding an overall declining incidence of bronchiolitis associated hospitalizations in the USA (that does not apply to other countries), regarding lower RSV mortality rates than previously reported, regarding an only moderate influence on reduced wheezing episodes following palivizumab prophylaxis (even in the presence of significant study findings), regarding questionable benefit for cystic fibrosis or Down syndrome patients, regarding reports on palivizumab resistant RSV isolates from palivizumab recipients, and regarding cost analysis studies (most of them were done with the manufacturer´s support, and independent studies revealed high costs for a small benefit). This is only to provide information on a still ongoing debate regarding recommendations for palivizumab prophylaxis. Since 20 y palivizumab remains the only licensed tool against RSV in high-risk populations. A further discussion is beyond this review. Some recent publications either provide relevant epidemiological data on different subgroups of high-risk infants regarding RSV hospitalization ratesCitation71-74 or give some insights in the debate on the new AAP guidelines and its consequences to the reader.Citation75-79
Recommendations for palivizumab prophylaxis differ by far from country to country; details are beyond this product review. Since the introduction of palivizumab in to clinical use the discussion concerning the high costs of the product and its incomplete efficacy remains to be a never ending story.
From the manufacturer´s view palivizumab is licensed for preterm infants of 35 weeks of gestational age and younger with and without bronchopulmonary dysplasia and for infants with hemodynamically significant congenital heart disease according to the above mentioned trials.Citation44
An Italian consensus conference reviewed the evidence regarding efficacy and safety of palivizumab and its recommendations.Citation80 The conference members recommended palivizumab prophylaxis comparable to the latest AAP recommendations. Regarding other rare diseases – as discussed above – the conference members referred the task to specialists, who should have to evaluate the seriousness of the disease and to estimate the probability of a serious RSV infection.Citation80 This seems to be a difficult decision on the base of limited clinical evidence.
At least there raised discussion about the threshold protective serum palivizumab concentration suggesting that 25 to 30 µg/mL derived from the cotton rat model might be enough and about the number of monthly injections needed. Five injections have been given through all the studies and been recommended by the manufacturer,Citation44 but models suggest that 3 to 4 injections starting at a variable season dependent time point might be enough with cost savings of 20% and without increased RSV hospitalization rates.Citation81-83
Recent epidemiologic data
In a retrospective sequential 2 time period analysis comparing the rates of RSV hospitalization in patients younger than 2 y of age before and after implementation of the AAP 2014 guidelines on the use of palivizumab the rates did not differ between periods (5.37 versus 5.78 per 1000 children <24 months).Citation77 This effect was associated with significantly less use of palivizumab (21.7 doses of palivizumab decreased to 10.3 doses per 1000 children <24 months).Citation77 Very recently, RSV hospitalization rates have been reported that were (1) lower compared with historical reports 2 decades before for preterm infants and those with bronchopulmonary dysplasia,Citation84-87 (2) still remarkable regarding infants with congenital heart disease,Citation88,89 and (3) interesting regarding other rare diseases.Citation84,90,91
Recommendations to prevent nosocomial RSV infection
The role of palivizumab to prevent nosocomial spread of RSV is unclear as far as it is mainly used in multicomponent control strategies. Nosocomial RSV infection is known to be associated with severe courses of disease and high mortality, especially in case of underlying disease.Citation92 RSV transmission risk varies by hospital settings and has been reported to range from 6 to 56% (median 28·5%) in neonatal and/or pediatric settings.Citation93 Eleven studies used palivizumab as a measure to control nosocomial RSV outbreaks; their study designs lack a control /comparator group, therefor, reported success rates are difficult to interpret.Citation93
Palivizumab and motavizumab
A new humanized Mab derived from palivizumab was developed in the early 2000s.Citation94 Shortly, variants of palivizumab with enhanced in vitro neutralization of RSV were created. Testing of these modified Mabs in cotton rats resulted in an only marginally improvement in neutralization capacity compared with palivizumab. This surprising small effect resulted from unforeseeable broad tissue binding. Further analyses showed that small changes at the affinity binding region markedly improved the non-specific binding to various tissues. The resulting Mab, motavizumab, bound 70-fold better to RSV F than palivizumab; and showed a 20-fold better neutralization result in vitro. Motavizumab reduced pulmonary RSV titers in cotton rats by 100-fold lower levels compared with palivizumab at same concentrations; and highly potent stopped nasal viral replication.Citation94
Children hospitalized due to RSV LRTI received intravenous motavizumab at either3, 15, or 30 mg/kg or a placebo to test its safety and tolerability and to assess motavizumab concentrations and its immunogenicityCitation95 Motavizumab significantly reduced viral loads in the upper respiratory tract. No adverse events were associated with motavizumab administrations.Citation95 In a multinational study including 6635 preterm infants with and without bronchopulmonary dysplasia motavizumab showed a 26% relative reduction of RSV related hospitalizations compared with palivizumab, thus demonstrating non-inferiority.Citation96 This result of the in vivo performance of motavizumab was a little bit disappointing.
Between April 2006 and May 2006, 260 high-risk infants randomly received either 2 monthly injections of motavizumab followed by 3 injections of palivizumab or 2 times palivizumab followed by 3 times motavizumab or at least solely 5 times motavizumab that resulted in comparable adverse events rates, serum concentrations and presence of antidrug antibodies.Citation97 Motavizumab and palivizumab proved to be save and well tolerated in 1236 children with hemodynamically significant congenital heart disease up to the age of 2 years; only skin events were more often diagnosed in motavizumab recipients.Citation98 RSV outpatient medically attended LRTI occurred at similar rates between groups.
Interestingly, motavizumab was tested in term Native American infants up to the age of 6 months (randomized 2:1 placebo-controlled) resulting in an 87% relative reduction of RSV hospitalizations (2% vs. 11%).Citation99 Adverse events occurred less common in the motavizumab group; but hypersensitivity reactions were more common seen (14.7 vs. 12.3%). No influence was found on wheezing episodes up to 3 y of age. This was an impressive result demonstrating the first use of RSV immunoprophylaxis in term infants.Citation99
The Antiviral Drugs Advisory Committee of the FDA did not approve motavizumab as requested from the company in 2010.Citation100 Concerns about safety, allergic reactions, and confusion about the sponsor's decision to show that the agent was non-inferior to palivizumab were the main reasons for the expert panel to vote against approval.
Latest monoclonal antibodies against RSV
Recently, the development of RSV Mab went toward extension of the serum half-life of the Mab.Citation101 Such a Mab would lead to less frequent dosing and might be a milestone in RSV immunoprophylaxis. This new Mab named motavizumab-YTE was studied in 31 healthy adults who received a single intravenous dose compared with motavizumab at different doses (0.3, 3, 15, or 30 mg/kg) over a 240 d follow-up.Citation102 The half-life of motavizumab-YTE was 2 to 4 times longer compared with motavizumab as was the clearance lower (71 compared with 86%). Peak concentrations and distribution properties were similar independent from motavizumab dosages. Motavizumab-YTE serum concentrations necessary for RSV neutralization persisted for 240 d compared with 90 d for motavizumab. Safety and presence of antidrug antibodies did not differ between both MAbs.Citation102
The latest development is REGN-2222, a completely human IgG MAb targeting the F protein.Citation103 This Mab showed in vitro (in cotton rats) a 36-fold better inhibition of RSV fusion to the cells and a 10-40-fold better reduction of viral loads both in the lung and the upper respiratory tract compared with palivizumab. A phase-I dose escalation study conducted in healthy adults of 18–60 y of age showed a longer half-life associated with low immunogenicity. A phase-III study (www.clinicaltrials.gov; NCT02325791) including preterm infants of chronological age below 6 months is currently underway. REGN-2222 is administered intramuscularly and is expected to be dosed only one or 2 times over the RSV season.Citation103
The challenging task of adherence and compliance to palivizumab dosing
Palivizumab does not reduce the risk of RSV infection by 100%. As shown by data from the Palivizumab Outcomes Registry 46% of all hospitalizations occur following the first injection, and 29% after the second injection; thereafter rates vary between 4 and 10% at 35-days intervals.Citation104 Hence, it might have played a role that palivizumab trough serum concentrations were less than 40 µg/mL following the first palivizumab dose in about 33% of the infants; and following their second or third dose up to 14%.Citation46 Another critical point are the recommended time-intervals between palivizumab injections of 28 to 30 d.Citation44
Delays in the injection scheme sometimes add to the challenge of adherence to the palivizumab injection scheme. Some outpatient doctors erroneously argue that interim infections with fever are contraindications for palivizumab injections as this is the case for routine vaccine administration. Another reason for compliance problems is missing parental education regarding RSV infection, thorough hand hygiene and practical application of palivizumab prophylaxis.Citation105 Compliance was significantly improved by a home-based compared with the normal office palivizumab administration regimen (compliance 98% vs. 89%, p < 0.001) resulting in a significant reduction of RSV hospitalizations (0.93% vs. 3.57%, p < 0.001).Citation106 Nevertheless, this regimen is not realizable and probably not affordable for daily practice. A lot of measures including reminder telephone calls, extensive counseling programs, reminder calendars, and education in the respective native language led to increased compliance, but results rarely were significant.Citation107 Several studies recommend education of parents on palivizumab prophylaxis, support on transportation, transcultural and language difficulties, and assistance in recognizing severity of RSV disease to improve compliance. In a large review including 10,390 infants identified from disposal records provided by a pharmaceutic company, RSV hospitalization rates were significantly lower in the compliant group (1.4% vs. 3.1%).Citation107 This data was confirmed by a large study from the Canadian registry of palivizumab reporting that adherence to the monthly injection regimen was significantly associated with a lower incidence of RSV infections.Citation108
Commercial issues and product availability
Palivizumab was available as Synagis 50 mg or 100 mg powder and solvent for solution for injection until 2016 (lyophilized palivizumab). Now palivizumab is available as single-dose liquid solution vials at 50 mg per 0.5 ml and 100 mg per 1.0 ml (liquid palivizumab) for intramuscular injection. Two clinical studies compared liquid to lyophilized palivizumab in preterm infants and found comparable safety, efficacy, tolerability, immunogenicity, and pharmacokinetics.Citation109,110
Caution should be given in case of moderate and severe thrombocytopenia and coagulation disorders.Citation44 Anaphylaxis (< 1 case per 100,000 patients) has rarely been reported. In 2002 (Medimmune letter dated November 26, 2002) following experience with 2,000,000 doses palivizumab administrations 2 cases of anaphylaxis had been reported. Adverse effects commonly found were URTI, otitis media, fever, rhinitis, hernia, and elevations of serum AST. In the former trial,Citation48 infants had received routine childhood vaccines, influenza vaccine, bronchodilators or corticosteroids during the study period in similar rates regarding study groups. Adverse reactions were not found to be increased. Thus, active immunization does not seem to interfere with palivizumab. There is no knowledge regarding carcinogenesis, mutagenesis and reproductive toxicity and no further experience of its prophylactic use in adults or pregnancy.Citation44,111
A review of deaths among children below 2 y of age having received palivizumab revealed 133 cases over a 4-year study period (1998 – 2001).Citation112 The median age of the cohort was 5 months, and 54% were male. Most of the deaths were associated with congenital anomalies in 38% (singular anomalies were reported in 64%, multiple anomalies in 44%) or with respiratory infections in 23%. Palivizumab was not found to further elevate the risk of death.Citation112
The manufacturer further states, that palivizumab is not licensed for the treatment of RSV disease.Citation44 A phase I/II study using different palivizumab single intravenous doses for the treatment of RSV disease failed to demonstrate clinical benefits.Citation113 Hence, palivizumab is no treatment option for established RSV disease.
Conclusions
Palivizumab is a safe and well tolerated Mab for the prophylactic use to reduce the risk of severe RSV infection needing hospitalization in high-risk infants; i.e. including those born preterm (≤ 35 weeks of gestational age up to the chronological age of 12 months), and those with bronchopulmonary dysplasia and hemodynamically significant congenital heart disease (up to the chronological age of 24 months). Predominantly palivizumab is given intramuscularly at 5 doses of 15 mg/kg over the first RSV season; in cases needing treatment of their underlying disease (bronchopulmonary dysplasia, congenital heart disease) prophylaxis might be given over a second RSV season, and in selected cases of clinical rare pathologic conditions (cases with lung hypoplasia, neuromuscular impairment, tracheostomy, etc.) palivizumab is given at the decision of the specialist. Since nearly all RSV related deaths (99%) occur in developing, resource poor countries without the possibility of palivizumab prophylaxis due to the high costs of the product, palivizumab has limited potential to reduce the global burden of RSV disease.Citation114 As far as a vaccine still is not in sight and no simply and effective antiviral treatment of RSV exists, prophylactic palivizumab administrations remain the only tool in the prevention of severe RSV infection in high-risk infants. Long lasting Mabs like motavizumab-YTE are likely to replace palivizumab in the near future until a successful vaccine is on the market.
Disclosure of potential conflicts of interest
The author received honoraria for oral lectures on the topic of palivizumab and RSV disease from AbbVie Austria.
References
- Taylor G. Animal models of respiratory syncytial virus infection. Vaccine 2017; 35(3):469-80; PMID:27908639; https://doi.org/10.1016/j.vaccine.2016.11.054
- Rodriguez WJ. Respiratory syncytial virus infections. Pediatr Infect Dis J 1999; 10(3):161-8
- Tawar RG, Duquerroy S, Vonrhein C, Varela PF, Damier-Piolle L, Castagné N, MacLellan K, Bedouelle H, Bricogne G, Bhella D, et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science 2009; 326(5957):1279-83; PMID:19965480; https://doi.org/10.1126/science.1177634
- Simoes EA. Respiratory syncytial virus infection. Lancet 1999; 354(9181):847-52; PMID:10485741; https://doi.org/10.1016/S0140-6736(99)80040-3
- Bont L, Kimpen JL. Immunological mechanisms of severe respiratory syncytial virus bronchiolitis. Intensive Care Med 2002; 28(5):616-21; PMID:12029411; https://doi.org/10.1007/s00134-002-1256-z
- Bont L, Versteegh J, Swelsen WT, Heijnen CJ, Kavelaars A, Brus F, Draaisma JM, Pekelharing-Berghuis M, van Diemen-Steenvoorde RA, Kimpen JL. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr Res 2002; 52(3):363-7; PMID:12193668; https://doi.org/10.1203/00006450-200209000-00009
- Rossi GA, Colin AA. Respiratory syncytial virus - host interaction in the pathogenesis of bronchiolitis and its impact on respiratory morbidity in later life. Pediatr Allergy Immunol 2017 [Epub ahead of print]; https://doi.org/10.1111/pai.12716.
- Rey-Jurado E, Kalergis AM. Immunological features of respiratory syncytial virus-caused pneumonia-implications for vaccine design. Int J Mol Sci 2017; 18(3). pii: E556; PMID:28561768; https://doi.org/10.3390/ijms18030556
- Bont L, Baraldi E, Fauroux B, Greenough A, Heikkinen T, Manzoni P, Martinón-Torres F, Nair H, Papadopoulos NG, ReSViNET. RSV–still more questions than answers. Pediatr Infect Dis J 2014; 33(11):1177-9; PMID:25162928; https://doi.org/10.1097/INF.0000000000000535
- Sommer C, Resch B, Simões EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J 2011; 5:144-54; PMID:22262987; https://doi.org/10.2174/1874285801105010144
- Resch B. Burden of respiratory syncytial virus infection in young children. World J Clin Pediatr 2012; 1(3):8-12; PMID:25254161; https://doi.org/10.5409/wjcp.v1.i3.8
- Resch B, Sommer C, Nuijten MJ, Seidinger S, Walter E, Schoellbauer V, Mueller WD. Cost-effectiveness of palivizumab for respiratory syncytial virus infection in high-risk children, based on long-term epidemiologic data from Austria. Pediatr Infect Dis J 2012; 31(1):e1-8; PMID:21960187; https://doi.org/10.1097/INF.0b013e318235455b
- Hall CB, Simőes EA, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Curr Top Microbiol Immunol 2013; 372:39-57; PMID:24362683; https://doi.org/10.1007/978-3-642-38919-1_2
- Meissner HC. Viral bronchiolitis in children. N Engl J Med 2016; 374:62-72; PMID:26735994; https://doi.org/10.1056/NEJMra1413456
- Bont L, Checchia PA, Fauroux B, Figueras-Aloy J, Manzoni P, Paes B, Simões EA, Carbonell-Estrany X. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther 2016; 5(3):271-98; PMID:27480325; https://doi.org/10.1007/s40121-016-0123-0
- Haynes AK, Prill MM, Iwane MK, Gerber SI. Respiratory syncytial virus—United States, July 2012–June 2014. MMWR Morb Mortal Wkly Rep 2014; 63:1133-36; PMID:25474034
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010; 375:1545-55; PMID:20399493; https://doi.org/10.1016/S0140-6736(10)60206-1
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360(6):588-98; PMID:19196675; https://doi.org/10.1056/NEJMoa0804877
- Ralston S, Hill V. Incidence of apnea in infants hospitalized with respiratory syncytial virus bronchiolitis: A systematic review. J Pediatr 2009; 155(5):728-33; PMID:19647839; https://doi.org/10.1016/j.jpeds.2009.04.063
- Morichi S, Morishita N, Ishida Y, Oana S, Yamanaka G, Kashiwagi Y, Kawashima H. Examination of neurological prognostic markers in patients with respiratory syncytial virus-associated encephalopathy. Int J Neurosci 2017; 127(1):44-50; PMID:26732732; https://doi.org/10.3109/00207454.2016.1138951
- Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, Gustafsson PM. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65(12):1045-52; PMID:20581410; https://doi.org/10.1136/thx.2009.121582
- Mejias A, Ramilo O. New options in the treatment of respiratory syncytial virus disease. J Infect 2015; 71(Suppl 1):S80-7; PMID:25922289; https://doi.org/10.1016/j.jinf.2015.04.025
- Flamant C, Hallalel F, Nolent P, Chevalier JY, Renolleau S. Severe respiratory syncytial virus bronchiolitis in children: From short mechanical ventilation to extracorporeal membrane oxygenation. Eur J Pediatr 2005; 164(2):93-8; PMID:15703980; https://doi.org/10.1007/s00431-004-1580-0
- Liu F, Ouyang J, Sharma AN, Liu S, Yang B, Xiong W, Xu R. Leukotriene inhibitors for bronchiolitis in infants and young children. Cochrane Database Syst Rev 2015; (3):CD010636; PMID:25773054; https://doi.org/10.1002/14651858.CD010636.pub2.
- Ventre K, Randolph A. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev 2010; (5):CD000181; PMID:20464715; https://doi.org/10.1002/14651858.CD000181.pub4
- Taylor G. Animal models of respiratory syncytial virus infection. Vaccine 2017; 35(3):469-80; PMID:27908639; https://doi.org/10.1016/j.vaccine.2016.11.054
- Hemming VG, Prince GA. Intravenous immunoglobulin G in viral respiratory infections for newborns and infants. Pediatr Infect Dis 1986; 5(3 Suppl):S204-6; PMID:3714525
- Prince GA, Hemming VG, Chanock RM. The use of purified immunoglobulin in the therapy of respiratory syncytial virus infection. Pediatr Infect Dis 1986; 5(3 Suppl):S201-3; PMID:3714524
- Groothuis JR, Levin MJ, Rodriguez W, Hall CB, Long CE, Kim HW, Lauer BA, Hemming VG. Use of intravenous gamma globulin to passively immunize high-risk children against respiratory syncytial virus: Safety and pharmacokinetics. The RSVIG Study Group. Antimicrob Agents Chemother 1991; 35(7):1469-73; PMID:1718213; https://doi.org/10.1128/AAC.35.7.1469
- Groothuis JR, Simoes EAF, Levin MJ, Hall CB, Long CE, Rodriguez WJ, Arrobio J, Meissner HC, Fulton DR, Welliver RC, et al. Hemming VG: Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N Engl J Med 1993; 329:1524-30; PMID:8413475; https://doi.org/10.1056/NEJM199311183292102
- Simoes EA, Sondheimer HM, Top FH Jr, Meissner HC, Welliver RC, Kramer AA, Groothuis JR. Respiratory syncytial virus immune globulin for prophylaxis against respiratory syncytial virus disease in infants and children with congenital heart disease. The Cardiac Study Group. J Pediatr 1998; 133:492-9; PMID:9787686; https://doi.org/10.1016/S0022-3476(98)70056-3
- American Academy of Paediatrics. Respiratory syncytial virus immune globulin intravenous: Indications for use. Pediatrics 1997; 99:645-50; PMID:9093323; https://doi.org/10.1542/peds.99.4.645
- Resch B. Palivizumab for the prophylaxis of respiratory syncytial virus infection. Pediatric Health 2008; 2(3):265-78; https://doi.org/10.2217/17455111.2.3.265
- Beeler JA, van Wyke Coelingh K. Neutralizing epitoptes of the F glycoprotein of respiratory syncytial virus: Effect of mutation upon fusion function. J Virol 1989; 63:2941-50; PMID:2470922
- Young J. Development of a potent respiratory syncytial virus-specific monoclonal antibody for the prevention of serious lower respiratory tract disease in infants. Respir Med 2002; 96(Suppl B):S31-35; PMID:11996402; https://doi.org/10.1053/rmed.2002.1298
- Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis 1997; 176:1215-24; PMID:9359721; https://doi.org/10.1086/514115
- DeVincenzo JP, Hall CB, Kimberlin DW, Sánchez PJ, Rodriguez WJ, Jantausch BA, Corey L, Kahn JS, Englund JA, Suzich JA, et al. Surveillance of clinical isolates of respiratory syncytial virus for palivizumab (Synagis)-resistant mutants. J Infect Dis 2004; 190(5):975-8; PMID:15295704; https://doi.org/10.1086/423213
- Johnson S, Griego SD, Pfarr DS, Doyle ML, Woods R, Carlin D, Prince GA, Koenig S, Young JF, Dillon SB. A direct comparison of the activities of two humanized respiratory syncytial virus monoclonal antibodies: MEDI-493 and RSHZ19. J Infect Dis 1999; 180:35-40; PMID:10353858; https://doi.org/10.1086/314846
- Papenburg J, Carbonneau J, Hamelin ME, Isabel S, Bouhy X, Ohoumanne N, Déry P, Paes BA, Corbeil J, Bergeron MG, et al. Molecular evolution of respiratory syncytial virus fusion gene, Canada, 2006-2010. Emerg Infect Dis 2012; 18(1):120-4; PMID:22264682; https://doi.org/10.3201/eid1801.110515
- Piedimonte G, King KA, Holmgren NL, Bertrand PJ, Rodriguez MM, Hirsch RL. A humanized monoclonal antibody against respiratory syncytial virus (palivizumab) inhibits RSV-induced neurogenic-mediated inflammation in rat airways. Pediatr Res 2000; 47(3):351-6; PMID:10709734; https://doi.org/10.1203/00006450-200003000-00011
- DeVincenzo JP, Aitken J, Harrison L. Respiratory syncytial virus (RSV) loads in premature infants with and without prophylactic RSV fusion protein monoclonal antibody. J Pediatr 2003; 143:123-6; PMID:12915838; https://doi.org/10.1016/S0022-3476(03)00213-0
- Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner HC, Gruber WC, Sanchez PJ, Jafri H, Balsley J, Carlin D, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis 1998; 178:1555-61; PMID:9815203; https://doi.org/10.1086/314523
- Sáez-Llorens X, Castaño E, Null D, Steichen J, Sánchez PJ, Ramilo O, Top FH Jr, Connor E. Safety and pharmacokinetics of an intramuscular humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. The MEDI-493 study group. Pediatr Infect Dis J 1998; 17(9):787-91; PMID:9779762; https://doi.org/10.1097/00006454-199809000-00007
- MedImmune Inc. Synagis® (palivizumab) [online]. Available from URL: https://www.synagis.com/ [accessed 2017 Feb 7]
- Feltes TF, Cabalka AK, Meissner C, Piazza FM, Carlin DA, Top FH Jr, Connor EM, Sondheimer HM. Palivizumab prophylaxis reduces hospitalisation due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003; 143:532-40; PMID:14571236; https://doi.org/10.1067/S0022-3476(03)00454-2
- Fenton C, Scott LJ, Plosker GL. Palivizumab as prophylaxis for respiratory syncytial virus. Pediatric Drugs 2004; 6:177-97; PMID:15170364; https://doi.org/10.2165/00148581-200406030-00004
- Subramanian KN, Weisman LE, Rhodes T, Ariagno R, Sánchez PJ, Steichen J, Givner LB, Jennings TL, Top FH Jr, Carlin D, et al. Safety, tolerance and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. MEDI-493 study group. Pediatr Infect Dis J 1998; 17(2):110-5; PMID:9493805; https://doi.org/10.1097/00006454-199802000-00006
- [No authors listed]. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 1998; 102(3 Pt 1):531-7.
- Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH Jr, Connor EM, Sondheimer HM, Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003; 143(4):532-40; PMID:14571236; https://doi.org/10.1067/S0022-3476(03)00454-2
- Groothuis JR. Safety and tolerance of palivizumab administration in a large Northern Hemisphere trial. Northern Hemisphere expanded access study group. Pediatr Infect Dis J 2001; 20(6):628-30; PMID:11419509; https://doi.org/10.1097/00006454-200106000-00018
- Groothuis JR. Safety of palivizumab in preterm infants 29 to 32 weeks' gestational age without chronic lung disease to prevent serious respiratory syncytial virus infection. Eur J Clin Microbiol Infect Dis 2003; 22(7):414-7; PMID:12827537; https://doi.org/10.1007/s10096-003-0961-z
- Lacaze-Masmonteil T, Seidenberg J, Mitchell I, Cossey V, Cihar M, Csader M, Baarsma R, Valido M, Pollack PF, Groothuis JR, et al. Evaluation of the safety of palivizumab in the second season of exposure in young children at risk for severe respiratory syncytial virus infection. Drug Saf 2003; 26(4):283-91; PMID:12608889; https://doi.org/10.2165/00002018-200326040-00005
- Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 2013; (4):CD006602; PMID:23633336; https://doi.org/10.1002/14651858.CD006602.pub4
- Wang D, Bayliss S, Meads C. Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: A systematic review and additional economic modelling of subgroup analyses. Health Technol Assess 2011; 15(5):iii-iv, 1-124; PMID:21281564; https://doi.org/10.3310/hta15050
- Checchia PA, Nalysnyk L, Fernandes AW, Mahadevia PJ, Xu Y, Fahrbach K, Welliver RC Sr. Mortality and morbidity among infants at high risk for severe respiratory syncytial virus infection receiving prophylaxis with palivizumab: A systematic literature review and meta-analysis. Pediatr Crit Care Med 2011; 12(5):580-8; PMID:21200358; https://doi.org/10.1097/PCC.0b013e3182070990
- Simoes EA, Groothuis JR. Respiratory syncytial virus prophylaxis–the story so far. Respir Med 2002; 96(Suppl B):S15-24; PMID:11996400; https://doi.org/10.1053/rmed.2002.1296
- [No authors listed]. Prevention of respiratory syncytial virus infections: Indications for the use of palivizumab and update on the use of RSV-IGIV. American academy of pediatrics committee on infectious diseases and committee of fetus and newborn. Pediatrics 1998; 102(5):1211-6; PMID:9794957; https://doi.org/10.1542/peds.102.5.1211
- American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics 2003; 112(6 Pt 1):1442-6; PMID:14654627
- Committee on Infectious Diseases. From the American Academy of Pediatrics: Policy statements–Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics 2009; 124(6):1694-701; PMID:19736258; https://doi.org/10.1542/peds.2009-2345
- Resch B. Palivizumab in preventing respiratory syncytial virus-related hospitalization in high-risk infants. Expert Rev Pharmacoecon Outcomes Res 2008; 8(6):529-38; PMID:20528363; https://doi.org/10.1586/14737167.8.6.529
- American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134(2):415-20; PMID:25070315; https://doi.org/10.1542/peds.2014-1665
- Resch B. Respiratory syncytial virus infection in high-risk infants - an update on palivizumab prophylaxis. Open Microbiol J 2014; 8:71-7; PMID:25132870; https://doi.org/10.2174/1874285801408010071
- Manzoni P, Paes B, Resch B, Carbonell-Estrany X, Bont L. High risk for RSV bronchiolitis in late preterms and selected infants affected by rare disorders: A dilemma of specific prevention. Early Hum Dev 2012; 88(Suppl 2):S34-41; PMID:22633511; https://doi.org/10.1016/S0378-3782(12)70012-9
- Paes B, Mitchell I, Li A, Lanctôt KL. Respiratory hospitalizations and respiratory syncytial virus prophylaxis in special populations. Eur J Pediatr 2012; 171(5):833-41; PMID:22203430; https://doi.org/10.1007/s00431-011-1654-8
- Resch B, Paes B. Are late preterm infants as susceptible to RSV infection as full term infants? Early Hum Dev 2011; 87(Suppl 1):S47-9; PMID:21276672; https://doi.org/10.1016/j.earlhumdev.2011.01.010
- Resch B, Manzoni P, Lanari M. Severe respiratory syncytial virus (RSV) infection in infants with neuromuscular diseases and immune deficiency syndromes. Paediatr Respir Rev 2009; 10(3):148-53; PMID:19651386; https://doi.org/10.1016/j.prrv.2009.06.003
- van Beek D, Paes B, Bont L. Increased risk of RSV infection in children with Down's syndrome: Clinical implementation of prophylaxis in the European Union. Clin Dev Immunol 2013; 2013:801581; PMID:23878586; https://doi.org/10.1155/2013/801581
- Yi H, Lanctôt KL, Bont L, Bloemers BL, Weijerman M, Broers C, Li A, Kiss A, Mitchell I, Paes B, et al. Respiratory syncytial virus prophylaxis in Down syndrome: A prospective cohort study. Pediatrics 2014; 133(6):1031-7; PMID:24799541; https://doi.org/10.1542/peds.2013-3916
- Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, Bont L, Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368(19):1791-9; PMID:23656644; https://doi.org/10.1056/NEJMoa1211917
- American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134(2):e620-38; PMID:25070304; https://doi.org/10.1542/peds.2014-1666
- Anderson EJ, Krilov LR, DeVincenzo JP, Checchia PA, Halasa N, Simões EA, Domachowske JB, Forbes ML, Pannaraj PS, McBride SJ, et al. SENTINEL1: An observational study of respiratory syncytial virus hospitalizations among U.S. Infants Born at 29 to 35 weeks' gestational age not receiving immunoprophylaxis. Am J Perinatol 2017; 34(1):51-61; PMID:27233106; https://doi.org/10.1055/s-0036-1584147
- Zuccotti G, Fabiano V. Indications to respiratory syncytial virus immunoprophylaxis in the 29-32 wGA group: Is there still room for debating? Ital J Pediatr 2017; 43(1):17; PMID:28257653; https://doi.org/10.1186/s13052-017-0341-4
- Cetinkaya M, Oral TK, Karatekin S, Cebeci B, Babayigit A, Yesil Y. Efficacy of palivizumab prophylaxis on the frequency of RSV-associated lower respiratory tract infections in preterm infants: Determination of the ideal target population for prophylaxis. Eur J Clin Microbiol Infect Dis 2017; PMID:28391538; https://doi.org/10.1007/s10096-017-2976-x
- Rajah B, Sánchez PJ, Garcia-Maurino C, Leber A, Ramilo O, Mejias A. Impact of the updated guidance for palivizumab prophylaxis against respiratory syncytial virus infection: A single center experience. J Pediatr 2017; 181:183-8. e1 reporting increased morbidity 29-34; PMID:27855996; https://doi.org/10.1016/j.jpeds.2016.10.074
- Yogev R, Krilov LR, Fergie JE, Weiner LB. Re-evaluating the new committee on infectious diseases recommendations for palivizumab use in premature infants. Pediatr Infect Dis J 2015; 34(9):958-60; PMID:26107347; https://doi.org/10.1097/INF.0000000000000808
- Grindeland CJ, Mauriello CT, Leedahl DD, Richter LM, Meyer AC. Association between updated guideline-based palivizumab administration and hospitalizations for respiratory syncytial virus infections. Pediatr Infect Dis J 2016; 35(7):728-32; PMID:27078122; https://doi.org/10.1097/INF.0000000000001150
- Ambrose CS. Statistical power to detect an association between guideline-based palivizumab administration and hospitalizations for respiratory syncytial virus infections. Pediatr Infect Dis J 2017; 36(3):348; PMID:28187116; https://doi.org/10.1097/INF.0000000000001432
- Grindeland CJ, Leedahl DD. In reply: Statistical power to detect an association between guideline-based palivizumab administration and hospitalizations for respiratory syncytial virus infections. Pediatr Infect Dis J 2017; 36(3):348-9; PMID:28187117; https://doi.org/10.1097/INF.0000000000001433
- Newby B, Sorokan T. Respiratory syncytial virus infection rates with limited use of palivizumab for infants born at 29 to 31+6/7 weeks gestational age. Can J Hosp Pharm 2017; 70(1):13-8; PMID:28348428
- Pignotti MS, Carmela Leo M, Pugi A, De Masi S, Biermann KP, Galli L, Vitali Rosati G, Buonocore G, Mugelli A, Dani C, et al. Consensus conference on the appropriateness of palivizumab prophylaxis in respiratory syncytial virus disease. Pediatr Pulmonol 2016; 51(10):1088-96; PMID:27618642; https://doi.org/10.1002/ppul.23561
- Robbie GJ, Zhao L, Mondick J, Losonsky G, Roskos LK. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob Agents Chemother 2012; 56(9):4927-36; PMID:22802243; https://doi.org/10.1128/AAC.06446-11
- Gutfraind A, Galvani AP, Meyers LA. Efficacy and optimization of palivizumab injection regimens against respiratory syncytial virus infection. JAMA Pediatr 2015; 169(4):341-8; PMID:25706618; https://doi.org/10.1001/jamapediatrics.2014.3804
- Lavoie PM, Solimano A, Taylor R, Kwan E, Claydon J, Turvey SE, Marr N. Outcomes of respiratory syncytial virus immunoprophylaxis in infants using an abbreviated dosing regimen of palivizumab. JAMA Pediatr 2016; 170(2):174-6; PMID:26720836; https://doi.org/10.1001/jamapediatrics.2015.3235
- Manzoni P, Paes B, Lanctôt KL, Dall'Agnola A, Mitchell I, Calabrese S, Maule M, Girardi E, Harimoto T, Li A. Outcomes of infants receiving palivizumab prophylaxis for respiratory syncytial virus in canada and italy: An international, prospective cohort study. Pediatr Infect Dis J 2017; 36(1):2-8; PMID:27649365; https://doi.org/10.1097/INF.0000000000001340
- Resch B, Egger B, Kurath-Koller S, Urlesberger B. Respiratory syncytial virus hospitalizations in infants of 28 weeks gestational age and less in the palivizumab era. Int J Infect Dis 2017; 57:50-3; PMID:28163166; https://doi.org/10.1016/j.ijid.2017.01.034
- Resch B, Bramreiter VS, Kurath-Koller S, Freidl T, Urlesberger B. Respiratory syncytial virus associated hospitalizations in preterm infants of 29 to 32 weeks gestational age using a risk score tool for palivizumab prophylaxis. Eur J Clin Microbiol Infect Dis 2017; 36(6):1057-1062; PMID:28078558; https://doi.org/10.1007/s10096-016-2891-6
- Wang DY, Li A, Paes B, Mitchell I, Lanctôt KL, CARESS Investigators. First versus second year respiratory syncytial virus prophylaxis in chronic lung disease (2005-2015). Eur J Pediatr 2017; 176(3):413-22. 2.3 – 3.9% RSVH rate; PMID:28105526; https://doi.org/10.1007/s00431-017-2849-4
- Resch B, Kurath-Koller S, Hahn J, Raith W, Köstenberger M, Gamillscheg A. Respiratory syncytial virus-associated hospitalizations over three consecutive seasons in children with congenital heart disease. Eur J Clin Microbiol Infect Dis 2016; 35(7):1165-9; PMID:27126331; https://doi.org/10.1007/s10096-016-2649-1
- Kim AY, Jung SY, Choi JY, Kim GB, Kim YH, Shim WS, Kang IS, Jung JW. Retrospective multicenter study of respiratory syncytial virus prophylaxis in korean children with congenital heart diseases. Korean Circ J 2016; 46(5):719-26; PMID:27721865; https://doi.org/10.4070/kcj.2016.46.5.719
- Sánchez-Luna M, Medrano C, Lirio J, RISK-21 Study Group. Down syndrome as risk factor for respiratory syncytial virus hospitalization: A prospective multicenter epidemiological study. Influenza Other Respir Viruses 2017; 11(2):157-64. 9.7% RSV hosp rate; PMID:27611835; https://doi.org/10.1111/irv.12431
- Metz J, Eber E, Resch B. Respiratory syncytial virus infection associated hospitalization rates in infants and children with cystic fibrosis. Pediatr Infect Dis J 2017; 36(6):545-548.
- Thorburn K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Arch Dis Child 2009; 94(2):99-103; PMID:18653625; https://doi.org/10.1136/adc.2008.139188
- French CE, McKenzie BC, Coope C, Rajanaidu S, Paranthaman K, Pebody R, Nguyen-Van-Tam JS, Noso-RSV Study Group, Higgins JP, Beck CR. Risk of nosocomial respiratory syncytial virus infection and effectiveness of control measures to prevent transmission events: A systematic review. Influenza Other Respir Viruses 2016; 10(4):268-90; PMID:26901358; https://doi.org/10.1111/irv.12379
- Wu H, Pfarr DS, Johnson S, Brewah YA, Woods RM, Patel NK, White WI, Young JF, Kiener PA. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol 2007; 368(3):652-65; PMID:17362988; https://doi.org/10.1016/j.jmb.2007.02.024
- Lagos R, DeVincenzo JP, Muñoz A, Hultquist M, Suzich J, Connor EM, Losonsky GA. Safety and antiviral activity of motavizumab, a respiratory syncytial virus (RSV)-specific humanized monoclonal antibody, when administered to RSV-infected children. Pediatr Infect Dis J 2009; 28(9):835-7; PMID:19636278; https://doi.org/10.1097/INF.0b013e3181a165e4
- Carbonell-Estrany X, Simões EA, Dagan R, Hall CB, Harris B, Hultquist M, Connor EM, Losonsky GA, Motavizumab Study Group. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: A noninferiority trial. Pediatrics 2010; 125(1):e35-51; PMID:20008423; https://doi.org/10.1542/peds.2008-1036
- Fernández P, Trenholme A, Abarca K, Griffin MP, Hultquist M, Harris B, Losonsky GA, Motavizumab Study Group. A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season. BMC Pediatr 2010; 10:38; PMID:20525274; https://doi.org/10.1186/1471-2431-10-38
- Feltes TF, Sondheimer HM, Tulloh RM, Harris BS, Jensen KM, Losonsky GA, Griffin MP, Motavizumab Cardiac Study Group. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatr Res 2011; 70(2):186-91; PMID:21522037; https://doi.org/10.1203/PDR.0b013e318220a553
- O'Brien KL, Chandran A, Weatherholtz R, Jafri HS, Griffin MP, Bellamy T, Millar EV, Jensen KM, Harris BS, Reid R, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: A phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis 2015; 15(12):1398-408; PMID:26511956; https://doi.org/10.1016/S1473-3099(15)00247-9
- Medscape Medical News. FDA panel nixes licensing request for motavizumab. 2010. Available at http://www.medscape.com/viewarticle/722903 [accessed March 30, 2017]
- Wu H, Pfarr DS, Losonsky GA, Kiener PA. Immunoprophylaxis of RSV infection: Advancing from RSV-IGIV to palivizumab and motavizumab. Curr Top Microbiol Immunol 2008; 317:103-23; PMID:17990791
- Robbie GJ, Criste R, Dall'acqua WF, Jensen K, Patel NK, Losonsky GA, Griffin MP. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother 2013; 57(12):6147-53; PMID:24080653; https://doi.org/10.1128/AAC.01285-13
- Mejias A, Garcia-Maurino C, Rodriguez-Fernandez R, Peeples ME, Ramilo O. Development and clinical applications of novel antibodies for prevention and treatment of respiratory syncytial virus infection. Vaccine 2017; 35:496-502; PMID:27692523; https://doi.org/10.1016/j.vaccine.2016.09.026
- Parnes C, Guillermin J, Habersang R, Nicholes P, Chawla V, Kelly T, Fishbein J, McRae P, Goessler M, Gatti A, et al. Palivizumab outcomes registry study group. Palivizumab prophylaxis of respiratory syncytial virus disease in 2000-2001: Results from the palivizumab outcomes registry. Pediatr Pulmonol 2003; 35(6):484-9; PMID:12746948; https://doi.org/10.1002/ppul.10288
- Fenton C, Scott LJ, Plosker GL. Palivizumab: A review of its use as prophylaxis for serious respiratory syncytial virus infection. Paediatr Drugs 2004; 6(3):177-97; PMID:15170364; https://doi.org/10.2165/00148581-200406030-00004
- Golombek SG, Berning F, Lagamma EF. Compliance with prophylaxis for respiratory syncytial virus infection in a home setting. Pediatr Infect Dis J 2004; 23(4):318-22; PMID:15071285; https://doi.org/10.1097/00006454-200404000-00008
- Frogel MP, Stewart DL, Hoopes M, Fernandes AW, Mahadevia PJ. A systematic review of compliance with palivizumab administration for RSV immunoprophylaxis. J Manag Care Pharm 2010; 16(1):46-58; PMID:20131495; https://doi.org/10.18553/jmcp.2010.16.1.46
- Chan P, Li A, Paes B, Abraha H, Mitchell I, Lanctôt KL, CARESS investigators. Adherence to palivizumab for respiratory syncytial virus prevention in the canadian registry of palivizumab. Pediatr Infect Dis J 2015; 34(12):e290-7; PMID:26780032; https://doi.org/10.1097/INF.0000000000000922
- Robbie GJ, Makari D, Harris B, Losonsky GA, Jafri HS. Randomized, double-blind study of the pharmacokinetics and safety of palivizumab liquid formulation compared with lyophilized formulation. Infect Dis Ther 2014; 3(2):203-14; PMID:25269648; https://doi.org/10.1007/s40121-014-0042-x
- Makari D, Jensen KM, Harris B, Jafri HS. Randomized, double-blind study of the safety of the liquid versus lyophilized formulation of palivizumab in premature infants and children with chronic lung disease of prematurity. Infect Dis Ther 2014; 3(2):339-47; PMID:25156956; https://doi.org/10.1007/s40121-014-0033-y
- FDA Synagis (Palivizumab) [online] Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/palimed102302LB.pdf
- Mohan AK, Braum M, Ellenberg S, Hedje J, Cote TR. Deaths among children less than two years of age receiving palivizumab: An analysis of comorbiditeis. Pediatr Infect Dis J 2004; 23:342-45; PMID:15071290; https://doi.org/10.1097/00006454-200404000-00013
- Sáez-Llorens X, Moreno MT, Ramilo O, Sánchez PJ, Top FH Jr, Connor EM, MEDI-493 Study Group. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatr Infect Dis J 2004; 23(8):707-12; PMID:15295219; https://doi.org/10.1097/01.inf.0000133165.85909.08
- Murray J, Saxena S, Sharland M. Preventing severe respiratory syncytial virus disease: passive, active immunisation and new antivirals. Arch Dis Child 2014; 99(5):469-73; PMID:24464977; https://doi.org/10.1136/archdischild-2013-303764
- RSV Vaccine and mAb Snapshot. 2016. Available from http://www.path.org/vaccineresources/files/RSV-snapshot-December2016.pdf (accessed 7 Feb 2017)
