ABSTRACT
Background: Genetic immunization is expected to induce the expression of antigens in a native form. The encoded peptide epitopes are presented on endogenous MHC molecules, mimicking antigen presentation during a viral infection. We have explored the potential of enfuvirtide (T20), a short HIV peptide with antiviral properties, to enhance immune response to HIV antigens. To generate an expression vector, the T20 sequence was cloned into a conventional plasmid, the novel minicircle construct, and a replicon plasmid. In addition, 3 conventional plasmids that express the envelope of HIV-1 subtypes A, B and C and contain T20 in their gp41 sequences were also tested. Results: All combinations induced HIV-specific antibodies and cellular responses. The addition of T20 as a peptide and as an expression cassette in the 3 DNA vectors enhanced antibody responses. The highest anti-HIV-1 Env titers were obtained by the replicon T20 construct. This demonstrates that besides its known antiviral activity, T20 promotes immune responses. We also confirm that the combination of slightly divergent antigens improves immune responses. Conclusions: The antiretroviral T20 HIV-1 sequence can be used as an immunogen to elicit binding and neutralizing antibodies against HIV-1. These, or similarly modified gp41 genes/peptides, can be used as priming or boosting components for induction of broadly neutralizing anti-HIV antibodies. Future comparative studies will reveal the optimal mode of T20 administration.
Introduction
In this paper, we investigate the dual nature of the T20 peptide, also known as enfuvirtide. It is the first HIV-1 fusion inhibitor peptide-based drug that was approved for treatment of AIDS patients, with the brand name Fuzeon.Citation1,2 T20 consists of a 36 amino acid (aa) peptide mimicking the C-terminal heptad helix sequence close to the membrane`s proximal external region (MPER) of the HIV-1 envelope.Citation3 T20 blocks one of the early steps of the viral life cycle before reverse transcription. It can prevent de novo infection and cell-to-cell viral transmission. The T20 peptide inserts itself between 2 viral heptad regions of the transmembrane antigen gp41 and blocks fusion of the virion to the cell membrane.
The properties of the T20 sequence to mimic the C-terminal heptad region and bind to the N-terminal heptad region were considered by us to give T20 an advantage when accumulated in a single molecule. We have previously demonstrated that T20 multimerization via DOCK-AND-LOCK®-mediated conjugation to an IgG-carrier increases anti-retroviral activity 10–100-fold.Citation4 The property of site-specific binding of an antigen is also useful to induce immune responses, since complexes of antigens usually improve immunogenicity.
The MPER of gp41 in HIV is considered to have low immunogenicity, related to its position close to lipid viral and cellular membranes.Citation5 The T20 peptide might be recognized as an HIV antigen. The reason is that T20 contains a conserved peptide (ELDKWA), the so called 2F5 epitope.Citation6 Antibody 2F5 was isolated from a patient with long-standing HIV infection, and was shown to have broadly neutralizing capacities and clinical efficacy.Citation7 It has been shown that gp41 antibodies do not essentially impair T20 antiviral effects in the treatment of HIV-infected patients,Citation8 but little is known about the immunoprotective properties of antibodies directed to the T20 peptide.
Several authors have attempted induction of antibodies to the conserved regions of gp41. This was done by using purified proteins, peptides, and peptide mimetics representing the MPER, or viral-like particles (VLP). Citation9-13 None of those gp41 immunogens succeeded in inducing antibodies with the breadth and neutralizing properties of human monoclonal antibodies (hmAb) 2F5, 4E10 or 10E8, recovered from long-term infected individuals.Citation14,15 On the other hand, a lipid-modified gp41 peptide, gp41int-Cys representing 100 amino acids from the MPER region of HIV subtype B, was shown to induce both highly binding antibodies and neutralization.Citation10
We demonstrate that immunization with plasmid-based constructs encoding the short T20 peptide results in induction of antibodies to the peptide. This occurs when the epitope is present in the full HIV-1 envelope, as well as when displayed as a short peptide. Using a minicircle (MC) construct and a replicon-based vector to express T20, we have demonstrated that the MC-T20, used as enhancer together with an HIV-DNA envelope plasmid combination (pCMV-EnvABC), strongly enhanced end-point specific antibody titers as well as cellular T20-specific interferon gamma (IFNγ) responses. The replicon construct or the MC-T20 together with a heterogeneous plasmid mix of pCMV-EnvABC induced antibodies with neutralizing activity against several subtypes of HIV-1.
Results
Construction of MC and pCMVEE vectors and the design of the immunization studies
To obtain native configuration and long-term stable expression of the T20 peptide, the DNA encoding the peptide sequence was cloned into the pMC parental plasmid and processed to obtain MC-T20. The same coding sequence was cloned into the replicon vector pCMVEE. Schematic representations of MC-T20, pCMV-T20, pCMVEE-T20 and the peptide sequence of T20 are shown in . In vitro verification of T20 expression was shown by western blotting in Fig. S1A for MC-T20 and pCMV-T20, and in Fig. S1B for pCMVEE-T20. The design of immunization studies performed in mice with various vectors and combinations is provided in and Table S1.
Figure 1. Schematic overview of T20 constructs. New constructs representing MC-T20, pCMV-T20, pCMVEE-T20 and the T20 peptide amino acid sequence with the 2F5 epitope in bold letters.
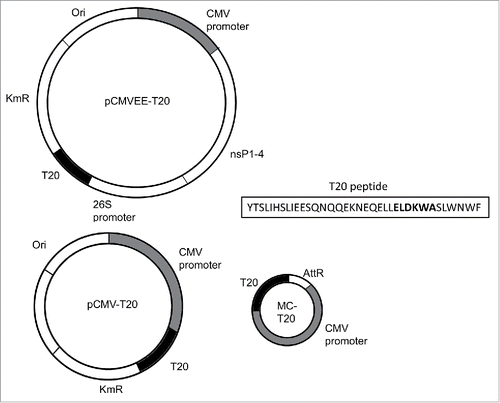
Table 1. Overview of immunization schedules for T20 constructs.
T20 peptide immunogenicity
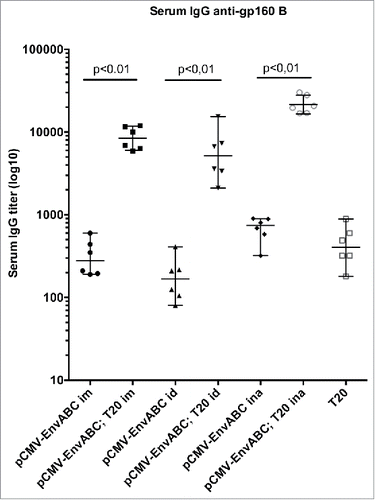
As shown in , immunization of mice with the pCMV-EnvABC induced binding antibodies to HIV-1 Env B at the same titers as immunization with the T20 peptide. Addition of the T20 peptide to the pCMV-EnvABC immunization increased antibody titers significantly. The additive effect of the T20 peptide was seen whether the intramuscular (im), intradermal (id) or intranasal (ina) immunization routes were used. The highest serum anti-gp160 B titers of over 20 000 were noted after intranasal immunization and boosting with the T20 peptides ( and Table S1, Group I). Thus, priming with pCMV-EnvABC and boosting with T20 peptide gave increased binding antibody levels to the gp160 protein subtype B, compared with pCMV-EnvABC alone.
Figure 2. Antibody titers to HIV-1 gp160 subtype (B) following pCMV-EnvABC and T20 peptide immunization. Six mice in each group were immunized with pCMV-EnvABC (20 µg) with T20 peptide (2 µg). Immunogens were given intramuscularly (im), intradermally (id) or intranasally (ina). Sera or tissue antibody levels were assayed by ELISA. Median IgG titers and ranges are shown.
IgG serum titers to HIV-1 Gag antigen were not significantly changed by T20 peptide boosting (not shown, the Gag antigen served as a control antigen in the binding assay). This result was expected, since neither T20 nor the pCMV-EnvABC constructs contain Gag sequences.
Attempts to verify viral neutralization (see below) with the sera from demonstrated neutralizing titers around 200 for subtype B. Sera from these animals (Table S1, Group I) did not permit extended studies.
Binding antibodies induced by MC-T20 and pCMVEE-T20
Antibody binding induced by MC-T20 and pCMV-EnvABC were assayed in a cohort of mice (Table S1, Group II). Binding antibodies to Env C and T20 of low titers (150–830) appeared after immunization with MC-T20 alone. These titers were amplified by the replicon vector pCMVEE-T20 (600–10400) and by co-delivery of MC-T20 with pCMV-EnvABC (600–5000) (Table S1). Avidity indices of the murine sera were high in pooled sera of the group that received pCMV-EnvABC together with the MC-T20 (0.90) and lower in groups receiving MC-T20 or pCMVEE-T20 alone (0.51 and 0.38 respectively, not shown in figures).
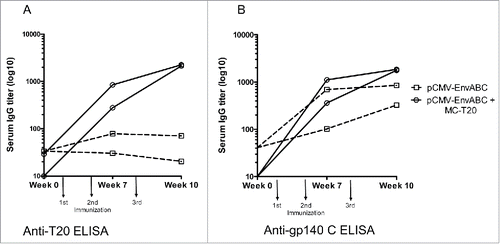
The development over time of antibodies to HIV-1 Env and T20 is illustrated in . The pCMV-EnvABC, which contains the T20 subtype B sequence in all 3 constructs induced antibody to gp140 subtype C. This reactivity was significantly enhanced by the addition of MC-T20. When testing the same sera against the T20 peptide antigen, it is notable that the MC-T20 construct induces antibodies to the T20 peptide. Antibodies of these magnitudes were not induced by pCMV-EnvABC without the added MC-T20 construct ( and Table S1 Group III).
Figure 3. A-B. Anti-gp140 (C) and anti-T20 antibody titers following immunizations with pCMV-EnvABC with and without MC-T20. Five mice in each group were immunized with pCMV-EnvABC (20 µg) with MC-T20 (20 µg); or pCMV-EnvABC (20 µg) with pCMV-DNA (20 µg). Sera were taken and antibodies against gp140 C and T20 peptide measured by ELISA. (See also Table S1, Group II.)
Neutralizing antibodies induced by T20 DNA constructs
HIV-1 strains of various subtypes were subjected to neutralization by the murine sera. Neutralization of HIV-1 strains was demonstrated more homogeneously in groups immunized with pCMV-EnvABC together with MC-T20, than in groups which received pCMV-EnvABC alone ( and ).
Figure 4. A-D. Neutralization titers to HIV-1 of subtypes A, B, (C) and CRF_AE. Five mice in each group were immunized with pCMV-EnvABC (20 µg) and MC-T20 20 µg; pCMV-EnvABC (20 µg) and pCMV-T20 (20 µg); pCMV-EnvABC (20 µg) and T20 peptide (10 µg); pCMV-EnvABC (20 µg) and non-coding pCMV-DNA (20 µg); or non-coding pCMV-DNA (40 µg). The p24 ELISA was used to detect subtype A, B and C viral antigen inhibition, the RT assay to detect CRF_AE viral antigen inhibition. (See also Table S1, Group III.)
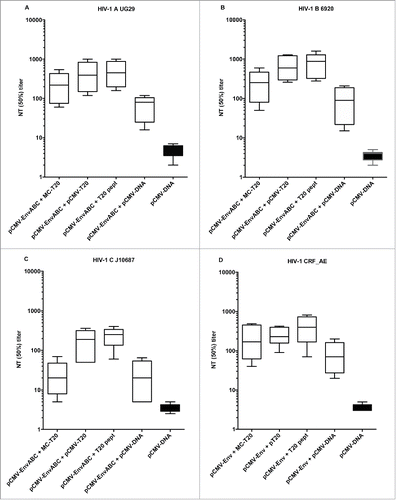
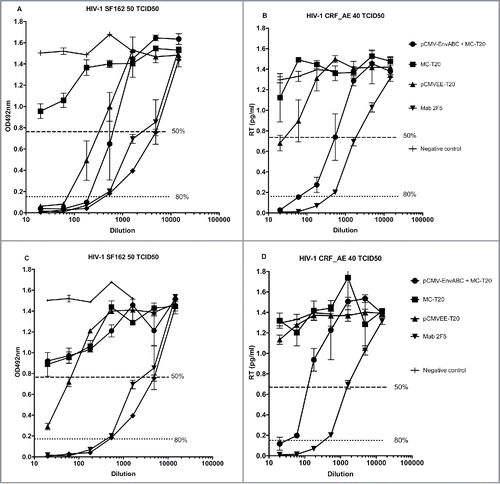
Figure 5. A-D. Neutralization titers to HIV-1 of subtypes (B) and CRF_AE. Five mice in each group were immunized with pCMV-EnvABC (20 µg) with MC-T20 (20 µg); or MC-T20 (20 µg); or pCMVEE-T20 (2 µg). Sera were taken, divided into pools of sera with higher ELISA titers (A and B) and lower binding titers (C and D), and neutralization performed. The p24 ELISA was used to detect subtype B viral antigen inhibition, the RT assay to detect CRF_AE viral antigen inhibition. Positive sera consisted of hmAb 2F5 (inverted triangles) and 4E10 (diamonds). (See also Table S1, Group II.)
The T20 was added to pCMV-EnvABC as MC-T20, pCMV-T20 and T20 peptide (). When such T20 additions were made, titers were generally higher than when pCMV-EnvABC was given with control DNA such as noncoding pCMV-DNA (). HIV-1 of subtypes A, B, C and CRF_AE were neutralized (). This indicates that the T20 acts as a co-immunogen to plasmids carrying the full Env sequences.
In , pools of sera with high ( and ) or low ( and ) binding titers in ELISA were used for neutralization of HIV-1 subtypes B and CRF_AE. Neutralization by these serum pools demonstrated a pattern with low neutralizing titers after immunization with MC-T20 alone. Titers were increased by the replicon vector pCMVEE-T20 and by the addition of multi-plasmid pCMV-EnvABC ( and Table S1, Group III).
Neutralizing antibodies induced by pCMV-EnvABC given alone (pCMV-EnvABC with noncoding pCMV-DNA, ) is therefore most likely due to antibodies induced against other epitopes of the HIV-1 envelope than those found at T20 of subtype B.
Human mab 2F5 was used as a comparator in both binding and neutralization assays. Binding specificities to coated T20 as well as neutralization of the selected HIV subtypes occurred in a similar fashion with samples from mice immunized with pCMVEE-T20 as with the site-specific monoclonal antibody ( and not shown).
Cell-mediated immune responses
Cellular immune responses could be demonstrated by ELISpot assays of spleen cells from mice receiving pCMV-EnvABC with and without MC-T20. High IFNγ production with the T20 peptide as a stimulating agent occurred with pCMV-EnvABC with MC-T20 and pCMVEE-T20 (Fig. S2). Cell mediated responses to the T20 antigen were higher with the pCMVEE-T20 than with the MC-T20. The MC in combination with pCMV-EnvABC elicited an even higher cellular response. Cellular reactivities to the gp140 C protein were, however, low (Fig. S2). It is possible that the MC-T20 construct preferentially directed responses to B-cells. Therefore,the constructs should not be mixed for induction of broad cellular responses.
A cytokine analysis of individual blood samples was performed to reveal the type of cellular immune responses. Individuals receiving MC-T20 alone or combined with pCMV-EnvABC preferentially had increased IL-2, TNFα, IFNγ and IL-12p70, indicating long-standing T-cell activation. The group receiving pCMVEE-T20 had a different pattern with increased blood levels of IL-4, IL-5 and IL-10, indicating mainly B-cell activation (data not shown).
Discussion
Strategies used in HIV vaccine research include the use of plasmids, viral vectors and proteins that encode or represent the envelope glycoprotein. Prime-boosting strategies have shown the advantage of inducing strong and broad humoral and cellular responses. Priming by DNA followed by boosting with viral vectors is the most widely-researched approach, and poxvirus- and adeno-virus-based vectors have reached the clinical phase. The moderately successful HIV-1 vaccine RV144 study used a combination of an avian pox vector together with adjuvanted Env proteins. There are still ongoing analyses of correlates of protection from the RV144 study, although antibodies reactive with viral envelope components (V1V2 loops of HIV-1) and antibodies mediating antibody-dependent cellular cytotoxicity (ADCC) that related to protection were described.Citation27,28 It will therefore be of further interest to investigate whether antibody functionality differs when induced by antigens in various expression systems. It has been established that conventional plasmids followed by vaccinia-vector boosts induced strong and long-lived ADCC antibodies,Citation29-31 although these properties are difficult to investigate in the murine system.
Our attempts to improve antibody induction to the HIV virion and infected cell surface were directed at a conserved amino acid stretch that also has potent anti-retroviral effects.Citation4,32 The T20 peptide is located at the gp41 transmembrane protein which is important for HIV-1 fusion. This part of the virion is available for direct immune attack during just a short period of the viral life cycle, and antibodies may have to utilize cell/virion lipid membranes for access to bind and neutralize.Citation33 To enhance native-like immune responses to the gp41 antigen of HIV, and particularly the small T20 peptide, we constructed vectors of different types: the very small minicircle MC-T20 and the DNA-launched replicon pCMVEE-T20. The T20-expressing DNAs were administered either alone or in combination with a plasmid DNA vaccine expressing full-length HIV envelope genes. The full-length HIV envelope plasmids were also used in prime-boost regimens together with the T20 antiviral peptide.
When used in mice, MC-T20 induced production of antibodies to the T20 peptide. This pepide harbors an epitope (2F5) to which broadly neutralizing antibodies are directed. MC-T20, given together with pCMV-EnvABC, the HIV-DNA plasmid combination, strongly enhanced end point titers against HIV-1 Env gp160 B, gp140 C, and T20. Sera from mice immunized with pCMV-EnvABC and the T20 plasmid resulted in increased antibody titers also against other sites of the gp160 HIV protein. Immunization with the pCMVEE-T20 vector resulted in high titers of HIV-1-binding and -neutralizing antibodies. For induction of functionally neutralizing antibody it is likely that the T20, 2F5 and other epitopes of the MPER region should be presented as DNA, preferably in combination with the full Env sequences, i.e. also other epitopes of Env.Citation34,35 Naturally, using epitopes expressed as peptides from a plasmid as adjuvant to constructs expressing the full length HIV proteins can be supplemented with other more traditional adjuvants.Citation12,30
It appears that expression of the small fragment of gp41 represented by T20 has a favorable configuration to induce neutralizing antibodies, compared with the same amino acid region in its native configuration in the full gp160 or gp140 sequence or as a virus-like particle. The most likely explanation is that the amino acid stretch represented by T20 is less exposed due to hydrophobicity or location within or close to sequences that adhere strongly to one another.Citation36 The pCMV-EnvABC expresses several subtype representatives of Env, and the extent of such responses was enhanced by adding constructs expressing solely a single peptide of the gp41, the T20 sequence. These added specificities may not be enough to make a very broad antibody response, but might benefit from addition of T20 sequences from yet other subtypes of HIV-1. A further path we will take is to express T20-like fragments from other subtypes of HIV-1, to augment broadness of the polyclonal antibody population.
The final constructs and doses to choose for such exposure in humans is not yet clear. The T20 peptide has been used as an anti-retroviral drug for many years, the MC-T20 is a small compound with stable but limited expression, and the pCMVEE-T20 construct includes the expression of the replicase, leading to augmented adaptive immunity.Citation18,37 Due to the different nature of these constructs, the doses of T20 might not have been equivalent in our studies, and it is quite possible that increased doses of either compound, or other immunization intervals, could have led to increased immune responses.
The MC vector is devoid of the bacterial sequences needed for propagation in the production bacterial strain, such as origin of replication and the antibiotic-resistance genes commonly used as selection markers. This results in a vector that consists only of the expression cassette.Citation38,39 The MC has been shown to give a higher and more stable expression of the transgene compared with the corresponding plasmid.Citation40 This is not only due to the possibility of giving a higher dose of therapeutic DNA when using an MC as compared with a plasmid; it also relates to increased robustness and diminished heterochromatin silencing of the vector.Citation41
The MC has been shown to hold promise as a DNA vaccine vector.Citation42,43 In a challenge experiment, the MC vector conferred better protection and elicited a stronger antigen specific CD8+ T-cell response than plasmid DNA.Citation42 When used in mice as a vector for HIV DNA vaccine, the humoral and cellular immune response elicited by the MC was improved as compared with a conventional plasmid vector.Citation44 We have also shown previously that an MC containing a small cassette can withstand shearing forces to a greater extent than a larger construct.Citation18 Recently, a promising technique was published showing that production in a ligase-mediated and bending protein-assisted circularization reaction at high DNA concentration may greatly amplify MC production.Citation45
The second vector used in this study is the novel non-integrative αvirus replicon. The αvirus genus is a group of enveloped viruses that belongs to the Togaviridae family. When the virus' structural genes are replaced by foreign genes of interest, they generate self-replicating RNA (replicons) that express foreign gene(s) in transfected cells. Replicons have been shown to produce high amounts of heterologous proteins in transfected cells. The mRNA downstream of the 26S promoter is produced in 10-fold molar excess when compared with genomic RNA.Citation21 In the present paper, pCMVEE-T20 constructs launched from plasmid DNA with a CMV promoter had the highest T20 peptide expression and induced the highest antibody titers binding to the T20 peptide.
A few publications have described expression of HIV-1 gp41 fragments. Dervillez et al. have previously expressed the C46 T20-like peptide in vitro as a multimeric fusion protein, which was shown to have an antiviral effect in vitro.Citation46 They also expressed the peptide as a monomer and could show expression but no secretion of the peptide. However, they did not study the immunogenicity of their peptide construct. For immunostimulation, secretion is not crucial, since the transfected cells present the endogenously-produced antigen on MHC molecules. Expression of peptides as short as 36 amino acids is not trivial; in nature they are often produced as part of a pre-protein or as multimers which are then cleaved. Here, we show that the T20 peptide can be expressed from different DNA-based vectors and induce HIV-specific immune responses.
In humans, adaptive antibody responses to HIV-1 envelope antigens of gp41 and gp120 occur weeks after primary HIV-infection. The quality of these antibodies is important for determining viral set-point levels, since a continuous escape of both antibody and cellular immune reactivity makes antibodies to variable sites of the virus only partially effective in reducing viral loads. Antibodies that broadly neutralize several tiers and subtypes of HIV-1 are usually directed toward less variable sites, and occur late in disease.Citation15,47
Although the effective time for antibodies to bind and neutralize via the MPER- regions is short, it was demonstrated that broadly neutralizing antibodies properties decreased viral load for a considerable time in HIV-infected patients.Citation7 The studies of passive immunization appear to have an increased effect when a combination of antibodies is used. This may be due to an altered configuration of the envelope gp120 trimer,Citation4 improved by the combination of antibodies with different targets on the Env protein.Citation48,49
The MPER region of gp41 is considered to be of low immunogenicity, which is possibly related to its position close to viral and cellular lipid membranes.Citation5,50,12,49 Our induced immune reactivity to the T20 peptide of MPER was considered to result from the fact that the peptide mimics residues of the HIV-1 glycoprotein gp41. The CD4+ molecule acts as the primary receptor of HIV-1. The antigen gp41 binds to the target cell through integration of the gp41 fusion peptide, before the formation of a 6-helix bundle. This permits viral attachment to the CCR5 cell surface co-receptor. Thus, antibodies or cellular reactivity formed against the T20 peptide or its epitopes will also find epitopes at the HIV-1 transmembrane protein of the virion during infection. The same properties that make T20 a very efficient antiviral during long-term exposureCitation4 may thus be the reason that gene-expressed T20 induces neutralizing antibodies when administered as DNA.
In these studies we used neutralization assays with viruses of different subtypes, which replicate well in vitro. Other types of assays such as pseudovirion inhibition or antibody-dependent assays would have made our results more comparable with those of human or non-primate immunization studies. The sample sizes from mice did not permit further analyses. We have however shown previously that results from our murine studies mirror the broadness and subtype reactivities seen in immunized humans from several countries and with various genetic backgrounds.Citation17,26,34,35 The possibility to assay T20 or sequences homologous to gp41 of SIV as immunogens might be further explored in non-human primates. In this situation, the gp41 fragments may act independently to boost antiviral responses to the homologous transmembrane regions.
The murine Fab has a different configuration from human IgG, but it was still possible to induce and identify stem cells with genes for the desired broadly neutralizing activities from HIV-immunized mice which had been knocked-in for human Ig production.Citation51 The T20 antigen is not very immunogenic when given as a peptide to infected humans,Citation8 and induction of neutralizing antibody has not been shown. The individuals who receive the T20 treatment are highly immunosuppressed. Therefore, it is likely that T20 or similar molecules expressed intracellularly by DNA constructs in immunocompetent humans might induce or boost functional antibodies with variable Fab chains similar to the stem cell variants described by Sok et al.Citation51
The expression of small, defined sequences of hidden epitopes from HIV can thus possibly lead to development of new DNA drugs, and might be used to prime the induction of broadly neutralizing antibodies.
Materials and methods
Peptides and vectors
Peptide (): The T20 peptide derived from HXB2 of HIV-1 subtype B is a linear 36 amino acid peptide used as a fusion inhibitor in anti-retroviral therapy (Roche Molecular Systems, USA). The peptide amino acid sequence is as follows: YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWF; with the 2F5 epitope in bold.
pCMV-EnvABC: This compound consists of 3 plasmids containing gp160 genes from HIV-1 subtypes A, B, and C.Citation16 They were produced by homologous recombination where 75% of the original subtype B of pCMV-gp160 (pCMV-EnvB) was substituted for sequences found in subtypes A and C.Citation16,17 The constructs also contain a CMV promoter, an HPV16 polyA signal, and a kanamycin resistance gene (KmR). One of these plasmids, pCMV-EnvB, was used as a donor vector for the expression cassette to produce pCMV-T20 and MC-T20 (see below). pCMV-DNA, which does not contain any expression cassette, was used for control immunizations.
Minicircle MC-T20 (): The nucleotide (nt) sequence for the T20 peptide with a secretion signal (ss) from the V-J2-C region of the mouse Ig kappa-chain for efficient secretion of recombinant proteins (Immunomedics Inc., Morris Plains, NJ, USA)Citation4 was cloned into the pCMV-T20 plasmid (see pCMV-EnvABC) using Fse I and EcoRV restriction sites, by enzymes from New England Biolabs (R0588S and R0195S, NEB). PCR was performed to amplify the ss-T20 gene fragment (Eurofins Genomics, DE). The whole expression cassette was cloned into a pMC-vector, using its restriction sitesCitation18 to produce MC-T20. The resulting construct was transformed into the ZYCY10P3S2T bacterial strain and expanded overnight. MC-T20 induction and purification were performed according to Kay et al.Citation19 The cloned sequences were confirmed by sequencing (Eurofins Genomics). The resulting MC-T20 was 1.1 kb which is less than one third of a corresponding conventional plasmid.
To assess expression, pCMV-T20 and MC-T20 were transfected into HeLa, HEK293 and U2OS cells by polyethyleneimine (PEI) or lipofectamine (BMS1003 and 11668027, ThermoFisher Scientific). Crude cell lysate was run on a 20% TBE gel. After transfer to a nitrocellulose membrane by an iBlotter (Invitrogen Life Technologies, SE), the membrane was incubated with anti-HIV Env human monoclonal antibodies hmAb 2F5 or 4E10 (generous gifts from H. and D. Katinger, Polymun Scientific), and then with goat-anti-human IgG labeled with IRDye 680LT (925–68078 LI-COR).
pCMVEE-T20 (): We cloned the HIV T20 sequence used in the pCMV-T20 and MC-T20 constructs above into the multiple cloning site (MCS) of the pCMVEE vector to generate the pCMVEE-T20 plasmid using the EcoRV and Fse I restriction sites. pCMVEE is a DNA-launched αvirus replicon vector based on the Venezuelan Equine Encephalitis virus (VEE).Citation20 The replicon is under the control of the CMV promoter and encodes the viral replicase, the internal 26S promoter followed by the T20 gene and the viral polyA. After transcription of the replicon mRNA, the 5′ end of the parental genome encoding the viral replicase is translated in the cytoplasm. Next, the replicase catalyzes the transcription of a negative sense copy of the genome, which serves as a template for new replicon RNA, as well as for high level transcription of sub-genomic mRNA from the internal 26S promoter.Citation21 pCMVEE-T20 cloning was confirmed by sequencing (Eurofins Genomics).
Eukaryotic expression of T20 from pCMVEE-T20 was confirmed in baby hamster kidney (BHK) cells. Transfection was performed using lipofectamine 2000 (11668027, ThermoFisher Scientific) according to the manufacturer's instructions. After 24 h, cells and supernatants were harvested, denatured and run on an SDS-PAGE, and blotted to a PVDF membrane before T20 was detected using hmAb 2F5 (AB001, Polymun Scientific), followed by HRP-conjugated rabbit anti-human IgG (Sigma-Aldrich, MO, USA). The membranes were developed with enhanced chemiluminescent substrate (Pierce ECL Western Blotting Substrate, 32106 Thermo Fisher Scientific), and visualised with a ChemiDoc MP imaging system (Bio-Rad, CA, USA).
Murine immunization
Female BALB/c mice 8–12 weeks of age were purchased from Scanbur Research (Sollentuna, Sweden) and kept at the Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet. A summary of the immunization studies is provided in and Supplementary, with additional parameters described in the figure legends. All mice were of the same strain, from the same deliverer, of the same gender and similar ages. They were were immunized with T20 peptide, MC-T20, pCMV-T20, pCMV-EnvABC, pCMVEE-T20 and/or control pCMV-DNA to assess the serological and cellular responses. The mice were given protein/peptides intramuscularly (im), intradermally (id) or intranasally (ina), and gene constructs intradermally by needle (id) followed by electroporation (EP). EP was performed by DermaVax skin delivery system (Cellectis, Paris, FR), which has a needle array electrode with two parallel rows of four 2-mm pins.Citation22,23
Immunological assays
Enzyme-linked immunosorbent assay
ELISA was performed with baculovirus recombinant protein subtype B gp160/LAV (2000 LAV, Protein Sciences), recombinant HIV-1 antigen CN54 gp140 C (ENV002, Polymun) the gp41 peptides T20 and P18 from subtype B and HIV-1 Gag p24 antigen (Los Alamos database, http://www.hiv.lanl.gov/ and Thermohybaid, DE). Microplates (243656, Nalge-Nunc) were coated with proteins (1 µg/well) or peptide (10 µg/well) in 200 µl/well and sera were assayed as described.Citation24 Avidity indices were determined after urea elution of antibodies using 7 M urea. ELISA titers were determined by interpolating the dilution at which the dilution curve reaches the defined cutoff value using GraphPad Prism software (CA, USA).
Neutralization assay
Viral isolates were derived from subtype A 92UG29/WHO strain, subtype B strain IIIB LAI X4, subtype B SF162 R5, B/6920 R5, HIV-1 B/6794 × 4, HIV-1 C J10687 R5 and HIV-1 CRF01_AE 1545 (http://www.hiv.lanl.gov). The panel was selected to include both X4 and R5 strains and to be related to the pCMV-EnvABC plasmid immunogen.Citation25 Sera were diluted in RPMI 1640 supplemented with 5% FCS and antibiotics (61870–010, ThermoFisher Scientific) in 96-well tissue culture plates (260860, ThermoFisher Scientific). Each dilution was mixed with virus of a simultaneously determined TCID50 and incubated at 37°C for 1 h followed by the addition of 105 human PBMCs activated by phytohemagglutinin (PHA) and rIL-2 (200–02, PeproTech) or C8166.CCR5 receptor-rich cells. The cells were incubated over night at 37°C in 5% CO2 in air, and washed twice with RPMI 1640 before they received new medium. After 5–7 d of culture, the presence of HIV-1 p24 antigen in the culture medium was measured by ELISA for HIV-1 subtypes A and B or the Lenti-RT assay (51010, CavidiTech) for HIV-1 subtypes C and CRF01_AE. The background of the p24 ELISA was determined for each plate and subtracted from all wells. The percentage of neutralization was determined as [1-(mean p24 OD in the presence of test serum/mean p24 OD in the absence of test serum)] × 100. CavidiTech results were calculated as pg of RT/ml.
ELISpot assays
Interferon gamma (IFNγ) Enzyme Linked ImmunoSpot (ELISpot) was used to determine the frequency of IFNγ producing T cells after stimulation with proteins or peptides. Plates (MAIPSWU10, Millipore) were coated with IFN-γ capture antibodies according to the manufacturer's instructions (3420–3–250, MabTech). Splenocytes were prepared as described.Citation24,26 A total of 2 × 105 cells were plated per well and stimulated for 21 +/- 2 h with peptides/proteins of HIV-1. Concanavalin A (C5275–5MG, Sigma-Aldrich) was used as a positive control, and culture medium (MDEM with 2 mM L-glutamine, 1% Penicillin-Streptomycin and 5% bovine calf serum) as negative control. ELISpots were developed with biotinylated detection Mab R4–6A2 (1 μg/ml) followed by streptavidine alkaline phosphatase (ALP) and 5-bromo-4-chloro-3-indolyl phosphate-nitroblue tetrazolium (BCIP/NBT) substrate. The number of spot-forming cells (SFCs) was determined using an ELISpot reader (BioReader Autoplate 5000, BioSys, Karben, DR). Cytokines secreted by spleen cells were measured by BioPlex Pro Mouse Cytokine assay TH1/TH2 panel 8-Plex according to the manufacturer's protocol (BioRad, Richmond, CA, USA). In brief, 25 µl of serum mixed with assay diluent was added to cytokine beads labeled with anti-TH1 and anti-TH2 cytokine antibodies. Standard curves for each cytokine were used to determine cytokine concentration/ml of serum. Control serum was derived from a pool of non-immunized mice.
Statistical analysis
Statistical analyses were performed using Prism 5 GraphPad Software, (CA, USA). Statistical methods used were the non-parametric Mann-Whitney U test and Kruskal-Wallis non-parametric analysis with Dunn´s correction.
Disclosure of potential conflicts of interest
C.-H. C. and D. M. G. are employees and stockholders of Immunomedics. DMG is an inventor of relevant patents assigned to Immunomedics. SS and BW share a patent with Immunomedics. The Immunomedics company did not influence planning or interpretations.
Supplemental_Material.zip
Download Zip (660.9 KB)Acknowledgements
We acknowledge the valuable participation and competence of Nicoleta Eserblom during the performance of the T-20 characterization and immunizations.
Funding
Funding was obtained from Physicians against AIDS, and EU-grants for projects EAVI, Epiical and Vactrain. Funding for Lena Hansen was obtained from Bergen University and the Karolinska Institutet.
References
- Matthews T, Salgo M, Greenberg M, Chung J, DeMasi R, Bolognesi D. Enfuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat Rev Drug Discov. 2004;3(3):215-25. doi:10.1038/nrd1331. PMID:15031735
- Makinson A, Reynes J. The fusion inhibitor enfuvirtide in recent antiretroviral strategies. Curr Opin HIV AIDS. 2009;4(2):150-8. doi:10.1097/COH.0b013e32832498d8. PMID:19339955
- Qiu Z, Chong H, Yao X, Su Y, Cui S, He Y. Identification and characterization of a subpocket on the N-trimer of HIV-1 Gp41: implication for viral entry and drug target. AIDS. 2015;29(9):1015-24. doi:10.1097/QAD.0000000000000683. PMID:26125136
- Chang CH, Hinkula J, Loo M, Falkeborn T, Li R, Cardillo TM, Rossi EA, Goldenberg DM, Wahren B. A novel class of anti-HIV agents with multiple copies of enfuvirtide enhances inhibition of viral replication and cellular transmission in vitro. PLoS One. 2012;7(7):e41235. doi:10.1371/journal.pone.0041235. PMID:22844444
- Montero M, van Houten NE, Wang X, Scott JK, The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design. Microbiol Mol Biol Rev. 2008;72(1):54-84, table of contents. doi:10.1128/MMBR.00020-07. PMID:18322034
- Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Rüker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67(11):6642-7; PMID:7692082
- Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11(6):615-22. doi:10.1038/nm1244. PMID:15880120
- Walmsley S, Henry K, Katlama C, Nelson M, Castagna A, Reynes J, Clotet B, Hui J, Salgo M, DeMasi R, et al. Enfuvirtide (T-20) cross-reactive glycoprotein 41 antibody does not impair the efficacy or safety of enfuvirtide. J Infect Dis. 2003;188(12):1827-33. doi:10.1086/379810. PMID:14673761
- Bianchi E, Joyce JG, Miller MD, Finnefrock AC, Liang X, Finotto M, Ingallinella P, McKenna P, Citron M, Ottinger E, et al. Vaccination with peptide mimetics of the gp41 prehairpin fusion intermediate yields neutralizing antisera against HIV-1 isolates. Proc Natl Acad Sci U S A. 2010;107(23):10655-60. doi:10.1073/pnas.1004261107. PMID:20483992
- Lai RP, Hock M, Radzimanowski J, Tonks P, Hulsik DL, Effantin G, Seilly DJ, Dreja H, Kliche A, Wagner R, et al. A fusion intermediate gp41 immunogen elicits neutralizing antibodies to HIV-1. J Biol Chem. 2014;289(43):29912-26. doi:10.1074/jbc.M114.569566. PMID:25160627
- Benen TD, Tonks P, Kliche A, Kapzan R, Heeney JL, Wagner R. Development and immunological assessment of VLP-based immunogens exposing the membrane-proximal region of the HIV-1 gp41 protein. J Biomed Sci. 2014;21:79. doi:10.1186/s12929-014-0079-x. PMID:25160824
- Hanson MC, Abraham W, Crespo MP, Chen SH, Liu H, Szeto GL, Kim M, Reinherz EL, Irvine DJ. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine. 2015;33(7):861-8. doi:10.1016/j.vaccine.2014.12.045. PMID:25559188
- Vassell R, He Y, Vennakalanti P, Dey AK, Zhuang M, Wang W, Sun Y, Biron-Sorek Z, Srivastava IK, LaBranche CC, et al. Immunogens Modeling a Fusion-Intermediate Conformation of gp41 Elicit Antibodies to the Membrane Proximal External Region of the HIV Envelope Glycoprotein. PLoS One. 2015;10(6):e0128562. doi:10.1371/journal.pone.0128562. PMID:26087072
- Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J Virol. 1994;68(6):4031-4; PMID:7514684
- Borrow P, Moody MA. Immunologic characteristics of HIV-infected individuals who make broadly neutralizing antibodies. Immunol Rev. 2017;275(1):62-78. doi:10.1111/imr.12504. PMID:28133804
- Ljungberg K, Rollman E, Eriksson L, Hinkula J, Wahren B. Enhanced immune responses after DNA vaccination with combined envelope genes from different HIV-1 subtypes. Virology. 2002;302(1):44-57. doi:10.1006/viro.2002.1547. PMID:12429515
- Nilsson C, Hejdeman B, Godoy-Ramirez K, Tecleab T, Scarlatti G, Bråve A, Earl PL, Stout RR, Robb ML, Shattock RJ, et al. HIV-DNA Given with or without Intradermal Electroporation Is Safe and Highly Immunogenic in Healthy Swedish HIV-1 DNA/MVA Vaccinees: A Phase I Randomized Trial. PLoS One. 2015;10(6):e0131748. doi:10.1371/journal.pone.0131748. PMID:26121679
- Stenler S, Wiklander OP, Badal-Tejedor M, Turunen J, Nordin JZ, Hallengärd D, Wahren B, Andaloussi SE, Rutland MW, Smith CI, et al. Micro-minicircle Gene Therapy: Implications of Size on Fermentation, Complexation, Shearing Resistance, and Expression. Mol Ther Nucleic Acids. 2014;2:e140. doi:10.1038/mtna.2013.67. PMID:24399204
- Kay MA, He CY, Chen ZY, A robust system for production of minicircle DNA vectors. Nat Biotechnol. 2010;28(12):1287-9. doi:10.1038/nbt.1708. PMID:21102455
- Ljungberg K, Whitmore AC, Fluet ME, Moran TP, Shabman RS, Collier ML, Kraus AA, Thompson JM, Montefiori DC, Beard C, et al. Increased immunogenicity of a DNA-launched Venezuelan equine encephalitis virus-based replicon DNA vaccine. J Virol. 2007;81(24):13412-23. doi:10.1128/JVI.01799-07. PMID:17913817
- Strauss JH, Strauss EG, The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58(3):491-562; PMID:7968923
- Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, Bråve A, Wahren B, Pisa P. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS One. 2009;4(9):e7226. doi:10.1371/journal.pone.0007226. PMID:19789652
- Hallengard D, Bråve A, Isaguliants M, Blomberg P, Enger J, Stout R, King A, Wahren B. A combination of intradermal jet-injection and electroporation overcomes in vivo dose restriction of DNA vaccines. Genet Vaccines Ther. 2012;10(1):5. doi:10.1186/1479-0556-10-5. PMID:22873174
- Hallengard D, Wahren B, Brave A. A truncated plasmid-encoded HIV-1 reverse transcriptase displays strong immunogenicity. Viral Immunol. 2013;26(2):163-6. doi:10.1089/vim.2012.0083. PMID:23573980
- Brave A, Boberg A, Gudmundsdotter L, Rollman E, Hallermalm K, Ljungberg K, Blomberg P, Stout R, Paulie S, Sandström E, et al. A new multi-clade DNA prime/recombinant MVA boost vaccine induces broad and high levels of HIV-1-specific CD8(+) T-cell and humoral responses in mice. Mol Ther. 2007;15(9):1724-33. doi:10.1038/sj.mt.6300235. PMID:17579577
- Brave A, Gudmundsdotter L, Sandström E, Haller BK, Hallengärd D, Maltais AK, King AD, Stout RR, Blomberg P, Höglund U, et al. Biodistribution, persistence and lack of integration of a multigene HIV vaccine delivered by needle-free intradermal injection and electroporation. Vaccine. 2010;28(51):8203-9. doi:10.1016/j.vaccine.2010.08.108. PMID:20951666
- Zolla-Pazner S, deCamp AC, Cardozo T, Karasavvas N, Gottardo R, Williams C, Morris DE, Tomaras G, Rao M, Billings E, et al. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS One. 2013;8(1):e53629. doi:10.1371/journal.pone.0053629. PMID:23349725
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275-86. doi:10.1056/NEJMoa1113425. PMID:22475592
- Joachim A, Nilsson C, Aboud S, Bakari M, Lyamuya EF, Robb ML, Marovich MA, Earl P, Moss B, Ochsenbauer C, et al. Potent functional antibody responses elicited by HIV-I DNA priming and boosting with heterologous HIV-1 recombinant MVA in healthy Tanzanian adults. PLoS One. 2015;10(4):e0118486. doi:10.1371/journal.pone.0118486. PMID:25874723
- Wise MC, Hutnick NA, Pollara J, Myles DJ, Williams C, Yan J, LaBranche CC, Khan AS, Sardesai NY, Montefiori D, et al. An Enhanced Synthetic Multiclade DNA Prime Induces Improved Cross-Clade-Reactive Functional Antibodies when Combined with an Adjuvanted Protein Boost in Nonhuman Primates. J Virol. 2015;89(18):9154-66. doi:10.1128/JVI.00652-15. PMID:26085155
- Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, Donahue DA, Lorin V, Casartelli N, Noël N, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun. 2016;7:10844. doi:10.1038/ncomms10844. PMID:26936020
- Qiu S, Yi H, Hu J, Cao Z, Wu Y, Li W. The binding mode of fusion inhibitor T20 onto HIV-1 gp41 and relevant T20-resistant mechanisms explored by computational study. Curr HIV Res. 2012;10(2):182-94. doi:10.2174/157016212799937191. PMID:22339124
- Veiga AS, Pattenden LK, Fletcher JM, Castanho MA, Aguilar MI. Interactions of HIV-1 antibodies 2F5 and 4E10 with a gp41 epitope prebound to host and viral membrane model systems. Chembiochem. 2009;10(6):1032-44. doi:10.1002/cbic.200800609. PMID:19283693
- Rollman E, Hinkula J, Arteaga J, Zuber B, Kjerrström A, Liu M, Wahren B, Ljungberg K. Multi-subtype gp160 DNA immunization induces broadly neutralizing anti-HIV antibodies. Gene Ther. 2004;11(14):1146-54. doi:10.1038/sj.gt.3302275. PMID:15103320
- Nilsson C, Godoy-Ramirez K, Hejdeman B, Bråve A, Gudmundsdotter L, Hallengärd D, Currier JR, Wieczorek L, Hasselrot K, Earl PL, et al. Broad and potent cellular and humoral immune responses after a second late HIV-modified vaccinia virus ankara vaccination in HIV-DNA-primed and HIV-modified vaccinia virus Ankara-boosted Swedish vaccinees. AIDS Res Hum Retroviruses. 2014;30(3):299-311. doi:10.1089/aid.2013.0149. PMID:24090081
- Frey G, Chen J, Rits-Volloch S, Freeman MM, Zolla-Pazner S, Chen B. Distinct conformational states of HIV-1 gp41 are recognized by neutralizing and non-neutralizing antibodies. Nat Struct Mol Biol. 2010;17(12):1486-91. doi:10.1038/nsmb.1950. PMID:21076402
- Knudsen ML, Ljungberg K, Tatoud R, Weber J, Esteban M, Liljeström P. Alphavirus replicon DNA expressing HIV antigens is an excellent prime for boosting with recombinant modified vaccinia Ankara (MVA) or with HIV gp140 protein antigen. PLoS One. 2015;10(2):e0117042. doi:10.1371/journal.pone.0117042. PMID:25643354
- Darquet AM, Cameron B, Wils P, Scherman D, Crouzet J. A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Ther. 1997;4(12):1341-9. doi:10.1038/sj.gt.3300540. PMID:9472558
- Bigger BW, Tolmachov O, Collombet JM, Fragkos M, Palaszewski I, Coutelle C. An araC-controlled bacterial cre expression system to produce DNA minicircle vectors for nuclear and mitochondrial gene therapy. J Biol Chem. 2001;276(25):23018-27. doi:10.1074/jbc.M010873200. PMID:11304530
- Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther. 2003;8(3):495-500. doi:10.1016/S1525-0016(03)00168-0. PMID:12946323
- Riu E, Chen ZY, Xu H, He CY, Kay MA. Histone modifications are associated with the persistence or silencing of vector-mediated transgene expression in vivo. Mol Ther. 2007;15(7):1348-55. doi:10.1038/sj.mt.6300177. PMID:17457320
- Dietz WM, Skinner NE, Hamilton SE, Jund MD, Heitfeld SM, Litterman AJ, Hwu P, Chen ZY, Salazar AM, Ohlfest JR, et al. Minicircle DNA is superior to plasmid DNA in eliciting antigen-specific CD8+ T-cell responses. Mol Ther. 2013;21(8):1526-35. doi:10.1038/mt.2013.85. PMID:23689601
- Schleef M, Schirmbeck R, Reiser M, Michel ML, Schmeer M. Minicircle: Next Generation DNA Vectors for Vaccination. Methods Mol Biol. 2015;1317:327-39; PMID:26072415
- Wang Q, Jiang W, Chen Y, Liu P, Sheng C, Chen S, Zhang H, Pan C, Gao S, Huang W. In vivo electroporation of minicircle DNA as a novel method of vaccine delivery to enhance HIV-1-specific immune responses. J Virol. 2014;88(4):1924-34. doi:10.1128/JVI.02757-13. PMID:24284319
- Thibault T, Degrouard J, Baril P, Pichon C, Midoux P, Malinge JM. Production of DNA minicircles less than 250 base pairs through a novel concentrated DNA circularization assay enabling minicircle design with NF-κB inhibition activity. Nucleic Acids Res 2017;45(5):e26. doi:10.1093/nar/gkw1034
- Dervillez X, Hüther A, Schuhmacher J, Griesinger C, Cohen JH, von Laer D, Dietrich U. Stable expression of soluble therapeutic peptides in eukaryotic cells by multimerisation: application to the HIV-1 fusion inhibitory peptide C46. ChemMedChem. 2006;1(3):330-9. doi:10.1002/cmdc.200500062. PMID:16892368
- Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10(4):359-69. doi:10.1089/aid.1994.10.359. PMID:7520721
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466-70. doi:10.1038/nature10373. PMID:21849977
- Krebs SJ, McBurney SP, Kovarik DN, Waddell CD, Jaworski JP, Sutton WF, Gomes MM, Trovato M, Waagmeester G, Barnett SJ, et al. Multimeric scaffolds displaying the HIV-1 envelope MPER induce MPER-specific antibodies and cross-neutralizing antibodies when co-immunized with gp160 DNA. PLoS One. 2014;9(12):e113463. doi:10.1371/journal.pone.0113463. PMID:25514675
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308(5730):1906-8. doi:10.1126/science.1111781. PMID:15860590
- Sok D, Briney B, Jardine JG, Kulp DW, Menis S, Pauthner M, Wood A, Lee EC, Le KM, Jones M, et al. Priming HIV-1 broadly neutralizing antibody precursors in human Ig loci transgenic mice. Science. 2016;353(6307):1557-1560. doi:10.1126/science.aah3945. PMID:27608668
