ABSTRACT
Background and aims: Routine childhood vaccinations schedules recommend that children receive the vaccine doses at specific ages. Vaccination coverage data are conventionally reported by the up-to-date method. We aimed to assess vaccination timeliness by the age-appropriate method and compare with the up-to-date vaccination coverage. Methods: Assessment of age-appropriate and up-to-date vaccination coverage among children born in Israel in 2009 and followed to age 48 months (national representative sample, n = 3892). The vaccinations included: Hepatitis B vaccine (HBV), Diphtheria-Tetanus-acellular Pertussis-Polio-Haemophilus-influenzae-b (DTaP-IPV-Hib), Pneumococcal conjugate vaccine (PCV), Measles-mumps-rubella-varicella vaccine (MMR/MMRV) and Hepatitis A vaccine (HAV). The categories defined: age-appropriate (at the recommended age and up to 1 month), delayed less than 6 months, delayed 6 months and above and unvaccinated (48 months). Results: The age-specific vaccinations assessment showed considerable delay in receipt of routine vaccination. While most (96%, 95%, 91%, 96%, 94% and 86%) children were vaccinated up-to-date for HBV3, DTaP-IPV-Hib4, PCV3, MMR/MMRV1, HAV1and HAV2 vaccine doses; only 26%, 29%, 47%, 64%, 55% and 12% were vaccinated age-appropriate. Vaccination delay was more common in vaccines with multiple doses. Vaccination delay was associated with high child's birth order, low socio-economic rank, ethnicity (delay more common in Jews vs. Arabs), season of birth (winter) and delayed receipt of DTaP-IPV-Hib vaccine 1st dose. Conclusions: This study assessed age-appropriate childhood vaccination coverage in a national cohort of children. While the overall vaccination coverage stands in line with the WHO goals, vaccination timeliness and equity are inadequate and targeted public health intervention programs aimed at vaccination timeliness are necessary.
Background
Routine immunization programs for young children are considered among the foremost beneficial and cost-effective primary prevention strategies in public health; hence, vaccination coverage is an established child's health indicator.Citation1-5 Immunizations during the first months of life are aimed to induce immunity, essential for protection against infectious pathogens to which infants are susceptible, that are particularly hazardous and cause significant burden in early childhood.Citation6-10 The overall concept of routine childhood vaccination programs stated by the World Health Organization (WHO) and public health agencies is that vaccine doses should be given at defined ages, according to the schedule, and delay is undesired.Citation11-14 However, delay and incompleteness of routine vaccinations are very frequent and have been reported in high income countries and in middle and low income countries worldwide.Citation15-24 Monitoring immunization coverage is essential for planning actions to improve acceptance of recommended childhood vaccines and to evaluate the program impact. Country –level immunization coverage reports are predominantly based on periodical surveys and cumulative data. The validity of immunization coverage estimates has been reported to be inappropriate; the officially reported national data of coverage rates for DTP3 vaccine (Diphtheria-Tetanus-Pertussis vaccine, third dose) were higher than those reported from surveys in many countries.Citation25,26 Additionally, national figures conceal immunization coverage inequities e.g. in socio-economically deprived or in minority groups.Citation27,28 Therefore, individually - based immunisation registers (an immunisation registry is defined as a secure database which records information about vaccinations given to individuals) and information systems have been or are being developed in many countries.Citation29-31
The overall national immunization coverage reported in Israel is adequate according to the WHO immunization coverage targets.Citation32 However, some sub-populations may be under-immunized, as highlighted by outbreaks of vaccine-preventable diseases which emerged in specific communities (e.g., Arab Bedouin and Jewish ultra-orthodox) in the country.Citation27,28,33,34 The setting of a national immunization registry enabled assessment of vaccinations receipt patterns.Citation35 The conventional method of evaluating vaccination coverage is up-to-date (proportion of vaccinated children at pre-defined ages). The up-to-date method does not reflect vaccination timeliness, which is better evaluated by the age-appropriate method. In the age-appropriate method, the age at each vaccine dose is recorded. The age-appropriate method is complex, requires sequential data collection, and therefore is not routinely used.Citation15,23,36
The study aim is to assess timeliness and completeness of childhood vaccinations according to the recommended schedule in Israel, by applying the 2 methods (age-appropriate and up-to-date) and to explore factors associated with vaccination acceptance and timeliness.
Results
The general characteristics of the children in the study group (n = 3892, born in 2009 and followed 48 months) are presented in . The rate of age-specific vaccinations was determined at monthly intervals. The cumulative proportion of vaccination uptake using the inverse Kaplan-Meier curves is presented in , for the vaccine doses HBV3, DTaP-IPV-Hib4, PCV3, MMR/MMRV1, HAV1 and HAV2. Age–specific coverage data at 3 age points – first - one month post the age recommended for the vaccine dose in the schedule (defined as age-appropriate), and then at further 2 age points, were extracted from the cumulative curves. The age–specific coverage for the hepatitis B vaccine (HBV: at birth, 1 and 6 months) 3rd dose, HBV3: 26%, 79% and 89% were vaccinated at age 7, 9 and 12 months. For the Diphtheria, Tetanus, acellular Pertussis, Polio and Haemophilus influenzae type b (DTaP-IPV-Hib at age 2, 4, 6 and 12 months) fourth dose, DTaP4: 29%, 73% and 90% were vaccinated at age 13, 18 and 24 months. For the pneumococcal conjugate vaccine (PCV at age 2, 4 and 12 months, introduced into the routine immunization schedule in 2009) third dose, PCV3: 47%, 80% and 88% were vaccinated at age 13, 18 and 24 months. For the measles-mumps-rubella-varicella vaccine (MMR/MMRV at age 12 months) first dose, 64%, 89% and 95% were vaccinated with MMR/MMRV1 at age 13, 18 and 24 months. For the hepatitis A vaccine (HAV at age 18 and 24 months) first dose, HAV1 at 19 and 24 months, 55% and 86%, respectively and for second dose, HAV2 at 25 and 30 months, 12% and 65%, respectively. The combination vaccine DTaP-IPV-Hib (pentavalent vaccine) is scheduled and provided in the routine immunization program. In the study group most children received the DTaP-IPV-Hib combination; 42 children (1.08%) were reported with any split of the 5 antigens and 9 children (0.23%) received DT vaccine. The MMRV vaccine replaced MMR vaccine in the routine immunization schedule Israel in 2008 and had been provided either as combination or separately. In the study group most children received the MMRV vaccine; 157 children (4.03%) received MMR vaccine.
Table 1. General characteristics of the study population (n = 3892 children).
Figure 1. The cumulative proportion of vaccination uptake, using the inverse Kaplan-Meier curves, for the vaccine doses: Hepatitis B vaccine third dose (HBV3, scheduled at 6 months), Diphtheria- Tetanus- acellular Pertussis- Polio- Haemophilus influenzae type b vaccine fourth dose (DTaP-IPV-Hib4, scheduled at 12 months), Pneumococcal conjugate vaccine third dose (PCV3, scheduled at 12 months), Measles-mumps-rubella-varicella first dose (MMR/MMRV1, scheduled at 12 months), Hepatitis A vaccine first and second doses (HAV1 and HAV2, scheduled at 18 and 24 months, respectively).
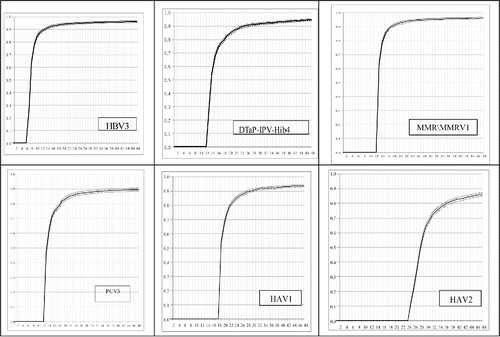
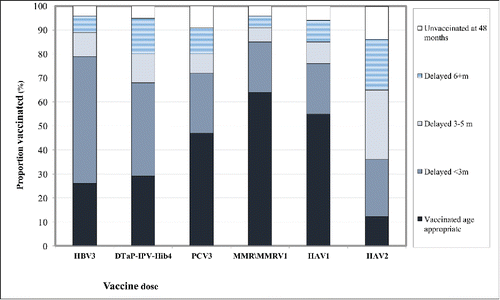
The assessment of vaccination coverage rates by categories revealed a substantial gap between the up-to-date vaccination status and the age-appropriate vaccination status. The distribution of vaccination coverage rates by categories is presented in . At the end of follow-up (age 48 months) 96%, 95%, 91%, 96%, 94% and 86% of the children were defined as vaccinated up-to-date for the HBV3, DTaP-IPV-Hib4, PCV3, MMR/MMRV1, HAV1 and HAV2 vaccine doses, respectively. The assessment of age-specific vaccinations status showed considerable delay in receipt of routine childhood vaccines; 26%, 29%, 47%, 64%, 55% and 12% of the children were defined as vaccinated age-appropriate, according to the recommended schedule for the above vaccine doses. Most delays were categorized as “mild/moderate” (less than 3 months and 3–5 months). The fraction of “severe delay” ranged from 5% for MMR/MMRV1 to 21% for HAV2. The fraction of “unvaccinated at 48 months” ranged from 4% for MMR/MMRV1 to 14% for HAV2.
Figure 2. The distribution of vaccination coverage rates by categories at the end of follow-up (age 48 months) for the vaccine doses: Hepatitis B vaccine third dose (HBV3), Diphtheria- Tetanus- acellular Pertussis- Polio- Haemophilus influenzae type b vaccine fourth dose (DTaP-IPV-Hib4), Pneumococcal conjugate vaccine third dose (PCV3), Measles-mumps-rubella-varicella first dose (MMR/MMRV1), Hepatitis A vaccine first and second doses (HAV1 and HAV2).
Cox Proportional Hazards regression was performed to analyze markers for timeliness of HBV3, DTaP-IPV-Hib4 and MMR/MMRV1 vaccines (). The risk markers significantly associated with delayed vaccinations included: a child's birth order of fourth and above, ethnicity (delay was more common among Jewish vs. Arab children), a lower socio-economic status of the locality of the family residence (the association with delayed childhood vaccinations was evident for the multiple doses vaccines HBV3 and DTaP-IPV-Hib4 and less for the first dose of MMR/MMRV1 vaccine) and delayed acceptance of the first dose of DTaP-IPV-Hib vaccine (DTaP-IPV-Hib1).
Table 2. Cox Proportional Hazards regression model (Hazard ratio; HR, and 95% confidence interval; CI) for vaccine doses: Hepatitis B vaccine third dose (HBV3), Diphtheria- Tetanus- acellular Pertussis- Polio- Haemophilus influenzae type b vaccine fourth dose (DTaP-IPV-Hib4) and Measles-mumps- rubella-varicella first dose (MMR/MMRV1).
The multiple logistic regression analysis models with the dependent variable being “vaccinated up-to-date” status, a compound outcome defined specifically for the ages of 12, 24 and 48 months are presented in . The up-to-date vaccination status was defined for age 12 months (including the vaccine doses HBV3, DTaP-IPV-Hib 3 and PCV2), for age 24 months (including the vaccine doses HBV3, DTaP-IPV-Hib4, PCV3 and MMR/MMRV1) and for age 48 months (same as for 24 months combined with HAV2). The proportion defined as “vaccinated up-to-date” at 48 months was 86% for all vaccines and 92.3% excluding the vaccine doses of HAV2 (often delayed) and PCV3 (introduced in 2009). Prior to the multiple regression with the compound outcome, regression analysis of the vaccine doses of HBV3, PCV3, DTaP-IPV-Hib4 and MMR/MMRV1 was performed separately for each vaccine with the demographic and socio economic variables. The independent variables significantly associated with “vaccinated up-to-date” status in the final model were: child's birth order (increasing), ethnicity (up-to-date status more common in Arab vs. Jewish children), socio-economic status of the locality of residence (lower) and birth season (lower probability of children born in winter months, January to March, to be vaccinated up-to-date). The maternal characteristics which were not included in the final model were age (highly correlated with the child's birth order) and maternal education (missing data in 46% of files). In the separate analysis the child's birthweight was associated only with delayed HBV3 vaccine (at age 7 months). Notably, delayed acceptance of DTaP-IPV-Hib1, (scheduled at age 2 months) was a significant predicting factor for a child to be unvaccinated “up to date” at future ages. An additional multiple logistic regression model with the dependent variable ” delayed acceptance of DTaP-IPV-Hib1 ” revealed 2 risk markers: low birthweight <2500 g (Odds ratio 1.59, 95%CI 1.1–2.31, p = 0.014) and child's birth order of fourth and above (Odds ratio 2.11, 95%CI 1.64–2.71, p = 0.0001).
Table 3. Multiple logistic regression model - dependent variable “vaccinated up-to-date” at age 12, 24 and 48 months.
As the socio-economic status of the locality was correlated with ethnicity (lowest socio-economic rank 1 includes only Arab Bedouin and Jewish ultra-orthodox localities), the multiple logistic regression analysis models were subsequently performed separately for the ethnic sub-groups of Arabs and Jewish children. The risk markers among Arab children were the birth order, mother's status (higher risk in unmarried), birth season, socio-economic rank of the locality and delayed acceptance of DTaP-IPV-Hib1. The risk markers among Jewish children were the birth order, birth season and delayed acceptance of DTaP-IPV-Hib1.
The 4 vaccination status categories (vaccinated age-appropriate, delayed less than 6 months, delayed 6 months and above and unvaccinated at 48 months) were compared as to the mean socio-economic rank. The categories “age-appropriate” (mean rank 4.58 ± 0.20) and “delayed less than 6 months” (mean rank 4.56 ± 0.13) did not differ compared with the other categories. The categories ” delayed 6 months and above - severe delay” (mean rank 4.25 ± 0.19) and “unvaccinated at 48 months” (mean rank 4.91 ± 0.25) were found to be significantly different (Tukey Test, p <0.01).
Comparison of vaccination coverage rates (age 2 years) in the study group and the national reported vaccination coverage rates for the 2009 cohort is presented in . The vaccination coverage rates found in the study group were consistently lower compared with the national reported vaccination coverage rates, with a range from 2.4% for the MMR1 vaccine to 6.6% for the HAV1 vaccine.
Table 4. Up-to-date routine childhood immunization coverage rates at age 2 years: comparison of coverage rates in the study group and coverage rates in the ministry of health national report.Citation38
Discussion
In the current study assessment of age-appropriate vaccination status of children yielded considerable gaps from up-to-date vaccination status. Appropriate estimate of vaccination coverage is essential to assess children's health status, to identify trends, to detect disparities and to plan intervention programs.Citation5,14,15,25,26,28 A major goal of vaccination programs is to obtain the highest levels of protection against vaccine-preventable diseases at the earliest age possible.Citation1 Inadequate timeliness of childhood immunizations have been reported in several settings and countriesCitation15,19-24 Therefore, even in countries with high up-to-date coverage, evaluation of timeliness through age-appropriate vaccine receipt provides valuable insights. Routine vaccination timeliness and completeness are still a public health challenge as disparities and delays prevail worldwide. The estimated global coverage of MCV1 (measles containing vaccine 1st dose) and of DTP3 (diphtheria-tetanus-pertussis 3rd dose) was 82% in 2009 and 85%, 86%, respectively, in 2014, both below the Global Vaccine Action Plan (GVAP) targets of 90% or more.Citation37
The up-to-date routine childhood vaccination coverage in Israel is well in line with the WHO set goals.Citation32,38 However, routine vaccination delay was frequent with similar proportion of children in the “mild - moderate delays” and “age-appropriate” categories for multiple doses vaccines (e.g., HBV3, DTaP-IPV-Hib4 and PCV3). The MMR/MMRV1 vaccine was considerably less delayed. This can be attributed to MMR1 being a single dose vaccine not depending on timing of previous doses, as well as a vaccine upon which major efforts are made to ensure proper timeliness, due to emergence of measles and mumps outbreaks in Israel during the last decade.Citation33,34 The age-appropriate method is useful to appraise coverage rates goals and to monitor implementation of new vaccines. During 2009 the 7-valent pneumococcal conjugated vaccine (PCV7) was introduced into the routine vaccination schedule in Israel (2, 4 and 12 months); in 2010, PCV13 replaced PCV7.Citation8 We found that PCV vaccination coverage in the first year (2009 cohort) was similar to other multiple doses vaccines.
Early vaccinations delay is a strong predictor of future routine vaccinations delay. In our study, children with delayed receipt of DTaP-IPV-Hib1 vaccine (scheduled at 2 months) were significantly less likely to be up-to-date at 12, 24 and 48 months. Similarly, children with early vaccines delay (at 3 months) had significantly lower up-to-date coverage at 19 to 35 months, compared with children without early delay, in a US survey.Citation39 Accordingly, as pertussis infection is specifically hazardous in early infancy, combining strategies (immunization in pregnancy and timely initiation of the pertussis vaccine series) are necessary.Citation9 In a US model, timely administration of infant pertussis vaccine doses can potentially reduce morbidity and mortality in infants under 1 y.Citation40 Low birthweight infants were prone for delayed DTaP-IPV-Hib vaccine first dose. Vaccinations timeliness is essential as low birthweight infants are at increased risk for pertussis-related morbidity.Citation9,41
Recognized risk markers of vaccine delay in our study included high child's birth order,,Citation15,19,20,22,23,24,33 and low socioeconomic rank.Citation16,19,24 However, the higher socio-economic rank of children unvaccinated at 48 months, compared with the low socio-economic rank of children vaccinated but with severe delay, requires further research. Similarly, lower compliance with a national campaign of bivalent oral polio vaccine, was evident in higher socio-economic localities in 2013 in Israel.Citation42,43 Vaccination coverage rates were also significantly associated with ethnicity; consistently higher rates and timeliness among Arab compared with Jewish children. This finding differs from studies in which minority groups were at risk for delay and incompleteness of childhood vaccinations.Citation20,24,39 In a study on vaccinations attitudes in Israel, Arabs are more likely than Jews to adhere to recommendations for vaccinations.Citation44 The mother's marital status, described as a risk factor,Citation22 was found only in Arab children. The season of birth (delay rates higher in infants born in winter) is plausible, as causes for vaccine delays (mostly unfounded) often include acute respiratory infection.Citation45
Parental views on vaccine timeliness include perceiving delay as unimportant and not affecting child's health,Citation45 or even perceiving vaccination delay as a safer alternative than the recommended schedules of childhood vaccination.Citation46 Patterns of vaccine acceptance are influenced by a wide array of elements, including the target population (children and their guardians) and the health services attributes (infra-structure, expenditures and access to services).Citation47 Parental decision-making on childhood vaccinations is an increasingly complex process, may include elements of vaccine hesitancy and presents a challenge for health professionals.Citation47,48 The present approach to vaccine hesitancy comprises a range of possible expressions - complete vaccination decline, partial (or differential) acceptance, alternative schedules of vaccine doses and timing (mild, moderate and considerable delays) and acceptance according to the recommended schedule albeit involving parental concerns and anxiety.Citation44-48 In Israel, our findings suggest that the most common form of vaccine hesitancy is expressed in relation to timing with prevailing procrastination.
The setting of a national immunization registry enabled assessment of vaccinations receipt patterns in Israel.Citation35 The vaccination coverage rates in the study were lower than the national reported vaccination coverage rates at age 2 y. This may be attributed to data collected retrospectively and not necessarily precisely at age 2 y. Similarly, surveys in various countries revealed lower vaccination coverage rates compared with the officially reported national data.Citation25,26 Developing national or regional immunisation registers and information systems may contribute to evaluations based on accurate and detailed data.Citation29-31 Registry-based data may provide insights on population vaccination status and trends that would not be revealed by the traditional methods.Citation15 Accurate estimates enable modeling of population susceptibility, disease burden and program evaluation.
The results of this study are subject to limitations. Only children with full vaccination and demographic data were included and this may have resulted in an underestimate of vaccination delay. However, our choice to count only vaccine doses that were documented in the database was the only way to obtain accurate vaccination dates. Although this was the most accurate method to obtain full details, even computerized records may be incomplete, and some dates may have not been documented, resulting in an over-estimate of the prevalence of vaccination delay. Children who died or moved were not included in the survey, and they might have been vaccinated or not; hence, there may be a potential bias in estimates of the cumulative proportion of vaccination. As for the factors affecting vaccination receipt patterns, we included mainly socio-demographic factors from the national newborn registry. We were not able to include in our study health-related parameters and factors related to the preventive services health supplier side, which should be further evaluated.
In summary, applying survival analysis methods provided the age-appropriate vaccination coverage and relevant information on vaccination timeliness. These data are essential for policy makers and for health-care providers in detecting risk groups, in reducing missed opportunities and in setting targets and priorities for immunization programs. It has been postulated earlier that “for better immunisation coverage, measure coverage better.”Citation28 In special circumstances, such as outbreak settings and mass vaccination campaigns, this method can support both planning and evaluation. Assessment of age-appropriate vaccination coverage can be used to estimate person-time at risk for vaccine-preventable diseases and to appraise attainment of national vaccination goals, with emphasis on vaccination timeliness and completeness.
Methods
Routine childhood vaccinations are included in the preventive services according to the National Health Insurance Law in Israel. Childhood vaccinations are not compulsory. The recommended vaccination schedule is outlined by the National Immunization Technical Advisory Group.Citation49 The routine childhood vaccinations are offered in community-based maternal and child health (MCH) clinics without charge to all children, regardless of civil status. The reported utilization rate of the services provided by MCH clinics for young children in Israel is high (96%).Citation50
The study population included children born from January 1st 2009 to December 31st 2009 and followed to age 48 months. The population registration ordinance in Israel requires all births to be notified to the ministry of the interior and infants receive personal identification numbers (ID) shortly after birth. The total population of Israel in 2009 was 7.6 million with 161,042 live births reported nationally.Citation51 A random sample was selected from the national newborn registry. The sample size calculation assumptions were: proportion of unvaccinated children (age 2 years, estimated range 5% – 25%),Citation9,33,34 precision- 1.5%, confidence interval 95%, population size 161,042. After adjustment the sample was set at 4000 children. Inclusion criteria included: being born in Israel, having a unique identifier (allowing data matching) and surviving to 48 months. Exclusion criteria included: born abroad (different vaccination schedules), lacking unique identifier and not surviving to 48 months.
The routine vaccination schedule (0–2 y 2009) included: Hepatitis B vaccine (HBV) at birth, 1 and 6 months, Diphtheria- Tetanus- acellular Pertussis- Polio- Haemophilus influenzae type b (DTaP-IPV-Hib) at 2, 4, 6, and 12 months, pneumococcal conjugate vaccine (PCV) at 2, 4, and 12 months, Measles-mumps-rubella-varicella (MMR/MMRV) at 12 months and hepatitis A vaccine (HAV) at 18 and 24 months. The varicella vaccine was introduced in 2008 and the pneumococcal vaccine in 2009.
The children's unique identifiers (personal ID, date of birth) were crossed against the national immunization database. The variables collected included: 1) child socio-demographics; date of birth, gender, ethnicity, birth order, birthweight; 2) maternal socio-demographics; age, birth country, marital status and socio-economic (SE) rank of the locality in which the family resides. The SE rank used was the Central Bureau of Statistics localities classification, an ordinal scale of 1 (lowest) to 10 (highest).Citation52 The socio-demographic variables of the children in the study group were found similar to those of the 2009 general birth cohort.Citation51 Data on vaccine doses including dates were assembled for the study cohort. Vaccine doses were defined as valid according to the ministry of health guidelines for minimum ages and intervals between doses. After data incorporation, immunization records were available for 3892/4000 (97.3%) children. The general characteristics (gender, birthweight, birth order, ethnicity, SE rank) of children with available vs. unavailable immunization records were comparable.
Data analysis
Data analysis was performed with Statistical Package for Social Sciences software version 21.0 (SPSS Inc., Chicago, IL). Vaccination coverage rates were assessed by up-to-date and age-appropriate methods. Vaccine uptake was estimated by survival analysis. The age-specific immunization coverage was retrieved from cumulative proportion of vaccinated children by that age and plotted as an inverse Kaplan-Meier curve. Days of delay were converted to months as 30.5 days/month. Vaccine doses received up to one month post the recommended age were considered timely (no delay). The following categories were defined for the children vaccination status: a. Vaccinated age-appropriate, b. Delayed less than 6 months, divided to 2 sub-groups (mild - delayed less than 3 months, moderate- delayed 3 – 5 months), c. Delayed 6 months and above (severe delay) and d. Unvaccinated (at the age of 48 months). Comparison of the 4 vaccination status categories as to the mean socio-economic rank was performed with Tukey test. Analysis of delay in timely vaccination was performed using Cox Proportional Hazards model. The survival time to the event (vaccination) was grouped into the 4 categories defined. Not vaccinated at all were considered censored observations. The results are presented as Hazard ratio (HR) with a 95% Confidence Interval and a p-value resulting from Wald test. A multiple regression analysis model was performed for “up-to-date” vaccination status at age 12, 24 and 48 months. Associations between variables and the vaccination status are presented as Odds Ratio with a 95% Confidence Interval. A p-value of < 0.05 was considered significant for all comparisons.
Ethical approval
The study was approved by the Israel ministry of health Institutional Review Board and conducted according to the relevant ministry of health instructions.
Abbreviations
| WHO | = | World Health Organization |
| MCH | = | Maternal and Child Health |
| GVAP | = | Global Vaccine Action Plan |
| SE rank | = | Socio economic rank of the locality of residence |
| DTaP-IPV-Hib | = | Diphtheria-Tetanus-acellular Pertussis-Polio-Haemophilus influenza B combined vaccine |
| HBV | = | Hepatitis B vaccine |
| PCV | = | Pneumococcal conjugate vaccine |
| MMRV | = | Measles-Mumps-Rubella-Varicella vaccine |
| HAV | = | Hepatitis A vaccine |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to acknowledge the support of Ziona Haklai MA, head of Health Information Division, Ministry of health, Hanna Shoob MPH, Jerusalem district health office, Nesia Cohen, Computing Division, Ministry of health, Dr. Mario Baras, Braun School of Public and Community Medicine, Hebrew university, and Prof Itamar Grotto, Head of Public Health Services, Ministry of health, Jerusalem Israel. The authors would like to acknowledge the dedicated public health nurses in the Mother and Child health clinics in Israel.
References
- Plotkin SA. Vaccines: past, present and future. Nat Med 2005; 11(4 Suppl):S5-11
- Pickering LK, Baker CJ, Freed GL, Gall SA, Grogg SE, Poland GA, Rodewald LE, Schaffner W, Stinchfield P, Tan L, et al. Immunization programs for infants, children, adolescents, and adults: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(6):817-40; https://doi.org/10.1086/605430
- Rappuoli R, Pizza M, Del Giudice G, De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci U S A 2014; 111(34):12288-93; https://doi.org/10.1073/pnas.1402981111
- Frieden TR. The Future of Public Health. N Engl J Med 2015; 373:1748-54; https://doi.org/10.1056/NEJMsa1511248
- Cutts FT, Izurieta HS, Rhoda DA. Measuring coverage in MNCH: design, implementation, and interpretation challenges associated with tracking vaccination coverage using household surveys. PLoS Med 2013; 10(5):e1001404; PMID:23667334; https://doi.org/10.1371/journal.pmed.1001404
- PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol 2011; 12(3):189-94; PMID:21321588; https://doi.org/10.1038/ni0311-189
- Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev 2000; 13(2):302-17; PMID:10756001; https://doi.org/10.1128/CMR.13.2.302-317.2000
- Ben-Shimol S, Greenberg D, Givon-Lavi N, Schlesinger Y, Somekh E, Aviner S, Miron D, Dagan R. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: an active prospective nationwide surveillance. Vaccine 2014; 32(27):3452-9; https://doi.org/10.1016/j.vaccine.2014.03.065
- Zamir CS, Dahan DB, Shoob H. Pertussis in infants under one-year-old: risk markers and vaccination status–a case-control study. Vaccine 2015; 33(17):2073-8; https://doi.org/10.1016/j.vaccine.2015.02.050
- Zamir C, Dagan R, Zamir D, Rishpon S, Fraser D, Rimon N, Ben Porath E. Evaluation of screening for hepatitis B surface antigen during pregnancy in a population with a high prevalence of hepatitis B surface antigen-positive/hepatitis B e antigen-negative carriers. Pediatr Infect Dis J 1999; 18(3):262-6; PMID:10093949; https://doi.org/10.1097/00006454-199903000-00011
- Ad Hoc Working Group for the Development of Standards for Pediatric Immunization Practices. Standards for pediatric immunization practices. JAMA 1993; 269:1817-22; PMID:8459514; https://doi.org/10.1001/jama.1993.03500140069038
- World Health Organization. “ Immunization surveillance, assessment and monitoring” http://www.who.int/immunization/monitoring_surveillance/en/ accessed Dec 20, 2016
- Zinkernagel RM. Advances in immunology: maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med 2001; 345:1331-5; PMID:11794153; https://doi.org/10.1056/NEJMra012493
- Falagas ME, Zarkadoulia E. Factors associated with suboptimal compliance to vaccinations in children in developed countries: a systematic review. Curr Med Res Opin 2008; 24(6):1719-41; https://doi.org/10.1185/03007990802085692
- Dayan GH, Shaw KM, Baughman AL, Orellana LC, Forlenza R, Ellis A, Chaui J, Kaplan S, Strebel P. Assessment of delay in age-appropriate vaccination using survival analysis. Am J Epidemiol 2006; 163(6):561-70; https://doi.org/10.1093/aje/kwj074
- Jessop LJ, Kelleher CC, Murrin C, Lotya J, Clarke AT, O'Mahony D, Fallon UB, Johnson H, Bury G, Murphy AW; Lifeways Cohort Study Steering Group. Determinants of partial or no primary immunisations. Arch Dis Child 2010; 95(8):603-5; https://doi.org/10.1136/adc.2009.161810
- Smith PJ, Humiston SG, Marcuse EK, Zhao Z, Dorell CG, Howes C, Hibbs B. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public Health Rep 2011; 126(Suppl 2):135-46; https://doi.org/10.1177/00333549111260S215
- Gilkey MB, McRee AL, Brewer NT. Forgone vaccination during childhood and adolescence: Findings of a statewide survey of parents. Prev Med 2013; 56(3–4):202-6; https://doi.org/10.1016/j.ypmed.2012.12.019
- Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health 2012; 66(7):e14; https://doi.org/10.1136/jech.2010.124651
- Luman ET, Barker LE, Shaw KM, McCauley MM, Buehler JW, Pickering LK. Timeliness of childhood vaccinations in the United States: days undervaccinated and number of vaccines delayed. JAMA 2005; 293(10):1204-11; https://doi.org/10.1001/jama.293.10.1204
- Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet 2009; 373 (9674):1543-9; https://doi.org/10.1016/S0140-6736(09)60317-2
- Dombkowski KJ, Lantz PM, Freed GL. Risk factors for delay in age-appropriate vaccination. Public Health Rep 2004; 119(2):144-55; https://doi.org/10.1177/003335490411900207
- Pavlopoulou ID, Michail KA, Samoli E, Tsiftis G, Tsoumakas K. Immunization coverage and predictive factors for complete and age-appropriate vaccination among preschoolers in Athens, Greece: a cross–sectional study. BMC Public Health 2013; 13:908; https://doi.org/10.1186/1471-2458-13-908
- Lernout T, Theeten H, Hens N, Braeckman T, Roelants M, Hoppenbrouwers K, Van Damme P. Timeliness of infant vaccination and factors related with delay in Flanders, Belgium. Vaccine 2014; 32(2):284-9; https://doi.org/10.1016/j.vaccine.2013.10.084
- Murray CJ, Shengelia B, Gupta N, Moussavi S, Tandon A, Thieren M. Validity of reported vaccination coverage in 45 countries. Lancet 2003; 362(9389):1022-7; https://doi.org/10.1016/S0140-6736(03)14411-X
- Lim SS, Stein DB, Charrow A, Murray CJ. Tracking progress towards universal childhood immunisation and the impact of global initiatives: a systematic analysis of three-dose diphtheria, tetanus, and pertussis immunisation coverage. Lancet 2008; 372(9655):2031-46; https://doi.org/10.1016/S0140-6736(08)61869-3
- Belmaker I, Dukhan L, Elgrici M, Yosef Y, Shahar-Rotberg L. Reduction of vaccine-preventable communicable diseases in a Bedouin population: summary of a community-based intervention programme. Lancet 2006; 367(9515):987-91; PMID:16564360; https://doi.org/10.1016/S0140-6736(06)68425-0
- Papania M, Rodewald L. For better immunisation coverage, measure coverage better. Lancet 2006; 367(9515):965-6; PMID:16564344; https://doi.org/10.1016/S0140-6736(06)68403-1
- Johansen K, Lopalco PL, Giesecke J. Immunisation registers–important for vaccinated individuals, vaccinators and public health. Euro Surveill 2012; 17(16)
- Groom H, Hopkins DP, Pabst LJ, Murphy Morgan J, Patel M, Calonge N, Coyle R, Dombkowski K, Groom AV, Kurilo MB, et al. Immunization information systems to increase vaccination rates: a community guide systematic review. J Public Health Manag Pract 2015; 21(3):227-48; https://doi.org/10.1097/PHH.0000000000000069
- Community Preventive Services Task Force. Recommendation for use of immunization information systems to increase vaccination rates. J Public Health Manag Pract 2015; 21(3):249-52
- Israel: WHO and UNICEF estimates of immunization coverage: 2015 revision. July 6, 2016. http://www.who.int/immunization/monitoring_surveillance/data/isr.pdf. accessed Dec 23, 2016
- Stein-Zamir C, Shoob H, Abramson N, Zentner G. Who are the children at risk? Lessons learned from measles outbreaks. Epidemiol Infect 2012; 140(9):1578-88; https://doi.org/10.1017/S095026881100238X
- Stein-Zamir C, Schroeder H, Shoob H, Abramson N, Zentner G. Characteristics of a large mumps outbreak: Clinical severity, complications and association with vaccination status of mumps outbreak cases. Hum Vaccin Immunother 2015; 11(6):1413-7; PMID:25874726; https://doi.org/10.1080/21645515.2015.1021522
- Stein-Zamir C, Zentner G, Tallen-Gozani E, Grotto I. The Israel National Immunization Registry. Isr Med Assoc J 2010; 12(5):296-300
- Laubereau B, Hermann M, Schmitt HJ, Weil J, von Kries R. Detection of delayed vaccinations: a new approach to visualize vaccine uptake. Epidemiol Infect 2002; 128(2):185-92; https://doi.org/10.1017/S0950268801006550
- Subaiya S, Dumolard L, Lydon P, Gacic-Dobo M, Eggers R, Conklin L. Centers for Disease Control and Prevention (CDC). Global routine vaccination coverage 2014. MMWR Morb Mortal Wkly Rep 2015; 64(44):1252-5; https://doi.org/10.15585/mmwr.mm6444a5
- Central Bureau of Statistics Israel. Children immunized out of those registered in the mother and infant centers. Available at: http://www.cbs.gov.il/shnaton66/st06_12.pdf Statistical Abstract of Israel, Table 6.12 Central Bureau of Statistics, Jerusalem, Israel, 2016
- Rosenthal J, Rodewald L, McCauley M, Berman S, Irigoyen M, Sawyer M, Yusuf H, Davis R, Kalton G. Immunization coverage levels among 19- to 35-month-old children in 4 diverse, medically underserved areas of the United States. Pediatrics 2004; 113(4):e296-302; https://doi.org/10.1542/peds.113.4.e296
- Curran D, Terlinden A, Poirrier JE, Masseria C, Krishnarajah G. Vaccine Timeliness: A Cost Analysis of the Potential Implications of Delayed Pertussis Vaccination in the US. Pediatr Infect Dis J 2016; 35(5):542-7; https://doi.org/10.1097/INF.0000000000001071
- Riise ØR, Laake I, Vestrheim D, Flem E, Moster D, Bergsaker MA, Storsæter J. Risk of pertussis in relation to degree of prematurity in children < 2 years of age. Pediatr Infect Dis J 2017; 36(5):e151-e156
- Binyaminy B, Bilenko N, Haas EJ, Grotto I, Gdalevich M. Socioeconomic status and vaccine coverage during wild-type poliovirus emergence in Israel. Epidemiol Infect 2016; 144(13):2840-7; https://doi.org/10.1017/S0950268816000844
- Stein Zamir C, Grotto I. The detection of wild poliovirus in environmental samples in Israel and the following public health investigation and response. Oral presentation, e-poster. ESPID, European Society for Paediatric Infectious Diseases, 32nd Annual Meeting; May 2014. Dublin, Ireland. http://espid.meetingxpert.net/espid945/faculty2517731/faculty.aspx
- Velan B, Boyko V, Lerner-Geva L, Ziv A, Yadgar Y, Kaplan G. Individualism, acceptance and differentiation as attitude traits in the public's response to vaccination. Hum Vaccin Immunother 2012; 9(8):1272-82; https://doi.org/10.4161/hv.21183
- Stein Zamir C, Israeli A. Knowledge, attitudes and perceptions about routine childhood vaccinations among jewish Ultra-Orthodox mothers residing in communities with low vaccination coverage in the jerusalem district. Matern Child Health J 2017; 21(5):1010-7; https://doi.org/10.1007/s10995-017-2272-5
- Dempsey AF1, Schaffer S, Singer D, Butchart A, Davis M, Freed GL. Alternative vaccination schedule preferences among parents of young children. Pediatrics 2011; 128(5):848-56; https://doi.org/10.1542/peds.2011-0400
- Larson HJ. Negotiating vaccine acceptance in an era of reluctance. Hum Vaccin Immunother 2013; 8:1779-81; https://doi.org/10.4161/hv.25932
- Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother 2013; 9(8):1763-73; https://doi.org/10.4161/hv.24657
- Adjagba A, Senouci K, Biellik R, Batmunkh N, Faye PC, Durupt A, Gessner BD, da Silva A. Supporting countries in establishing and strengthening NITAGs: lessons learned from 5 years of the SIVAC initiative. Vaccine 2015; 33(5):588-95; https://doi.org/10.1016/j.vaccine.2014.12.026
- Palti H, Gofin R, Adler B. Evaluation of utilization of preventive services for infants in Israel–personal and organizational determinants. Harefuah 2004; 143(3):184-8, 247.(Heb)
- Central Bureau of Statistics Israel. Number of live births in Israel 2002–2013. Available at: http://www.cbs.gov.il/publications/lidot/lidot_all_1.pdf accessed Dec 23, 2016. Central Bureau of Statistics, Jerusalem, Israel, 2016
- Central Bureau of Statistics. Characterization and Classification of Local Authorities by the Socio-Economic Level of the Population 2006. Available at: http://www.cbs.gov.il/publications/local_authorities06/local_authorities_e.htm. Central Bureau of Statistics, Jerusalem, Israel, 2014
