ABSTRACT
An open-label, randomized, multi-center trial was conducted to compare the immunogenicity and safety of an indigenously developed tetravalent DTwP-Hib vaccine, Easyfour®-TT with a commercially available vaccine, Quadrovax®. A total of 244 infants in good health, aged 6–10 weeks, were randomized in a 1:1 allocation to receive three doses of the test or comparator vaccine. Immunogenicity of the vaccines was determined by measuring the baseline and post-vaccination antibody response against the vaccine antigens; safety was evaluated in terms of local and systemic reactions (solicited and unsolicited) reported during the trial. Similar levels of seroprotection/seroresponse were achieved, 4 weeks after receiving 3 doses of Easyfour®-TT and Quadrovax®, and the antibody response of Easyfour®-TT was found non-inferior to Quadrovax®, against all four vaccine antigens. Both vaccines were well tolerated and had similar reactogenicity profiles, with a significantly lower occurrence of local (redness at injection site) and systemic reactions (irritability post-vaccination) with Easyfour®-TT vaccine as compared to Quadrovax® (p < 0.05). All adverse events resolved completely with no sequelae. All through the study, only one serious adverse event was observed that completely resolved upon treatment and was deemed unrelated to the vaccine administered. This study demonstrated that Easyfour®-TT vaccine was safe and immunogenic.
Clinical trial registration number: CTRI/2014/12/005326 (registered with the Clinical Trial Registry of India (CTRI)).
Introduction
World Health Organization and Indian Academy of Pediatrics have promoted use of combination vaccines for immunisation programs. The main advantages of combination vaccines are reduced discomfort to infants because of lesser number of Injection /doses and lower cost. This would lead to increased compliance with the recommended immunization schedule.Citation1-3 The other advantages include reduction of extra visits to hospitals as well as the cost of stocking and administration of individual vaccines.Citation4-5
A trivalent combination vaccine (DTP) against diphtheria, tetanus, and pertussis was licensed in United States as early as 1949 and effectively integrated into the immunisation programs.Citation6 Addition of new antigens, such as Haemophilus influenzae type b (Hib) to the existing DTP vaccines, then, resulted in the advent of tetravalent vaccines to combat more childhood vaccine preventable diseases.Citation7
DTwP-Hib combination vaccines are generally available as a composite of lyophilized Hib reconstituted in liquid DTwP vaccine.Citation8-14 Since a fully liquid tetravalent combination vaccine does not require reconstitution and is more convenient to handle, Panacea Biotec Ltd. has indigenously developed Easyfour®-TT, a fully liquid tetravalent vaccine.
The present study was designed to compare and evaluate the immune response and safety of Easyfour®-TT (comprising of diphtheria toxoid, tetanus toxoid, inactivated whole cell- Bordetella pertussis and Hib) with Quadrovax® (Serum Institute of India Ltd.), a commercially available tetravalent DTwP-Hib formulation containing lyophilized Hib.
The immune responses were quantitated as percent of subjects who attained protective antibody levels against diphtheria, tetanus, pertussis and Hib, four weeks after conclusion of vaccination series. The safety of the vaccines was evaluated by assessing the occurrence and intensity of local and systemic reactions, following each vaccine dose.
Results
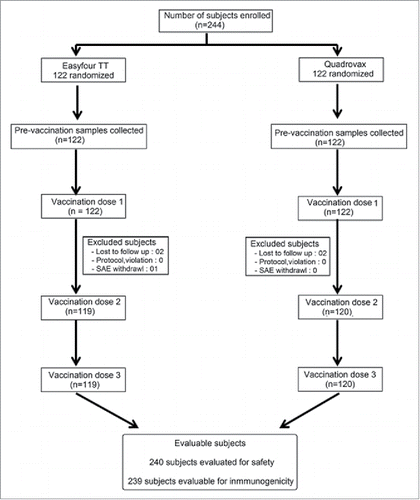
A total of 244 healthy infants, 6–10 weeks of age were enrolled in the study and randomized at a 1:1 ratio (122 subjects per group) to receive three doses of either the test (Easyfour®-TT) or comparator (Quadrovax®) vaccine, at an interval of four weeks between doses (). Demographic characteristics (age, gender, weight and length) were comparable between the study cohorts (). A total of 239 subjects (119 and 120 subjects in the Easyfour®-TT and Quadrovax® group, respectively), received all three vaccine doses and comprised the per protocol immunogenicity analyses cohort. For safety analyses, 240 subjects (120 in each vaccine group) were included, 4 subjects (2 subjects in each group) were not available for the safety analysis due to loss to follow-up after the 1st study visit. The entire study, starting from enrollment to last follow-up visits, was conducted from January to May 2015.
Table 1. Demographic profile of subjects.
Immunogenicity evaluation
Seroprotection rate (SPR) against diphtheria, tetanus, Hib; seroresponsiveness against pertussis and Geometric Mean Titres (GMTs) against all antigens were estimated, four weeks post immunisation with the dose 3 of the respective vaccine. The seroprotection/ seroresponse for all four components in the Easyfour®-TT were non-inferior when compared to those achieved in the comparator vaccine group (). GMTs were also not significantly different between the groups ().
Table 2. Seroprotection/ Seroconversion rates (SPR) 4 weeks post-vaccination.
Table 3. Geometric mean titer (GMT) and 95% CI.
Analysis of baseline, pre-vaccination blood samples revealed seroprotection (≥0.1 IU/ml for anti-tetanus toxoid antibodies) rate of 97.47% in the Easyfour®-TT and 99.16% in the Quadrovax® group. This baseline seroprotection is possibly due to passive transfer of antibodies to neonates from mothers who were administered of tetanus toxoid vaccine during pregnancy, consistent with the previously published literature.Citation7-15 100% subjects in the Easyfour®-TT and Quadrovax® groups achieved seroprotection against tetanus toxoid post-vaccination. The post vaccination anti-tetanus GMTs were similar between recipients of Easyfour®-TT(GMT 1.51, [1.30–1.75]) and Quadrovax® (GMT 1.33, [1.18–1.49]) (p = 0.069) ().
Four weeks after the third dose of vaccine, 73.95% (65.4–81.8) subjects in the Easyfour®-TT compared to 72.50% (63.91–79.71) in the Quadrovax® group were seroconverted against pertussis anti-PT (p = 0.96). The seroconversion rate of Easyfour®-TT against pertussis anti-PT was non-inferior to Quadrovax® with a difference in seroconversion by −1.45 and the upper limit (9.78) of the 95% CI was lower than the pre-specified non-inferiority margin of 10% (). Further, the post vaccination pertussis anti-PT GMTs were similar among recipients of Easyfour®-TT (GMT 54.33, [42.37–69.67]) and Quadrovax® (GMT 52.55, [41.92–65.88]) (p value = 0.213) ().
The anti-diphtheria seroprotection rate, four weeks after the third dose of vaccination was 99.16% (95.39–99.85) in the Easyfour®-TT group compared to 99.17% (95.44–99.85) in the Quadrovax® group (p value = 0.99). The difference in seroprotection was 0.01 and the upper limit (2.32) of the 95% CI was lower than the pre-specified non-inferiority margin of 10%, demonstrating that Easyfour®-TT was non-inferior to Quadrovax® in terms of protection against diphtheria (). A post vaccination GMT of 1.7 [1.42–2.04] was achieved with Easyfour®-TT, which was not statistically significant when compared to Quadrovax® GMT 2.07, [1.7–2.53], (p value = 0.069).
Post-vaccination short-term PRP (Hib) seroprotection (≥0.15 μg/mL for anti-PRP antibodies) rate was 100% for both the groups, whereas the long-term PRP (Hib) seroprotection rate (anti-PRP antibody titer ≥1.0 μg/mL) for Easyfour®-TT and Quadrovax® were 95.80% (90.54–98.19) and 96.67% (91.75–98.70), respectively. With a difference in seroconversion by 0.87 and the upper limit of 95% CI (5.7) was lower than the pre-specified non-inferiority margin of 10%, demonstrating that Easyfour®-TT was non-inferior to Quadrovax® for Hib immune response (). The GMTs for anti-PRP (short-term and long-term) in recipients of Easyfour®-TT (GMT 14.42, [11.15–18.65]) and Quadrovax® (GMT 15.27, [12.04–20.53]) were also found to be comparable (p value = 0.149).
Safety
Overall 718 doses of the vaccines were administered; 358 in Easyfour®-TT and 360 in Quadrovax® groups, respectively. All solicited local and systemic reactions were recorded for up to 30 min, immediately after vaccination and thereafter followed up for next 3 days after vaccination.
The solicited local reactions reported after dose I were 79 (65.83%) and 89 (74.16%) in Easyfour®-TT and Quadrovax® group, respectively. After dose II, solicited local reactions reported were 45 (37.81%) and 68 (56.66%) in Easyfour®-TT and Quadrovax® group, respectively. After dose III, solicited local reactions reported were 33 (27.73%) and 42 (35%) in Easyfour®-TT and Quadrovax® group, respectively. During 3 day post vaccination follow-up, solicited local reactions were pain, redness and swelling at the injection site.
The solicited systemic reactions reported after dose I were 53(44.16%) and 79(65.83%) in Easyfour®-TT and Quadrovax® group, respectively. After dose II, solicited systemic reactions reported were 33(27.73%) and 55(45.83%) in Easyfour®-TT and Quadrovax® group, respectively. After dose III, solicited systemic reactions reported were 26(21.84%) and 31(25.83%) in Easyfour®-TT and Quadrovax® group respectively. During 3 day post vaccination follow-up, the solicited systemic reactions observed included Fever, Acute allergic reaction, Irritability/Restlessness/Fussiness, Excessive sleepiness/Drowsiness, Vomiting and Diarrhea (). Frequencies of Redness and Irritability/Restlessness/Fussiness were significantly lower in Easyfour®-TT group compared to those observed in the Quadrovax® group (p < 0.05). Most of the AEs were mild in nature and resolved without sequelae.
Table 4. Total incidences of solicited local and systemic AEs reported during the study period (number and percentage of subjects experiencing at least one AE).
Unsolicited AEs were reported in 0.83% and 2.5% subjects in Easyfour®-TT and Quadrovax® group, respectively, during the four week extended follow-up interlude between each vaccine administration. Two cases of upper respiratory tract infection and one case of hypotonia were reported in Quadrovax® group. All unsolicited AEs were mild/moderate in intensity and resolved completely during the study period. There was no fatality reported during the trial.
One non-fatal Serious Adverse Event (SAE) of hospitalisation following seizures was reported after administration of first dose of Easyfour®-TT. The subject experienced mild fever only on the day of vaccination and she experienced seizures after 4 days of vaccine administration. She recovered completely without sequelae after treatment and was subsequently withdrawn from the study. As per WHO vaccine causality assessment criteria, the event was considered unrelated to the vaccination. Overall, the reactogenicity profiles for both vaccines were similar and were found to be safe and well tolerated by the subjects.
Discussion
The number of immunisation recommended in early childhood has exponentially increased over the years. To overcome the potential challenge of incorporating new vaccines into an already complex immunisation schedule, vaccines containing combination of protective antigens against multiple diseases have been introduced. This strategy reduces the number of vaccination per infant, the global cost of immunisation and improves disease control with the possibility of eradicating pathogens thus eliminating the disease. The new combination vaccines are stable, less immunogenic or unsafe when compared to previously licensed uncombined, individual, single antigen vaccines. Furthermore, antigenic interference due to immunological, physical and chemical interactions among the collective products as well as consolidation of different immunisation schedules should not negatively impact the immunogenicity or safety. Multiple studies have demonstrated that none of the DTwP based vaccine in combination with any conjugate Hib vaccines were either less efficacious or more reactogenic than DTwP vaccines alone. The vaccines were immunogenic for all vaccine constituents including PRP (Hib).Citation13 In Canada, a decrease in the rate of invasive Hib diseases was observed following license and application of DTwP/PRP-T vaccines.Citation16
In the current study, conducted in India, Easyfour®-TT a fully liquid tetravalent combination vaccine manufactured by Panacea Biotec Ltd. administered at 6, 10 and 14 weeks has been evaluated. The Efficacy and safety of Easyfour®-TT, a fully liquid tetravalent vaccine was compared to Quadrovax®, a licensed tetravalent vaccinecontaining lyophilized Hib. The results demonstrated a comparable immunogenicity and reactogenicity profile for Easyfour®-TT with Quadrovax®.
Easyfour®-TT was well tolerated and similar safety profiles were observed in both groups for local and systemic AEs. Severe adverse events such as Hypotonic–hyporesponsive episode (HHE),Citation18,19 Encephalopathy,Citation20,Citation22 Dravet's SyndromeCitation23,24 and AnaphylaxisCitation25,26 associated with DTwP vaccines were not observed in current study.
In the present study, long-term seroprotection (> 1.0 µg/ml) of anti PRP-T in Easyfour®-TT, 4 weeks after the third dose (in a schedule of 6, 10 and 14 weeks) was 95.8% in comparison to Quadrovax (96.67%). These results were also comparable to other published studies of DTwP-Hib such as TETRAct-Hib (97.7%)Citation13 and Shan 4(92.68%).Citation7
Easyfour®-TT will be particularly useful when Hepatitis B is to be administered at birth followed by subsequent doses at 1 and 6 months of age, In such a case, it will be administered in primary immunisation schedule at 6, 10 and 14 weeksCitation7 as per Expanded Program of Immunization (EPI) recommendations. If, Hepatitis B is to be administered at 6, 10 and 14 weeks as a combination vaccine as per EPI, a tetravalent vaccine containing Hib such as Easyfour®-TT, can be administered as booster dose at 15–18 months.Citation13
Material and methods
Trial design and subjects
This was a phase III randomized, open-label clinical trial to evaluate Immunogenicity and Safety of an indigenously developed DTwP-Hib Tetravalent Combination Vaccine (Easyfour®-TT) with Quadrovax® in Indian infants (www.ctri.nic.in, Registration Number: CTRI/2014/12/005326). The study was conducted at four study centers in India, namely King George Hospital -Visakhapatnam, Sant Dnyaneshwar Medical Education Research Centre -Pune, Calcutta Medical College- Kolkata and Bharti Vidyapeeth Medical College and Hospital-Pune. 244 subjects were enrolled (122 in each group) who received at least one vaccine dose. Four subjects (02 in each group) did not return after 1st visit and their data was not available (they did not return diary card). Hence safety analysis was done on 240 subjects (120 in each group). 239 subjects completed the study. Four subjects (02 in each group) did not return after 1st visit and one (01) subject was dropped out because of SAE experienced in Easyfour®-TT group. So Immunogenicity analysis was done on 239 subjects (119 in Easyfour®-TT group and 120 in Quadrovax® group). Protocols along with other study-related documents were pre-approved by the Drugs Controller General of India and local institutional ethics committees, prior to the start of the trial. The study complied with guidelines issued in Declaration of Helsinki, Good Clinical Practice as per Central Drug Standard Control Organisation and Ethical Guidelines for Biomedical Research on Human Subjects by Indian Council of Medical Research). A written, informed consent from the parents or guardians was a prerequisite for subject recruitment.
244 healthy, full-term (>36 weeks gestation period) infants aged 6 to 10 weeks were recruited in the trial, in compliance with inclusion and exclusion criteria. All subjects in good physical condition as established by their medical history and regular health check-up; and who were able to follow-up with all trial procedures were included. Exclusion criteria included history of infection (potentially related to pathogens targeted by the DPT-Hib vaccine), presence of neurological disorder, history of seizures before receiving the vaccine, temperature of >38°C in past 3 days, any evidence of acute illness within past 7 days, known or suspected immune disorder (congenital or hereditary), history of anaphylaxis, allergy to vaccine or any of its component, evidence of thrombocytopenia or a bleeding disorder, any clinically significant chronic disease (cardiac, pulmonary, renal, gastrointestinal, hepatic, endocrine, cancer, skin or autoimmune disease) or major congenital defects; administration of immunoglobulin, blood products, cytotoxic agents or radiotherapy since birth; and use of any investigational or un-registered drug/vaccine (except OPV, BCG and Hepatitis B vaccine) before commencement or during the trial. Subjects who had already received 1st dose of hepatitis B vaccine at birth were not excluded; the event was recorded and the participants were administered the remaining doses as per the respective study center schedule. Infants, who were not immunised with birth dose of hepatitis B vaccine, were vaccinated for hepatitis B along with the study vaccines, as per the respective study center schedule.
Investigators randomly allocated the subjects to the treatment groups in a 1:1 ratio, based on the randomization list supplied by the sponsor. A block randomization method was applied by biostatistician of sponsor (Panacea Biotec Ltd.) to prepare a randomization list, using computerised codes (generated by Sealed Envelope Ltd. 2014). Details of block size were not disclosed to conceal subject allocations from the investigators and prevent biasness. Treatment groups were assigned to subjects to prevent biasness.
Vaccines
Randomized subject received either three dose (0.5 mL) primary vaccination series of new DTwP-Hib tetravalent combination Easyfour®-TT vaccine (Panacea Biotec Ltd) or commercially licensed tetravalent DTwP/Hib vaccine Quadrovax® (Serum Institute of India Ltd.) by intramuscular route (anterolateral aspect of thigh) through needle of size 0.55 × 25 mm (24 gauge). The vaccinations were done at 0, 4 and 8 weeks of enrollment. Subjects were also immunised with Oral Polio Vaccine (OPV) as per the Universal Immunisation program. A single dose of Easyfour®-TT contained Diphtheria toxoid ≥30 IU, Tetanus toxoid ≥60 IU, inactivated B. pertussis (whole cell) ≥4.0 IU and Hib (PRP-TT) 10 µg.
Serological analysis for immunogenicity
3 mL blood sample was collected for immunogenicity analyses immediately prior to vaccination and at 4 weeks subsequent to conclusion of the three dose vaccination course. In order to conduct all the serological analyses in a blinded fashion, test samples (sera) were identified using a unique code designated at the study centers. Serum concentrations of antibodies against each vaccine constituents (both test and comparator vaccine) were estimated by Enzyme Linked Immunosorbent Assay (ELISA). IgG antibodies against diphtheria toxin and tetanus toxoid were determined using Demeditec ELISA kit (Demeditec Diagnostics GmbH, Germany); for both antibodies, an antibody titer ≥0.1 IU/mL was considered seroprotective. For detecting IgG antibodies specifically against type b capsular polysaccharide of Haemophilus influenzae (anti-PRP) Hib ELISA kit (Demeditec Diagnostics GmbH, Germany) was used. Both short-term and long-term protective seroresponse were defined as anti-PRP titer ≥0.15 μg/ mL and ≥1.0 μg/mL, respectively. As no correlate of protection is established for pertussis till date, seroresponsiveness was measured for anti PT ≥ 4 –fold increase from baseline. IgG antibodies against B.pertussis were determined using Demeditec ELISA kit (Demeditec Diagnostics GmbH, Germany). All the analyses were conducted at Panacea's Drug Discovery R & D center accredited by Govt. of india's Department for Scientific & Industrial Research.
Safety assessments
Subjects were observed for minimum of 30 minutes after vaccination and any reaction detected were treated and recorded. Parents or Legally acceptable Representative (LAR) were provided with a diary card to record solicited reactions, both local and systemic, during three days following immunisation. Occurrence of unsolicited adverse events (AEs) up to 4 weeks after each injection was also recorded. Sponsor-supplied digital thermometer was used to daily record body temperature, measured in an axillary position. Reactions at the injection site (solicited local) included erythema (redness), swelling and tenderness; solicited systemic reactions comprised of fever, irritability, restlessness, fussiness, vomiting, diarrhea, and excessive sleepiness/drowsiness. The reactions were graded as mild (grade 1), moderate (grade 2) or severe (grade 3) based on the severity.
Pain was classified as mild if it was minor reaction to touch; moderate if the subject cried or protested on touch and severe if the subject cried when the limb was moved or was painful spontaneously. Redness and swelling were classified as mild, if size was between 2.5 to 5.0 cm; moderate, if the size varied between 5.1 to 10 cm and severe, if the size was more than 10 cm. Fever was classified as Mild, if it varied between 38–38.40C (100.4–101.1 0F); Moderate, if it varied between 38.5–38.90C (101.2–102.00F), Severe if it was greater than 39.00C (102.10F). Diarrhea was classified as Mild, if transient or intermittent episodes of unformed stools or increase of ≤ 3 stools over baseline per 24 hour period, Moderate, if persistent episodes of unformed to watery stools or increase of 4–6 stools over baseline per 24 hour period and Severe, if bloody diarrhea or increase of ≥ 7 stools over baseline per 24 hour period or if Intra venous fluid replacement was indicated. Fever, swelling and diarrhea were assessed as per the guidelines by Brighton Collaboration.Citation17-19
Any adverse or SAEs occurred between the enrollments to last follow-up visit were also documented. The parents /LAR were instructed to immediately communicate about the development of any serious symptom, to the investigator.
Statistical analysis
The primary objective of the study was to demonstrate that the test vaccine, Easyfour®-TT is non-inferior to the comparator, Quadrovax®, with respect to antibodies against diphtheria and tetanus toxoid, Hib, and Pertussis immune response 4 week after the 3-dose primary vaccination course. Data for demographic characteristics (age, weight and length) were represented as range and mean ± standard deviation (SD); gender distribution was represented as counts with percentages. Frequencies of AEs were compared by means of Chi-square or Fischer's exact test. Antibody Geometric Mean Titres (GMTs) in post-vaccination samples were compared based on 95% CI of difference in seroconversion/seroprotection rates If the upper limit of the 95% CI of the difference in SPRs was < 10% for individual vaccine antigens, the study vaccine was considered non-inferior to comparator vaccine.Citation13,20
Two-sided 95% confidence of intervals (CIs) with upper and lower limits and p-values were also calculated. All statistical analyses were, performed using SAS® software (version 9.3) and p-values < 0.05 were considered statistically significant.To prove non-inferiority of Easyfour®-TT to comparator vaccine, a minimal sample size of 110 subjects per group was determined essential, with 90% power and alpha (type-I error) set at 0.05. Considering a possible attrition rate of 10%, the final sample size was estimated as 122 subjects per group and 244 subjects as the total sample size for the entire study.
Immunogenicity analyses were performed on all participants that completed the study and provided blood samples before and after vaccination, but safety was analyzed in all participants receiving at least one dose of the either vaccine.
Disclosure of potential conflicts of interest
LM, AS, SK, CP, BB, AG, DC and SP are employees of Panacea Biotec. Drs. PV, AD, TKS, SKL had no financial interests in the vaccine or the manufacturer but received research funding to undertake the study. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions
LM, SS, SP and CP conceived and designed the study. LM, AS, BB, SK and CP contributed to the analysis and interpretation of data. All the authors were involved in the initial drafting of the manuscript. LM, AS, SS and BB were involved in the finalization of the manuscript.
Acknowledgments
The authors would like to thank the volunteers who participated in the trial and their families for their support Satyender Kumar, member of clinical data management team, principal investigators and their staff who oversaw the conduct of the study at their respective sites: P. Venugopal, King George Hospital, Visakhapatnam, Ashish Dhongade, Sant Dnyaneshwar Medical Education Research Centre, Pune, Tapas Kumar Sabui, Calcutta Medical College, Kolkata, Sanjay Kewalchand Lalwani, Bharti Vidyapeeth Medical College and Hospital, Pune.
Funding
This work was funded by Panacea Biotec Limited.
References
- Arístegui J, Usonis V, Coovadia H, Riedemann S, Win KM, Gatchalian S, Bock HL. Facilitating the WHO expanded program of immunization: the clinical profile of a combined diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae type b vaccine. Int J Infect Dis 2003; 7(2):143-51; PMID:12839717; https://doi.org/10.1016/S1201-9712(03)90011-7
- Li Y, Li RC, Ye Q, Li C, Liu YP, Ma X, Li Y, Zhao H, Chen X, Assudani D, et al. Safety, immunogenicity and persistence of immune response to the combined diphtheria, tetanus, acellular pertussis, poliovirus and Haemophilus influenzae type b conjugate vaccine (DTPa-IPV/Hib) administered in Chinese infants. Hum Vaccines Immunother 2016; 13(3):588-98 (just-accepted):00-
- Hansen J, Timbol J, Lewis N, Pool V, Decker MD, Greenberg DP, Klein NP. Safety of DTaP-IPV/Hib vaccine administered routinely to infants and toddlers. Vaccine 2016; 34(35):4172-9; PMID:27373595; https://doi.org/10.1016/j.vaccine.2016.06.062
- Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, Russell A. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J 2007; 26(6):496-500; https://doi.org/10.1097/INF.0b013e31805d7f17
- Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt HJ, von Kries R. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J 2006; 25(6):507-12; https://doi.org/10.1097/01.inf.0000222413.47344.23
- Mitchell VS, Philipose NM, Sanford JP, editors. The Children's Vaccine Initiative: Planning Alternative Strategies. Washington DC: National Academy Press; 1993, p. 196-203
- Dhingra MS, Rao R, Bhat S, Joshi R, Kalra V, Parikh HR, Rao SN, Sethi GR, Shah N, Muzaffaruddin M. Evaluation of the immunogenicity and safety of an indigenously developed DTwP-Hib tetravalent combination vaccine (Shan 4) with EasyFourTM in Indian infants administered per EPI schedule: A phase III trial. Hum Vaccines 2010; 6(7):572-7; PMID:20421723; https://doi.org/10.4161/hv.6.7.11817
- Watemberg N, Dagan R, Arbelli Y, Belmaker I, Morag A, Hessel L, Fritzell B, Bajard A, Peyron L. Safety and immunogenicity of Haemophilus type b-tetanus protein conjugate vaccine, mixed in the same syringe with diphtheria-tetanus-pertussis vaccine in young infants. Pediatr Infect Dis J 1991; 10(10):758-63; https://doi.org/10.1097/00006454-199110000-00008
- Barra A, Dagan R, Preud'homme JL, Bajart A, Danve B, Fritzell B. Characterization of the serum antibody response induced by Haemophilus influenzae type b tetanus protein-conjugate vaccine in infants receiving a DTP-combined vaccine from 2 months of age. Vaccine 1993; 11(10):1003-6; PMID:8212818; https://doi.org/10.1016/0264-410X(93)90124-G
- Dagan R, Botujansky C, Watemberg N, Arbelli Y, Belmaker I, Ethevenaux C, Fritzell B. Safety and immunogenicity in young infants of Haemophilus b-tetanus protein conjugate vaccine, mixed in the same syringe with diphtheria-tetanus-pertussis-enhanced inactivated poliovirus vaccine. Pediatr Infect Dis J 1994; 13(5):356-62; https://doi.org/10.1097/00006454-199405000-00005
- Amir J, Melamed R, Bader J, Ethevenaux C, Fritzell B, Cartier JR, Arminjon F, Dagan R. Immunogenicity and safety of a liquid combination of DTP-PRP-T [corrected] vs lyophilized PRP-T reconstituted with DTP. Vaccine 1997; 15(2):149-54; PMID:9066031
- Kaplan SL, Lauer BA, Ward MA, Wiedermann BL, Boyer KM, Dukes CM, Schaffer DM, Paisley J, Mendelson R, Pedreira F, et al. Immunogenicity and safety of Haemophilus influenzae type b-tetanus protein conjugate vaccine alone or mixed with diphtheria-tetanus-pertussis vaccine in infants. J Pediatr 1994; 124(2):323-7; PMID:8301447
- Sharma H, Yadav S, Patil V, Chacko B, Kapre S, Jadhav S, Ravetkar S, Bahl S, Parekh S, Chakravarty A, et al. A phase III randomized, controlled study to assess and compare the immunogenicity and tolerability of single and multi-dose vials of DTwP-Hib, a fully liquid quadravalent vaccine and their comparison with TETRAct-Hib vaccine in Indian infants aged 6–14 weeks. Vaccine 2011; 29(48):8773-9; PMID:21968445
- Kanra G, Silier T, Yurdakok K, Yavuz T, Baskan S, Ulukol B, Ceyhan M, Ozmert E, Türkay F, Pehlivan T. Immunogenicity study of a combined diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis vaccine used to reconstitute a freeze-dried Haemophilus influenzae type b vaccine (DTaP-IPV//PRP-T) administered simultaneously with a hepatitis B vaccine at two, three and four months of life. Vaccine 1999; 18(9-10):947-54; PMID:10580209
- Rao R, Dhingra MS, Bavdekar S, Behera N, Daga SR, Dutta AK, Kundu R, Maiya P, Mishra P, Shah R, et al. A comparison of immunogenicity and safety of indigenously developed liquid (DTwPHB-Hib) pentavalent combination vaccine (Shan 5) with Easyfive (liq) and TritanrixHB + Hiberix (lyo) in Indian infants administered according to the EPI schedule. Hum Vaccine 2009; 5(6):425-9
- Scheifele DW. Recent trends in pediatric Haemophilus influenzae type B infections in Canada. Immunization Monitoring Program, Active (IMPACT) of the Canadian Paediatric Society and the Laboratory Centre for Disease Control. Canadian Med Association J 1996; 154(7):1041-7; PMID:8625025
- Marcy SM, Kohl KS, Dagan R, Nalin D, Blum M, Jones MC, Hansen J, Labadie J, Lee L, Martin BL, et al. Fever as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine 2004; 22(5):551; PMID:14741143
- Kohl KS, Walop W, Gidudu J, Ball L, Halperin S, Hammer SJ, Heath P, Varricchio F, Rothstein E, Schuind A, et al. Swelling at or near injection site: case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine 2007; 25(31):5858; PMID:17548132
- Gidudu JF, Walco GA, Taddio A, Zempsky WT, Halperin SA, Calugar A, Gibbs NA, Hennig R, Jovancevic M, Netterlid E, et al. Immunization site pain: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2012; 30(30):4558-77; PMID:22521267
- Donken R, De Melker H, Rots N, Berbers G, Knol M. Comparing vaccines: A systematic review of the use of the non-inferiority margin in vaccine trials. Vaccine 2015; 33(12):1426-32; PMID:25659273; https://doi.org/10.1016/j.vaccine.2015.01.072
- Patel MK, Patel TK, Tripathi CB. Diphtheria, pertussis (whooping cough), and tetanus vaccine induced recurrent seizures and acute encephalopathy in a pediatric patient: Possibly due to pertussis fraction. J Pharmacol Pharmacother 2012; 3:71-3
- Howson CP, Fineberg HV. Adverse events following pertussis and rubella vaccines. Summary of a report of the Institute of Medicine. JAMA 1992; 267:392-6
- Tro-Baumann B, von Spiczak S, Lotte J, Bast T, Haberlandt E, Sassen R, et al. A retrospective study of the relation between vaccination and occurrence of seizures in Dravet syndrome. Epilepsia 2011; 52:175-8
- McIntosh AM, McMahon J, Dibbens LM, Iona X, Mulley JC, Scheffer IE, Berkovic SF. Effects of vaccination on onset and outcome of Dravet syndrome: A retrospective study. Lancet Neurol 2010; 9:592-8
- Edwards KM, Decker MD, Mortimer EA Jr. Pertussis Vaccine. In: Plotkin SA, Orenstein WA, eds. Vaccines. Philadelphia: W.B. Saunders Company; 1999
- Bohlke K, Davis RL, Marcy SM, Braun MM, DeStefano F, Black SB, Mullooly JP, Thompson RS; Vaccine Safety Datalink Team. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics 2003; 112:815-20