ABSTRACT
Background: To determine the safety and immunogenicity of a novel recombinant adenovirus type 5 vector based Ebola virus disease vaccine (Ad5-EBOV) in Africans in China.
Methods: A phase 1, dose-escalation, open-label trial was conducted. 61 healthy Africans were sequentially enrolled, with 31 participants receiving one shot intramuscular injection and 30 participants receiving a double-shot regimen. Primary and secondary end points related to safety and immunogenicity were assessed within 28 d after vaccination. This study was registered with ClinicalTrials.gov (NCT02401373).
Results: Ad5-EBOV is well tolerated and no adverse reaction of grade 3 or above was observed. 53 (86.89%) participants reported at least one adverse reaction within 28 d of vaccination. The most common reaction was fever and the mild pain at injection site, and there were no significant difference between these 2 groups. Ebola glycoprotein-specific antibodies appeared in all 61 participants and antibodies titers peaked after 28 d of vaccination. The geometric mean titres (GMTs) were similar between these 2 groups (1919.01 vs 1684.70 P = 0.5562). The glycoprotein-specific T-cell responses rapidly peaked after 14 d of vaccination and then decreased, however, the percentage of subjects with responses were much higher in the high-dose group (60.00% vs 9.68%, P = 0.0014). Pre-existing Ad5 neutralizing antibodies could significantly dampen the specific humoral immune response and cellular response to the vaccine.
Conclusion: The application of Ad5-EBOV demonstrated safe in Africans in China and a specific GP antibody and T-cell response could occur 14 d after the first immunization. This acceptable safety profile provides a reliable basis to proceed with trials in Africa.
Introduction
Since the first outbreak of Ebola virus disease in West Africa in 1976, there have been 22 intermittent outbreaks till 2014.Citation1 During those almost 40 y of scientific studies, medical researchers have not found any effective methods to prevent or treat Ebola virus. Since the outbreak in 2014, the mortality rate steadily increased and the number of deaths was far greater than the total of the previous 37 years, regardless of the progress made in controlling of the outbreak.Citation2 Scientists around the world are dedicated to identifying an Ebola virus vaccine, and some studies have entered phase II clinical trials. However, most vaccines are developed based on the 1976 outbreak Zaire strain.Citation3 After comparing of the Zaire-type sequence included in GenBank, the envelope glycoprotein (GP) protein homology of Zaire-type in the 1976 outbreak and the Zaire-type in the 2014 outbreak was of 97% similarity. Therefore, 2014 outbreak Zaire-type strain is considered as a new epidemic one.Citation4-6 Results from a clinical study on chimpanzee adenoviral vector Ebola vaccine have also indicated that the vaccine-elicited antibody responses to the 1976 Zaire antigen were significantly higher than responses to the 2014 Zaire-Guinea strain.Citation4 Thus we could expect that the vaccine based on the Zaire-Guinea (2014) type is more beneficial than that based on the 1976 Zaire strain. In previous studies, we developed an Ad5-EBOV vaccine based on 2014 Zaire type Ebola virus. In clinical trials, 120 participants were selected and divided into the low-dose (4 × 1010 vp), high-dose (1.6 × 1011 vp), and placebo groups (n = 40 each).Citation7 And the results indicated that this vaccine was safe among healthy Chinese populations with strong immunogenicity. The high-dose trial vaccine had relatively better immunogenicity than the low-dose trial vaccine. One injection of high-dose Ad5-EBOV vaccine could activate specific humoral immunity that produced antibodies against Ebola virus infection on the 14th day.Citation7 In this study, we evaluated the safety, tolerability, and immunogenicity of 8 × 1010 vp and 1.6 × 1011 vp doses of Ad5-EBOV in Africans in China, to provide a reliable basis to proceed with trials in Africa.
Results
As shown in , from March 30, 2015 to August 21, 2015, a total of 118 healthy African volunteers in China (with half males and half females) were screened; ultimately, 61 participants were enrolled. 31 people were selected for the low-dose group (15 men and 16 women) with a mean age of 21.61 y and mean body mass index (BMI) of 24.81 kg·m−2. 30 people were selected for the high-dose group (16 men and 14 women) with a mean age of 23.18 y and mean BMI of 24.56 kg·m−2. All participants completed the 28-day follow-up period. Baseline characteristics and pre-existing adenovirus type 5 antibody titers in 2 groups are shown in . No significant difference was seen between the 2 groups.
Figure 1. Screening, enrollment, vaccinations, and follow-up in the study. The low dose group and high dose group were enrolled sequentially according to a doseescalation protocol. All the 61 participants completed the studyinjection regimen and 28 d of followup. All available study data were used for final analysis.
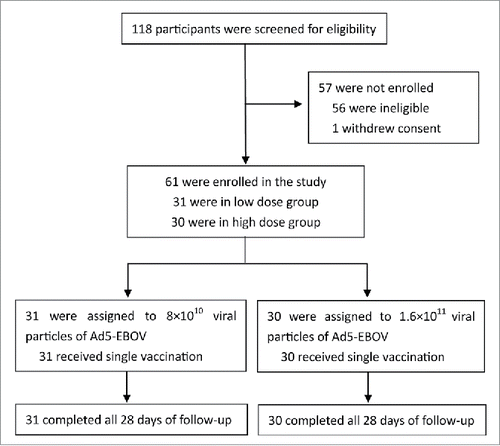
Table 1. Baseline characteristics and titres of pre-existing adenovirus type-5 neutralising antibodies.
Safety evaluation
The overall incidence for reported adverse events during the 28-day safety observation period in this study was 86.89% (53/61). No solicited or unsolicited adverse reaction of grade 3 and above was observed. There were also no severe adverse events. The difference in the incidence of adverse events between the low- and high-dose groups was not statistically significant (see Appendix 1 in Supplemental Material). Within 7 d after inoculation, there were 41 cases of grade 1 (67.21%) and 12 cases of grade 2 (19.67%) solicited adverse reactions occurred. In this study, local solicited adverse reactions mainly included injection site pain, swelling, induration, and redness with incidences of 52.46%, 39.34%, 26.23%, and 21.31%, respectively. Others showed local skin and mucous membrane damage (9.84%), itching (4.92%), and rash (3.28%). There was no statistical difference between the low and high dose groups except local induration.
Systemic solicited adverse reactions in this study mainly consisted of fever (44.26%), headache (40.98%), fatigue (29.51%) and cough (19.67%). Other systemic adverse reactions were muscle pain (11.48%), sore throat (9.84%), nausea (8.20%), diarrhea (8.20%) and joint pain (6.56%). There were 5 cases (16.13%) showed fatigue in low dose group, significantly lower than the 13 cases in high dose groups (43.33%). There was no statistical difference in the remaining systemic adverse reactions between these 2 groups. Within 28 d after inoculation, 6 (9.83%) and 5 (8.20%) cases reported grade1 and grade 2 unsolicited adverse events, respectively, and there was no statistically significant difference between these 2 groups ().
Table 2. Comparison of solicited adverse reactions and unsolicited adverse reactions occurring within 28 d after vaccination.
In the 28-day post-immunization observation period, the overall incidence of laboratory abnormalities was 26.23% and no abnormalities of grade 3 and above were observed. The difference in the incidence of laboratory abnormalities between the 2 groups was not statistically significant. Detailed laboratory abnormalities included leukopenia (7 cases, 11.48%), hemoglobin decrease (6 cases, 9.84%), prolonged prothrombin time (2 cases, 3.28%) and elevated alanine aminotransferase (one case, 1.64%). Lymphocyte count decrease or thrombocytopenia was not observed. Leukocyte count and hemoglobin level abnormality were both grade 1 adverse events. Elevated alanine transaminase, and prolonged prothrombin time were only observed in the high-dose group. Abnormalities in the low-dose group all belonged to grade 1. In the high-dose group, one case of grade 1 prolonged prothrombin time, and one case of grade 2 prolonged prothrombin time were observed (see Appendix 2 in Supplemental Material). Special treatments were not conducted for the above laboratory abnormalities, and close monitoring indicated that they could return to normal without intervention.
Immunogenicity evaluation
For the 61 participants, Ebola glycoprotein-specific antibody was not detected in the serum pre-immunization (day 0, baseline). At 7, 14, and 28 day post-immunization, the serum Ebola glycoprotein-specific antibody seroconversion rates were 9.84%, 98.36%, and 100%, respectively. Ebola glycoprotein-specific ELISA EC90 titres, expressed as geometric mean titres (GMTs), were 6.68, 1374.25 and 1919.01 for the low-dose group at 7, 14, and 28 d post-immunization, respectively. As for the high-dose group, the ELISA EC90 titres were 7.27, 1185.51 and 1684.70 at day 7, 14, 28, respectively. There was no statistically significant difference among the low dose group and the high dose group. ELISA EC90 titres in terms of GMTs increased rapidly 14 d post-immunization for both groups, and gradually reached the peak at day 28 after immunization. But the differences in GMTs value between 14 and 28 d post-immunization were not significant (see ). In this study, the participants’ serum anti-human adenovirus type 5 (Ad5) neutralizing antibody levels before and after immunization were evaluated. There were 17 negative pre-existing neutralizing antibody cases (27.87%) and 44 positive pre-existing neutralizing antibody cases (72.13%) in the 61 subjects. Twenty-two participants (36.07%) had a low level of pre-existing Ad5 neutralizing antibody (Ad5 antibody level <200), while 39 participants (63.93%) had a high level (Ad5 antibody level ≥ 200). The distribution of pre-existing Ad5 neutralizing antibodies was similar to that in clinical studies in healthy Chinese participants, in which 33 (41.25%) had a low level and 47 (58.75%) had a high level. The GMTs value of pre-existing Ad5 neutralizing antibody was 118.47 and 191.44 respectively in 31 cases of low dose group and 30 cases of high dose group. There was no statistical significance in pre-existing Ad5 neutralizing antibody between the low and high dose groups. In the low dose group, the GMTs value of Ad5 neutralizing antibody at day 7, 14 and 28 post-immunization was 317.62, 955.75 and 674.68, respectively. However, there was one participant who had not been detected the Ad5 neutralizing antibody during the period of study. In the high dose group, the GMTs value of Ad5 neutralizing antibody at day 7, 14 and 28 post-immunization was 778.09, 2381.20 and 1618.35, respectively. The level of Ad5 neutralizing antibody reached peak at 14 d post-immunization and then decreased for both groups, while the value at day 28 after immunization was lower than that at day 14, but the difference was not statistically significant.
Table 3. Geometric mean titres and responders by treatment groups.
Both in the low-dose group or high-dose group, the vaccine-elicited glycoprotein-specific antibody response on day 28 after immunization was significantly higher in pre-existing Ad5 neutralizing antibody negative participants than the ones with high-level or positive pre-existing Ad5 neutralizing antibody. However, the ELISA EC90 titres value on day 28 after immunization was 1402.85 and 1479.61, respectively in participants with positive pre-existing Ad5 neutralizing antibody who vaccinated the high-dose and low-dose vaccine, and there was no significant difference between these 2 dose groups. Additionally, for participants with low-level of pre-existing Ad5 neutralizing antibody, ELISA EC90 titres after high-dose vaccination was 3153.17, which was comparative to that after low-dose inoculation (2774.80); for participants with high-level of pre-existing Ad5 antibody, ELISA EC90 titres after inoculation of high-dose vaccine was 1287.82, which was close to that after the low-dose vaccine (1470.31).
Before vaccination, 64.52–80% of participants had a positive Ad5 neutralizing antibody and 58.06–70% of participants had a high level of pre-existing Ad5 antibody (). Presence of pre-existing Ad5 neutralizing antibody weakened the vaccine-induced GP-specific antibody response, irrespective of vaccine dose in this study. Correlation analysis showed that the levels of pre-existing Ad5 neutralizing antibody were inversely correlated with the glycoprotein-specific antibody response, with the correlation coefficient was -0.5200 (P = 0.0028) in the low dose group and -0.5100 (P = 0.0038) in the high dose group.
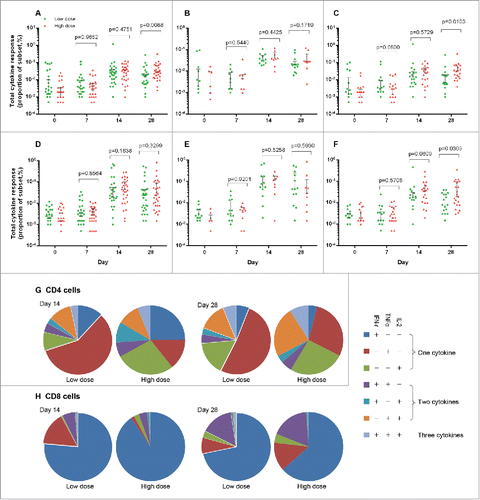
Glycoprotein-specific cellular immune responses were evaluated by interferon (IFN)-γ ELISPOT on day 0, 14, 28 and intracellular cytokine staining (ICS) on day 0, 7, 14 and 28. ELISPOT IFN-γ responses significantly increased after vaccination and got a similar magnitude at day 14 and day 28, with a median of 10 spot-forming cells (Interquatile range, IQR, 30) in participants in the low-dose group and 60 spot-forming cells (IQR, 80) in participants in the high-dose group at day 14, slightly increased to 20 spot-forming cells per 106 PBMCs (IQR, 50) and 90 spot-forming cells per 106 PBMCs (IQR, 200), respectively, at day 28. ELISPOT IFN-γ responses were significantly higher in participants in the high-dose group than in those in the low-dose group, at both days 14 and 28 (). The pre-existing Ad5 neutralizing antibody significantly diminished the IFN-γ response in both vaccine groups. The ICS was expressed as frequencies of CD4 and CD8 cells producing IFN-γ, tumor necrosis factor (TNF) α or interleukin 2 after stimulation with Ebola Zaire-Makona glycoprotein peptides. Both CD4 and CD8 T-cell responses increased significantly after vaccination and peaked at day 14, with no significant difference between doses, except CD4 T-cell response at day 28 (). The magnitude of response was greater in participants with low or negative pre-existing Ad5 neutralizing antibody than in those with high pre-existing antibody in low-dose group, however, there were no obvious difference in high-dose group. Similar kinetics was shown in the specific CD4 and CD8 T-cell responses with CD69 expression (see Appendix 3 in Supplemental Material). Both CD4 and CD8 T cells presented polyfunctional and monofunctional phenotypes. The CD8 cell response consisted mainly of IFN-γ-producing cells at day 14, among which the IFN-γ TNFα co-producing subsets increased significant at day 28 ().
Figure 2. Glycoprotein-specific T-cell response measured by Enzyme-Linked ImmunoSpot at different time points pre- and post-vaccination. IFN-γ expressing T-cells per 106 PBMC in the all participants (A, n = 31 in the low dose group and n = 30 in the high dose group), and those with pre-existing adenovirus type-5 neutralising antibody titres ≤ 1:200 (B, n = 13 in the low dose group and n = 9 in the high dose group) or >1:200 (C, n = 18 in the low dose group and n = 21 in the high dose group). Cases with undetected T cell response were not shown. The line at median with 75th percentiles (3rd quatile, Q3) were shown in the figure. IFN = interferon. PBMC = peripheral blood mononuclear cells.
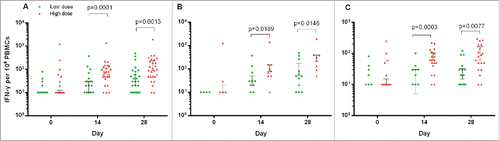
Figure 3. Glycoprotein-specific T-cell response measured by flow cytometry with intracellular cytokine staining (ICS) at different time points pre- and post-vaccination. CD4 T-cell response in all participants (A, n = 31 in the low dose group and n = 30 in the high dose group) and those with pre-existing adenovirus type-5 neutralising antibody titres ≤1:200 (B, n = 13 in the low dose group and n = 9 in the high dose group) or >1:200 (C, n = 18 in the low dose group and n = 21 in the high dose group), and CD8 T-cell response in all participants (D, n = 31 in the low dose group and n = 30 in the high dose group) and those with pre-existing adenovirus type-5 neutralising antibody titres ≤1:200 (E, n = 13 in the low dose group and n = 9 in the high dose group) or >1:200 (F, n = 18 in the low dose group and n = 21 in the high dose group). Proportions of glycoprotein-specific CD4 (G) and CD8 (H) cells that produce any combination of the three cytokines at day14 and day 28. TNF = tumour necrosis factor. IL = interleukin. IFN = interferon. Cases with undetected T cell response were not shown. The line at median with 75th percentiles (3rd quatile, Q3) were shown in the Figure.
Discussion
Our findings show that healthy Africans in China have good Ad5-EBOV tolerability and all the adverse reactions are mild or moderate. The incidence of adverse reactions was similar to those reported in other studies on Ebola virus disease vaccine.Citation5,Citation8,Citation9 The most common adverse reaction for Africans in China was injection site pain, which was the same as those in study on healthy Chinese populations.Citation7 Moreover, the incidence of adverse reactions obviously increased as the dose increased. In this study on Africans in China, the number of cases of minor localized indurations reported in the high-dose group was far higher than that in the low-dose group. Since the participants in the high-dose group were injected on both left and right arms and those in the low-dose group were only injected in the left arm, double injection in high-dose group may have also increased the number of cases of localized adverse reactions.Citation9-10 Among the participants with the same (high) injection dose, the incidence of fever and headache in Africans was higher than that in healthy Chinese people.Citation7 We speculated that this might due to the difference in ages, combined with published report.Citation11 The average age of healthy Chinese people was higher than that in Africans. Africans were all young people at around 20 y of age, in which case they might have different subjective feelings of fever, headache, and fatigue. However, these adverse reactions were all less serious than grade 3, indicating that healthy Africans could well-tolerate the high-dose vaccine.Citation11-12
Adenovirus type-5 Ebola vaccine produced a considerable GP antibody response and GP-specific T cell response in healthy African participants in China after 14 d. GP antibody response peaked at day 28, but T cell response decreased. Pre-existing Ad5 neutralizing antibodies could significantly weaken antibody response and T cell response to the vaccine.Citation13-14 In our previous studies, pre-existing Ad5 neutralizing antibody could impact serum GP antibody level, while a dose increase (from 4 × 1010 vp to 1.6 × 1011 vp) could overcome this negative effect.Citation7 In this study, in the dose range of 8 × 1010–1.6 × 1011 vp, increasing the inoculated vaccine dose could not increase the GP antibody response level, which indicated that within a certain dose range, an increase in inoculated vaccine dose could not increase GP antibody response level. Yet, we noted increasing the vaccine dose could increase the response of specific T cells, predominantly CD4+T cells; Pre-existing Ad5 neutralizing antibodies could suppress specific T cells responses, while the increases of inoculated vaccine dose could still compensate for this negative effect.
In this study, we did not find that the proportion of adults with pre-existing Ad5 neutralizing antibody in healthy Africans in China was greater than that of healthy Chinese people. After the same dose inoculation of vaccine, the produced serum GP antibody level was similar. Considering GP antibody titers is well correlated with protection efficacy in NHPs and there exists the similar GP antibody titers in high-dose and low-dose group in our study, we highly recommended 8 × 1010 vp as an optimal dose for the next clinical trial study.
The shortages of our research were that: 1) lack of normal control groups; 2) the data of half a year and one year was not compared and discussed here.
Anyway, we plan to assess the persistence of the specific immune response by following up the vaccine recipients of this study. On the basis of our findings, we suggested that the application of low-dose Ad5-EBOV was safe in Africans in China. This acceptable safety profile provides a reliable basis to proceed with trials in Africa.
Materials and methods
Study design and participants
It was a single-site, phase 1, dose escalation clinical trial conducted at the First affiliated Hospital of Zhejiang University. Participants were black individuals from African adults who live in China. Recruitment was predominantly by presentations and communications at health fairs, medical clinics, and colleges. We enrolled non-pregnant, healthy adults aged 18–40 y. Ethnicity/nationality was self-reported and collected, which was confirmed by passport information. Additional inclusion and exclusion criteria are detailed at ClinicalTrials.gov (NCT02401373).
Study approval
The protocol and informed consent documents were reviewed and approved by the ethics committee of The First affiliated Hospital of Zhejiang University (approval No. 2015–EC-24). The principles of the current revision of the Declaration of Helsinki concerning medical research in humans, the International Conference on Harmonization Guideline for Good Clinical Practice, and the Guideline for vaccine clinical trials recommended by China State Food and Drug Administration (CFDA) were well followed in this study. Written informed consent was obtained from all participants before inclusion in the study. Participants should be identified by number, not by name.
Interventions
This experimental vaccine was a replication-defective adenovirus type-5 vector-based vaccine and was constructed with the Admax system (Microbix Biosystem, ON, Canada). Vaccines were manufactured as lyophilised white powder containing 4.0 × 1010 adenovirus type-5 viral particles per vial. Two groups of volunteers were sequentially enrolled in this dose escalation trial. Doses of 8 × 1010 and 1.6 × 1011 viral particles were evaluated, and each dose group was designed to enroll 30 vaccinees. Participants in low dose group received one shot intramuscularly in the left arm with 2 vials of the vaccine (a total of 8.0 × 1010 viral particles) dissolved in 1 mL sterile water for injection. When the safety of the vaccine within 7 d after vaccination in the last participant in low dose group was confirmed, volunteers for high dose group began to be enrolled. In high dose group, participants received a double-shot regimen of the vaccine with one shot of 8.0 × 1010 viral particles in each arm.
Measurements
Participants were under close clinical observation for the incidence of any adverse reactions for at least 6 h after the vaccination. Then, any adverse events were self-recorded on diary cards in the following 28 d. All participants were asked to return to the site on day 3, 7, 14, and 28 after the vaccination to undergo a safety assessment and give a blood sample for immunogenicity evaluation.
Safety assessment
Safety monitoring included clinical evaluations and laboratory examinations at scheduled study visits. Participants were given a diary and asked to record all solicited (prompted in the diary and by investigators at study visits) and unsolicited adverse events. Solicited adverse events included local reactions and systemic events. Solicited local reactions were injection-site pain, induration, redness, swelling, itching, and rash. Solicited systemic events were fever, headache, fatigue, nausea or vomiting, diarrhea, myalgia, arthralgia, pharyngalgia, and cough. Adverse events occurring within 7 d after injection were referred to as reactogenicity. Severity was assessed according to the standard guidelines for adverse reactions grading of vaccine clinical trials recommended by CFDA, using a graded severity scale ranging from 0 to 4: mild (grade 1), moderate (grade 2), severe (grade 3), life-threatening (grade 4). Adverse events were listed per vaccinee and were reported individually and in aggregate.
Laboratory examinations included routine blood tests (hemoglobin, white blood cell count, lymphocyte count, platelets count), liver and kidney function (alanine transaminase, total bilirubin, and creatinine) and blood coagulation function test (prothrombin time and activated partial thromboplastin time).
Immunogenicity assessment
Immunogenicity were assessed using the same methods described previously by Zhu et al.Citation7 Specifically, titers of antibody directed against the vaccine-matched 2014 Zaire Makonaglycoprotein (GP) were determined by ELISA, and reported as the ELISA 90% effective concentration (EC90; the most dilute serum concentration at which there is a 90% decrease in antigen binding). It was calculated by GraphPad Prism (version 5) with a subtraction of the pre-vaccination optical density. Therefore, the Ebola glycoprotein-specific antibody pre-vaccination was regarded as negative in all the participants. ELISA EC90 titres higher than 10 was recognized as positive response. Anti-adenovirus type-5 neutralising antibody titres were detected with a serum neutralisation assay before and after vaccination. T cell responses to GP were detected by ELISpot and Intracellular cytokine staining (ICS).
Statistics
Safety and immunogenicity were the primary end point. We further stratified the patients’ baseline Ad5 neutralizing antibody titers (negative [≤ 1:18] or positive [≥ 1:18], low [≤ 1:200] or high [≥ 1:200]). The safety analysis was mainly descriptive analysis of the incidence of adverse reactions/adverse events, vital signs and laboratory biochemical index. The Chi-square tests could be used when compared in those groups, moreover, exact Fisher's method could be used when necessary. Immunogenicity analysis of antibody levels needs logarithmic transformation. t test was used mainly to compare difference between groups. The antibody positive rate, T cell response rate comparison between groups could be analyzed by Chi-square tests, or Fisher's exact probability method.
Immunogenicity index data using GraphPad Prism 5 and SPICE5.3 software for processing. All statistical calculations were performed by an independent statistician using SAS9.1. The hypothesis test for safety analysis was processed by bilateral inspection (2 - side test). When compared between groups, inspection level α = 0.05. And P≤ 0.05 was set as the difference was statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Author contributions
Prof. LJL, WC and LHW designed the trial and the study protocol. YQQ, JFS, NPW, LHW, HNG, JZST, JL, GJL, MHL and GLW participated in the site work, including the recruitment, follow-up, and data collection. ZZ, HPY, XXW, YHL, LW, JJL, LHH, SPW and QX contributed to participate in the laboratory analyses, data interpretation. YZ contributed to the vaccine management. PL, LL, GLW, and HLO contributed to the statistical analysis. HNG drafted the manuscript. LHW, LHH, MHL and JL modified and revised the manuscript. WC and LJL supervised the whole process of study and took the responsibility for all the data gathered, revised the manuscript. Prof. LJL decided to publish the paper. All authors reviewed and approved the final version of the report.
2017HV0149R-s01.docx
Download MS Word (229.2 KB)Acknowledgments
The authors appreciate the generous cooperation received from the staff of the Research Center for Clinical Pharmacy, The First Affiliated Hospital of Zhejiang University.
Funding
This work was supported by the National Major Study Plan of China under Grant (No. 2016YFC1200204); and the Public Welfare Projects of Zhejiang Province, China under Grant (No. 2015C33166).
References
- Nanclares C, Kapetshi J, Lionetto F, de la Rosa O, Tamfun JJ, Alia M, Kobinger G, Bernasconi A. Ebola virus disease, democratic republic of the Congo, 2014. Emerg Infect Dis 2016; 22(9):1579-86; PMID:27533284; https://doi.org/10.3201/eid2209.160354
- Hersey S, Martel LD, Jambai A, Keita S, Yoti Z, Meyer E, Seeman S, Bennett S, Ratto J, Morgan O, et al. Ebola Virus Disease–Sierra Leone and Guinea, August 2015. MMWR Morb Mortal Wkly Rep 2015; 64(35):981-4; PMID:26355422; https://doi.org/10.15585/mmwr.mm6435a6
- Martins KA, Jahrling PB, Bavari S, Kuhn JH. Ebola virus disease candidate vaccines under evaluation in clinical trials. Expert Rev Vaccines 2016; 15(9):1101-12; PMID:27160784; https://doi.org/10.1080/14760584.2016.1187566
- Ledgerwood JE, DeZure AD, Stanley DA, Coates EE, Novik L, Enama ME, Berkowitz NM, Hu Z, Joshi G, Ploquin A, et al. Chimpanzee adenovirus vector ebola vaccine. N Engl J Med 2017; 376(10):928-38; PMID:25426834; https://doi.org/10.1056/NEJMoa1410863
- De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, Cochet C, Estoppey D, Steiner-Monard V, Lonchampt S, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 2016; 16(3):311-20; PMID:26725450; https://doi.org/10.1016/S1473-3099(15)00486-7
- Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keita S, De Clerck H, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 2014; 371(15):1418-25; PMID:24738640; https://doi.org/10.1056/NEJMoa1404505
- Zhu FC, Hou LH, Li JX, Wu SP, Liu P, Zhang GR, Hu YM, Meng FY, Xu JJ, Tang R, et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet 2015; 385(9984):2272-9; PMID:25817373; https://doi.org/10.1016/S0140-6736(15)60553-0
- Ewer K, Rampling T, Venkatraman N, Bowyer G, Wright D, Lambe T, Imoukhuede EB, Payne R, Fehling SK, Strecker T, et al. A Monovalent Chimpanzee Adenovirus Ebola Vaccine Boosted with MVA. N Engl J Med 2016; 374(17):1635-46; PMID:25629663; https://doi.org/10.1056/NEJMoa1411627
- Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386(9996):857-66; PMID:26248676; https://doi.org/10.1016/S0140-6736(15)61117-5
- Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, Yerly S, Dayer JA, Kraehling V, Kasonta R, et al. Phase 1 Trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med 2016; 374(17):1647-60; PMID:25830326; https://doi.org/10.1056/NEJMoa1502924
- Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, Doumbia M, Traore A, Coulibaly F, Kodio M, Onwuchekwa U, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2016; 16(1):31-42; PMID:26546548; https://doi.org/10.1016/S1473-3099(15)00362-X
- Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J, Eickmann M, Finckh A, Goncalves AR, Hooper JW, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 2015; 15(10):1156-66; PMID:26248510; https://doi.org/10.1016/S1473-3099(15)00154-1
- Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine 2010; 29(2):304-13; PMID:21034824; https://doi.org/10.1016/j.vaccine.2010.10.037
- Sumida SM, Truitt DM, Kishko MG, Arthur JC, Jackson SS, Gorgone DA, Lifton MA, Koudstaal W, Pau MG, Kostense S, et al. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J Virol 2004; 78(6):2666-73; PMID:14990686
