ABSTRACT
Pneumococcal diseases are associated with a significant clinical and economic burden. The 7-valent pneumococcal conjugate vaccine (PCV-7) has been used for the immunization of newborns against invasive pneumococcal diseases (IPD) in Italy while now, the pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) and the 13-valent pneumococcal conjugate vaccine (PCV-13) are available.
The aim of this analysis was to compare the estimated health benefits, cost and cost-effectiveness of immunization strategies vs. non-vaccination in Italy using the concept of overall vaccine effectiveness.
A published Markov model was adapted using local data wherever available to compare the impact of neonatal pneumococcal vaccination on epidemiological and economic burden of invasive and non-invasive pneumococcal diseases, within a cohort of newborns from the Italian National Health Service (NHS) perspective. A 18-year and a 5-year time horizon were considered for the base-case and scenario analysis, respectively.
PHiD-CV and PCV-13 are associated with the most important reduction of the clinical burden, with a potential marginal advantage of PHiD-CV over PCV-13. Compared with no vaccination, PHiD-CV is found on the higher limit of the usually indicated willingness to pay range (30,000 - 50,000€/quality-adjusted life year [QALY] gained), while the incremental cost-effectiveness ratio (ICER) for PCV-13 is slightly above. Compared with PCV-13, PHiD-CV would provide better health outcomes and reduce costs even at parity price, solely due to its differential effect on the incidence of NTHi acute otitis media (AOM). The analysis on a shorter time horizon confirms the direction of the base-case.
Introduction
Pneumococcal diseases still represent a major public health issue associated with significant clinical and economic burden worldwide. Among young, elderly, and immunocompromised individuals, infection with Streptococcus pneumoniae constitutes an important risk factor for several diseases including non-invasive (pneumonia and acute otitis media [AOM]) and invasive pneumococcal diseases (IPD) (mainly meningitis and bacteremia). Citation1
Significant changes in pneumococcal disease epidemiology have been observed since the USA Citation2 and European countries, Citation3–Citation5 including Italy, have introduced pneumococcal conjugate vaccines (PCVs) into national childhood immunization plans.
In Italy, the first PCV (7-valent pneumococcal conjugate vaccine [PCV-7]) was licensed in 2002 and has been included in regional childhood immunization schedules between 2006 and 2010. Citation6 Routine childhood immunization program with PCV-7 has significantly influenced the epidemiology of pneumococcal diseases. Despite an observed increase of non-vaccine serotype (NVT) IPD cases, due to serotype replacement, pneumococcal immunization plan resulted in marked incidence reductions of any-serotype IPD, in all age groups. Citation7–Citation9 In 2008–2014, in the 0–4 age group, IPD incidence for all serotypes decreased from 7.1 to 2.9/100,000; incidence for vaccine serotypes (VT) decreased from 5.5 to 1.1/100,000, while incidence for NVT increased from 1.6 to 2.0/100,000 (2.5 in 2013). In the >64 age group, IPD incidence increased from 5.3 to 7.5/100,000; VT incidence decreased from 3.9 to 3.2 (4.9 in 2010 and 4.3 in 2013), whereas NVT incidence increased from 1.4 to 4.4/100,000.10
To improve immunization against pneumococcal diseases, 2 second-generation higher-valent PCVs (pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine [PHiD-CV, Synflorix, GSK, serotype 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F] and 13-valent pneumococcal conjugate vaccine [PCV-13, Prevenar 13, Pfizer, serotype 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F]) were developed. In 2010, a national recommendation by the Italian Ministry of Health replaced PCV-7 with its successor vaccine PCV-13. Citation11 Finally, with the aim to achieve and maintain pneumococcal vaccination coverage in newborns ≥ 95%, the National Vaccination Plan 2012–2014 included the active offer of PCV to all newborns at 3, 5–6 and 11–13 months of age. Citation12
Currently in Italy, a 23-valent pneumococcal polysaccharide vaccine (PPV23) for the immunization against IPD in adults and children ≥ 2 years is also available.
At the time of initial licensure of the second-generation PCVs, the effectiveness was evaluated on the basis of criteria of non-inferiority to PCV-7 using serological end-points. However, the overall impact of a PCV cannot be predicted solely on the basis of immunological parameters related to the serotypes actually included in the formulation (VT), as there may be differences in the efficacy against each VT, in their level of cross-protection against NVT, in the ability to induce an indirect protection (herd immunity) and in the level of associated serotype replacement. Citation13 The persistence of immunity is another variable in estimating impact of vaccination; for PCV, the clinical relevance of immunological response to some PHiD-CV and PCV-13 serotypes is still not clear, and the association between surrogate markers of protection and clinical protection was not always consistent across serotypes and studies.
In fact, in 2012, the WHO position paper stated that the superiority of a vaccine should not be based on the number of serotypes included unless there is evidence that the inclusion of additional serotypes can enhance its effectiveness in specific epidemiological conditions. Citation1 Although it may be tempting to consider the clinical impact of a PCV as simply proportional to the number of serotypes in a formulation, the overall impact on invasive disease is in fact a combination of 3 distinct effects: effect on VT disease, effect (if any) on vaccine-related types (VRTs), and degree of NVT disease variation. Citation14 The ability of specific PCV to influence each of these 3 effects is a function not only of the VT composition, but also of their conjugation chemistry, the dosage level of each serotype in the conjugate vaccine, and carrier proteins, making predictions difficult. Citation14
For the reasons above, in the literature, vaccine effectiveness is increasingly expressed in terms of overall IPD incidence reduction, regardless of the causative serotype, which is the “hard outcome” of high interest to health policymakers. Citation3 , Citation13 , Citation15–Citation17
Furthermore, randomized controlled trials (RCTs) and observational studies have confirmed the protection of PCV-7 and PHiD-CV against cross-reactive serotypes, 6A and 19A respectively, and an overall effectiveness of PHiD-CV. Citation3 , Citation13 , Citation18 Based on these findings, in 2015–2016, Belgium, Luxembourg and New Zealand have recognized that both PHiD-CV and PCV-13 vaccines are suitable for inclusion on the National Immunization Schedule. Citation18–Citation20 In July 2015, the European Medicines Agency's Committee for Medicinal Products for Human Use has expanded the indications of PHiD-CV by including the cross-protection against serotype 19A. Citation21 A systematic review of the literature on the impact and effectiveness of PHiD-CV and PCV-13 in Latin America, performed by the Pan American Health Organization (PAHO), has found no evidence of the superiority of one vaccine over the other with regards to impact and effectiveness on hospitalization and mortality reduction in children under 5 years old, considering the outcomes studied, namely death or hospitalizations due to IPD, pneumonia (X-ray-confirmed pneumonia, consolidated, and clinical pneumonia), meningitis or sepsis. Citation22
The aim of the present study was to compare the estimated health benefits, cost and cost-effectiveness of childhood immunization strategies with PCV-7, PHiD-CV, and PCV-13 vs. non-vaccination in the Italian context (despite the fact that PCV7 is no longer on the market, it was considered adequate to compare all possible vaccine strategies). Rather than serotype-specific immunization, we have chosen to use overall vaccine effectiveness (OVE) data, as well described by Hausdorff et al. and considered as a new relevant public health parameter. Citation14 , Citation22
Results
Base-case (18-year time horizon)
Expected total clinical events and health care costs are shown in .
Table 1. Base-case analysis results (18-year time horizon).
The least effective strategy is to not vaccinate, while higher valency vaccines are associated with the most important reduction of the clinical burden, with a potential marginal advantage of PHiD-CV over PCV-13 determined solely when effectiveness in preventing NTHi-caused AOM episodes is taken into account. On the economic side, the least costly strategy is to not vaccinate, while the most expensive is to vaccinate with PCV-13 – nothing can be said for PCV-7, given the absence of a unit cost for the vaccine, no longer available.
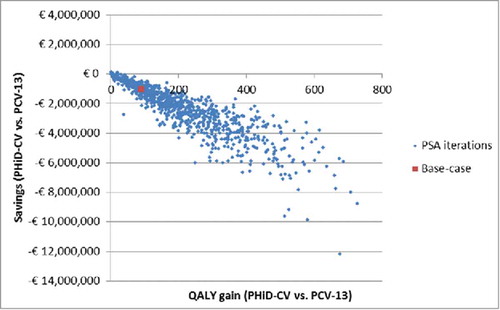
ICERs have been calculated for PHiD-CV and PCV-13 vs. no vaccination, and for PHiD-CV vs. PCV-13 ().
Compared with no vaccination, PHiD-CV is found on the higher limit of the usually indicated willingness to pay (WTP) range (30,000 - 50,000€/QALY gained), while the ICER for PCV-13 is slightly above.
Comparing the 2 higher valency vaccines, PHiD-CV is expected “dominant," according to the pharmacoeconomics jargon, providing better health outcomes and reducing costs even when considering the parity price. As indeed expected from the inputs, the 2 strategies differ only in their effect on AOM. The reduced AOM incidence is expected to be associated with some 90 incremental QALYs and with savings of over one million Euros for an Italian newborn cohort followed over 18 y.
Based on the base-case results, a threshold analysis indicated that PCV-7 would be cost-effective at a WTP of 50,000€/QALY for unit prices up to €8.09. It may be worth indicating that the ICERs vs. PCV-7 at these price levels would be around 57,000 and 50,000€/QALY for PCV-13 and PHiD-CV, respectively.
Scenario analysis (5-year time horizon)
The analysis on a shorter time horizon confirms the direction of the base-case, and indicates how most of the expected disease burden, in terms of QALY losses and costs, accumulates in the first years. In the incremental analysis of PHiD-CV vs. PCV-13, reduced AOM incidence is expected to be associated with 64 incremental QALYs and with about €900,000 savings for an Italian newborn cohort followed over 5 y (details in Appendix section 1 in Supplemental Material).
Sensitivity analysis
Probabilistic sensitivity analysis (PSA)
The distribution of expected differences among the active strategies is presented in as scatterplot of the incremental results of the 1,000 iterations of the PSA. The high correlation among QALY and cost differences is not surprising if one considers that both are related to AOM prevention.
Deterministic sensitivity analysis
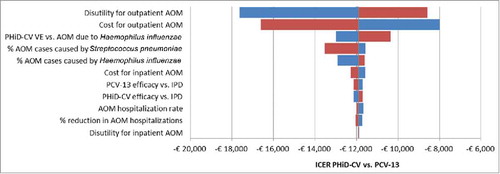
The tornado diagram depicted in shows the influence on the output of the model, expressed in terms of ICER of PHiD-CV vs. PCV-13, of variations in input parameters to their extreme values (details in Appendix section – section 1 in Supplemental Material). Parameters are sorted in order of decreasing sensibility of the results to their variations: the most influential parameters, not surprisingly, are those related to the cost and utility of AOM, which is the driver of the expected differences between PHiD-CV and PCV-13. For all values tested, the ICER remains negative, indicating pharmacoeconomic dominance.
Figure 2. DSA: Tornado diagram of ICER of PHiD-CV vs. PCV-13.
AOM: Acute otitis media; DSA: Deterministic sensitivity analysis; ICER: Incremental cost-effectiveness ratio; IPD: Invasive pneumococcal disease; PHiD-CV: Pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine; PCV-13: 13-valent pneumococcal conjugate vaccine; VE: Vaccine effectiveness.
Discussion
Despite the implementation of vaccination programs, pneumococcal diseases continue to be associated with mortality, morbidity and direct medical costs.
Our economic analysis suggests that from the NHS perspective, considering direct costs only in the direct comparison with PCV-13, the vaccination with PHiD-CV is a more slightly cost-effective strategy for infants in Italy, even at parity price. The advantage of PHiD-CV over PCV-13 is determined solely by its superior effectiveness in preventing AOM episodes that is expected to be associated with some 90 incremental QALYs and with savings of over €1 million accruing over 18 y in a single newborns cohort.
The main limitation of our analysis is the absence of direct comparison data between some of the compared strategies; this, however, is inherent with the type of clinical effectiveness studies in public health, and relates to the surrogate parameters sufficient for regulatory purposes. However, we believe to have taken a conservative approach when selecting the input data, and are therefore confident in the direction indicated by our results.
The efficacy of PHiD-CV against AOM has been shown in the COMPAS study Citation16 and supported by the study of Prymula et al. Citation23 in which a PHiD-CV precursor vaccine (11Pn-PD) was associated with reductions of 33.6%, 51.5%, and 35.6% for all causes AOM, Streptococcus pneumoniae-related AOM, and NTHi-related AOM, respectively. Those results have been confirmed by preclinical and clinical studies. Citation16 , Citation24 Furthermore, the study of Camilli et al. Citation25 reported that vaccination with PCV-7 and PCV-13 have led to a change in the nasopharyngeal flora of children under 6 y of age, increasing the incidence of AOM caused by NTHi. Based on these evidences, although the indication is not present in the European Summary of product characteristics, we decided in this analysis to consider some effectiveness of PHiD-CV against NTHi-related AOM. This is consistent with economic evaluation methodology which recommended to consider all differential aspects of the compared alternatives. Citation26 In fact, as indicated by the results of the sensitivity analyses, from a purely economic point of view, the scale of non-invasive pneumococcal disease overwhelms the effects on IPD, for prevention of which universal children vaccination is mainly advocated and implemented. It may be said that the reduction of AOM and pneumonia cases in early childhood is a concomitant health benefit, albeit less clinically crucial, of PCVs, characterized by an impressive payback that allows for the cost-effectiveness of the vaccination programs.
To our knowledge, this is the first study assessing cost-effectiveness of PCVs using OVE as alternative public health parameter, instead of serotype-specific effectiveness, according to the most recent studies which have questioned the evaluation of vaccines based solely on the number of serotypes. Differences in the effectiveness of VT, in the cross-protection, but also in the induction of indirect protection and in the level of replacement may in fact occur. Citation14 , Citation27
To date, observational studies of real life impact are available, and confirm the protection of PHiD-CV against cross-reactive serotypes, 19A in particular. This leads to an OVE similar to PCV-13: Citation3 , Citation13 , Citation28–Citation30 it has already been said that the protection against IPD is now more a matter of vaccination coverage than of selection of one over the other higher valency PCVs. Citation14 Consistently with these findings, some European countries, such as Austria, Finland, Iceland, the Netherlands, Belgium and Sweden (Regions), have considered PHiD-CV equivalent to PCV-13 in terms of IPD protection and therefore adopted vaccination with PHiD-CV.
The use of OVE instead of serotype-specific data has prompted the need for changing some of the methodology of the original simulation model; these changes were mainly related to the overcoming of the need for some assumptions. In particular, there was no further need to assume the size and duration of the indirect protective effect, which was estimated as a balance between cross-protection, herd immunity, and serotype replacement phenomena in earlier, serotype-specific data fed versions of the model. OVE data capture this balance directly. Furthermore, no assumptions on the dynamics of the “ramp-up” of VE were needed anymore, as the OVE data used already represent the mean effectiveness in the 0–4 y population. A possible drawback of these changes, to be taken into consideration when interpreting the results, is that the levels of OVE are applied to a population with potentially different epidemiology than the one in which it was observed.
As for any study, the results presented have to be interpreted in light of their limitations. We already addressed the main ones, namely the absence of direct comparison data, the use of effectiveness data stemming from countries with a possibly different epidemiology than Italy's, and the fact that differences between the 2 high valency vaccines are completely driven by their effect on AOM, which is not their main clinical indication. Furthermore, we underline that the use of a static, as compared with a dynamic, simulation model precludes the possibility to accurately capture the long-term evolution of the epidemiology brought by vaccination programs. However, we took measures to minimize their potential to introduce bias, as described, and are confident in the direction and main conclusion indicated by our results.
Conclusions
The results of our analysis further corroborate the above mentioned public health choices, indicating that at an equal level of protection against IPD and pneumonia, PHiD-CV may offer additional slight advantages over PCV-13 in terms of AOM prevention. PHiD-CV may allow for slight economic savings, contributing to the sustainability of the vaccination programs from the NHS perspective, even at parity price.
Methods
A previously published Markov model Citation31 was adapted to simulate the impact of neonatal pneumococcal vaccination on epidemiological and economic burden of meningitis, bacteremia, pneumonia, and AOM, within a cohort of newborns in Italy.
Local data were used to populate the model wherever available, and all the assumptions and data sources were based on an in-depth evidence review. The analysis was conducted from the Italian National Health Service (NHS) perspective and compares 2 + 1 regimes of PHiD-CV (Synflorix, GSK) and PCV-13 (Prevenar 13, Pfizer) (doses administered at 3, 5, and 11 months) vs. PCV-7 (equaled to a strategy able to maintain current epidemiology) and no vaccination (in which a rebound to pre-PCV epidemiology is expected).
Given the uncertainty associated with epidemiological evolution and future available strategies, the usual lifetime horizon was considered to be too long and barely relevant. The time horizon was limited to the whole pediatric age (up to 18 years) for the base-case and to pre-scholar age (up to 5 years, the follow-up time of the clinical studies from which inputs were obtained) as scenario analysis.
Model structure
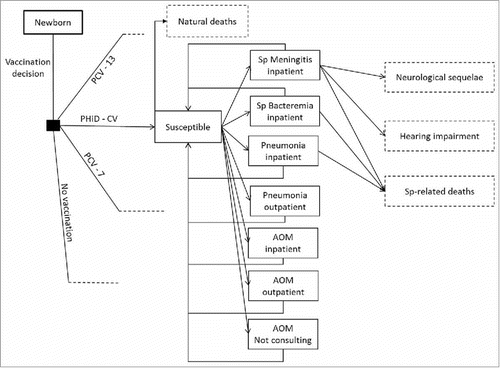
The model is a static age-stratified Markov model, considering 12 health states: no disease, meningitis, bacteremia, pneumonia (inpatient and outpatient), AOM (inpatient, outpatient and not-consulting), meningitis-related neurological sequelae and hearing impairment, Streptococcus pneumoniae-related and natural deaths (). Individuals of the birth cohort move to health states according to estimated transition probabilities determined by age-specific disease incidence rates (cycle length is one year). Vaccination decreases disease incidence rates and transition probabilities in accordance with vaccine effectiveness for IPD, pneumonia, and AOM. Vaccine effectiveness translates into cost offsets, reduced disutilities, and mortality decline. Costs and quality-adjusted life years (QALYs) specific to each health state were estimated and summarized over the time horizon and converted into a corresponding incremental cost-effectiveness ratio (ICER).
Figure 3. Model flow diagram. Rectangles represent mutually exclusive health states. Dotted rectangles represent absorbing health states and represent the proportion of the population removed from the model. Age-specific incidences are applied monthly to the susceptible population, after accounting for arm-specific VE. Costs and benefits are computed monthly and aggregated over the analyzed time horizon. Non-consulting AOM are accounted for in the quality-of-life impact calculation. No Vaccination: is a counterfactual scenario, in which universal vaccination is not fostered by the health system. It allows assessing the absolute value of PCV vaccination programs, and not only the comparison between 2 specific vaccination products.
AOM: Acute otitis media; PCV-7: 7-valent pneumococcal conjugate vaccine; PCV-13: 13-valent pneumococcal conjugate vaccine; PHiD-CV: Pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine; Sp: Streptococcus pneumonia.
Population
The population assessed in the model is represented by the cohort of 496,627 newborns in Italy in 2014, as reported by the Italian National Statistics Institute (ISTAT). Citation32 Background age-specific mortality rates during the simulation are also taken from ISTAT. Citation33
According to the Italian Ministry of Health, PCV vaccine coverage is set at 87.46%. Citation34
Epidemiological data
As detailed in the below Vaccine effectiveness section, we use OVE data; therefore incidence of IPD, pneumonia, and AOM epidemiological data used in the model include all serotypes.
The assumption on which epidemiology and vaccine effectiveness inputs are based is that the current epidemiology is a reflection of a quasi-steady-state subsequent to 10 y of pneumococcal conjugate vaccination with PCV-7: the current incidence would be maintained if continuing universal vaccination with PCV-7, it would rebound to pre-PCV-7 levels in case of interruption of the vaccination program, and can be further reduced with vaccines of higher valency, as shown in the clinical studies on which effectiveness inputs are sourced.
IPD
To avoid the known underestimation in the nation-wide figures, the age-specific annual incidence of pneumococcal meningitis and bacteremia (referring to 2011) rates were derived from the 7 Italian Regions with active surveillance systems. Citation10 In absence of local data, the frequency of meningitis long-term sequelae, and the case fatality rates for meningitis and bacteremia derived from the UK Health Protection Agency Citation35 ().
Table 2. Clinical inputs.
Community-acquired pneumonia (CAP)
The overall incidence rate for hospitalized all-cause pneumonia was calculated from age- and gender-specific incidence rates in the Veneto Region during 2004–2012, Citation36 weighted for the relative age-group specific proportion of males and females in Italy. Citation32 Age-specific case-fatality ratios for hospitalized pneumonia were also taken from Baldo et al. Citation36 (). The incidence rates for non-hospitalized CAP were derived from national general practitioners (GP) and pediatricians databases Citation37 , Citation38 ().
AOM
The proportion of national AOM cases due to Streptococcus pneumoniae (32%) or non-typeable Haemophilus influenzae (NTHi – 43%) was taken from the study by Camilli et al. Citation25
Incidence of AOM in 0–6-year-olds was elaborated from age-specific incidence rates of AOM in Italy from one local and one multinational study. Citation39 , Citation40 In the model, GP consultation rate is corrected with an adjustment factor (1.25) to estimate the true incidence of AOM taking into account the percentage of cases not associated with a visit ( - details in Appendix section 2 in Supplemental Material).
The hospitalization rates for AOM in 0–2-year-olds were taken from Ansaldi et al. Citation7 ; for older groups it has been calculated from the AOM hospitalization rate reported by the Pedianet 2006 report Citation37 ().
Vaccine effectiveness
Effectiveness against IPD
Based on in depth review of latest evidence showing equivalent protection of PHiD-CV and PCV-13 for IPD and pneumonia, we use OVE data, more accessible than serotype-specific immunization data and a relevant alternative in particular from the Public health perspective for the decision makers.
The Quebec experience was considered as the most appropriate source to extract OVE (75% for PHiD-CV, 65% for PCV-13, 50% for PCV-7) since it includes data for the different vaccines and because these vaccines have been subsequently used in the same healthcare system.13 However, in absence of a direct comparison study between PHiD-CV and PCV-13, we deemed a conservative approach to be preferable, and attributed them equal OVE (70%, mean of the 2). This is aligned with other non-comparative studies on PHiD-CV, Citation3 , Citation16 and PCV-13, Citation17 , Citation41 which report similar OVE (e.g., for PHiD-CV in Jokinen et al. = 80%; Citation3 for PCV-13 in Waight et al. = 77% and 74% in 0–2 and 2–4-year-olds, respectively Citation17 ).
Reported OVE was recalculated in consideration of the fact that the assumed 70% OVE refers to the comparison with no vaccination. The effect of PCV-13 and PHiD-CV in PCV-7-pre-exposed populations is less (56%). Citation17 Current incidence in Italy reflects about 10 y of universal vaccination: the assumption is that these represent a 50% remaining incidence vs. pre-PCV-7 (50% is the OVE of PCV-7 recorded in Quebec Citation13 ).
Conversely, a “no vaccination” strategy would be expected to result in a return to the pre-PCV epidemiology, with a rebound due to higher circulation of the invasive serotypes now controlled by the herd immunity and ongoing vaccination of newborns. Corrected OVE vs. IPD are presented in (details in Appendix section 3 in Supplemental Material).
In accordance with the OVE source used (referring to the entire age group 0–4-year-olds), we attributed a plateau phase of full effectiveness from 0 to 4 y included (the phase is linear), and a declining phase until complete waning at 10 y. This implies that no further reduction in IPD incidence beyond that age is expected when compared with the current epidemiology.
Effectiveness against pneumonia
In the absence of comparative vaccine effectiveness estimates on pneumonia, PHiD-CV and PCV-13 are attributed the same reduction in hospitalizations (23%) and in GP visits (7.3%), taken from COMPAS. Citation16 This effectiveness level refers to a universal vaccination-naïve population; consistently with the recalculation of vaccine effectiveness against IPD, we adjusted effectiveness taking into account reduced pneumonia incidence following PCV-7 Citation42 ( - details in Appendix section 4 in Supplemental Material).
Effectiveness against AOM
Vaccine effectiveness against all-cause AOM was calculated by multiplying pathogen-specific vaccine effectiveness by relative prevalence of the causative pathogens as reported in Camilli et al. (32% and 43% of AOM cases are attributable to Streptococcus pneumoniae and NTHi, respectively). Citation25 For PHiD-CV, the effectiveness against AOM caused by NTHi was taken from COMPAS Citation16 while in absence of data, PCV-13 effectiveness against NTHi-caused AOM was assumed equal to PCV-7 effectiveness. Citation43
Effectiveness data in Streptococcus pneumoniae-caused episodes prevention come from an observational study for PCV-7 and PCV-13, Citation44 and from COMPAS for PHiD-CV, Citation16 corrected for current epidemiology ( - details in Appendix section 5 in Supplemental Material).
Disutilities
Specific disutilities to each health state were used in the model ().
Table 3. Disutilities used in the model.
Costs
Consistently with the perspective of the Italian NHS, the analysis estimated direct medical costs only (related to vaccine and administration, inpatient/outpatient disease-related treatment and long-term sequelae) (). All costs were measured in € (2015 values). In the model, the same price of €47.73 per vaccine dose for both PHiD-CV and PCV-13 was applied (maximum price for NHS Citation50 , Citation51 ), because in Italy, the regional tender is based on price only. Health effects and costs were discounted at 3%/year.
Table 4. Direct costs estimated in the model.
Trademark statement
Synflorix is a trade mark owned by the GSK group of companies. Prevenar is a trade mark of Wyeth LLC.
Abbreviations
| AOM | = |
acute otitis media |
| CAP | = |
community-acquired pneumonia |
| GP | = |
general practitioner |
| ICER | = |
incremental cost-effectiveness ratio |
| IPD | = |
invasive pneumococcal disease |
| ISTAT | = |
Istituto nazionale di statistica (Italian National Statistics Institute) |
| NHS | = |
National Health Service |
| NVT | = |
non-vaccine serotype |
| NTHi | = |
non-typeable Haemophilus influenzae |
| OVE | = |
overall vaccine effectiveness |
| PCV | = |
pneumococcal conjugate vaccine |
| PCV-7 | = |
7-valent pneumococcal conjugate vaccine |
| PCV-13 | = |
13-valent pneumococcal conjugate vaccine, |
| PHiD-CV | = |
pneumococcal Haemophilus influenzae protein D conjugate vaccine |
| PPV-23 | = |
23-valent pneumococcal polysaccharide vaccine |
| PSA | = |
probabilistic sensitivity analysis |
| QALY | = |
quality-adjusted life years |
| RCT | = |
randomized controlled trials |
| VT | = |
vaccine serotype |
| WHO | = |
World Health Organization |
| WTP | = |
willingness to pay |
Disclosure of potential conflicts of interest
SC and VF are employees of the GSK group of companies (the manufacturer of PHiD-CV, Synflorix) and SC reports ownership of stocks from this entity. PC reports personal fees and non-financial support from the GSK group of companies during the conduct of the study as well as personal fees and non-financial support from the GSK group of companies, Novartis, Pfizer and Sanofi and grants from Pfizer, unrelated to the present work. LP received grant from the GSK group of companies for the conduct of this analysis and received grants from the GSK group of companies, Fresenius Kabi, Roche, Amgen, Sanofi, Novartis, Gilead, Chiesi, CSL Behring, Janssen-Cilag, Leo Pharma, Merck Sharp and Dohme, Bristol-Myers Squibb, and ViiV Healthcare and personal fees from Fresenius Kabi, outside of the submitted work. SE received research grants from the GSK group of companies, DMG, Novartis Vaccines, Pfizer, Sanofi and Scioderm as well as payment for lectures and advisory board participation from the GSK group of companies, Pfizer, Valeas, and Vifor. GP reports personal fees and non-financial support from the GSK group of companies during the conduct of the study.
Contributorship
All authors participated in the design or implementation or analysis, and interpretation of the study; and the development of this manuscript. All authors had full access to the data and gave final approval before submission
KHVI_A_1343773_supp.docx
Download MS Word (40.6 KB)Acknowledgments
Authors would like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Stephanie Garcia coordinated manuscript development and editorial support. The authors also thank Ombretta Bandi (SEED medical publishers, on behalf of GlaxoSmithKline S.p.A.) for providing medical writing support.
Funding
GlaxoSmithKline S.p.A. (Verona, Italy) funded this study (GSK study identifier: HO-15–16068) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline S.p.A. (Verona, Italy) and GlaxoSmithKline Biologicals SA took in charge all costs associated with the development and publication of this manuscript.
References
- World Health Organization (WHO) . Pneumococcal vaccines WHO position paper 2012. Weekly Epidemiological Record 2012; 87:129-44; PMID:24340399
- Moore MR , Link-Gelles R , Schaffner W , Lynfield R , Lexau C , Bennett NM , Petit S , Zansky SM , Harrison LH , Reingold A , et al. Impact of 13-valent pneumococcal conjugate vaccine used in children on invasive pneumococcal disease in children and adults in the United States: Analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301–9; PMID:25656600; https://doi.org/10.1016/S1473-3099(14)71081-3
- Jokinen J , Rinta-Kokko H , Siira L , Palmu AA , Virtanen MJ , Nohynek H , Virolainen-Julkunen A , Toropainen M , Nuorti JP. Impact of Ten-Valent Pneumococcal Conjugate vaccination on Invasive Pneumococcal disease in Finnish children - A population-based study. PLoS One 2015; 10:e0120290; PMID:25781031; https://doi.org/10.1371/journal.pone.0120290
- Moreira M , Castro O , Palmieri M , Efklidou S , Castagna S , Hoet B . A reflection on invasive pneumococcal disease and pneumococcal conjugate vaccination coverage in children in Southern Europe (2009–2016). Human Vaccines & Immunotherapeutics 2017; 13:1242–53; https://dx.doi.org/10.1080/21645515.2016.1263409
- Savulescu C , Krizova P , Lepoutre A , Mereckiene J , Vestrheim DF , Ciruela P , Ordobas M , Guevara M , McDonald E , Morfeldt E , et al. Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: an observational multicentre study. The Lancet Respiratory Medicine 2017; 5:648–56; https://doi.org/10.1016/S2213-2600(17)30110-8
- Alfonsi V , D'Ancona F , Giambi C , Nacca G , Rota MC . Current immunization policies for pneumococcal, meningococcal C, varicella and rotavirus vaccinations in Italy. Health Policy 2011; 103:176-83; PMID:22030308; https://doi.org/10.1016/j.healthpol.2011.10.002
- Ansaldi F , Sticchi L , Durando P , Carloni R , Oreste P , Vercelli M , Crovari P , Icardi G . Decline in pneumonia and acute Otitis Media after the Introduction of childhood Pneumococcal vaccination in Liguria, Italy. J Int Med Res 2008; 36:1255-60; PMID:19094434; https://doi.org/10.1177/147323000803600612
- D'Ambrosio F , Del Grosso M , Camilli R , Ingrosso L , Grazia Caporalli M , D'Ancona F , Pantosti A . Surveillance of invasive diseases caused by Streptococcus pneumoniae in Italy: evolution of serotypes and antibiotic resistance in different age groups before and after implementation of PCV7. Microbiologia Medica 2013; 28(1):17–20; https://doi.org/10.4081/mm.2013.2275
- Durando P , Alicino C , De Florentiis D , Martini M , Icardi G . Improving the protection against Streptococcus pneumoniae with the new generation 13-valent pneumococcal conjugate vaccine. J Prev Med Hyg 2012; 53:68-77; PMID:23240163
- Istituto Superiore di Sanita . Dati di sorveglianza delle malattie batteriche invasive aggiornati al 12 agosto 2015. 2015 [accessed 2015 Dec 22]. http://www.iss.it/binary/mabi/cont/Report_MBI_20150812_V5.pdf
- Ministero della Salute . Indicazioni in merito alla somministrazione del vaccino antipneumococcico Prevenar 13 in età pediatrica, Circolare del 27 maggio 2010, prot.n.111432/72AF. 2010 [accessed 2015 Dec 24]. http://www.fimpcalabria.org/public/vaccinazioni/indicazioni%20in%20merito%20alla%20somministrazione%20del%20vaccino%20antipneumococcico%20prevenar%2013%20in%20et%C3%A0%20pediatrica%20%282%29.pdf
- Ministero della Salute . Piano Nazionale Prevenzione Vaccinale (PNPV) 2012-2014. Intesa Stato-Regioni del 22 febbraio 2012. Supplemento ordinario alla “Gazzetta Ufficiale„ n. 60 del 12 marzo 2012 - Serie generale. http://www.gazzettaufficiale.it/do/gazzetta/serie_generale/3/pdfPaginato?dataPubblicazioneGazzetta=20120312&numeroGazzetta=60&tipoSerie=SG&tipoSupplemento=SO&numeroSupplemento=47&numPagina=1&edizione=0
- Deceuninck G , De Serres G , Boulianne N , Lefebvre B , De Wals P . Effectiveness of three pneumococcal conjugate vaccines to prevent invasive pneumococcal disease in Quebec, Canada. Vaccine 2015; 33:2684-9; PMID:25887086; https://doi.org/10.1016/j.vaccine.2015.04.005
- Hausdorff WP , Hoet B , Adegbola RA . Predicting the impact of new pneumococcal conjugate vaccines: serotype composition is not enough. Expert Rev Vaccines 2015; 14(3):413-28; https://doi.org/10.1586/14760584.2015.965160
- Palmu AA , Jokinen J , Borys D , Nieminen H , Ruokokoski E , Siira L , Puumalainen T , Lommel P , Hezareh M , Moreira M , et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet 2013; 381(9862):214-22; PMID:23158882; https://doi.org/10.1016/S0140-6736(12)61854-6
- Tregnaghi MW , Sàez-Llorens X , López P , Abate H , Smith E , Pósleman A , Calvo A , Wong D , Cortes-Barbosa C , Ceballos A , et al. Efficacy of Pneumococcal Nontypable Haemophilus influenzae Protein D Conjugate Vaccine (PHiD-CV) in young Latin American children: A double-blind randomized controlled trial. PLoS Med 2014; 11:e1001657; PMID:24892763; https://doi.org/10.1371/journal.pmed.1001657
- Waight PA , Andrews NJ , Ladhani SN , Sheppard CL , Slack MPE , Miller E . Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 2015; 15(6):535-43; https://doi.org/10.1016/S1473-3099(15)70044-7
- Superior Health Council of Belgium . Avis du conseil supérieur de la santé N° 8813: Vaccination de l'enfant et de l'adolescent contre le pneumocoque. 2015 [accessed 2016 Dec 16]. http://www.health.belgium.be/internet2Prd/groups/public/@public/@shc/documents/ie2divers/19103438.pdf
- Conseil Supérieur des Maladies Infectieuses . Avis du CSMI pour la vaccination universelle contre le pneumocoque : vaccin 10 ou 13-valent ? 2016 [accessed 2016 Nov 16]. http://www.sante.public.lu/fr/espace-professionnel/recommandations/conseil-maladies-infectieuses/infection-pneumocoques/2016-09-20-vaccination-pneumocoque.pdf
- Immunisation Subcommittee of PTAC . Record of the Immunisation Subcommittee of the Pharmacology and Therapeutics Committee (PTAC) meeting held at PHARMAC on 28 October 2015. 2016 [accessed 2016 Nov 17]. https://www.pharmac.govt.nz/assets/ptac-immunisation-subcommittee-minutes-2015-10.pdf
- European Medicines Agency (EMA) . Summary of product characteristics (Synflorix). 2015 [accessed 2015 Dec 24]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000973/WC500054346.pdf
- de Oliveira LH , Camacho LA , Coutinho ESF , Martinez-Silveira MS , Carvalho AF , Ruiz-Matus C , Toscano CM . Impact and Effectiveness of 10 and 13-Valent Pneumococcal Conjugate Vaccines on Hospitalization and Mortality in Children Aged Less than 5 Years in Latin American Countries: A Systematic Review. PLoS One 2016; 11:e0166736; PMID:27941979; https://doi.org/10.1371/journal.pone.0166736
- Prymula R , Peeters P , Chrobok V , Kriz P , Novakova E , Kaliskova E , Kohl I , Lommel P , Poolman J , Prieels JP , et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006; 367(9512):740-8; PMID:16517274; https://doi.org/10.1016/S0140-6736(06)68304-9
- Palmu AA , Jokinen J , Nieminen H , Rinta-Kokko H , Ruokokoski E , Puumalainen T , Borys D , Lommel P , Traskine M , Moreira M . Effect of pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) on outpatient antimicrobial purchases: a double-blind, cluster randomised phase 3-4 trial. Lancet Infect Dis 2014; 14:205-12; PMID:24287186; https://doi.org/10.1016/S1473-3099(13)70338-4
- Camilli R , Vescio MF , Giufré M , Daprai L , Garlaschi ML , Cerquetti M , Pantosti A . Carriage of Haemophilus influenzae is associated with pneumococcal vaccination in Italian children. Vaccine 2015; 33:4559-64; PMID:26190092; https://doi.org/10.1016/j.vaccine.2015.07.009
- Drummond MF , Schulpher MJ , Claxton K , Stoddart GL , Torrance GW . Methods for the economic evaluation of health care programmes. 4th edition. Oxford: Oxford University Press; 2015.
- Poolman J , Frasch C , Nurkka A , Käyhty H , Biemans R , Schuerman L . Impact of the Conjugation Method on the Immunogenicity of Streptococcus pneumoniae Serotype 19F Polysaccharide in Conjugate Vaccines. Clin Vaccine Immunol 2011; 18:327-36; PMID:21123523; https://doi.org/10.1128/CVI.00402-10
- Domingues CMA , Verani JR , Montenegro Renoiner EI , de Cunto Brandileone MC , Flannery B , de Oliveira LH , Santos JB , de Moraes JC . Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. The Lancet Respiratory Medicine 2014; 2:464–71. https://doi.org/10.1016/S2213-6702600(14)70060-8
- Knol MJ , Wagenvoort GH , Sanders EA , Elberse K , Vlaminckx BJ , de Melker HE , van der Ende A . Invasive pneumococcal disease 3 years after introduction of 10-Valent Pneumococcal Conjugate Vaccine, the Netherlands. Emerg Infect Dis 2015; 21:2040-4; PMID:26488415; https://doi.org/10.3201/eid2111.140780
- Verani JR , Santos Domingues CMA , Cassio de Moraes J , Group tBPCVES . Indirect cohort analysis of 10-valent pneumococcal conjugate vaccine effectiveness against vaccine-type and vaccine-related invasive pneumococcal disease. Vaccine 2015; 33:6145-8; PMID:26469718; https://doi.org/10.1016/j.vaccine.2015.10.007
- Knerer G , Ismaila A , Pearce D . Health and economic impact of PHiD-CV in Canada and the UK: a Markov modelling exercise. J Medical Economics 2012; 15:61-76; ; https://doi.org/10.3111/13696998.2011.622323
- ISTAT - Istituto Nazionale di Statistica . Popolazione residente in Italia al 1° Gennaio 2015 per età, sesso e stato civile. 2015 [accessed 2015 Dec 22]. http://demo.istat.it/pop2015/index.html
- ISTAT - Istituto Nazionale di Statistica . Tavole di mortalità della popolazione italiana. 2015 [accessed 2015 Dec 22]. http://demo.istat.it/index.html
- Ministero della Salute . Ufficio V - Malattie infettive e profilassi internazionale - DG Prevenzione Sanitaria. 2016 [accessed 2016 Apr 12]. http://www.salute.gov.it/imgs/C_17_tavole_20_allegati_iitemAllegati_2_fileAllegati_itemFile_0_file.pdf
- Health Protection Agency (HPA) . Pneumococcal serotype distribution for samples referred for serotyping epidemiological years (July–June): 2000/1 to 2005/6. 2009 [accessed 2015 Dec 22]. http://webarchivenationalarchivesgov.uk/20100304045635/http://wwwhpaorguk/webw/HPAweb&HPAwebStandard/HPAweb_C/1195733824984?p=1203409671918
- Baldo V , Cocchio S , Baldovin T , Buja A , Furlan P , Bertoncello C , Russo F , Saia M . A population-based study on the impact of hospitalization for pneumonia in different age groups. BMC Infect Dis 2014; 14:485; PMID:25192701; https://doi.org/10.1186/1471-2334-14-485
- Pedianet . Report Dati Pedianet Anno 2006. http://www.pedianet.it/bin=1039/Report-2006.pdf, 2006
- Sterrantino C , Trifirò G , Lapi F , Pasqua A , Mazzaglia G , Piccinni C , Cricelli C , Rossi A , Blasi F . Burden of community-acquired pneumonia in Italian general practice. Eur Respiratory J 2013; 42:1739-42; PMID:23949958; https://doi.org/10.1183/09031936.00128713
- Liese J , Silfverdal S , Giaquinto C , Carmona A , Larcombe J , Garcia-Sicilia J , Fuat A , Garces-Sanchez M , Basanta ML , Hiraldo EM , et al. Incidence and clinical presentation of acute otitis media in children aged <6 years in European medical practices. Epidemiol Infect 2014; 142:1778-88; PMID:24330917; https://doi.org/10.1017/S0950268813002744
- Marchisio P , Cantarutti L , Sturkenboom M , Girotto S , Picelli G , Dona D , Scamarcia A , Villa M , Giaquinto C . Burden of acute otitis media in primary care pediatrics in Italy: a secondary data analysis from the Pedianet database. BMC Pediatrics 2012; 12:1-8; PMID:22208358; https://doi.org/10.1186/1471-2431-12-185
- Fortunato F , Martinelli D , Cappelli MG , Cozza V , Prato R . Impact of Pneumococcal Conjugate Universal Routine Vaccination on Pneumococcal Disease in Italian Children. J Immunol Res 2015; 2015:206757; PMID:26351644; https://doi.org/10.1155/2015/206757
- Black SB , Shinefield HR , Ling S , Hansen J , Fireman B , Spring D , Noyes J , Lewis E , Ray P , Lee J , et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002; 21:810-5; PMID:12352800; https://doi.org/10.1097/00006454-200209000-00005
- Eskola J , Kilpi T , Palmu A , Jokinen J , Eerola M , Haapakoski J , Herva E , Takala A , Käyhty H , Karma P , et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Eng J Med: Massachusetts Medical Society 2001; 344:403–9; https://doi.org/10.1056/NEJM200102083440602
- Ben-Shimol S , Givon-Lavi N , Leibovitz E , Raiz S , Greenberg D , Dagan R . Near-Elimination of Otitis Media Caused by 13-Valent Pneumococcal Conjugate Vaccine (PCV) Serotypes in Southern Israel Shortly After Sequential Introduction of 7-Valent/13-Valent PCV. Clin Infect Dis 2014; 59:1724-32; PMID:25159581; https://doi.org/10.1093/cid/ciu683
- Bennett JE , Sumner W, II , Downs SM , Jaffe DM . Parents' utilities for outcomes of occult bacteremia. Arch Pediatrics Adolescent Med 2000; 154:43-8
- Oh PI , Maerov P , Pritchard D , Knowles SR , Einarson TR , Shear NH . A cost-utility analysis of second-line antibiotics in the treatment of acute otitis media in children. Clin Therapeutics 1996; 18:160-82; PMID:8851461; https://doi.org/10.1016/S0149-2918(96)80188-3
- Morrow A , De Wals P , Petit G , Guay M , Erickson LJ . The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol 2007; 18:121-7; PMID:18923713
- Cheng AK , Niparko JK . Cost-utility of the cochlear implant in adults: A meta-analysis. Arch Otolaryngol Head Neck Surg 1999; 125:1214-8; https://doi.org/10.1001/archotol.125.11.1214
- Oostenbrink R , Oostenbrink JB , Moons KGM , Derksen-Lubsen G , Essink-Bot ML , Grobbee DE , Redekop WK , Moll HA . Cost-utility analysis of patient care in children with meningeal signs. Int J Technol Assessment Health Care 2002; 18:485-96; PMID:12391942
- Decreto-Legge 8 luglio 1974, n. 264 - Norme per l'estinzione dei 760 debiti degli enti mutualistici nei confronti degli enti ospedalieri, il finanziamento della spesa ospedaliera e l'avvio della riforma sanitaria. (GU n.181 del 11-7-1974) Decreto-Legge convertito con modificazioni dalla L. 17 agosto 1974, n. 386 (in G.U. 29/08/1974, n.225). 1976 [accessed 2016 Jan 15]. http://www.normattiva.it/atto/caricaDettaglioAtto?atto.dataPubblicazioneGazzettaD1974-07-11&attocodiceRedazionaleD074U0264¤tPageD1
- Federazione nazionale unitaria titolari di farmacia . Prevenar 13. 2016 [accessed 2016 Jan 15]. http://www.federfarma.it/Farmaci-e-farmacie/Cerca-un-farmaco
- Ministero della Salute . Remunerazione prestazioni di assistenza ospedaliera per acuti, assistenza ospedaliera di riabilitazione e di lungodegenza post acuzie e di assistenza specialistica ambulatoriale. Gazzetta Ufficiale Serie Generale 2013; 23. http://www.gazzettaufficiale.it/do/gazzetta/serie_generale/3/pdfPaginato?dataPubblicazioneGazzetta=20130128&numeroGazzetta=23&tipoSerie=SG&tipoSupplemento=GU&numeroSupplemento=0&numPagina=1&edizione=0
- Mantovani LG , de Portu S , Cortesi PA , Belisari A . Valutazione economica del vaccino coniugato 13-valente. Italian J Public Health 2010; 7:36-45