ABSTRACT
Frequent mismatches between the predominant circulating B strain lineage and the B strain lineage in trivalent influenza vaccines have resulted in missed opportunities to prevent influenza illness. Quadrivalent influenza vaccines containing B strains from each of the 2 lineages have been developed for improved prevention of influenza B infections. Here, we describe the results of a phase III, randomized, double-blind, active-controlled, multicenter trial examining the safety and immunogenicity of a split-virion inactivated quadrivalent influenza vaccine (IIV4) in 675 adults ≥ 65 y of age (NCT01218646). Participants were randomly assigned 1:1:1 to receive a single intramuscular injection with the investigational IIV4, or one of 2 split-virion trivalent inactivated influenza vaccines (IIV3s): a licensed IIV3 containing a B Victoria-lineage strain or an investigational IIV3 containing a B Yamagata-lineage strain. Post-vaccination (day 21) hemagglutinin inhibition titers to all strains induced by IIV4 were statistically non-inferior to those induced by the 2 IIV3s. In addition, for each B strain, rates of seroconversion in the IIV4 group were superior to those induced by the comparator IIV3 not containing that B strain. For all vaccines, the most common solicited reaction was injection-site pain, and most reactions were mild to moderate in intensity and transient. Overall safety profiles were similar between IIV4 and the IIV3s, and no vaccine-related serious adverse events were reported. These results confirm that in adults ≥ 65 y of age, IIV4 was well tolerated and immunogenic against the additional B lineage strain without compromising the immunogenicity of the other 3 vaccine strains.
Introduction
Since 2001, 2 antigenically distinct lineages of influenza B have co-circulated globally, leading to frequent mismatches between the predominant circulating B strain and the single B strain in trivalent influenza vaccines.Citation1,2 The result has been a missed opportunity to reduce morbidity and mortality related to seasonal influenza, a disease that contributes to an estimated 3000 to 49,000 deaths and 55,000 to 431,000 hospitalizations each year in the US.Citation1,2 Quadrivalent influenza vaccines containing B strains from both linages have been developed to provide improved protection against influenza and have been available in the US since the 2013–2014 influenza season.Citation3 Economic modeling suggests that if quadrivalent vaccines had been used instead of trivalent vaccines in the US during the 1999–2000 to 2008–2009 influenza seasons, an additional 2.7 million influenza cases, 21,440 influenza-related hospitalizations, and 1371 influenza-related deaths could have been prevented,Citation4 with substantial savings to society and third-party payers.Citation5
A split-virion quadrivalent influenza vaccine (IIV4; Fluzone® Quadrivalent, Sanofi Pasteur) was approved in the US in 2013 for individuals ≥ 6 months of age.Citation6 A phase II trial in healthy adults demonstrated the safety and immunogenicity of IIV4.Citation7 In that study, IIV4 was compared with 2 split-virion trivalent influenza vaccines (IIV3s), each lacking one of the 2 B strains included in IIV4. Hemagglutination inhibition (HAI) antibody titers induced by IIV4 were statistically non-inferior to those induced by the comparator IIV3 for the 2 A strains and the B strain present in each of the comparators. Also, IIV4 induced higher HAI antibody titers to each B strain than the control IIV3 lacking the same B strain. The study found no differences in the incidence or severity of solicited reactions or unsolicited adverse events (AEs) between the quadrivalent and trivalent formulations.
Because the phase II study included a limited number of older adults (≥ 65 y of age), a phase III study was conducted to better assess safety and immunogenicity of IIV4 in this age group, which accounts for approximately 63% of influenza-related hospitalizations and 90% of influenza-related deaths.Citation8,9 The study also included a small open-label cohort to confirm the safety and immunogenicity of the same year's formulation of IIV3 in adults 18–64 y of age.
Results
Randomized cohort
Participants
The study included 675 adults ≥ 65 y of age randomized (n = 225 per group) to receive IIV4, the licensed IIV3 containing a B strain of the Victoria lineage (IIV3–1), or an investigational IIV3 containing a B strain of the Yamagata lineage (IIV3–2) (). All but 3 randomized participants completed the study. Baseline characteristics were generally similar in the 3 vaccine groups ().
Figure 1. Participant disposition and study flow in the randomized cohort (adults ≥ 65 y of age). A total of 675 adults ≥ 65 y of age were randomly assigned 1:1:1 to receive the quadrivalent split-virion influenza vaccine (IIV4), the licensed trivalent split-virion influenza vaccine containing the B Victoria-lineage strain (IIV3–1), or an investigational split-virion IIV3 containing the B Yamagata-lineage strain (IIV3–2). AE, adverse event; n, number of participants in the group.
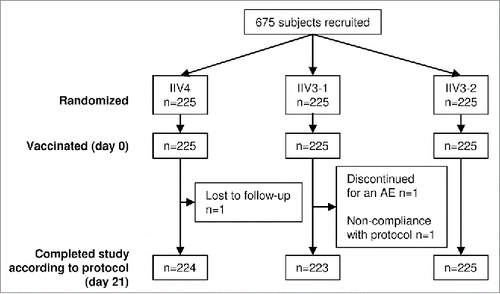
Table 1. Participant characteristics in the randomized cohort (≥ 65 years).
Immunogenicity
This study met its primary objective, which was to demonstrate non-inferiority of post-vaccination HAI geometric mean titers (GMTs) for IIV4 vs. the pooled IIV3s for all 4 strains (). Also, for the B Yamagata-lineage strain (but not the B Victoria-lineage strain), the HAI GMT in participants vaccinated with IIV4 was superior to that in participants vaccinated with the IIV3 lacking the homologous B strain. In addition, for the A/H3N2, B Yamagata-lineage, and B Victoria-lineage strains, post-vaccination seroconversion rates for IIV4 were non-inferior to those for the pooled IIV3s, and for each B lineage strain, seroconversion rates were superior to those for the IIV3 lacking the homologous B strain.
Table 2. Immunogenicity comparisons in the randomized cohort (≥ 65 years).
HAI titers at baseline were similar in all study groups (). Immunization with all 3 vaccines increased HAI GMTs by at least 5-fold against the 2 A strains and by 1.8 to 2.6-fold against the homologous B strains. Post-vaccination seroprotection rates were ≥ 91% against the A strains and 67% to 78% against the B strains, and seroconversion occurred in > 55% of participants for the A strains and in 19% to 33% for the B strains in each vaccine. Interestingly, 9.1% (95% confidence interval [CI], 5.7 to 13.8) of participants vaccinated with IIV3–1, which contained a Victoria-lineage B strain, seroconverted against the B Yamagata-lineage strain, while 8.6% (95% CI, 5.3 to 13.1) of participants vaccinated with IIV3–2 seroconverted against the B Victoria-lineage strain.
Table 3. Immunogenicity measures in the randomized cohort (≥ 65 years).
Solicited reactions and AEs
Pain was the most common injection-site reaction and myalgia the most common systemic reaction for all vaccines (). Most solicited reactions were grade 1 or 2. Grade 3 solicited reactions included injection-site pain, fever, headache, malaise, and myalgia, each reported by no more than 2 participants (< 2%) per group. All solicited reactions resolved within 8 d (data not shown). Proportions reporting solicited injection-site and systemic reactions were similar in participants vaccinated with IIV4 or IIV3.
Table 4. Proportions reporting solicited reactions in the randomized cohort (≥ 65 years).
No vaccine-related serious adverse events (SAEs), no AEs of special interest, and no immediate (< 20 min) unsolicited AEs were reported (). Proportions of participants reporting unsolicited AEs considered to be vaccine-related were similar for the 3 vaccines. The most common AEs considered to be vaccine-related were at the injection site (< 2% per group) and included hematoma, induration, pain, and pruritus. One participant vaccinated with IIV3–1 discontinued for events considered to be vaccine-related (diarrhea and injection-site pruritus).
Table 5. Proportions reporting unsolicited adverse events in the randomized cohort (≥ 65 years).
Open-label cohort
In addition to the 3 randomized cohorts, the study included an open-label cohort in which 64 healthy adults 18–64 y of age were vaccinated with the licensed IIV3 to document its immunogenicity and safety in this age group as part of the manufacturer's annual vaccine assessment. Results from this cohort are presented in the supplemental online material.
Discussion
This study, performed during the 2010–2011 influenza season in the US, showed that in adults ≥ 65 y of age, IIV4 induced non-inferior antibody titers compared with control IIV3s for all 4 vaccine strains. This finding is in line with the results of a phase II trial using similar IIV4 and IIV3s performed during the 2009–2010 influenza season in adults ≥ 18 y of age.Citation7 The current study further showed that, in adults ≥ 65 y of age, IIV4 induced superior HAI antibody titers for the B Yamagata-lineage strain when compared with the IIV3 lacking the homologous strain and that IIV4 was as well tolerated as the IIV3s.
Although HAI GMTs were superior for the B Yamagata-lineage strain, they were not for the B Victoria-lineage strain. This lack of superiority for the B Victoria-lineage strain might have been due to interference by existing antibody, cross-reactivity between the B strains, or other unknown factors. Low-level B-strain cross-reactivity has been documented in adults,Citation7,10 and was also observed in this study along with high baseline seroprotection rates against the B strains. The ability to detect superiority might have also been limited by the well-documented weaker immune responses in older adults as a result of immunosenescence.Citation11 Irrespective of the reason, the failure to meet the superiority criterion for the B Victoria-lineage strain is not expected to adversely affect clinical protection because IIV4 was non-inferior to the IIV3s for post-vaccination HAI GMTs. The study also showed that seroconversion rates were non-inferior for IIV4 vs. IIV3 for all vaccine strains except for A/H1N1. This failure to reach non-inferiority for the A/H1N1 strain is not expected to affect protection provided by IIV4 because the seroprotection rate for this strain was 91%.
In this study, IIV4 induced post-vaccination seroprotection rates in the older adult participants that were at least 91% for the A strains and at least 73% for the B strains. For this study, we used the widely accepted HAI titer of at least 1:40 to define seroprotection, but whether this definition and serum antibody titers in general are valid correlates of protection continues to be debated.Citation12–16 According to current US guidelines, however, the HI antibody response remains an acceptable surrogate marker that is likely to predict clinical benefit.Citation17
Although this study met its primary objective of showing non-inferior antibody titers in older adults for IIV4 vs. IIV3, immune responses in this population were substantially lower than those observed in the younger open-label cohort that received licensed IIV3–1. Reduced immunogenicity of influenza vaccines in older adults is well documentedCitation11 and is why a high-dose split-virion inactivated influenza vaccine (Fluzone High-Dose, Sanofi Pasteur) containing 60 µg hemagglutinin per strainCitation18 and a subunit vaccine containing a squalene-based oil-in-water emulsion as an adjuvant (Fluad, Sequirus)Citation19 have been developed specifically for this age group. These vaccines are currently trivalent, but quadrivalent formulations of both are under clinical development.
In addition to confirming non-inferior antibody titers for IIV4 vs. IIV3, this study demonstrated that the IIV4 had a safety profile similar to that of IIV3. No important safety issues or substantial differences in the occurrence or severity of solicited reactions or AEs were detected.
One limitation of this study is that the trial population consisted of medically stable, community-dwelling older adults. Accordingly, safety and immunogenicity findings from this trial may not be generalizable to other older adults, such as those who are frail or institutionalized. However, because the immune responses (for homologous strains) and safety profile between IIV4 and the comparator IIV3s were similar in the current study, it is reasonable to expect that the performance of IIV4 relative to IIV3 would also be similar within specific subpopulations of older adults. Another limitation is that the size of the study population was too small to detect adverse reactions that could occur with relatively low frequency post-vaccination. Even so, given the extensive safety experience of IIV3 and similar reactogenicity being observed for IIV4 and IIV3, it is unlikely that the safety profile for IIV4 would be materially different from IIV3 with respect to any rare adverse reactions that might occur after influenza vaccination.
To conclude, this study showed that the addition of a second B strain in IIV4 did not affect tolerability or compromise the immunogenicity of the other vaccine strains in older adults.
Patients and methods
Study design
This was a phase III trial conducted at 12 sites in the US between October and December, 2010 (NCT01218646). The study included a double-blind, randomized, controlled cohort of older adults (≥ 65 y of age) and an open-label cohort of younger adults (18–64 y of age). The primary objective for the randomized cohorts was to demonstrate that IIV4 induced non-inferior antibody responses compared with those induced by comparator IIV3s containing matching A and B strains in adults ≥ 65 y of age. The objective of the open-label cohort study was to document the immunogenicity and safety of the 2010–2011 formulation of the licensed IIV3 in healthy adults.
Ethics
The study was approved by all institutional review boards and performed in accordance with International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Participants
Healthy older adults (≥ 65 y of age) were recruited for the randomized, double-blind cohort, and healthy younger adults (18–64 y of age) were recruited for the open-label cohort. Participants were excluded if they had an allergy to egg proteins, latex, or any vaccine component; a history of serious adverse reactions to any influenza vaccine; received any vaccine in the 4 weeks preceding study vaccination or an influenza vaccine after August 1, 2010; a history of Guillain-Barré syndrome; a known or suspected immunodeficiency; received immunosuppressive therapy within the preceding 6 months or long-term systemic corticosteroid therapy for more than 2 consecutive weeks within the past 3 months; a developmental delay, neurologic disorder, or seizure disorder; or received blood or blood-derived products in the past 3 months.
Vaccines
IIV4 contained A/California/07/2009 (H1N1), A/Victoria/210/2009 (H3N2), B/Brisbane/60/2008 (Victoria lineage), and B/Florida/04/2006 (Yamagata lineage) strains. IIV3–1 was the licensed 2010–2011 formulation of Fluzone (Sanofi Pasteur) containing A/California/07/2009 (H1N1), A/Victoria/210/2009 (H3N2), and B/Brisbane/60/2008. IIV3–2 was an investigational trivalent vaccine containing A/California/07/2009 (H1N1), A/Victoria/210/2009 (H3N2), and B/Florida/04/2006. All were split-virion inactivated vaccines provided in prefilled 0.5-mL single-dose syringes or in single-dose vials containing 15 µg hemagglutinin per strain.
Study conduct
Participants in the randomized cohort (adults aged 65 y and older) were randomly assigned using a pre-programmed interactive voice response system 1:1:1 to be vaccinated with a single dose of IIV4, IIV3–1, or IIV3–2. Neither participants nor investigators knew which vaccine was administered. Participants in the open-label cohort (adults aged 18–64 years) were all vaccinated with IIV3–1. All vaccines were administered by intramuscular injection into the deltoid muscle using 25 gauge, 1-inch (25 mm)-long needle.
HAI assay
HAI titers were measured at baseline (day 0) and 21 (window, 21–28) days after vaccination and were recorded as the reciprocal of the dilution as described previously.Citation20 The lower limit of quantitation was set at the reciprocal of the lowest dilution (10) and the upper limit of quantitation as the highest dilution (10,240) used in the assay. Seroprotection was defined as an HAI titer ≥ 40. Seroconversion rate was defined as a pre-vaccination titer < 10 and post-vaccination titer ≥ 40 or a pre-vaccination titer ≥ 10 and a ≥ 4-fold increase in post-vaccination titer.
Safety
Unsolicited AEs and SAEs were collected by investigators according to International Committee for Harmonisation Guideline (E2A) for Clinical Safety Data Management. Unsolicited AEs were collected up to day 21 post-vaccination. Immediate events were those occurring within 20 min after vaccination. SAEs and AEs of special interest were collected for 6 months after vaccination. AEs of special interest included Guillain-Barré syndrome, Bell's palsy, encephalitis/myelitis, optic neuritis, Stevens-Johnson syndrome, and toxic epidermal necrolysis.
Solicited injection-site and systemic reactions were recorded by participants for 7 d after vaccination using a diary card. Solicited injection-site reactions included pain, erythema, and swelling. Solicited systemic reactions included fever, headache, malaise, and myalgia. Swelling and erythema were assigned grade 1 for a diameter ≥ 25 to ≤ 50 mm, grade 2 for a diameter ≥ 51 to ≤ 100 mm, and grade 3 for a diameter > 100 mm. Fever was assigned grade 1 for ≥ 38.0°C to ≤ 38.4°C, grade 2 for ≥ 38.5°C to ≤ 38.9°C, and grade 3 for ≥ 39.0°C. All other AEs and solicited reactions were assigned grade 1 for no interference with daily activity, grade 2 for some interference with daily activity, and grade 3 for significant interference preventing daily activity.
Sample size calculation
A sample size calculation was performed only for the randomized, double-blind cohort. For each group, 225 participants were planned to be enrolled. Assuming an attrition rate no greater than 5%, this provided 90.3% power to demonstrate non-inferiority in GMTs between IIV4 and the IIV3s.
Statistical analysis
All analyses were performed using SAS® version 9.1 or higher (SAS Institute). Missing and incomplete data were not replaced and no search for outliers was performed. Safety was assessed in all participants who received a study or control vaccine. Immunogenicity was assessed in all participants who received the study or a control vaccine, had a valid post-vaccination serology result, and completed the study according to protocol.
Non-inferiority and superiority of IIV4 vs. comparator IIV3s was assessed for the randomized cohort. Non-inferiority of the HAI GMTs and seroconversion rates was assessed for all 4 viral strains in IIV4 compared with the control IIV3s containing the homologous B strains. For comparison of A/H1N1 and A/H3N2 responses, data were pooled for the 2 IIV3s. For non-inferiority comparisons of B-strain responses, IIV4 was compared with the respective IIV3 containing the same B-lineage strain. For each strain, the HAI GMT for IIV4 was considered non-inferior to that for the pooled IIV3s if the lower limit of the 2-sided 95% CI of the GMT ratio (IIV4 divided by IIV3) was > 0.66. Similarly, for each strain, the seroconversion rate for IIV4 was considered non-inferior to that for the pooled IIV3s if the lower limit of the 2-sided 95% CI of the difference in rates (IIV4 minus IIV3) was > −10%. Superiority of HAI GMTs and seroconversion rates was assessed for each B-lineage strain in IIV4 compared with each respective IIV3 lacking the matched B-lineage strain. For each B strain, the HAI GMT for IIV4 was considered superior to the that for the IIV3 lacking the homologous B strain if the lower limit of the 2-sided 95% CI of the GMT ratio (IIV4 divided by IIV3) was > 1.5, and the seroconversion rate for IIV4 was considered superior that for the IIV3 lacking the homologous B strain if the lower limit of the 2-sided 95% CI of the difference in rates (IIV4 minus IIV3) was > 10%.
Abbreviations
| AE | = | adverse event |
| CI | = | confidence interval |
| GMT | = | geometric mean titer |
| HAI | = | hemagglutination inhibition |
| IIV3 | = | trivalent split-virion inactivated influenza vaccine |
| IIV3–1 | = | licensed IIV3 containing a B strain of the Victoria lineage |
| IIV3–2 | = | investigational IIV3 containing a B strain of the Yamagata lineage |
| IIV4 | = | quadrivalent split-virion inactivated influenza vaccine |
Disclosure of potential conflicts of interest
D.P.G., C.A.R., and M.D.D. were employees of Sanofi Pasteur during the period of this study. H.K.T has received research support from Sanofi Pasteur, Gilead, and MedImmune and serves as an advisor for Seqirus and VaxInnate.
2017HV0122R1-s01.docx
Download MS Word (22.3 KB)Acknowledgment
Medical writing assistance in the preparation of this manuscript was provided by Dr. Phillip Leventhal of 4Clinics (Paris, France).
Funding
The study was funded by Sanofi Pasteur. This study funder, Sanofi Pasteur, contributed to the study design, data collection, analysis and interpretation, review of the manuscript, and decision to publish.
References
- Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother 2012; 8:81-8; PMID: 22252006; https://doi.org/10.4161/hv.8.1.17623
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010; 28 Suppl 4:D45-53; PMID: 20713260; https://doi.org/10.1016/j.vaccine.2010.08.028
- US Centers for Disease Conrol and Prevention. Prevention and control of influenza with vaccines: Interim recommendations of the Advisory Committee on Immunization Practices (ACIP), 2013. MMWR Morb Mortal Wkly Rep 2013; 62:356 PMID: 23657110
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993-8; PMID: 22226861; https://doi.org/10.1016/j.vaccine.2011.12.098
- Lee BY, Bartsch SM, Willig AM. The economic value of a quadrivalent versus trivalent influenza vaccine. Vaccine 2012; 30:7443-6; PMID: 23084849; https://doi.org/10.1016/j.vaccine.2012.10.025
- US Food and Drug Administration. Fluzone Quadrivalent. [accessed http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm356091.htm
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine 2013; 31:770-6; PMID: 23228813; https://doi.org/10.1016/j.vaccine.2012.11.074
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333-40; PMID: 15367555; https://doi.org/10.1001/jama.292.11.1333
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179-86; PMID: 12517228; https://doi.org/10.1001/jama.289.2.179
- Cadorna-Carlos JB, Nolan T, Borja-Tabora CF, Santos J, Montalban MC, de Looze FJ, Eizenberg P, Hall S, Dupuy M, Hutagalung Y, et al. Safety, immunogenicity, and lot-to-lot consistency of a quadrivalent inactivated influenza vaccine in children, adolescents, and adults: A randomized, controlled, phase III trial. Vaccine 2015; 33:2485-92; PMID: 25843270; https://doi.org/10.1016/j.vaccine.2015.03.065
- Haq K, McElhaney JE. Immunosenescence: Influenza vaccination and the elderly. Curr Opin Immunol 2014; 29:38-42; PMID: 24769424; https://doi.org/10.1016/j.coi.2014.03.008
- Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011; 204:1879-85; PMID: 21998477; https://doi.org/10.1093/infdis/jir661
- Petrie JG, Ohmit SE, Johnson E, Cross RT, Monto AS. Efficacy studies of influenza vaccines: Effect of end points used and characteristics of vaccine failures. J Infect Dis 2011; 203:1309-15; PMID: 21378375; https://doi.org/10.1093/infdis/jir015
- Trombetta CM, Montomoli E. Influenza immunology evaluation and correlates of protection: A focus on vaccines. Expert Rev Vaccines 2016; 15:967-76; PMID: 26954563; https://doi.org/10.1586/14760584.2016.1164046
- Reber A, Katz J. Immunological assessment of influenza vaccines and immune correlates of protection. Expert Rev Vaccines 2013; 12:519-36; PMID: 23659300; https://doi.org/10.1586/erv.13.35
- Cox RJ. Correlates of protection to influenza virus, where do we go from here?. Hum Vaccin Immunother 2013; 9:405-8; PMID: 23291930; https://doi.org/10.4161/hv.22908
- US Food and Drug Administration. Guidance for industry. Clinical data needed to support the licensure of seasonal inactivated influenza vaccines. Siliver Spring, MD: US FDA; 2015 Sep 15 [accessed 2017 Mar 2]. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074794.htm
- Robertson CA, DiazGranados CA, Decker MD, Chit A, Mercer M, Greenberg DP. Fluzone(R) High-Dose Influenza Vaccine. Expert Rev Vaccines 2016; 15:1495-505; PMID: 27813430; https://doi.org/10.1080/14760584.2016.1254044
- Frey SE, Reyes MR, Reynales H, Bermal NN, Nicolay U, Narasimhan V, Forleo-Neto E, Arora AK. Comparison of the safety and immunogenicity of an MF59(R)-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014; 32:5027-34; PMID: 25045825; https://doi.org/10.1016/j.vaccine.2014.07.013
- Pepin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine 2013; 31:5572-8; PMID: 24016810; https://doi.org/10.1016/j.vaccine.2013.08.069
