ABSTRACT
Infants younger than 6 months of age are at particular risk for serious illness from influenza infection. Currently available influenza vaccines are, however, not licensed for use in infants <6 months old. Influenza vaccination during pregnancy elicits robust antibody responses in the women that will protect the infants against influenza infection during the first few months of life. We aimed to determine the impact of influenza vaccination during pregnancy to prevent laboratory-confirmed influenza infection and influenza-associated hospitalisations in infants <6 months old.
An electronic search identified all studies assessing the proposed outcomes in infants after administration of influenza vaccine during pregnancy. Two meta-analyses were performed accordingly to studies restricting the evaluation to influenza-associated hospitalisations or not.
Four randomized control trials and 3 observational studies reported on the prevention of laboratory-confirmed influenza infection in infants <6 months old. Maternal influenza vaccination was associated with a 48% [95% confidence interval (CI): 33 to 59] reduced risk of infants having laboratory-confirmed influenza infection. Four observational studies reported on the prevention of hospitalizations associated with laboratory-confirmed influenza infection and the pool estimate was 72% (95%CI: 39% to 87%).
Receipt of influenza vaccine during pregnancy was associated with decreased risk of laboratory-confirmed influenza infection in the infants.
Introduction
Despite possible season to season fluctuation in influenza virus circulation and disease severity, infants <6 months of age have consistently been recognized at increased risk of developing complications from influenza infection. The highest incidence of influenza-associated hospitalizations is during the first year of life, with infants <6 months old at highest risk, because on being naïve to past influenza virus infection and immunologically immature.Citation1-4 In healthy infants, rates of hospitalizations attributable to influenza are similar to those of high-risk adults, and are even greater among infants with underlying medical conditions.Citation1 In the USA during some winters up to 10% of all infants seek medical care for influenza-associated illness, including hospitalization.Citation3,5 Data are more sparse from low-middle income countries, but a recent systematic analysis on the burden of influenza in pediatric respiratory hospitalizations worldwide estimated that influenza causes approximately 374,000 hospitalizations per year in children younger than 1 y of age, 228,000 of which occur in infants <6 months old.Citation6 Furthermore, influenza-associated hospitalization rates were more than 3 times higher in low-middle income than high-income countries.Citation6
Vaccination has been the main strategy to prevent and control seasonal and pandemic influenza disease for the past 60 y.Citation7 Nonetheless efforts to protect infants during their first 6 months of life from influenza infection through direct vaccination have been unsuccessful with current available vaccines; although safety and immunogenicity in young infants have been shown.Citation8 Conferring passive protection to the infants through maternal vaccination during pregnancy is an attractive alternative to direct immunization.Citation9 Healthy pregnant women are able to generate robust immune responses to influenza vaccines, and maternal influenza immunoglobulins G are efficiently transferred across the placenta,Citation10,11 which provide indirect protection against influenza infection at least for the first 2–3 months of life.Citation12,13 No safety concerns following influenza vaccination during pregnancy for the pregnant women, their infants and the fetus have been raised in the multiple studies that addressed this issue.Citation12,14-17
Influenza vaccination during pregnancy is increasingly being recognized as an important strategy for the prevention of influenza infection in the mothers themselves and the infants, with numerous public health organizations, including the World Health Organization, recommending that pregnant women be prioritized for seasonal influenza vaccination.Citation18 Several countries, mostly low and middle income with multiple competing public health priorities, have however not yet adopted maternal influenza vaccination into their national immunization programs, due to uncertainty on the burden of disease and effectiveness of maternal influenza immunization for protecting young infants.Citation19 We conducted a systematic review and meta-analysis to evaluate the effect of influenza vaccination during pregnancy to prevent laboratory-confirmed influenza infection and influenza-associated hospitalisations in infants during the first 6 months of life.
Results
Selection of studies and characteristics of included studies
The literature search identified a total of 764 potentially pertinent articles, and the full texts of 31 articles were reviewed. Data from one randomized control trial (RCT) was obtained directly from the authors ahead of publication.Citation17 Finally, 4 RCTsCitation12,15,17,20 and 5 observational studies were found to meet the inclusion criteria for the meta-analyses.Citation21-25 presents the study selection process. The characteristics of the included studies are described in .
Figure 1. Flow diagram of included and excluded studies. Of 764 citations, 31 full articles were reviewed to determine eligibility for inclusion, and 9 studies were included in the meta-analyses.
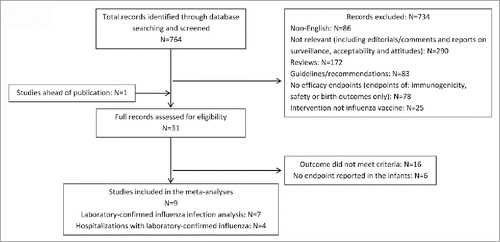
Table 1. Characteristics and reported measures of the studies included in the meta-analyses.
The RCTs included in total 10,099 participants and investigated the efficacy of influenza vaccination during pregnancy in preventing laboratory-confirmed influenza illness defined by either positivity on rapid-test in one trialCitation20 or polymerase chain reaction (PCR) confirmation in 3 trials.Citation12,15,17 No RCTs reported on prevention of hospitalization. In the control group, a placebo was used in 2 trialsCitation15,17 and another vaccine namely, the 23-valent pneumococcal polysaccharide vaccineCitation20 or the quadrivalent meningococcal vaccine,Citation12 in the other 2 trials. Two studies were conducted in AfricaCitation12,15 and 2 studies in Southern Asia.Citation10,20
Among the included observational studies 3 investigated whether influenza vaccination during pregnancy was associated with a reduced risk of infants having a medically-attended visit with laboratory-confirmed influenza infection.Citation22,23,25 Of these one was a retrospective population-based study in England that used the screening method and PCR detection to evaluate vaccine effectivenessCitation22 and 2, both from the USA, were cohort studies one each retrospective that used PCR, viral culture or direct fluorescent antibody testingCitation25 and prospective, which included viral culture, fourfold rise in antibody titers or rapid test as confirmation of influenza infection.Citation23 The study from England and the retrospective study from the USA also assessed the effect of influenza vaccination on the number of hospitalizations for laboratory-confirmed influenza among the infants together with 2 other studies from the USA;Citation21,22,24,25 one being a matched case-control with direct fluorescent antibody testingCitation21 and the second used active population-based surveillance and included viral culture or PCR as confirmation of influenza infection.Citation24 Overall the observational studies covered 14 different influenza seasons.
No studies stratified the outcome data according to the time between vaccination during pregnancy and timing of the local influenza virus circulation.
Quality of studies and quality of evidence
We rated all the RCTs as high quality with low risk of bias, . The 4 trials used randomization to allocate participants to the different study groups, one of the trials was double-blindCitation15 and the other 3 were observer-blind, with study personnel administering the vaccines not being masked to treatment allocation but all other study staff, investigators, and participants were masked.Citation10,12,20 In Bangladesh and Nepal intention to treat analysis excluded infants born within 2 weeks of maternal vaccination,Citation10,20 the other 2 RTCs included these infants in theirs intention to treat population.Citation12,15 In the 4 RCTs active weekly surveillance was performed until the infants reached 6 months of age to assess clinical respiratory symptoms and in all but one trialCitation20 influenza infection was confirmed by PCR.
Table 2. Quality of the studies included in the meta-analyses.
In the observational studies the testing of infant samples for influenza infection was mostly at the discretion of the clinician, which might have introduced bias, . Systematically testing of all eligible infants was just used in 2 studiesCitation23,24 and in the second part of the study by Benowitz et al.Citation21 The critical information regarding the maternal vaccination status was ascertained verbally in 2 of the studiesCitation24,25 and in the study by Eick et al. from the participants missing that information in the medical records.Citation23 All studies to different extend evaluated potential confounders of their analyses.
The quality of the evidence for medically-attended laboratory-confirmed influenza was assessed as high on the basis of data from RCTs. For the outcome of hospitalization with laboratory-confirmed influenza, the quality was judged as low based on data only being available from observational studies.
Medically-attended influenza illness
Randomized control trials
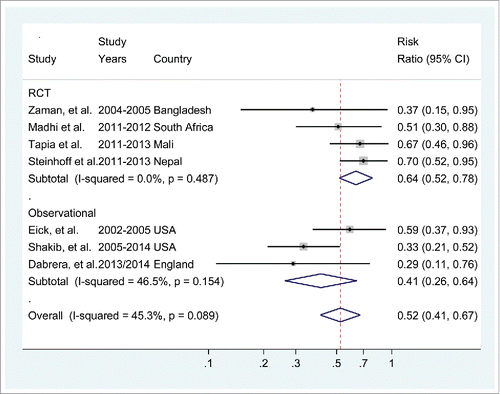
All 4 RCTs reported significant vaccine efficacy of influenza vaccination during pregnancy against laboratory-confirmed influenza in the infants during the first 6 months of life. The first randomized controlled trial that evaluated the effect of influenza vaccination during pregnancy on infant influenza illness was conducted in Dhaka, Bangladesh, from 2004 through 2005.Citation20 In this tropical country with year round influenza virus circulation, 340 women in the third trimester of pregnancy were randomly assigned to receive trivalent inactivated influenza vaccine (IIV3) (n = 172) or 23-valent pneumococcal polysaccharide vaccine (n = 168). 159 infants in the IIV3 group and 157 in the control group were included in the efficacy analysis. Even though only 120 of the 146 infants who had a clinic visit were tested for influenza (86% of 92 infants in the control group and 76% of 54 in the IIV3 group), this proportion of missed cases did not differ significantly and was unlikely to have influenced the vaccine efficacy estimate. Fewer rapid test-confirmed influenza cases were identified among infants of mothers who received IIV3 compared with those in the control group (6 vs. 16 infants, respectively) for a vaccine efficacy estimate of 63% (95%CI: 5% to 85%). In this study the 2004 IIV3 Southern Hemisphere vaccine formulation was used.
In South Africa 2 separate cohorts of HIV-uninfected pregnant women with an estimated gestation of 20 to 36 weeks were enrolled in 2011 (n = 1056) and 2012 (n = 1060) just prior the onset of the influenza seasons.Citation15 The trial was conducted in Soweto, an urban Black-African township outside of Johannesburg, and used the influenza vaccine recommended for the Southern Hemisphere, which was the same for both 2011 and 2012 influenza seasons. During the follow-up period, 19 episodes of PCR-confirmed influenza were detected in the 1026 infants in the IIV3 group compared with 37 cases in the 1023 infants born to mothers who received placebo, for a vaccine efficacy of 48.8% (95%CI: 11.6% to 70.4%).
In Mali, West-Africa, 4193 third trimester pregnant women were randomly assigned to receive IIV3 (n = 2108) or quadrivalent meningococcal vaccine (n = 2085).Citation12 2064 infants in the IIV3 group and 2041 infants in the quadrivalent meningococcal vaccine group were included in the analysis. During the entire study period 129 cases of PCR-confirmed influenza were recorded in infants; 52 in the influenza vaccine group and 77 in the control group, for an overall infant vaccine efficacy of 33.1% (95%CI: 3.7% to 53.9%). In this study continuous recruitment occurred from September 2011 to April 2013 and both Northern and Southern Hemisphere vaccine formulations were used.
The fourth RCT was undertaken in the rural subtropical plains of southern Nepal where influenza viruses circulates perennially.Citation17 In this trial 2 consecutive annual cohorts of pregnant women were enrolled from April 2011 through September 2013 and year-round maternal influenza vaccination was done using 2 IIV3 formulations for both the Northern and Southern Hemisphere. In total 3693 pregnant women (17–34 weeks gestation) were recruited and 3629 infants were included in the efficacy analysis. During the entire study period 74 cases of PCR-confirmed influenza were recorded from infants of mothers who received IIV3 compared with 105 in infants in the placebo group, for overall infant vaccine efficacy of 30% (95%CI: 5% to 48%).
Observational studies
All 3 observational studies that evaluated the effect of influenza vaccination during pregnancy in preventing laboratory-confirmed influenza in the infants that were included in the analysis reported a significant protective effect. Eick et al. conducted a prospective cohort study in the Navajo and Apache Indian reservations, USA, by following up 1160 mother-infant pairs from birth to 6 months over 3 influenza seasons from 2002 to 2005.Citation23 Maternal influenza vaccination status was based on medical record review or, if missing, by maternal report at enrolment. The outcome of interest was medically-attended influenza-like illness (ILI) with daily active surveillance for ILI episodes including review of the clinic, emergency department, and inpatient pediatric ward logs and parents were asked to contact research staff whenever a child had a medical visit for a respiratory, diarrheal, or febrile illness. Potential confounders were investigated but since no associations were found between these and the occurrence of the outcome only univariate regression models were presented. Forty-nine percent of infants were born to mothers who received influenza vaccine during pregnancy and 83 cases of laboratory-confirmed influenza infection in the infants were analyzed, of which 71 (86%) were confirmed by serology, 10 (12%) by viral culture, and 2 (2%) by rapid influenza testing. Using these 3 measures of influenza infection, the study reported a 41% reduction (risk ratio [RR] 0.59; 95%CI: 0.37 to 0.93) in the risk of laboratory-confirmed influenza for infants born to vaccinated women.
Using the screening method, Dabrera et al. estimated the effectiveness of IIV3 vaccination during pregnancy in preventing influenza virus infection in infants in England in the 2013/14 season.Citation22 Cases with PCR-confirmed influenza infection were retrospectively identified from a sentinel laboratory surveillance system which collected influenza testing data predominantly from secondary care settings. General practitioners of the identified cases were contacted and asked if the infant's mother had received influenza vaccination during pregnancy; the seasonal influenza vaccination coverage for the population of pregnant women in England was extracted from a national electronic reporting system used by general practices. Thirty-seven PCR-confirmed influenza cases were identified in infants <6 months old and included in the analysis; of these 5 (13.5%) mothers were reported to have received influenza vaccination during pregnancy compared with 39.8% of women nationally. The vaccine effectiveness adjusted for month of birth and region was 71% (95%CI: 24% to 89%).
In a large cohort of 249,387 infants born from 2005 to 2014 at different health care facilities from a healthcare network in the USA, Shakib et al. evaluated the effect of influenza vaccination during pregnancy on influenza infection confirmed by PCR, culture or direct fluorescent antibody testing (numbers detected by the different tests not mentioned) in infants <6 months old who visited the hospitals as outpatients and/or were hospitalized.Citation25 Vaccination status was ascertained verbally from the mother at delivery and overall 10% of the mothers reported influenza vaccination during pregnancy. Maternal comorbidities were compared in women who did and did not report influenza vaccination and multivariable adjusted analysis was performed. A total of 658 infants had laboratory-confirmed influenza, 20 born to women reporting vaccination and 638 born to unvaccinated women (adjusted RR 0.33; 95%CI: 0.21 to 0.52).
Meta-analysis
The pooled effect of all the studies included in the meta-analysis indicated a 48% (95%CI: 33% to 59%) reduction in infant influenza confirmed infection among those whose mothers were vaccinated during pregnancy, . No significant heterogeneity was detected among the chosen studies (I2 = 45.3%, p = 0.09). Stratifying by study type, the meta-analysis of the 4 RCTs revealed no heterogeneity (I2 = 0%, p = 0.49) and the pooled vaccine efficacy was 36% (95%CI: 22% to 48%). Restricting the meta-analysis to the 3 observational studies the pooled vaccine effectiveness was slightly higher 59% (95%CI: 36% to 74%) and the heterogeneity between studies was 46.5% (p = 0.15).
Influenza-associated hospitalizations
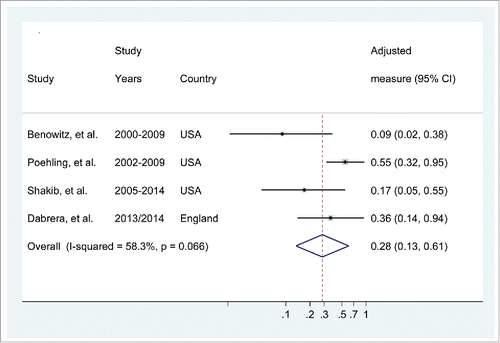
Four observational studies reported on the impact of maternal vaccination during pregnancy on laboratory-confirmed influenza hospitalizations in infants.Citation21,22,24,25 In the study in England by Dabreara et al., 32 PCR-confirmed influenza cases were hospitalised, with an adjusted vaccine effectiveness of 64% (95%CI: 6% to 86%).Citation22 The study by Shakib et al. evaluated 151 (23%) infants hospitalized for laboratory-confirmed influenza (results combined from 3 different tests), including 3 born to IIV vaccinated women and 148 born to unvaccinated women (adjusted RR 0.17; 95% CI, 0.05–0.55).
Benowitz et al. performed a matched case-control study at the New Haven Children's Hospital, USA, from 2000 to 2009.Citation21 Hospitalized infants with physician-ordered direct fluorescent antibody testing for seasonal influenza between 2000 and early 2007 were identified retrospectively, and enrolled telephonically. Furthermore, during the 2007–2008 and 2008–2009 influenza seasons, cases were identified prospectively. Women were considered vaccinated if there was written documentation in the medical records from primary medical providers, obstetricians, pharmacies, and anywhere else where they could have received influenza vaccination. Information about demographic characteristics, possible confounders, and comorbidities was collected by interviews and by reviewing medical records. No significant differences were noted in demographic characteristics between mothers who received influenza vaccine and those who did not. Of the 91 cases and the 156 controls matched by date of birth and date of hospitalization included in the analysis, 2 and 31 of their mothers, respectively, received influenza vaccine during pregnancy, for an adjusted vaccine effectiveness of 91.5% (95%CI: 61.7% to 98.1%).
Poehling et al. conducted a retrospective population-based surveillance study in infants hospitalized with fever or respiratory symptoms over 7 consecutive influenza seasons (2002–2003 through 2008–2009), and across 3 USA regions.Citation24 Maternal vaccination history was collected by interview at enrolment of the hospitalized infants and overall 19% of the mothers reported that they were vaccinated. Of the 1510 hospitalized infants, 151 had laboratory-confirmed influenza based on PCR or viral culture and 12% of their mothers reported being vaccinated compared with 20% of the mothers of influenza-negative infants. The authors performed adjusted analyses using 3 multivariate logistic regression models and found that hospitalized infants whose mothers received influenza vaccine during pregnancy were 45–48% less likely to have laboratory-confirmed influenza compared with infants of unvaccinated mothers. To avoid bias associated with clinician ordered testing, all infants in this study were tested by viral culture or PCR for influenza.
Meta-analysis
For the purpose of this meta-analysis, from the study by Poehling et al. we used the adjusted odds ratio (OR) reported using the multivariate logistic regression model-1 that included the covariates: age, sex, race/ethnicity, site, study year, and tertile of the influenza season. The pooled vaccine effectiveness of influenza vaccination during pregnancy in preventing infant influenza-associated hospitalizations was 72% (95%CI: 39% to 87%). The level of heterogeneity across the selected observational studies was moderate (I2 = 58.3%, p = 0.07), .
Discussion
In this report we summarized the current data on the effect of influenza vaccination during pregnancy to prevent laboratory-confirmed influenza infection and influenza-associated hospitalisations in infants during the first 6 months of life. Maternal influenza vaccination was associated with a 48% and 72% reduced risk of infants having laboratory-confirmed influenza infection and associated hospitalization, respectively. When analysis was restricted to RCTs the polled vaccine efficacy for laboratory-confirmed influenza infection was slightly lower with a point estimate of 36% and the heterogeneity between the studies was zero. The individual reported vaccine effects are shaped by the world regions where the studies were done with different viral circulation patterns and attack rates; by the different laboratory tests used for influenza detection, and the annual variation in influenza virus vs. the composition of the seasonal vaccines.
The 4 RCTs that investigated whether influenza vaccination during pregnancy was associated with a reduced risk of infants having laboratory-confirmed influenza were conducted in resource-limited settings, whereas the observational studies were from the USA and Europe. The lower pooled estimate obtained in the stratified analysis from the RCTs might truly reflect lower impact of vaccination on this end point in low-middle income countries or might be confounded by differences in study design. Moreover 3 of the RCTs came from parts of the world with perennial influenza virus circulation, in contrast to the trial from South Africa and the observational studies that were conducted in countries where influenza seasons are better defined during the Autumn-Winter months. It is, however, reassuring to observe that studies from different climates, with different temporal patterns of influenza circulation achieved comparable results.
There are several challenges regarding observational studies, including relying on self-reported information on vaccination during pregnancy, insufficient information on potential confounding variables that can be used to adjust the analyses and poor validation of the clinical diagnostics used to define the outcomes. In our analysis we included observational studies that allowed enrolment and testing based on clinical judgment, which may bias the vaccine effectiveness estimates if the decision to test was associated with the maternal vaccination status. Moreover studies using clinician-ordered testing to identify eligible infants normally had high proportion of infants with high-risk conditions.Citation21 The observational design of the studies included and the fact that 3 studies were retrospective,Citation21,22,25 lends to the risk of selection bias and residual confounding. Two observational studies exclusively documented self-reported influenza vaccination during pregnancy that is limited by recall bias.Citation24,25 In most studies demographic characteristics differed between pregnant women who did and did not report receiving the influenza vaccine, and despite studies reporting adjusted measures potential unmeasured factors may have influenced the estimates even after adjustments. It is possible that some of the benefits to the infant attributed to maternal vaccination in these studies may have resulted from different care-seeking behaviors in mothers who did and did not report vaccination.
Although we limited our analyses to studies that used laboratory confirmation as primary endpoints, different tests were used across the studies. PCR is the preferred laboratory test for influenza because of its high sensitivity and low likelihood of false positives. The use of less sensitive methods, such as viral culture or direct fluorescent antibody testing, might be bias to detect higher viral load and consequently a certain spectrum of disease. Less sensitivity and specific tests can nonetheless provide useful information especially when assessed in randomized and adequately control clinical trials. Three observational studies reported results combined from different assays, each with different sensitivities, to document influenza infection.Citation23-25 We have recently shown that the use of PCR and serology, alone or combined, as influenza infection endpoints in a RCT, yielded similar vaccine efficacy point estimates in pregnant women, albeit measuring different influenza infection illness spectrum.Citation26 The use of serology to document influenza illness in young infants might, however, be more problematic as their immune system is immature. Even though laboratory confirmation is a specific end point for influenza infection the use of this end point alone can under-estimate the true burden of influenza infection. Since primary influenza infections, including possibly sub-clinical or mild infections, may increase susceptibility to new bacterial acquisition, with disease from these bacteria only manifesting a few weeks later and beyond when influenza virus shedding has ceased.Citation27,28
The paucity of high-quality data on the effect of vaccination during pregnancy against hospitalizations associated with laboratory-confirmed influenza in infants is still a limitation for truly estimating the effect size of this intervention on more severe outcomes. Also most of the studies combined results across multiple influenza seasons, without reporting how well the vaccine matched the circulating influenza strains; stratification by season would be important to stablish the maximum and lowest effect that we can expect.
Although the effectiveness of influenza vaccination during pregnancy as a strategy for infant protection in non-RCT settings, in real-world situations, depends on the timing during pregnancy of vaccine administration, the time from vaccination to birth and the timing of influenza circulation,Citation29 our analysis strengthens the evidence that maternal vaccination provides passive protection against influenza to infants during the vulnerable period prior they are old enough to receive active immunization. Interestingly, both RCTs from Africa reported a much higher vaccine efficacy during the first period of follow-up (86% in South Africa for the first 8 weeks of life and 68% in Mali for the first 4 months) that gradually dropped with increasing age and that correlated with a decrease in maternally derived antibodies in the infants.Citation12,13 The observation that duration of protection is limited to the first 2–3 months of life suggests the need to find more immunogenic vaccines for pregnant women to increase the concentration of the antibodies transferred transplacentally which will last for a longer period in the infants.
Methods
Literature search and definitions
We performed a systematic literature search of Medline, the PubMed database, from its inception date to 31 January 2017 using the Medical Subject Heading (Mesh) terms vaccine, influenza, pregnancy, infant or child or baby. Authors of one study were contacted to request data that had been accepted for publication but not yet published at the time of our literature search (courtesy Mark Steinhoff). We aimed to identify all RCTs and observational studies investigating the efficacy or effectiveness of influenza vaccination during pregnancy in preventing laboratory-confirmed influenza in infants during the first 6 months of life. Studies were included in the meta-analyses if they compared infants born to women who received influenza vaccine at any stage during pregnancy to infants born to a control group (placebo, other vaccine or no intervention); reported on the outcome of laboratory-confirmed influenza illness (confirmation by polymerase chain reaction [PCR], culture or serology alone or in combination); effect estimates were reported against all circulating influenza viruses, and were published in English language. Studies were excluded if: the aim was only to investigate the immunogenicity, safety or cost-effectiveness of maternal vaccination; they reported on non-specific outcomes such as mortality, hospital admissions or influenza-like illness without laboratory confirmation; the subjects were patients with an immunocompromising condition, such as HIV infection. Two outcomes were assessed separately: laboratory-confirmed influenza events detected in infants during medically-attended respiratory illness visits and laboratory-confirmed influenza detection in hospitalized infants. Both authors assessed the articles selected for retrieval for methodological validity.
Quality assessment
To judge the quality of the studies, information about randomization, allocation concealment, intention to treat analysis, and blinding was extracted from RCTs; from the observational studies definitions on the outcomes used, how maternal vaccination status was assessed, potential confounding factors and any resulting adjustments were also recorded. The quality of the body of evidence for each outcome was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework according to which randomized trials and observational studies were initially assumed to have high- and low-quality evidence, respectively.Citation30,31 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist items in accordance with the PRISMA statement was used to report the study's findings in the meta-analysis.Citation32
Statistical analyses
Wherever studies reported vaccine efficacy or effectiveness, Risk Ratios (RR) or Odds Ratios (OR) and the respective confidence intervals were calculated. From the observational studies, when possible, adjusted effect sizes were used. Efficacy analysis was based on the intention to treat population. Two separate meta-analyses were performed to assess the effect of influenza vaccination during pregnancy on medically-attended respiratory illness visits with laboratory-confirmed influenza and on hospital admissions with laboratory-confirmed influenza in the infants during the first 6 months of life. The first meta-analysis was further stratified by study design. The associations between outcomes and maternal vaccination status were represented in Forest-plots where each study is displayed as a square and horizontal lines representing the relative effect measure and its 95% confidence intervals (95% CI), the area of the square represents the weight that the study contributes to the meta-analysis; the combined effect measures and its 95% CI were represented by a diamond. Combined effect measures and corresponding 95% CI were calculated using the DerSimonian and Laird method to allow for between study heterogeneity. The percentage of total variation across studies due to heterogeneity, was assessed by means of the I2-statistic and the corresponding p-values were calculated with log-relative effect measures using a random-effects model.Citation33 P-values < 0.05 were considered to be statistically significant in the meta-analysis. All meta-analysis procedures were performed using STATA, version 13.1 (College Station, TX, USA).
Disclosure of potential conflicts of interest
Authors declare no conflict of interest.
Acknowledgments
The authors would like to thank Stefano Tempia from the National Institute for Communicable Diseases, South Africa, Mark C Steinhoff from Cincinnati Children's Hospital Medical Center, USA and Saad Omer from Emory University, USA.
Funding
The authors had partial support from the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation in Vaccine Preventable Diseases; and the Medical Research Council: Respiratory and Meningeal Pathogens Research Unit. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of their institutions or organizations or of the sponsors.
References
- Neuzil KM, Mellen BG, Wright PF, Mitchel EF Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342(4):225-31; PMID:10648763; https://doi.org/10.1056/NEJM200001273420401
- Chiu SS, Lau YL, Chan KH, Wong WH, Peiris JS. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med 2002; 347(26):2097-103; PMID:12501221; https://doi.org/10.1056/NEJMoa020546
- Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, Bridges CB, Grijalva CG, Zhu Y, Bernstein DI, et al. The underrecognized burden of influenza in young children. N Engl J Med 2006; 355(1):31-40; PMID:16822994; https://doi.org/10.1056/NEJMoa054869
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004; 4(7):553-64; PMID:15229474; https://doi.org/10.1038/nri1394
- O'Brien MA, Uyeki TM, Shay DK, Thompson WW, Kleinman K, McAdam A, Yu XJ, Platt R, Lieu TA. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics 2004; 113(3 Pt 1):585-93; PMID:14993554; https://doi.org/10.1542/peds.113.3.585
- Lafond KE, Nair H, Rasooly MH, Valente F, Booy R, Rahman M, Kitsutani P, Yu H, Guzman G, Coulibaly D, et al. Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A systematic analysis. PLoS Med 2016; 13(3):e1001977; PMID:27011229; https://doi.org/10.1371/journal.pmed.1001977
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59(RR-8):1-62; PMID:20689501
- Englund JA, Walter E, Black S, Blatter M, Nyberg J, Ruben FL, Decker MD, GRC28 Study Team. Safety and immunogenicity of trivalent inactivated influenza vaccine in infants: A randomized double-blind placebo-controlled study. Pediatr Infect Dis J 2010; 29(2):105-10; PMID:19934787; https://doi.org/10.1097/INF.0b013e3181b84c34
- Jones C, Heath P. Antenatal immunization. Hum Vaccin Immunother 2014; 10(7):2118-22; PMID:25424828; https://doi.org/10.4161/hv.29610
- Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Altaye M, Breiman RF, M B B S KZ. Influenza immunization in pregnancy–antibody responses in mothers and infants. N Engl J Med 2010; 362(17):1644-6; PMID:20427817; https://doi.org/10.1056/NEJMc0912599
- Nunes MC, Cutland CL, Dighero B, Bate J, Jones S, Hugo A, van Niekerk N, Kuwanda L, Izu A, Weinberg A, et al. Kinetics of hemagglutination-inhibiting antibodies following maternal influenza vaccination among mothers with and those without HIV infection and their infants. J Infect Dis 2015; 212(12):1976-87; PMID:26080370; https://doi.org/10.1093/infdis/jiv339
- Tapia MD, Sow SO, Tamboura B, Tégueté I, Pasetti MF, Kodio M, Onwuchekwa U, Tennant SM, Blackwelder WC, Coulibaly F, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: A prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis 2016; 16(9):1026-35; PMID:27261067; https://doi.org/10.1016/S1473-3099(16)30054-8
- Nunes MC, Cutland CL, Jones S, Hugo A, Madimabe R, Simões EA, Weinberg A, Madhi SA, Maternal Flu Trial Team. Duration of infant protection against influenza illness conferred by maternal immunization: Secondary analysis of a randomized clinical trial. JAMA Pediatr 2016; 170(9):840-7; PMID:27380464; https://doi.org/10.1001/jamapediatrics.2016.0921
- Bratton KN, Wardle MT, Orenstein WA, Omer SB. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: A systematic review and meta-analysis. Clin Infect Dis 2015; 60(5):e11-9; PMID:25409473; https://doi.org/10.1093/cid/ciu915
- Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, Adrian PV, van Niekerk N, Treurnicht F, Ortiz JR, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014; 371(10):918-31; PMID:25184864; https://doi.org/10.1056/NEJMoa1401480
- Nunes MC, Aqil AR, Omer SB, Madhi SA. The effects of influenza vaccination during pregnancy on birth outcomes: A systematic review and meta-analysis. Am J Perinatol 2016; 33(11):1104-14; PMID:27603545; https://doi.org/10.1055/s-0036-1586101
- Steinhoff MC, Katz J, Englund JA, Khatry SK, Shrestha L, Kuypers J, Stewart L, Mullany LC, Chu HY, LeClerq SC, et al. Year-round influenza immunisation during pregnancy in Nepal: A phase 4, randomised, placebo-controlled trial. Lancet Infect Dis 2017.
- Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec 2012; 87(47):461-76; PMID:23210147
- Lambach P, Hombach J, Ortiz JR. A global perspective of maternal influenza immunization. Vaccine 2015; 33(47):6376-9; PMID:26319068; https://doi.org/10.1016/j.vaccine.2015.08.036
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359(15):1555-64; PMID:18799552; https://doi.org/10.1056/NEJMoa0708630
- Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vázquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis 2010; 51(12):1355-61; PMID:21058908; https://doi.org/10.1086/657309
- Dabrera G, Zhao H, Andrews N, Begum F, Green H, Ellis J, Elias K, Donati M, Zambon M, Pebody R. Effectiveness of seasonal influenza vaccination during pregnancy in preventing influenza infection in infants, England, 2013/14. Euro Surveill 2014; 19(45):20959; PMID:25411687
- Eick AA, Uyeki TM, Klimov A, Hall H, Reid R, Santosham M, O'Brien KL. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med 2011; 165(2):104-11; PMID:20921345; https://doi.org/10.1001/archpediatrics.2010.192
- Poehling KA, Szilagyi PG, Staat MA, Snively BM, Payne DC, Bridges CB, Chu SY, Light LS, Prill MM, Finelli L, et al. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol 2011; 204(6 Suppl 1):S141-8; PMID:21492825; https://doi.org/10.1016/j.ajog.2011.02.042
- Shakib JH, Korgenski K, Presson AP, Sheng X, Varner MW, Pavia AT, Byington CL. Influenza in infants born to women vaccinated during pregnancy. Pediatrics 2016; 137(6):e20152360; PMID:27244843; https://doi.org/10.1542/peds.2015-2360
- Madhi SA, Nunes MC, Weinberg A, Kuwanda L, Hugo A, Jones S, van Niekerk N, Ortiz JR, Neuzil KM, Klugman KP, et al. Contribution of serology assays in the evaluation of influenza virus infection rates and vaccine efficacy in pregnant women: Report from randomized controlled trials. Clin Infect Dis 2017; 64(12):1773-9; PMID:28369198; https://doi.org/10.1093/cid/cix241
- McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: Characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis 2002; 186(3):341-50; PMID:12134230; https://doi.org/10.1086/341462
- Weinberger DM, Simonsen L, Jordan R, Steiner C, Miller M, Viboud C. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis 2012; 205(3):458-65; PMID:22158564; https://doi.org/10.1093/infdis/jir749
- Hutcheon JA, Savitz DA. Invited commentary: Influenza, influenza immunization, and pregnancy-it's about time. Am J Epidemiol 2016; 184(3):187-91; PMID:27449413; https://doi.org/10.1093/aje/kww042
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. Author information, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64(4):401-6; PMID:21208779; https://doi.org/10.1016/j.jclinepi.2010.07.015
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64(4):383-94; PMID:21195583; https://doi.org/10.1016/j.jclinepi.2010.04.026
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009; 339:b2535; PMID:19622551; https://doi.org/10.1136/bmj.b2535
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11):1539-58; PMID:12111919; https://doi.org/10.1002/sim.1186