ABSTRACT
Human papillomavirus is considered the causative factor for cervical cancer, which accounts for approximately 5% of the global cancer burden and more than 600,000 new cases annually that are attributable to HPV infection worldwide. The first-generation prophylactic HPV vaccines, Gardasil® and Cervarix®, were licensed approximately a decade ago. Both vaccines contain the most prevalent high-risk types, HPV16 and 18, which are associated with 70% of cervical cancer. To further increase the type coverage, 5 additional oncogenic HPV types (31, 33, 45, 52 and 58) were added to the existing Gardasil-4 to develop a 9-valent HPV vaccine (9vHPV), Gardasil 9®, increasing the potential level of protection from ∼70% to ∼90%. The efficacy of the vaccine lies primarily in its ability to elicit type-specific and neutralizing antibodies to fend off the viral infection. Therefore, type-specific and neutralizing murine monoclonal antibodies (mAbs) were used to quantitate the antigenicity of the individual vaccine antigens and to measure the antibody levels in the serum samples from vaccinees in a type- and epitope-specific manner in a competitive immunoassay. Assays for 9vHPV are extended from the proven platform used for 4vHPV by developing and adding new mAbs against the additional types. In Phase III clinical trials, comparable safety profile and immunogenicity against the original 4 types were demonstrated for the 9vHPV vaccine, and these were comparable to the 4vHPV vaccine. The efficacy of the 9vHPV vaccine was established in trials with young women. Immunobridging for younger boys and girls was performed, and the results showed higher immunogenicity in the younger age group. In a subsequent clinical trial, the 2-dose regimen of the 9vHPV vaccine used among girls and boys aged 9–14 y showed non-inferior immunogenicity to the regular 3-dose regimen for young women (aged 16–26 years). Overall, the clinical data and cost-effectiveness analysis for the 9vHPV vaccine support its widespread use to maximize the impact of this important, life-saving vaccine.
Introduction
Human papillomavirus (HPV) is a small, non-enveloped capsid virus that has double-stranded circular DNA genomes. Citation1,Citation2 In the 1970s, Harald zur Hausen and his colleagues first introduced the concept that the precancerous lesions and cancer were caused by persistent infection with HPV. Citation3,Citation4 It was well known that HPV is a causative factor in cervical cancer, which is the fourth most frequent cancer in women, Citation5,Citation6 and accounts for approximately 5% of the global cancer burden worldwide. Citation7
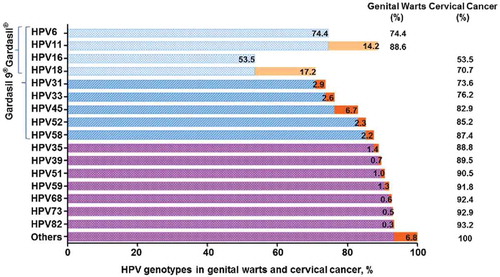
Cervical cancer is one of the few cancers that can be prevented using prophylactic vaccines. The first-generation vaccines, 4-valent HPV vaccine (referred to as 4vHPV vaccine or Gardasil®, Merck and Co., Inc.) and bivalent HPV vaccine (referred to as 2vHPV vaccine or Cervarix®, GlaxoSmithKline), were licensed in 2006 (in the US) and 2007 (in the EU), respectively. Citation8 Both vaccines were shown to effectively protect against infection from 2 high-risk HPV types (HPV16 and 18), which are responsible for causing approximately 70% of cervical cancers. Citation9,Citation10 The 4vHPV vaccine can also prevent against infection with 2 low-risk HPV genotypes (HPV6 and 11), which are responsible for approximately 90% of genital warts. Citation11,Citation12 Beyond the HPV types covered in the first generation vaccines, oncogenic HPV types 31, 33, 45, 52 and 58 comprise the next group of types with clinical significance because they account for nearly an additional 20% of cervical cancers worldwide (). Citation13,Citation14 Both HPV vaccines are shown to have some cross-protection against HPV types that are not included in the vaccines. In a meta-analysis, Citation15 cross-protection was shown that in the 2vHPV vaccine trial was higher than it was in the 4vHPV vaccine trial against persistent infections with HPV31 (77.1% vs 46.2%) and HPV45 (79.0% vs 7.8%), and against high-grade cervical intraepithelial neoplasia (CIN2/3) associated with HPV33 (82.3% vs 24.0%) and HPV45 (100% vs −51.9%). However, for both vaccines, there was very little evidence of cross-protection against HPV52 and 58. Citation16 Furthermore, antibody titers remain generally high for HPV16 and 18, but levels for HPV31, 33, and 45 reach much lower titers after 2vHPV or 4vHPV vaccination and decline within 2 y to the levels seen with natural infection or lower than the limit of detection, Citation17,Citation18 which suggests a potential for waning of cross-protection. Therefore, a multivalent HPV vaccine, including HPV types 16 and 18 plus these 5 additional HPV types, has the potential to prevent approximately 90% of cervical cancers or genital warts.
Figure 1. Percentages of HPV-related genital warts or cervical cancer attributed to different HPV genotypes. The 2 most prevalent high-risk HPV genotypes (HPV16 and 18) and 2 low-risk genotypes (HPV6 and 11) are included in the 4vHPV vaccine (Gardasil®) licensed in 2006. In addition to the above 4 genotypes, 5 common high-risk genotypes (HPV31, 33, 45, 52 and 58) are contained in the 9vHPV vaccine (Gardasil 9®) that was more recently licensed in 2014. The data were collected from Mariani et al. Citation7
To broaden protection against HPV genotypes beyond HPV types 6, 11, 16 and 18, 3 clinical trials Citation19-Citation21 (Study V502–001, V503–001, V504–001) were subsequently initiated to evaluate the multivalent HPV vaccine formulations (8-valent, 9-valent, and 4-valent combination with 5-valent) for selecting the optimal formulations and further evaluation. Based on the results of 3 studies, each 0.5 mL dose of the formulation of the 9-valent HPV (9vHPV) vaccine containing 30, 40, 60, 40, 20, 20, 20 and 20 μg of HPV6, 11, 16, 18, 31, 33, 45, 52 and 58 VLPs, respectively, was eventually selected. Citation22
After more than a decade for the developmental cycle, the 9vHPV vaccine was licensed in the US in December 2014 with the trade name Gardasil 9® (). Citation23 The milestones in the late stage of clinical development and licensure of prophylactic HPV vaccines are shown in . Because the same technology was used for developing the VLPs for the additional HPV types, the 9vHPV vaccine, like the 4vHPV vaccine, is a non-infectious recombinant VLP-based vaccine. The 9vHPV vaccine is prepared from the highly-purified virus-like particles (VLPs) of the major capsid L1 protein including the same 4 HPV types in the 4vHPV vaccine and 5 additional oncogenic high-risk HPV types. Citation24 VLPs from 9 different types were purified and adjuvant-adsorbed separately and then mixed to formulate the final product. Basic information about 3 currently licensed prophylactic HPV vaccines by the Food and Drug Administration (FDA) is shown in . By preventing HPV persistent infection and diseases from the additional HPV types, the 9vHPV vaccine has a potential to increase the prevention rate of cervical cancer from ∼70% to ∼90% Citation25 while providing additional protection against low/high-grade cervical intraepithelial neoplasia (CIN1/CIN2/3) and other HPV-related cancers (). Citation26-Citation31
Table 1. Basic information on the globally licensed human papillomavirus (HPV) vaccines.
Figure 2. Milestones and timelines in the late stage clinical development and licensure of HPV vaccines. The upper part of the timeline is related to the information on the 9vHPV vaccine, with the 2vHPV and 4vHPV vaccines in the lower part. The 5 important clinical trials for HPV vaccines in the last decade with a red frame are shown in the illustration. The specific information for these 5 clinical trials can be found in the Supplemental Material (Table S2).
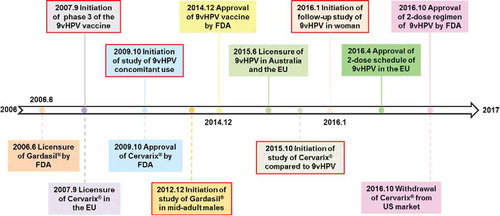
Table 2. Estimated contribution from different types covered in the 4vHPV and 9vHPV vaccine for HPV-related cancers and diseases worldwide.
Prevention of cervical cancer and other genital cancers by vaccination against oncogenic high-risk types has been a pivotal public health strategy in many countries and regions. Citation32 Due to the broader protection coverage, the 9vHPV vaccine has the potential to address an unmet medical need to prevent infection of the additional HPV types and associated cancers or pre-cancerous lesions. In this review, we introduced the bioanalytics of the 9vHPV vaccine to support product quality analysis and serological testing in clinical trials. The results on the 9vHPV vaccine safety and efficacy will be reviewed. The latest recommendation for the 9vHPV vaccination schedule (i.e., 2 doses for boys and girls aged 9–14 years) by the FDA will be discussed, which should improve the uptake of HPV vaccination with the 2-dose regimen.
Bioanalytics in support of vaccine development and commercialization
Since the integrity of the functional epitopes on the recombinant VLP surface is the basis for its function as an efficacious vaccine, it is essential to monitor HPV vaccine antigen type-specific conformational epitopes during vaccine processing and in the final formulation. The in vitro relative potency (IVRP) assay is considered a suitable replacement for the classical mouse potency assay and is an appropriate method for product lot-releasing and stability testing. Citation33-Citation35 Because most of the clinically relevant epitopes are type specific, an IVRP assay needs to be developed for each antigen. Most of the monoclonal antibodies (mAbs) used in IVRP assays are type specific and have neutralizing activity based on the pseudovirion-based neutralization assay. Citation36 The pair choices (normally chosen from a panel of mAbs) for each HPV vaccine type are listed in the . For a given HPV type, one mAb is used to capture the VLP on the ELISA plate, and another mAb is used for detection in a sandwich format () to yield quantitative information on the antigenicity of the VLPs by probing 2 different epitopes in the same assay.
Table 3. The type-specific and neutralizing monoclonal antibodies used for product potency analysis and for serological assay.
Figure 3. Schematic illustrations of IVRP for vaccine antigenicity on product quality and for serological assay for type-specific and epitope-focused determination of antibody titers elicited by vaccination. (A) IVRP for antigenicity. The IVRP assay is used for monitoring vaccine product quality during lot-release and stability testing. One monoclonal antibody is used to capture the type-specific VLP on the microplate, while the other monoclonal antibody is used for detection in a sandwich format. The final readout is performed with a horseradish peroxidase (HRP) or alkaline phosphatase (AP)-labeled secondary antibody that is specific to the subclass of the detection mAb. (B) Serological assay. The competitive Luminex immunoassay evaluates the level of functional antibody titers in vaccinees. The immunoassay quantitatively measures the ability of the type-specific antibody in serum to compete with a phycoerythrin (PE) labeled, HPV type-specific mAb for a given type () for binding to the same epitope. The Luminex microspheres were coupled with a given VLP type via covalent bonds. Owing to the competition with the labeled detection Ab, the fluorescent signals binding to the Luminex beads decrease if there are L1-specific neutralizing antibodies in serum samples. Abbreviations: mAb, monoclonal antibody; VLP, virus like particle; PE, phycoerythrin.
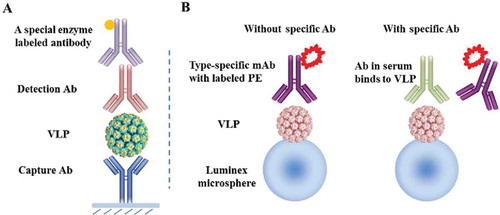
To support the clinical trials on the 9vHPV vaccine and evaluate the vaccine-induced immune responses in vaccinees, a 9-plexed HPV competitive Luminex immunoassay (HPV-9 cLIA) was developed. Citation41 The assay is based on the previous HPV-4 cLIA, which was used to measure the level of antibody response after administration of the 4vHPV vaccine. Citation42,Citation43 The HPV-9 cLIA uses VLPs that have been coupled to a set of 9 different florescent Luminex microspheres (or “barcoded” beads with a unique address for each bead set). Each type-specific antibody that binds to a specific Luminex microsphere is identified by distinct fluorescent dye spectral properties on the Luminex. The type-specific, conformational antibodies used in HPV-9 cLIA (of the 2 mAbs used in the IVRP assay for the same type) are shown in . Citation41,Citation44 To correlate the assay on vaccine antigenicity and the immune response elicited by vaccine administration, each of the HPV type-specific mAbs used in cLIA is one of the corresponding mAbs used in the IVRP assay for a given type. Because a detection Ab is more sensitively reflected in a sandwich assay than a capture Ab, most of the type-specific antibodies used in cLIA are matched to the detection antibodies in the IVRP assay except H6.M48, K11.B2 and H18.J5 ().
The antibody levels against vaccine targeting HPV types were measured in a competitive format on the Luminex platform (). The type-specific monoclonal antibody that labeled phycoerythrin competed with specific antibodies in individual serum samples for binding to conformational epitopes on the viral capsid protein. Citation42 The labeled fluorescent signals from the HPV type-specific monoclonal antibody that labeled phycoerythrin were inversely proportional to the antibody titers in vaccinees.
For the antigenicity measurement of the vaccine product, these conformational, type-specific mAbs were used to develop sandwich-format immunoassays for analyzing VLP antigens in aqueous solution or formulated antigens after dissolution treatment. The pairs of monoclonal antibodies of 9 different HPV types (listed in ) have been used in the assays for supporting vaccine product lot-releasing and stability testing. Citation40,Citation42 The bioanalytics tools described here have played critical roles in the licensure of 4vHPV and 9vHPV vaccines as well as the post licensure life cycle management.
Safety profile of the 9vHPV vaccine
Adding more adjuvant and antigen components to an existing vaccine can potentially impact its safety. In one preclinical toxicity study of the 9vHPV vaccine, 0.5 mL of vaccine formulation containing between 1.0- and −1.5-fold of each of 9vHPV antigen type was administered to female rats before mating and during gestation. In another toxicity study, rats were injected with a single human dose (0.5 mL) of the 9vHPV vaccine before mating and then during gestation and lactation. Both animal studies showed no evidence of fetus harm from the injection of the 9vHPV vaccine. Citation45
Clinically, the overall safety profile of the 9vHPV vaccine was assessed through 7 Phase III clinical trials (Study V503–001/002/003/005/006/007/009) Citation20,Citation46-Citation51 that were conducted in adolescents and younger men and women aged 9–26 y (). Overall, more than 15,000 subjects received at least one dose of the 9vHPV vaccine, and over 7,000 control subjects received the 4vHPV vaccine. The vaccination schedule was administered as a 3-dose regimen at Day 1, Month 2 and Month 6. Each subject in the studies obtained a report card to record non-serious or serious adverse events (AE or SAEs) and other medical conditions.
Table 4. The basic information of the 7 Phase III clinical trials of the 9vHPV vaccine.
The 9vHPV vaccine was generally well tolerated in adolescents (aged 9–15 years) and younger women (aged 16–26 years). Citation52 The AEs were largely injection-site AEs, such as local pain, swelling and erythema, but most of them were mild to moderate in intensity. Headache and pyrexia were the most frequent systemic AEs after vaccination with the 9vHPV vaccine. Rarely did subjects not complete the studies due to AEs and vaccine-related SAEs. Compared to the 4vHPV vaccine group, a slightly higher incidence of injection-site AEs was noted for the 9vHPV vaccine (90.7%) than for the 4vHPV vaccine (84.9%). Citation53
The most common SAEs in the 7 studies were elective or spontaneous abortions and appendicitis. Citation52 Among the subjects who received the 9vHPV vaccine, only 7 subjects reported vaccine-related SAEs. Seven subjects died during the studies. None of the deaths were considered vaccine-related. Therefore, the 9vHPV vaccine is generally well-tolerated in recipients who are 9–26 y of age, and the overall safety profile is comparable to the 4vHPV vaccine. Additionally, co-administration with other vaccines also slightly increases the incidence of local adverse reactions. Citation54 Due to a higher content of proteins and adjuvants in the 9vHPV vaccine, these results are consistent with the expected results. The favorable safety profile of the 9vHPV vaccine will also support widespread vaccination programs.
Efficacy and immunogenicity of the 9vHPV vaccine
With the availability of prophylactic HPV vaccines (4vHPV and 2vHPV vaccines) in many countries and regions, it is unacceptable to evaluate the efficacy and immunogenicity of the 9vHPV vaccine compared with placebo. Citation55 Therefore, the strategy of evaluation was implemented as described in a subsequent section.
Efficacy and immunogenicity against the original 4 types
Immunogenicity against the original 4 types (HPV6, 11, 16 and 18) was assessed in 3 clinical studies of the non-inferior immunogenicity of the 9vHPV compared with the 4vHPV vaccine in girls and boys aged 9–15 years, Citation56-Citation58 young women aged 16–26 years, Citation59 and men aged 16–26 y. Citation60,Citation61 No significant difference in the geometric mean titers (GMTs) of anti-HPV6, 11, 16 and 18 has been demonstrated in 3 clinical studies between the 9vHPV and clinically proven 4vHPV vaccine groups. The seroconversion rates of 4 HPV types at one month after the administration of the 3rd dose in the 9vHPV vaccine were comparable to those for the 4vHPV vaccine recipients.
Efficacy and immunogenicity against 5 additional types
A total of 14,204 women (9vHPV vaccine group, n = 7,099; 4vHPV vaccine group, n = 7,105) were followed up in a randomized, double-blind clinical trial. Citation62 The per-protocol efficacy (PPE) subjects included women who were seronegative on Day 1 and negative on polymerase chain reaction assays for the relevant HPV types from Day 1 to Month 7 during the study. The efficacy of the 9vHPV vaccine against a mixed end point of HPV 31, 33, 45, 52 and 58-related high-grade diseases in the PPE was 96.7% (1 case in the 9vHPV vaccine group versus 30 cases in the 4vHPV vaccine group). The efficacy against diseases, of any grade, related to the 5 new types was 97.1% (3 cases vs. 103 cases). The efficacy against 6-month persistent infection related to the 5 new types was 96.0% (35 cases vs. 810 cases). Citation62 Therefore, the 9vHPV vaccine is highly efficacious in preventing HPV31, 33, 45, 52 and 58-related persistent infection and diseases.
Immunobridging for different populations
In comparison to young girls and women aged 16–26 years, the immune response to the 9vHPV vaccine was assessed in adolescents aged 9–15 y and men aged 16–26 y. The seroconversion rates at Month 1 after the last dose were nearly 100% in all age groups. The GMTs to the 5 new types included in the 9vHPV vaccine is comparable in both sexes, while they are slightly higher in the younger age groups. Citation56 Based on the immune-bridging evaluation model, the efficacy of the 9vHPV vaccine against the new vaccine types is inferred from women aged 16–26 years, men aged 16–26 y and the younger age groups.
Overall, the 9vHPV vaccine induced a robust immune response against all related HPV types with the seroconversion rates close to 100% for all types. In addition, the antibody levels, reflected by GMTs determined by the competitive immunoassay with neutralizing mAbs as reporting molecules, were also higher in the younger groups (9–15 y of age) than in the older groups (16–26 y of age).
Immunogenicity of the 9vHPV vaccine with 2-dose vs. 3-dose regimen
Because the vaccine elicited a higher antibody titer in the younger population, they wondered whether a 2-dose regimen can elicit a sufficient immune response in the younger population to confer comparable immunity. They evaluated the immunogenicity of the 9vHPV vaccine among younger adolescents aged 9–14 y after receiving the 2-dose regimen compared with girls and young women aged 16–26 y receiving the 3-dose regimen. The clinical trial conducted at 52 sites in 15 countries was initiated in December 2013. Citation63
Immunogenicity was evaluated in a younger group of girls and boys aged 9–14 y who received the 2-dose schedule (Month 0 and 6 or 12) and an older group of women aged 16–26 y who received the 3-dose schedule (Month 0, 2 and 6) (). Citation64 Four weeks after the last dose of the 2-dose regimen, the seroconversion rates for all covered HPV types were between 99.3% and 100% in all groups. The GMTs for each type were higher in the younger group that received the 2-dose regimen than in women aged 16–26 y who received the 3-dose regimen. In the same bridging study, exploratory analysis in the group of girls aged 9–14 y indicated that the GMTs of the 2-dose schedule were lower than the 3-dose schedule at one month after the last dose for some vaccine types (e.g., HPV types 18, 31, 45 and 52 after Month 6 and HPV type 45 after Month 12). However, the clinical relevance of these findings remains unclear because the minimal antibody level required for protection is uncertain for each HPV type.
Table 5. Immunogenicity of the 9vHPV vaccine using 2-dose regimen in girls and boys vs. 3-dose in girls and young women.
In conclusion, among adolescents aged 9–14 y who received the 2-dose regimen of the 9vHPV vaccine on a schedule of Month 0 and Month 6 or 12, their immunogenicity was non-inferior to a 3-dose regimen in young women aged 16–26 y at 4 weeks after the last dose. However, further study is still needed to evaluate the persistent antibody immune responses and effectiveness in clinical trials.
Use of the 9vHPV vaccine in subjects previously vaccinated with the 4vHPV vaccine
The 4vHPV vaccine, a highly successful prophylactic vaccine, Citation65-Citation67 was licensed more than 8 y ahead of the 9vHPV vaccine. Therefore, the initial one or 2 doses (or even the full course of the 3-dose immunization) of the 4vHPV vaccine may have been administered before the availability of the 9vHPV vaccine. A Phase III, double-blind clinical study Citation49 was initiated to assess whether the 3 doses of the 9vHPV vaccine is well tolerated in girls and women aged 12–26 y who had previously been vaccinated with the 4vHPV vaccine. The results showed that 98.3–100% of the subjects previously vaccinated with the 4vHPV vaccine seroconverted to the new types covered in the 9vHPV vaccine. Likely due to a boosting effect, the GMTs of the original 4 types after vaccination with the 9vHPV vaccine were higher in women aged 12–26 y who were vaccinated with the 4vHPV vaccine than in those who received the 9vHPV vaccine alone. Citation68 Interestingly, the GMTs to the 5 new types were lower in those who were previously vaccinated with the 4vHPV vaccine. However, it is difficult to evaluate the significance of the findings because there is no categorical threshold for protection in terms of the minimum antibody titers.
Concomitant administration with other vaccines
To improve the uptake and reduce the cost of vaccination, concomitant administration with routine vaccines has been considered as an alternative in vaccination for pre-adolescents and adolescents. Previous data have showed that Gardasil® co-administered with Menactra™ (meningococcal vaccine) or Adacel™ (tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine) was well tolerated and did not interfere with the immune response to the respective vaccines. Citation69-Citation72 Both clinical studies (Studies V503–005 and V503–007) evaluated the tolerability and immunogenicity of administration of the first 9vHPV vaccine dose at the same time as Menactra™ and Adacel™ vs. administration of the 9vHPV vaccine one month before the administration of Menactra™ and Adacel™ in adolescents aged 9–14 y. Citation73-Citation76 The result demonstrated that both safety and the immune response to all components of the administered vaccines were non-inferior compared with non-concomitant administration (Table 1S).
Co-administration would reduce the number of visits required to deliver each dose of the vaccine. Additionally, it is estimated that co-administration of the 9vHPV vaccine with current routine vaccines could increase its uptake.
Recommendations for the 9vHPV vaccine
Since its licensure in the US in December 2014 (Canada in February 2014, the EU and Australia in June 2015), the 9vHPV vaccine has been used to prevent and decrease the incidence of HPV-related cancers and diseases in over 80 countries worldwide. However, the upper age range of recommendation differs among these countries. For instance, the vaccine is licensed in the US in women up to age 26 years, Citation77 in Australia and Canada up to age 45 years, Citation78 and in the EU without an upper age limit. In February 2015, the Advisory Committee on Immunization Practices (ACIP) included the 9vHPV vaccine in its recommendation for routine HPV vaccination of pre-adolescents aged 11 or 12 years, females aged 13–26 y and males aged 13–21 y who had not previously received 2vHPV or 4vHPV vaccination, including men who have sex with men, immunocompromised persons and those with HIV infection through the age of 26 y. Citation77
In April 2016, the European Medicines Agency approved the 2-dose regimen of the 9vHPV vaccine for young adolescents at the age of 9–14 years, and recommended that the second dose should be followed within Months 5 to 13 after the first dose. Citation79 In October 2016, the ACIP also changed the dosing schedule and recommended the HPV vaccine 2-dose regimen for individuals starting vaccination at the age of 9–14 y and recommending that the second dose be administered within Months 6 to 12 after the first dose. Citation80
Cost-effectiveness of the 9vHPV vaccination
At present, the social-economic burden of HPV-related infection and diseases is substantial in both developing and developed countries. However, most developed countries are gradually alleviating this burden with the introduction of Pap smear screening and national HPV vaccination programs. Citation81-Citation84 Some pharmacoeconomic analysis models have been used to evaluate whether the 9vHPV vaccine is likely to be cost-effective compared with the current vaccination strategies by providing broader protection against HPV infection. Citation85-Citation91
Cost-effectiveness analyses in several countries (such as the US, Germany and Austria) demonstrated that vaccination programs with the 9vHPV vaccine are likely to be cost-effective compared with the current national vaccination programs using the 2vHPV or 4vHPV vaccine. In the cost-effectiveness analysis of HPV vaccination programs in the US, Citation85-Citation87 the results indicated that switching to the 9vHPV vaccine was cost-effective for any age groups (pre-adolescents, adolescents or young women) despite the higher cost of the new vaccine. The 9vHPV vaccination yielded the same public health benefit as covering an additional 11% of adolescents with the 2vHPV or 4vHPV vaccination and it had a cost saving of $2.7 billion in the model. In Germany, Citation88 a dynamic transmission model was used to estimate the costs and quality adjusted life years (QALY) associated with vaccination strategies. Switching from the 2vHPV or 4vHPV vaccine to the 9vHPV vaccination program is highly cost-effective with an incremental cost-effectiveness ratio of €329 per QALY gained. Compared to the current strategy (4vHPV vaccination program), evaluation of the HPV vaccination program in Austria showed that vaccinating 60% of girls and 40% of boys at the age of 9-year old administered with one dose of the 9vHPV vaccine would substantially reduce the incidence of cervical cancer by additional 20%. Citation89 The vaccination strategy performed with the 9vHPV vaccine in the study was estimated to have an incremental cost-effectiveness ratio of €16,441per QALY gained compared with the 4vHPV vaccination program by considering the cost-effectiveness willingness-to-pay threshold of €30,000 per QALY gained.
Compared to the 4vHPV vaccination program, the 9vHPV vaccination was shown to be cost-effective and should significantly enhance public health benefits. Additionally, inclusion of boys in the 9vHPV vaccination program would be an efficient, cost-effective strategy to further reduce HPV-related cancers and diseases worldwide. Citation88,Citation89
Beyond the 9vHPV vaccine
There are still some oncogenic high-risk HPV types that are not covered by the 9vHPV vaccine, and these are responsible for another 10% of cervical cancer worldwide (). Citation92-Citation94 Compared with the normal population, some of uncommon high-risk HPV types are seen more frequently in the immunocompromised patients and HIV patients. Citation95 There is no doubt that cervical cancer and other HPV-related cancers screenings have to be continued in the general population, especially in high-risk groups. Unfortunately, these recommendations have not been well implemented in developing countries.
There are still significant populations with risks of HPV infection. In 2013, the Centers for Disease Control and Prevention in the US declared that only an average of 25.8% of teenagers aged 13–17 y completely received 3 doses of HPV vaccine, and 47.5% were vaccinated with at least one dose. Citation96 Such a low uptake of vaccination may be due to the inconvenience caused by needing 3 separate visits to receive all of the doses. This phenomenon also indirectly reflects the need to reduce the dose regimen of the vaccination. Citation97-Citation101 Furthermore, the 9vHPV vaccine is more expensive, costing roughly $325 to $533 for 3 doses in the United States. Citation102 Further reducing the costs of vaccine production could enhance the vaccine accessibility.
Currently, no HPV vaccine has been licensed with therapeutic efficacy, although there are some promising clinical data from some early phase studies using vectored DNA vaccine candidates. Citation103,Citation104 One clinical study conducted with the 9vHPV and 4vHPV vaccines showed that, compared with 0.87% of the recipients who were naive before vaccination, more than 7.9% of modified intention-to-treat recipients (HPV positive during the study) vaccinated with 9vHPV or 4vHPV still had HPV-related diseases and carcinoma in situ. Citation105 Therefore, the vaccine is only effective when administered before sexual debut or HPV infection. Considering the need for a broader coverage of the HPV vaccine, some researchers have been focused on developing a new generation HPV vaccine with a focus on the minor capsid protein, L2. Citation106-Citation109 Highly conserved peptides of the L2 protein was shown to induce cross-neutralizing and cross-protective antibody responses against multiple high-risk HPV types. Citation110-Citation112 It is promising that the HPV vaccine based on the L2 protein could be an effective new generation vaccine.
Conclusion
Over the last decade, the 4vHPV (and 2vHPV in some cases) vaccine has been successfully implemented across many countries for widespread vaccination among pre-adolescents and adolescent populations. This has likely prevented approximately 70% of cervical cancer cases that would occur in the future. The mechanism for protection is through the generation of type-specific neutralizing antibodies. By including an additional 5 oncogenic HPV types (31, 33, 45, 52 and 58) in the 2nd generation vaccine, another ∼20% of cervical cancer could be prevented with the 9vHPV vaccine.
The safety and efficacy of the 9vHPV vaccine was shown to be comparable to that of 4vHPV vaccine across multiple clinical trials in different populations. For the simplified dosing regimen of the 9vHPV vaccine, 2-dose administered in pre-adolescents and adolescents instead of 3-dose in young women is not only being cost-saving but also can improve the vaccine uptake. Because of the additional coverage, the widespread 9vHPV vaccination would improve health outcomes and be used worldwide.
The 9vHPV vaccine will play a pivotal role in worldwide public health efforts to prevent HPV-related cancers and diseases in the coming decades as the 2vHPV and 4vHPV vaccines having been withdrawn from the major markets like in the US. Further expanding the type coverage of a future generation vaccine would further increase the effectiveness of this powerful and life-saving public health tool. However, a creative approach may have to be taken other than simply adding more types of the L1-based antigen as the total antigen content in the 9vHPV vaccine is already at 270 μg per dose. Accessibility and vaccine uptake should be further improved collectively by the cooperation among vaccine manufacturers, law makers, governments and public health organizations.
Abbreviations
| ACIP | = |
advisory committee on immunization practices |
| AEs | = |
adverse events |
| CIN | = |
cervical intraepithelial neoplasia |
| cLIA | = |
competitive Luminex immunoassay |
| FDA | = |
food and drug administration |
| GMTs | = |
geometric mean titers |
| HPVs | = |
human papillomaviruses |
| IVRP | = |
in vitro relative potency |
| mAbs | = |
monoclonal antibodies |
| NCT | = |
national clinical trial |
| PE | = |
phycoerythrin |
| QALY | = |
quality adjusted life years |
| SAEs | = |
serious adverse events |
| VIN | = |
vulvar intraepithelial neoplasia |
| VLPs | = |
virus like-particles |
| WHO | = |
world health organization |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Material.zip
Download Zip (31.7 KB)Acknowledgment
We thank Mr. Xin Wang of Xiamen University for critical reading of the manuscript.
Funding
The writing was enabled with the supports from the National Natural Science Foundation of China (No.31670939 and No.81471934), Science and Technology Major Project of Fujian province (No.2015YZ0002) and the Scientific Research Foundation of State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics (Grant No. 2016ZY005).
References
- Brentjens MH , Yeung-Yue KA , Lee PC , Tyring SK. Human papillomavirus: a review. Dermatol Clin 2002; 20(2):315-31; PMID:12120445; https://doi.org/10.1016/S0733-8635(01)00028-6
- Wiley D , Masongsong E . Human papillomavirus: the burden of infection. Obstet Gynecol Surv 2006; 61(6 Suppl 1):S3-14; PMID:16729902; https://doi.org/10.1097/01.ogx.0000221010.82943.8c
- zur Hausen H , Gissmann L , Steiner W , Dippold W , Dreger I . Human papilloma viruses and cancer. Bibl Haematol 1975; (43):569-71; PMID:183728; https://doi.org/10.1159/000399220
- zur Hausen H . Oncogenic Herpes viruses. Biochim Biophys Acta 1975; 417(1):25-53; PMID:164249; https://doi.org/10.1016/0304-419X(75)90007-4
- World Health Organization . Human papillomavirus (HPV) and cervical cancer. Updated June 2016; http://www.who.int/mediacentre/factsheets/fs380/en/ (Accessed on 20 April 2017 )
- Ferlay J , Soerjomataram I , Dikshit R , Eser S , Mathers C , Rebelo M , Parkin DM , Forman D , Bray F . Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359-386; PMID:25220842; https://doi.org/10.1002/ijc.29210
- Mariani L , Preti M , Cristoforoni P , Stigliano CM , Perino A . Overview of the benefits and potential issues of the nonavalent HPV vaccine. Int J Gynaecol Obstet 2017; 136(3):258-65; PMID:28087890; https://doi.org/10.1002/ijgo.12075
- Harper DM . Currently approved prophylactic HPV vaccines. Expert Rev Vaccines 2009; 8(12):1663-79; PMID:19943762; https://doi.org/10.1586/erv.09.123
- Guan P , Howell-Jones R , Li N , Bruni L , de Sanjosé S , Franceschi S , Clifford GM . Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012; 131(10):2349-59; PMID:22323075; https://doi.org/10.1002/ijc.27485
- Wheeler CM , Hunt WC , Cuzick J , Langsfeld E , Pearse A , Montoya GD , Robertson M , Shearman CA , Castle PE . A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer 2013; 132(1):198-207; PMID:22532127; https://doi.org/10.1002/ijc.27608
- McCormack PL . Quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine (Gardasil®): a review of its use in the prevention of premalignant anogenital lesions, cervical and anal cancers, and genital warts. Drugs 2014; 74(11):1253-83; PMID:25022951; https://doi.org/10.1007/s40265-014-0255-z
- Garland SM , Kjaer SK , Muñoz N , Block SL , Brown DR , DiNubile MJ , Lindsay BR , Kuter BJ , Perez G , Dominiak-Felden G , et al. Impact and effectiveness of the Quadrivalent human papillomavirus vaccine: A systematic review of 10 years of real-world experience. Clin Infect Dis 2016; 63(4):519-27; PMID:27230391; https://doi.org/10.1093/cid/ciw354
- Muñoz N , Bosch FX , Castellsagué X , Díaz M , de Sanjose S , Hammouda D , Shah KV , Meijer CJ . Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer 2004; 111(2):278-85; PMID:15197783; https://doi.org/10.1002/ijc.20244
- de Sanjose S , Quint WG , Alemany L , Geraets DT , Klaustermeier JE , Lloveras B , Tous S , Felix A , Bravo LE , Shin HR , et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11(11):1048-56; PMID:20952254; https://doi.org/10.1016/S1470-2045(10)70230-8
- Malagón T , Drolet M , Boily MC , Franco EL , Jit M , Brisson J , Brisson M . Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12(10):781-9; PMID:22920953; https://doi.org/10.1016/S1473-3099(12)70187-1
- Dochez C , Bogers JJ , Verhelst R , Rees H . HPV vaccines to prevent cervical cancer and genital warts: an update. Vaccine 2014; 32(14):1595-601; PMID:24606637; https://doi.org/10.1016/j.vaccine.2013.10.081
- Einstein MH , Baron M , Levin MJ , Chatterjee A , Fox B , Scholar S , Rosen J , Chakhtoura N , Lebacq M , van der Most R , et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum Vaccin 2011; 7(12):1359-73; PMID:22048172; https://doi.org/10.4161/hv.7.12.18282
- Einstein MH , Baron M , Levin MJ , Chatterjee A , Fox B , Scholar S , Rosen J , Chakhtoura N , Meric D , Dessy FJ , et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum Vaccin 2011; 7(12):1343-58; PMID:22048173; https://doi.org/10.4161/hv.7.12.18281
- Broad spectrum HPV vaccine dose ranging study (V502-001) . ClinicalTrials.gov Identifier: NCT00260039; Last updated: 2015 June 5 ; [Accessed on 20 April 2017 ]. https://clinicaltrials.gov/ct2/show/NCT00260039?term=v502-001&rank=1
- Broad spectrum HPV (human papillomavirus) vaccine study in 16-to 26-year-old women (V503-001) . ClinicalTrials.gov Identifier: NCT00543543; Last updated: 2017 March 24 ; [Accessed on 20 April 2017 ]. https://clinicaltrials.gov/ct2/show/NCT00543543?term=v503-001&rank=2
- A study to evaluate tolerability and immunogenicity of V504 administered concomitantly with GARDASIL (V504-001). ClinicalTrials.gov Identifier: NCT00551187; Last updated: 2015 June 5 ; [Accessed on 20 April 2017 ]. https://clinicaltrials.gov/ct2/show/NCT00551187?term=v504-001&rank=1
- Luxembourg A , Brown D , Bouchard C , Giuliano AR , Iversen OE , Joura EA , Penny ME , Restrepo JA , Romaguera J , Maansson R , et al. Phase II studies to select the formulation of a multivalent HPV L1 virus-like particle (VLP) vaccine. Hum Vaccin Immunother 2015; 11(6):1313-22; PMID:25912208; https://doi.org/10.1080/21645515.2015.1012010
- Audisio RA , Icardi G , Isidori AM , Liverani CA , Lombardi A , Mariani L , Mennini FS , Mitchell DA , Peracino A , Pecorelli S , et al. Public health value of universal HPV vaccination. Crit Rev Oncol Hematol 2016; 97:157-67; PMID:26346895; https://doi.org/10.1016/j.critrevonc.2015.07.015
- Gupta G , Glueck R , Patel PR . HPV vaccines: Global perspectives. Hum Vaccin Immunother 2017; 13(6):1-4; PMID:28362244; https://doi.org/10.1080/21645515.2017.1289301
- Schuchat A . HPV “coverage.” N Engl J Med 2015; 372(8):775-6; PMID:25693018; https://doi.org/10.1056/NEJMe1415742
- Serrano B , Alemany L , Tous S , Bruni L , Clifford GM , Weiss T , Bosch FX , de Sanjosé S . Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer 2012; 7(1):38; PMID:23273245; https://doi.org/10.1186/1750-9378-7-38
- Joura EA , Ault KA , Bosch FX , Brown D , Cuzick J , Ferris D , Garland SM , Giuliano AR , Hernandez-Avila M , Huh W , et al. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev 2014; 23(10):1997-2008; PMID:25274978; https://doi.org/10.1158/1055-9965.EPI-14-0410
- Alemany L , Saunier M , Tinoco L , Quirós B , Alvarado-Cabrero I , Alejo M , Joura EA , Maldonado P , Klaustermeier J , Salmerón J , et al. Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur J Cancer 2014; 50(16):2846-54; PMID:25155250; https://doi.org/10.1016/j.ejca.2014.07.018
- de Sanjosé S , Alemany L , Ordi J , Tous S , Alejo M , Bigby SM , Joura EA , Maldonado P , Laco J , Bravo IG , et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer 2013; 49(16):3450-61; PMID:23886586; https://doi.org/10.1016/j.ejca.2013.06.033
- Alemany L , Saunier M , Alvarado-Cabrero I , Quirós B , Salmeron J , Shin HR , Pirog EC , Guimerà N , Hernandez-Suarez G , Felix A , et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer 2015; 136(1):98-107; PMID:24817381; https://doi.org/10.1002/ijc.28963
- Garland SM , Steben M , Sings HL , James M , Lu S , Railkar R , Barr E , Haupt RM , Joura EA . Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis 2009; 199(6):805-14; PMID:19199546; https://doi.org/10.1086/597071
- Bonanni P , Bechini A , Donato R , Capei R , Sacco C , Levi M , Boccalini S . Human papilloma virus vaccination: impact and recommendations across the world. Ther Adv Vaccines 2015; 3(1):3-12; PMID:25553242; https://doi.org/10.1177/2051013614557476
- Shank-Retzlaff ML , Wang F , Morley T , Anderson C , Hamm M , Brown M , Rowland K , Pancari G , Zorman J , Lowe R , et al. Correlation between mouse potency and in vitro relative potency for human papillomavirus Type 16 virus-like particles and Gardasil vaccine samples. Hum Vaccin 2005; 1(5):191-7; PMID:17012876; https://doi.org/10.4161/hv.1.5.2126
- Shank-Retzlaff ML , Zhao Q , Anderson C , Hamm M , High K , Nguyen M , Wang F , Wang N , Wang B , Wang Y , et al. Evaluation of the thermal stability of Gardasil. Hum Vaccin 2006; 2(4):147-54; PMID:17012891; https://doi.org/10.4161/hv.2.4.2989
- Nunnally BK , Turula VE , Sitrin RD . Vaccine Analysis: Strategies, Principles, and Control. Springer-Verlag Berlin Heidelberg; 2015; https://doi.org/10.1007/978-3-662-45024-6
- Smith JF , Kowalski R , Esser MT , Brown MJ , Bryan JT . Evolution of type-specific immunoassays to evaluate the functional immune response to Gardasil: a vaccine for human papillomavirus types 16, 18, 6 and 11. Hum Vaccin 2008; 4(2):134-42; PMID:18388490; https://doi.org/10.4161/hv.4.2.5261
- Christensen ND , Reed CA , Cladel NM , Hall K , Leiserowitz GS . Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type specific epitopes on HPV-6. Virology 1996; 224:477-86; PMID:8874508; https://doi.org/10.1006/viro.1996.0554
- Christensen ND , Kreider JW , Cladel NM , Patrick SD , Welsh PA . Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J Virol 1990; 64:5678-81; PMID:2170694; http://jvi.asm.org/content/64/11/5678.long
- Christensen ND , Dillner J , Eklund C , Carter JJ , Wipf GC , Reed CA , Cladel NM , Galloway DA . Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 1996; 223:174-84; PMID:8806551; https://doi.org/10.1006/viro.1996.0466
- Brown MJ , Seitx H , Towne V , Muller M , Finnefrock AC . Development of neutralizing monoclonal antibodies for oncogenic HPV types 31, 33, 45, 52, and 58. Clin Vaccine Immunol 2014; 21:587-93; PMID:24574536; https://doi.org/10.1128/CVI.00773-13
- Roberts C , Green T , Hess E , Matys K , Brown MJ , Haupt RM , Luxembourg A , Vuocolo S , Saah A , Antonello J . Development of a human papillomavirus competitive luminex immunoassay for 9 HPV types. Hum Vaccin Immunother 2014; 10(8):2168-74; PMID:25424920; https://doi.org/10.4161/hv.29205
- Opalka D , Lachman CE , MacMullen SA , Jansen KU , Smith JF , Chirmule N , Esser MT . Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol 2003; 10(1):108-15; PMID:12522048; http://cvi.asm.org/content/10/1/108.long
- Dias D , Van Doren J , Schlottmann S , Kelly S , Puchalski D , Ruiz W , Boerckel P , Kessler J , Antonello JM , Green T , et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol 2005; 12(8):959-69; PMID:16085914; https://doi.org/10.1128/CDLI.12.8.959-969.2005
- Zhao Q , Potter CS , Carragher B , Lander G , Sworen J , Towne V , Abraham D , Duncan P , Washabaugh MW , Sitrin RD . Characterization of virus-like particles in GARDASIL® by cryo transmission electron microscopy. Hum Vaccin Immunother 2014; 10(3):734-9; PMID:24299977; https://doi.org/10.4161/hv.27316
- Gardasil9(Human Papillomavirus 9-valent Vaccine, Recombinant) . Food and Drug Administration: Vaccines, Blood & Biologics; Vaccines; Approved products; Last Updated. 2016; [Accessed on 20 April 2017 ]. http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM426457.pdf
- A study of V503 (A multivalent human papillomavirus [HPV] L1 virus-like particle [VLP] Vaccine) in pre-adolescents and adolescents (V503-002). ClinicalTrials.gov Identifier: NCT00943722; Last updated: 2017 March 20 ; [Accessed on 20 April 2017 ]. https://clinicaltrials.gov/ct2/show/NCT00943722?term=V503-002&rank=1
- Multivalent HPV (human papillomavirus) vaccine study in 16- to 26-year old men and women (V503-003). ClinicalTrials.gov Identifier: NCT01651949; Last updated: 2017 March 23 ; [Accessed on 20 April 2017 ]. https://clinicaltrials.gov/ct2/show/NCT01651949?term=v503-003&rank=1
- A study of V503 given concomitantly with Menactra™ and Adacel™ in 11 to 15 year olds (V503-005). ClinicalTrials.gov Identifier: NCT00988884; Last updated: 2017 March 20 ; [Accessed on 20 April 2017 ]. https://clinicaltrials.gov/ct2/show/NCT00988884?term=v503-005&rank=1
- A study of V503, a 9-valent human papillomavirus (9vHPV) vaccine in females 12-26 years of age who have previously received GARDASIL™ (V503-006). ClinicalTrials.gov Identifier: NCT01047345; Last updated: 2017 March 23 ; [Accessed on 20 April 2017 ]. https://clinicaltrials.gov/ct2/show/NCT01047345?term=v503-006&rank=1
- A study of V503 vaccine given concomitantly with REPEVAX™ in 11 to 15 year olds (V503-007). ClinicalTrials.gov Identifier: NCT01073293; Last updated: 2017 March 20 ; [Accessed on 20 April 2017 ]. https://clinicaltrials.gov/ct2/show/NCT01073293?term=v503-007&rank=1
- Immunogenicity and tolerability of V503 versus GARDASIL (V503-009). ClinicalTrials.gov Identifier: NCT01304498; Last updated: 2017 March 7 ; [Accessed on 20 April 2017 ]. https://clinicaltrials.gov/ct2/show/NCT01304498?term=v503-009&rank=1
- Moreira ED, Jr , Block SL , Ferris D , Giuliano AR , Iversen OE , Joura EA , Kosalaraksa P , Schilling A , Van Damme P , Bornstein J , et al. Safety profile of the 9-Valent HPV vaccine: A Combined Analysis of 7 Phase III Clinical Trials. Pediatrics 2016; 138(2):e20154387; PMID:27422279; https://doi.org/10.1542/peds.2015-4387
- Pitisuttithum P , Velicer C , Luxembourg A . 9-Valent HPV vaccine for cancers, pre-cancers and genital warts related to HPV. Expert Rev Vaccines 2015; 14(11):1405-19; PMID:26366475; https://doi.org/10.1586/14760584.2015.1089174
- Lopalco PL . Spotlight on the 9-valent HPV vaccine. Drug Des Devel Ther 2016; 11:35-44; PMID:28053505; https://doi.org/10.2147/DDDT.S91018
- Luxembourg A , Bautista O , Moeller E , Ritter M , Chen J . Design of a large outcome trial for a multivalent human papillomavirus L1 virus-like particle vaccine. Contemp Clin Trials 2015; 42:18-25; PMID:25749310; https://doi.org/10.1016/j.cct.2015.02.009
- Vesikari T , Brodszki N , Van Damme P , Diez-Domingo J , Icardi G , Petersen LK , Tran C , Thomas S , Luxembourg A , Baudin M . A Randomized, Double-Blind, Phase III study of the immunogenicity and safety of a 9-Valent Human Papillomavirus L1 Virus-Like Particle Vaccine (V503) Versus Gardasil® in 9-15-Year-Old Girls. Pediatr Infect Dis J 2015; 34(9):992-8; PMID:26090572; https://doi.org/10.1097/INF.0000000000000773
- Drolet M , Bénard É , Boily MC , Ali H , Baandrup L , Bauer H , Beddows S , Brisson J , Brotherton JM , Cummings T , et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15(5):565-80; https://doi.org/10.1016/S1473-3099(14)71073-4
- Petersen LK , Restrepo J , Moreira ED, Jr , Iversen OE , Pitisuttithum P , Van Damme P , Joura EA , Olsson SE , Ferris D , Block S , et al. Impact of baseline covariates on the immunogenicity of the 9-valent HPV vaccine – A combined analysis of five phase III clinical trials. Papillomavirus Res 2017; 3:105-15; https://doi.org/10.1016/j.pvr.2017.03.002
- Van Damme P , Olsson SE , Block S , Castellsague X , Gray GE , Herrera T , Huang LM , Kim DS , Pitisuttithum P , Chen J , et al. Immunogenicity and Safety of a 9-Valent HPV Vaccine. Pediatrics 2015; 136(1):e28-39; PMID:26101366. https://doi.org/10.1542/peds.2014-3745
- Castellsagué X , Giuliano AR , Goldstone S , Guevara A , Mogensen O , Palefsky JM , Group T , Shields C , Liu K , Maansson R , et al. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine 2015; 33(48):6892-901; PMID:26144901; https://doi.org/10.1016/j.vaccine.2015.06.088
- Van Damme P , Meijer CJ , Kieninger D , Schuyleman A , Thomas S , Luxembourg A , Baudin M . A phase III clinical study to compare the immunogenicity and safety of the 9-valent and quadrivalent HPV vaccines in men. Vaccine 2016; 34(35):4205-12; PMID:27354258; https://doi.org/10.1016/j.vaccine.2016.06.056
- Chatterjee A . The next generation of HPV vaccines: nonavalent vaccine V503 on the horizon. Expert Rev Vaccines 2014; 13(11):1279-90; PMID:25256262; https://doi.org/10.1586/14760584.2014.963561
- A phase III study of a 2-dose regimen of a multivalent human papillomavirus (HPV) vaccine (V503), administered to 9 to 14 year-olds and compared to young women, 16 to 26 years old (V503-010). ClinicalTrials.gov Identifier: NCT01984697; Last updated: 2017 March 9 ; [Accessed on 20 April 2017 ]. https://clinicaltrials.gov/ct2/show/NCT01984697?term=v503-010&rank=1
- Iversen OE , Miranda MJ , Ulied A , Soerdal T , Lazarus E , Chokephaibulkit K , Block SL , Skrivanek A , Nur Azurah AG , Fong SM , et al. Immunogenicity of the 9-Valent HPV Vaccine Using 2-Dose Regimens in Girls and Boys vs a 3-Dose Regimen in Women. JAMA 2016; 316(22):2411-21; PMID:27893068; https://doi.org/10.1001/jama.2016.17615
- Tejada RA , Vargas KG , Benites-Zapata V , Mezones-Holguín E , Bolaños-Díaz R , Hernandez AV . Human papillomavirus vaccine efficacy in the prevention of anogenital warts: systematic review and meta-analysis. Salud Publica Mex 2017; 59(1):84-94; PMID:28423114; https://doi.org/10.21149/7824
- Hermann JS , Weckx LY , Monteiro Nürmberger J , Santos Junior GF , Campos Pignatari AC , Nagata Pignatari SS . Effectiveness of the human papillomavirus (types 6, 11, 16, and 18) vaccine in the treatment of children with recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol 2016; 83:94-8; PMID:26968061; https://doi.org/10.1016/j.ijporl.2016.01.032
- Tay SK , Hsu TY , Shcheprov A , Walia A , Kulkarni AS . The clinical and economic benefits of school-based quadrivalent HPV vaccination in Singapore. Int J Gynaecol Obstet 2017; 137(2):129-37; PMID:28190260; https://doi.org/10.1002/ijgo.12126
- Garland SM , Cheung TH , McNeill S , Petersen LK , Romaguera J , Vazquez-Narvaez J , Bautista O , Shields C , Vuocolo S , Luxembourg A . Safety and immunogenicity of a 9-valent HPV vaccine in females 12-26 years of age who previously received the quadrivalent HPV vaccine. Vaccine 2015; 33(48):6855-64; PMID:26411885; https://doi.org/10.1016/j.vaccine.2015.08.059
- Reisinger KS , Block SL , Collins-Ogle M , Marchant C , Catlett M , Radley D , Sings HL , Haupt RM , Garner EI , Protocol 025 Investigators . Safety, tolerability, and immunogenicity of gardasil given concomitantly with Menactra and Adacel. Pediatrics 2010; 125(6):1142-51; https://doi.org/10.1542/peds.2009-2336
- Harper DM , Vierthaler SL , Santee JA . Review of Gardasil. J Vaccines Vaccin 2010; 1(107) pii: 1000107; PMID:23805398; https://doi.org/10.4172/2157-7560.1000107
- Wheeler CM , Harvey BM , Pichichero ME , Simon MW , Combs SP , Blatter MM , Marshall GS , Catteau G , Dobbelaere K , Descamps D , et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011; 30(12):e225-234; PMID:21817954; https://doi.org/10.1097/INF.0b013e31822d28df
- Noronha AS , Markowitz LE , Dunne EF . Systematic review of human papillomavirus vaccine coadministration. Vaccine 2014; 32(23):2670-4; PMID:24412351; https://doi.org/10.1016/j.vaccine.2013.12.037
- Kosalaraksa P , Mehlsen J , Vesikari T , Forstén A , Helm K , Van Damme P , Joura EA , Ciprero K , Maansson R , Luxembourg A , et al. An open-label, randomized study of a 9-valent human papillomavirus vaccine given concomitantly with diphtheria, tetanus, pertussis and poliomyelitis vaccines to healthy adolescents 11-15 years of age. Pediatr Infect Dis J 2015; 34(6):627-34; PMID:25831420; https://doi.org/10.1097/INF.0000000000000694
- Schilling A , Parra MM , Gutierrez M , Restrepo J , Ucros S , Herrera T , Engel E , Huicho L , Shew M , Maansson R , et al. Coadministration of a 9-valent human papillomavirus vaccine with meningococcal and Tdap vaccines. Pediatrics 2015; 136(3):e563-572; PMID:26240207; https://doi.org/10.1542/peds.2014-4199
- Saslow D , Andrews KS , Manassaram-Baptiste D , Loomer L , Lam KE , Fisher-Borne M , Smith RA , Fontham ET , American Cancer Society Guideline Development Group . Human papillomavirus vaccination guideline update: American cancer society guideline endorsement. CA Cancer J Clin 2016; 66(5):375-85; PMID:27434803; https://doi.org/10.3322/caac.21355
- Pils S , Joura EA . From the monovalent to the nine-valent HPV vaccine. Clin Microbiol Infect 2015; 21(9):827-33; https://doi.org/10.1016/j.cmi.2015.05.001
- Petrosky E , Bocchini JA, Jr , Hariri S , Chesson H , Curtis CR , Saraiya M , Unger ER , Markowitz LE . Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015; 64(11):300-4; PMID:25811679; https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6411a3.html
- National Center for Immunization Research & Surveillance . Human papillomavirus (HPV) vaccines for Australians: information for immunization providers. Available from: http://ncirs.edu.au/assets/provider_resources/fact-sheets/human-papillomavirus-hpv-fact-sheet.pdf [Accessed on 20 April 2017 ]
- European Medicines Agency . Committee for Medicinal Products for Human Use (CHMP). Gardasil 9® International non-proprietary name: human papillomavirus 9-valent vaccine. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003852/WC500189113.pdf [Accessed on 20 April 2017 ]
- Meites E , Kempe A , Markowitz LE . Use of a 2-Dose Schedule for Human Papillomavirus vaccination-updated recommendations of the advisory committee on immunization practices. Am J Transplant 2017; 17(3):834-7; PMID:28240827; https://doi.org/10.1111/ajt.14206
- Eltoum IA , Roberson J . Impact of HPV testing, HPV vaccine development, and changing screening frequency on national Pap test volume: projections from the National Health Interview Survey (NHIS). Cancer 2007; 111(1):34-40; PMID:17262799; https://doi.org/10.1002/cncr.22487
- Kulasingam S , Connelly L , Conway E , Hocking JS , Myers E , Regan DG , Roder D , Ross J , Wain G . A cost-effectiveness analysis of adding a human papillomavirus vaccine to the Australian national cervical cancer screening program. Sex Health 2007; 4(3):165-75; PMID:17931529; https://doi.org/10.1071/SH07043
- Techakehakij W , Feldman RD . Cost-effectiveness of HPV vaccination compared with Pap smear screening on a national scale: a literature review. Vaccine 2008; 26(49):6258-65; PMID:18835313; https://doi.org/10.1016/j.vaccine.2008.09.036
- Brotherton JM , Fridman M , May CL , Chappell G , Saville AM , Gertig DM . Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011; 377(9783):2085-92; PMID:21684381; https://doi.org/10.1016/S0140-6736(11)60551-5
- Chesson HW , Markowitz LE , Hariri S , Ekwueme DU , Saraiya M . The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: Estimates from a simplified transmission model. Hum Vaccin Immunother 2016; 12(6):1363-72; PMID:26890978; https://doi.org/10.1080/21645515.2016.1140288
- Durham DP , Ndeffo-Mbah ML , Skrip LA , Jones FK , Bauch CT , Galvani AP . National- and state-level impact and cost-effectiveness of nonavalent HPV vaccination in the United States. Proc Natl Acad Sci USA 2016; 113(18):5107-12; PMID:27091978; https://doi.org/10.1073/pnas.1515528113
- Brisson M , Laprise JF , Chesson HW , Drolet M , Malagón T , Boily MC , Markowitz LE . Health and Economic Impact of Switching from a 4-Valent to a 9-Valent HPV Vaccination Program in the United States. J Natl Cancer Inst 2015; 108(1):djv282; PMID:26438574; https://doi.org/10.1093/jnci/djv282
- Largeron N , Petry KU , Jacob J , Bianic F , Anger D , Uhart M . An estimate of the public health impact and cost-effectiveness of universal vaccination with a 9-valent HPV vaccine in Germany. Expert Rev Pharmacoecon Outcomes Res 2017; 17(1):85-98; PMID:27366939; https://doi.org/10.1080/14737167.2016.1208087
- Boiron L , Joura E , Largeron N , Prager B , Uhart M . Estimating the cost-effectiveness profile of a universal vaccination programme with a nine-valent HPV vaccine in Austria. BMC Infect Dis 2016; 16:153; PMID:27084683; https://doi.org/10.1186/s12879-016-1483-5
- Drolet M , Laprise JF , Boily MC , Franco EL , Brisson M . Potential cost-effectiveness of the nonavalent human papillomavirus (HPV) vaccine. Int J Cancer 2014; 134(9):2264-8; PMID:24174175; https://doi.org/10.1002/ijc.28541
- Puig-Junoy J , Lopez-Valcarcel BG . Economic evaluations of massive HPV vaccination: within-study and between study variations in incremental cost per QALY gained. Prev Med 2009; 48(5):444-8; PMID:19232368; https://doi.org/10.1016/j.ypmed.2009.02.011
- Cuzick J , Ho L , Terry G , Kleeman M , Giddings M , Austin J , Cadman L , Ashdown-Barr L , Costa MJ , Szarewski A . Individual detection of 14 high risk human papilloma virus genotypes by the PapType test for the prediction of high grade cervical lesions. J Clin Virol 2014; 60(1):44-9; PMID:24630483; https://doi.org/10.1016/j.jcv.2014.02.002
- Cuzick J . Gardasil 9 joins the fight against cervix cancer. Expert Rev Vaccines 2015; 14(8):1047-9; PMID:26028344; https://doi.org/10.1586/14760584.2015.1051470
- Angioli R , Lopez S , Aloisi A , Terranova C , De Cicco C , Scaletta G , Capriglione S , Miranda A , Luvero D , Ricciardi R , et al. Ten years of HPV vaccines: State of art and controversies. Crit Rev Oncol Hematol 2016; 102:65-72; PMID:27066937; https://doi.org/10.1016/j.critrevonc.2016.03.020
- Lukai Z , Ebenezer T . Gardasil-9: A global survey of projected efficacy. Antiviral Res 2016; 130:101-9; PMID:27040313; https://doi.org/10.1016/j.antiviral.2016.03.016
- Stokley S , Jeyarajah J , Yankey D , Cano M , Gee J , Roark J , Robinette CC , Markowitz, L . Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014 — United States. Morb Mortal Wkly Rep 2014; 63(29):620-4; PMID:25055185; https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6329a3.htm [Accessed on 20 April 2017 ]
- Voss DS , Wofford LG . Human Papillomavirus vaccine uptake in Adolescent Boys: An evidence review. Worldviews Evid Based Nurs 2016; 13(5):390-5; PMID:27458811; https://doi.org/10.1111/wvn.12172
- Nanagas VC , Stolfi A , Nanagas MT , Eberhart GM , Alter SJ . Adolescent Male Human Papillomavirus Vaccination. Glob Pediatr Health 2016; 3:2333794X16642373; PMID:27336012; https://doi.org/10.1177/2333794X16642373
- Yang DY , Bracken K . Update on the new 9-valent vaccine for human papillomavirus prevention. Can Fam Physician 2016; 62(5):399-402; PMID:27255620; http://www.cfp.ca/content/cfp/62/5/399.full.pdf
- Bratic JS , Seyferth ER , Bocchini JA, Jr . Update on barriers to human papillomavirus vaccination and effective strategies to promote vaccine acceptance. Curr Opin Pediatr 2016; 28(3):407-12; PMID:27093354; https://doi.org/10.1097/MOP.0000000000000353
- Markowitz LE , Meites E , Unger ER . Two vs Three Doses of Human Papillomavirus Vaccine: New policy for the second decade of the vaccination program. JAMA 2016; 316(22):2370-2; PMID:27893046; https://doi.org/10.1001/jama.2016.16393
- Centers for Disease Control and Prevention (CDC) . Vaccines for Children Program (VFC). Prices last reviewed/updated: April 3, 2017; Available at: http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/ [Accessed on 20 April 2017 ]
- Massa S , Paolini F , Curzio G , Cordeiro MN , Illiano E , Demurtas OC , Franconi R , Venuti A . A plant protein signal sequence improved humoral immune response to HPV prophylactic and therapeutic DNA vaccines. Hum Vaccin Immunother 2017; 13(2):271-82; PMID:28118086; https://doi.org/10.1080/21645515.2017.1264766
- Wu CC , Wu FC , Hsu YT , Hsiao YC , Yang YC , Chang CA , Chang CL . Enhanced anti-tumor therapeutic efficacy of DNA vaccine by fusing the E7 gene to BAFF in treating human papillomavirus-associated cancer. Oncotarget 2017; 8(20):33024-36; PMID:28423693; https://doi.org/10.18632/oncotarget.16032
- Joura EA , Giuliano AR , Iversen OE , Bouchard C , Mao C , Mehlsen J , Moreira ED, Jr , Ngan Y , Petersen LK , Lazcano-Ponce E , et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372(8):711-23; PMID:25693011; https://doi.org/10.1056/NEJMoa1405044
- Pastrana DV , Gambhira R , Buck CB , Pang YY , Thompson CD , Culp TD , Christensen ND , Lowy DR , Schiller JT , Roden RB . Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology 2005; 337(2):365-72; PMID:15885736; https://doi.org/10.1016/j.virol.2005.04.011
- Seitz H , Canali E , Ribeiro-Müller L , Pàlfi A , Bolchi A , Tommasino M , Ottonello S , Müller M . A three component mix of thioredoxin-L2 antigens elicits broadly neutralizing responses against oncogenic human papillomaviruses. Vaccine 2014; 32(22):2610-7; PMID:24662712; https://doi.org/10.1016/j.vaccine.2014.03.033
- Schellenbacher C , Roden RB , Kirnbauer R . Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol 2009; 83(19):10085-95; PMID:19640991; https://doi.org/10.1128/JVI.01088-09
- Schellenbacher C , Roden RB , Kirnbauer R . Developments in L2-based human papillomavirus (HPV) vaccines. Virus Res 2017; 231:166-75; PMID:27889616; https://doi.org/10.1016/j.virusres.2016.11.020
- Roden RB , Yutzy WH , Fallon R , Inglis S , Lowy DR , Schiller JT . Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology 2000; 270(2):254-7; PMID:10792983; https://doi.org/10.1006/viro.2000.0272
- Tumban E , Peabody J , Peabody DS , Chackerian B . A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One 2011; 6(8):e23310; PMID:21858066; https://doi.org/10.1371/journal.pone.0023310
- Zhang X , Li S , Modis Y , Li Z , Zhang J , Xia N , Zhao Q . Functional assessment and structural basis of antibody binding to human papillomavirus capsid. Rev Med Virol 2016; 26(2):115-28; PMID:26676802; https://doi.org/10.1002/rmv.1867
