ABSTRACT
Psoriasis is a chronic, inflammatory, immune-mediated skin condition that affects 3 to 4% of the adult US population, characterized by well-demarcated, erythematous plaques with silver scale. Psoriasis is associated with many comorbidities including cardiometabolic disease and can have a negative impact on quality of life. The current armamentarium of psoriasis treatment includes topical therapies, phototherapy, oral immunosuppressive therapies, and biologic agents. Over the past 2 decades, there has been rapid development of novel biologic therapies for the treatment of moderate-to-severe plaque psoriasis. This article will review the role of IL-12, IL-23, and IL-17 in the pathogenesis of psoriasis and the monoclonal antibodies (ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab, tildrakizumab, and risankizumab) that target these cytokines in the treatment of this disease.
Introduction
Psoriasis is a chronic, inflammatory, immune-mediated skin condition that affects 3 to 4% of the adult US population. Citation1 Psoriasis is characterized by well-demarcated, erythematous plaques with silver scale, which can be associated with potential symptoms of pruritus, pain, skin tightness, bleeding, and flaking. Psoriasis is increasingly believed to be a systemic inflammatory disease and is associated with comorbidities such as psoriatic arthritis, cardiovascular disease, metabolic syndrome, kidney disease, malignancy, infection, and mood disorders. Citation2 Psoriasis can also have a significant negative impact on quality of life, including impairment in physical and mental functioning, psychological well-being, and work productivity. Citation3-Citation5
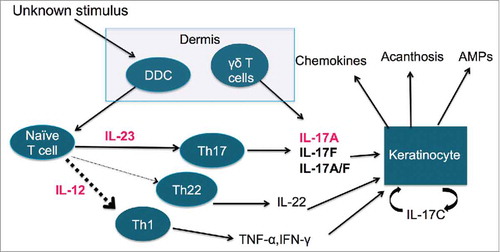
Although our knowledge of psoriasis has greatly expanded, the exact etiology of psoriasis remains unknown. Psoriasis involves an extremely complex immunologic pathogenesis of both the innate and adaptive immune systems (). Impaired T-cell activity contributes to hyperproliferation and abnormal differentiation of keratinocytes. Citation6 The keratinocytes then recruit dendritic cells to release interleukin (IL)-12 and 23. Citation7 IL-22 and IL-23 then activate 2 types of T-cells: T helper 1 (Th1) and T helper 17 (Th17), which release the psoriatic cytokines IL-17, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and IL-22. Citation8
Figure 1. The pathogenesis of psoriasis. Abbreviations: DDC – dermal dendritic cells, AMP – anti-microbial peptides, IL – interleukin, Th1 – T-helper type 1, Th17 – T-helper type 17, Th22 – T-helper type 22, TNF-α – tumor necrosis factor – α, INF-γ – interferon-gamma.
The current armamentarium of psoriasis treatment includes topical therapies, phototherapy, oral immunosuppressive therapies, and biologic agents. Citation9 There has been rapid development of novel therapies over the past 2 decades, especially biologic agents for the treatment of moderate-to-severe plaque psoriasis. TNFα inhibitors, such as the fusion protein etanercept (Enbrel) and the monoclonal antibodies adalimumab (Humira) and infliximab (Remicade), make up the first class of biologic agents. The next class to be approved by the Food and Drug Administration (FDA) was the monoclonal antibody ustekinumab, an IL-12/23 inhibitor. A more recent class of monoclonal antibodies are IL-17 inhibitors including secukinumab and ixekizumab, which block IL-17A, as well as brodalumab, which blocks the IL-17 receptor (IL-17RA). Lastly, a new class of biologics currently undergoing clinical trials includes the monoclonal antibody IL-23 inhibitors guselkumab, tildrakizumab, and risankizumab. This article will review the role of IL-12, IL-23, and IL-17 in the pathogenesis of psoriasis and the monoclonal antibodies (ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab, tildrakizumab, and risankizumab) that target these cytokines in the treatment of this disease.
IL-12 and IL-23 inhibitors
The role of IL-12 and IL-23 in psoriasis
Pre-clinical studies highlight the role of IL-12 and IL-23 in the pathogenesis of psoriasis. Citation10,Citation11 Binding of IL-12 to the IL-12R on CD4+ T cells results in differentiation to Th1 and subsequent increase in production of the pro-inflammatory cytokine IFN-γ. Citation12 IL-23 binds to the IL-23R on CD4+ T cells resulting in intracellular signaling for Th17 differentiation, which produce a multitude of cytokines including IL-17A, IL-17F, IL-22, IL-26, IFN-γ, CCL20, and TNF-α. Citation13,Citation14 Both IL-12 and IL-23 are hetereodimers that share the same p40 subunit necessary for binding to their receptor. Citation12,Citation13 The p40 subunit of IL-12 and IL-23 has been shown to be overexpressed in psoriasis plaques. Citation15 This commonality of the p40 subunit is a therapeutic target for psoriasis, whereby inhibiting the p40 subunit impedes the downstream effects of IL-12 and IL-23.
Ustekinumab
Ustekinumab (CNTO1275; Stelara®, Janssen Biotech Inc., Horsham, PA, USA) is a fully human IgG1 monoclonal antibody that binds with high affinity to the p40 subunit of IL-12 and IL-23 cytokines, neutralizing their activity and consequently blocking their downstream effects.
Dosing
Ustekinumab is dosed based on weight in which patients weighing 100 kg (220 lbs) or less receive 45 mg and those weighing more than 100 kg (220 lbs) receive 90 mg. Each subcutaneous (SC) dose of 45 or 90 mg dose is given at week 0, 4, then every 12 weeks there after Citation16 ().
Table 1. Dosing regimens of approved monoclonal antibodies targeting IL-12/23 and IL-17.
Phase I clinical trials
In a phase I, multi-center, double-blind, placebo-controlled, intra-cohort randomized, dose escalation study, a single subcutaneous dose of ustekinumab was administered to patients with moderate-to-severe plaque psoriasis. Citation17 Twenty-one subjects were randomly enrolled in a 1:4 ratio to receive placebo or ustekinumab 0.27 mg/kg, 0.675 mg/kg, 1.35 mg/kg, and 2.7 mg/kg. Patients receiving ustekinumab achieved rapid disease improvement with dose-dependent clinical response. At the higher dose of 2.7 mg/kg, all 4 subjects maintained an improvement of 67–89% in the Psoriasis Area and Severity Index (PASI) from baseline to week 12 and 24, which was the greatest and longest sustained response out of all groups. These findings were consistent with another Phase I open-label, sequential dose-escalation study examining ustekinumab IV administrations. Citation18
Phase II clinical trials
A Phase II, double-blind, multi-center, placebo-controlled, parallel-group study evaluated the safety and efficacy of ustekinumab in 320 moderate-to-severe psoriasis patients assigned to receive ustekinumab at a single 45 mg dose, a single 90 mg dose, 4 weekly 45 mg doses, 4 weekly 90 mg doses, or placebo. Citation19 Improvement of 75% or greater in PASI from baseline (PASI 75) at week 12 were observed in 52%, 59%, 67%, 81% and 2%, of patients respectively (P < 0.001, all comparisons vs. placebo). Improvement of 90% or greater in PASI from baseline (PASI 90) at week 12 were 23%, 30%, 44%, 52%, and 1%, respectively (P < 0.001, all comparisons vs. placebo). In general, response was sustained up to 24 weeks. Citation20
Phase III clinical trials
There were 2 pivotal Phase III studies, PHOENIX 1 and PHOENIX 2, investigating the safety and efficacy of ustekinumab for the treatment of moderate-to-severe psoriasis. Citation20-Citation23 PHOENIX 1 is a parallel, double-blind, multi-center, placebo-controlled trial of 766 patients with moderate-to-severe psoriasis randomly assigned to either 45 or 90 mg ustekinumab administered at week 0 and 4 and every 12 weeks thereafter, or placebo at week 0 and 4 followed by ustekinumab starting at week 12. Citation21 PASI 75 rates at week 12 were 67.1% and 66.4% of patients receiving 45 mg and 90 mg ustekinumab, respectively, which were significantly higher than placebo (3.1%, P < 0.0001 for both comparisons). Similarly, a greater number of patients in the ustekinumab group achieved PASI 90 at week 12 compared with placebo (36.7–41.6% vs. 2%). By week 24, PASI 75 rates peaked and were generally maintained until week 40. Long-term ustekinumab responders (PASI 75 at week 40) were re-randomized to continued maintenance dose or withdrawal. PASI 75 was maintained in a significantly greater number of patients continued on ustekinumab therapy compared with the withdrawal group (p < 0.0001). Initial clinical responses were generally maintained at week 76, Citation20 year 3, Citation21 and year 5. Citation22
PHOENIX 1 allowed for minimal dosing flexibility, thus PHOENIX 2 was designed to assess the long-term efficacy of ustekinumab with greater dosing flexibility. Citation23 At week 12, 66.7% of patients receiving ustekinumab 45 mg, 75.7% receiving ustekinumab 90 mg, and 3.7% receiving placebo achieved PASI 75 (p < 0.0001, vs. placebo for both comparisons). PASI 90 in the 45 mg and 90 mg dosing groups at week 12 was 32.3% and 50.9%, respectively, which was maintained at week 24. Partial responders (patients achieving PASI ≥ 50% but <75%) at week 28 were randomized to continue ustekinumab every 12 weeks or escalate dosing to every 8 weeks. A significantly greater number of partial responder receiving a dose escalation of 90 mg every 8 weeks reached PASI 75 at 52 weeks compared with those continued at 90 mg every 12 weeks (68.8% vs. 33.3%, respectively, P = 0.004). No difference in improvement was observed with dosing escalation from 45 mg every 12 weeks to every 8 weeks. Other Phase III clinical trials have been performed in specific psoriasis subpopulations with similar tolerability, safety, and efficacy responses. Citation24-Citation29
Safety and tolerability
Both short-term and long-term reports from Phase II and III trials regarding the safety profile of ustekinumab are favorable without observable dose-dependent adverse events (AEs). In a pooled assessment of Phase 2 trials, PHOENIX 1, and PHOENIX 2, rates of AEs were comparable among patients treated with placebo (50.4%), ustekinumab 45 mg (57.6%), or ustekinumab 90 mg (51.6%). Citation30 Similar rates of AEs were also found among ustekinumab and etanercept groups during the ACCEPT trial (etanercept: 70.0%; ustekinumab 45 mg: 66.0%; and ustekinumab 90 mg: 69.2%). Citation28 The most common AEs reported were headache, nasopharyngitis, upper respiratory tract infections (URTI), fatigue, pruritus, back pain, injection site reactions (ISR) and arthralgia. Citation30 Ustekinumab and placebo groups also had comparable rates per 100 patient-years for infections, serious infections, malignancies, and serous adverse events (SAEs). Citation31 Data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) has shown that unadjusted rates of serious infection for infliximab (2.91/100PY) and other biologics (1.91/100PY) were numerically higher compared with ustekinumab (0.93/100PY). Citation32,Citation33
Immunogenicity
In PHOENIX 1, of the 746 patients with available data at 76 weeks, 38 (5.1%) subjects developed antibodies to ustekinumab, predominantly with low titers (< 1/320). Citation20
IL-17 inhibitors
The role of IL-17 in psoriasis
IL-17 is a key player in the pathogenesis of psoriasis. IL-17 acts as a driver of inflammation and induces production of cytokines such as TNF-α, which recruit neutrophils and monocytes at the site of T-cell activation and leads to a self-sustaining keratinocyte hyperproliferation. Citation33 Among the many subtypes, IL-17A levels, in particular, are elevated in psoriatic lesions and can act directly on keratinocytes to induce expression of other pro-inflammatory molecules involved in psoriasis. Citation34,Citation35 IL-17A, IL-17F, and the IL-17A/F heterodimer stimulate a receptor complex consisting of IL-17RA and IL-17RC subunits. The 3 anti-IL-17 agents currently available for treatment of moderate-to-severe plaque psoriasis are ixekizumab, secukinumab, and brodalumab.
Secukinumab
Secukinumab (AIN475, Cosentyx®, Novartis Pharma AG, Basel, Switzerland) is a human IgG1κ monoclonal antibody that selectively targets and neutralizes IL-17A.
Dosing
Secukinumab is available as 150 mg/mL prefilled syringes (PFS) or auto-injector pens (AI). Secukinumab is usually given at a dose of 300 mg SC at weeks 0, 1, 2, 3, and 4, then every 4 weeks thereafter (). For some patients, 150 mg may be acceptable. Citation36
Phase I clinical trials
In a randomized, placebo-controlled, proof-of-concept Phase I clinical trial to demonstrate the safety and efficacy of secukinumab, 36 chronic plaque psoriasis patients were randomized to receive either a single intravenous 3 mg/kg dose of secukinumab or placebo. Citation37 Patients in the secukinumab group experienced relatively quick reduction in disease symptoms as early as 2 weeks after infusion. At week 12, the mean decrease in PASI score from baseline was 63% in the secukinumab group compared with 9% in the placebo group (P = 0.0005). Immunostains of patient skin biopsies revealed that the area occupied by dermal IL-17A+ CD3+ T cells was significantly smaller in secukinumab treated patients compared with placebo (P = 0.042). Analysis of skin samples using reverse transcription polymerase chain reaction (RT-PCR) showed significant downregulation of a variety of pro-inflammatory cytokines and chemokines including IL-17A, IL-21, CCL20, IL-12B, and INF-γ. Citation37
Phase II clinical trials
Four phase II studies with a total of 665 patients were conducted to determine the optimal dosing regimen, as well as efficacy and safety of secukinumab. In a study conducted by Papp et al., 4 subcutaneous doses of secukinumab (25 mg, 3 × 25 mg, 3 × 75 mg, and 3 × 150 mg) at 0, 4, and 8 weeks were compared with placebo over 36 weeks in 125 patients. Citation38 At week 12, the 3 × 150 mg and 3 × 75 mg dose groups resulted in significantly higher PASI 75 rates relative to placebo (82% and 57% vs. 9%; P < 0.001 and P = 0.002, respectively), which were maintained throughout the 36 weeks. Another phase II study by Rich et al. used 3 different induction regimens of secukinumab 150 mg (a single dose at week 0, early weekly regimen at weeks 0, 1, 2, 4, and monthly regimen at 0, 4, 8 weeks) compared with placebo in 404 psoriasis patients. Citation39 Following completion of the induction regimen, PASI 75 responders were re-randomized to a fixed interval maintenance regimen (secukinumab 150 mg at weeks 12 and 24) or treated at the start of relapse with 150 mg secukinumab. The early weekly and monthly regimens were associated with higher PASI 75 response rates compared with placebo. Maintenance of PASI 75 and 90 from weeks 20 to 28 were higher in the fixed interval regimen. Citation39
Phase III clinical trials
Two 52-week randomized, double-blinded, placebo-controlled Phase III clinical trials ERASURE (n = 738) and FIXTURE (n = 1306) investigated the efficacy of secukinumab in lyophilized powder formulation (LYO) at 300 mg and 150 mg doses administered once weekly for 5 weeks, then every 4 weeks. Citation40 In ERASURE, both doses of secukinumab showed significantly higher proportions of patients achieving PASI 75 at week 12 vs. placebo (300 mg: 81.6% and 150 mg: 71.6% vs. placebo: 4.5% P < 0.001). Citation40 In addition to a placebo group, FIXTURE used an active-control arm, comparing secukinumab to etanercept. Citation40 At week 12, a significantly greater proportion of patients achieved PASI 75 in the secukinumab group compared with etanercept and placebo (300 mg: 77.1%, 150 mg: 67.0%, etanercept: 44.0%, placebo: 4.9%; P < 0.001for all comparisons). PASI 75 was maintained through 52 weeks in 84.3% patients receiving secukinumab 150 mg (P < 0.001 vs. etanercept), 82.2% receiving secukinumab 300 mg (P = 0.009 vs. etanercept), 72.5% receiving etanercept, and 0% in placebo group. Citation40 Similar rates of PASI 75 were reached in other Phase III trials, JUNCTURE using secukinumab AI Citation41 and FEATURE using secukinumab in PFS. Citation42
Two other phase III clinical trials studied alternative dose regimens of secukinumab. In the SCULPTURE study, the fixed dose regimen achieved consistently higher PASI 75, 90, and 100 and IGA 0/1 responses vs. the retreatment as needed regimen. Citation43 In the STATURE study, partial responders in SCULPTURE (defined as ≥ 50% but < 75% improvement from baseline) showed improved treatment response with increased dosing regimen of both 300 mg and 10 mg/kg IV. Citation44
Safety and tolerability
The rate of AEs did not differ significantly between groups treated with 300 mg and 150 mg doses of secukinumab, placebo, and etanercept in all phase I, II, and III clinical trials. The most common AEs associated with secukinumab were nasopharyngitis, diarrhea, and URTI. Citation45 A pooled analysis of 10 phase II and III clinical studies of secukinumab showed that nasopharyngitis and URI's were significantly more common in patients who received long-term treatment with secukinumab compared with those who had placebo. Citation46 In addition, there was a dose-dependent increase in non-serious Candida and herpes virus infections in secukinumab-treated patients. Citation47 Rates of SAEs were comparable between patients who received secukinumab 300 mg and 150 mg and placebo (2%, 2%, 1.7%).
An AE of special interest with secukinumab is inflammatory bowel disease (IBD). A pooled analysis of all psoriasis patients who had been exposed to at least one dose of secukinumab showed that the exposure-adjusted incidence of IBD per 100 patient-years was comparable between patients receiving the 300 mg and 150 mg dose of secukinumab and etanercept (0.26, 0.35 and 0.34, respectively). However, a previous randomized, double-blind, placebo-controlled clinical trial of secukinumab for Crohn's disease revealed a significantly higher Crohn's Disease Activity Index (CDAI) and rate of AEs in the secukinumab treatment group relative to placebo. Citation48 Thus, there is concern for worsening or new onset Crohn's disease in patients with personal or family history of IBD.
Immunogenicity
Secukinumab has demonstrated low immunogenicity in both in vitro experiments and clinical trials. Citation49 Across the phase 3 clinical trials, 10 out of 2842 subjects (0.4%) treated with secukinumab developed anti-drug antibodies (ADA), most of which were non-neutralizing. Citation45,Citation47 There was no evidence of altered pharmacokinetics, safety, and efficacy with presence of ADA, although the small number of patients who developed ADA limited the power of such analyses.
Ixekizumab
Ixekizumab (LY2439821, Taltz®, Eli-Lilly and company, Indianapolis, IN, USA) is a humanized IgG subclass 4-kappa (IgG4-κ) anti-IL-17A monoclonal antibody.
Dosing
Ixekizumab comes as an 80 mg/mL PFS or AI. It is given as one 160 mg SC injection at week 0, then 80 mg every 2 weeks until week 12. Starting at week 16, 80 mg is administered every 4 weeks for maintenance Citation50 ().
Phase I clinical trials
In a 20-week, randomized, double-blind, placebo-controlled study, 40 patients with psoriasis received placebo or 5, 15, 50, or 150 mg of ixekizumab at weeks 0, 2, and 451. At week 6, PASI 75 was achieved by 0% (5 mg), 25% (15 mg), 71.4% (50 mg), and 100% (150 mg) of patients, while PASI 90 was achieved by 0% (5 mg), 12.5% (15 mg), 43% (50 mg), and 62.5% (150 mg) of patients. Sixteen weeks after the third dose, 74.1% of the patients treated with 150 mg of ixekizumab maintained PASI 90. Skin biopsies performed at week 2 and 6, when compared with baseline, showed significant and dose-dependent improvement of keratinocyte proliferation, epidermal hyperplasia, and number of inflammatory cells in the epidermis and dermis, with near normalization of skin by week 6 in patients treated with 50 mg and 150 mg of ixekizumab. Citation51
Phase II clinical trials
A total of 142 patients participated in the randomized, double-blind, placebo-controlled Phase II trial and received placebo or 10, 25, 75, or 150 mg of ixekizumab at weeks 0, 2, 4, 8, 12, and 16. Citation52 At week 12, PASI 75 was achieved by 29% (10 mg), 77% (25 mg), 83% (75 mg), and 82% (150 mg) of patients, while PASI 90 was achieved by 18% (10 mg), 50% (25 mg), 59% (75 mg), and 71% (150 mg) of patients. Of these, all except the 10 mg dose achieved significantly greater PASI 75 rates compared with placebo. In the open-label extension (OLE) study, patients who had not achieved PASI 75 were administered 120 mg of ixekizumab every 4 weeks, while patients who had achieved PASI 75 were withdrawn from treatment until loss of PASI 75 or until week 32, when they were treated with ixekizumab 120 mg every 4 weeks. At week 52, 77% of patients achieved PASI 75. Citation53
Phase III clinical trials
The pivotal clinical trials for ixekizumab were UNCOVER- 1, -2, and -3. Citation54-Citation56 These were large, randomized, double-blind, placebo-controlled trials. UNCOVER-1 evaluated ixekizumab dosed 160 mg at week 0 followed by 80 mg every 2 or 4 weeks compared with placebo, while UNCOVER-2 and -3 involved the additional etanercept treated arm.. In UNCOVER-1 and -2, patients achieving static physician global assessment (sPGA) 0/1 at week 12 were re-randomized to receive placebo or ixekizumab 80 mg every 4 or 12 weeks. Patients with relapse after re-randomization, defined as sPGA score of 3 or greater, were then treated with ixekizumab 80 mg every 4 weeks. Any patients with sPGA scores greater than 1 at week 12 were treated with 80 mg of ixekizumab administered every 4 weeks. At week 12 in UNCOVER-1, -2, and -3, patients treated with ixekizumab every 2 weeks had a PASI 75 between 87.3–89.7% and a PASI 90 of 68.1–70.9%, which were better than PASI 75 (77.5%-84.2%) and PASI 90 (59.7%-65.3%) of patients treated every 4 weeks. Citation54 Rapid treatment response was observed in UNCOVER-2 and -3; about 50% of patients achieved PASI 75 by week 4 in both ixekizumab treated groups. Citation55,Citation56
Safety and tolerability
Ixekizumab is generally well tolerated, with the most commonly reported AEs being nasopharyngitis, URTI, ISR/erythema/pain, pruritus, headache, and arthralgia. Overall frequency of serious infections was not increased in patients on ixekizumab during the first 12 weeks of therapy. Citation57 No cases of active or reactivated tuberculosis have been observed in the clinical trials involving ixekizumab. The rate of oral candidiasis was significantly greater in patients treated every 2 weeks compared with placebo, with most of these infections resolving with antifungal treatment. Rates of non-melanoma skin cancer (NMSC) and other malignancies were not significantly different than expected in the psoriasis population. Citation58 Major adverse cardiovascular events (MACE) were rare, with only one patient receiving ixekizumab every 4 weeks experiencing a stroke. The rate of MACE was less than 0.2% in the induction period with incidence rate less than 0.7 per 100 patient-years during the long-term component of the clinical trials.
Among 4209 clinical trials patients with a combined 6480 patient-exposure years, there were 29 patients with suspected IBD. Nineteen of these 29 were determined to be definite or probable, with incidence rates of 1.1/1000 and 1.9/1000 patient-exposure years for Crohn's disease and ulcerative colitis, respectively. Of these 19 patients, 15 patients had new onset of IBD, while 4 patients experienced flare up of their pre-existing IBD. Overall, at baseline, there were 16 patients who had a history of IBD, 4 of whom experienced flare up during clinical trials with ixekizumab. Citation59
Immunogenicity
Twenty-two percent of patients developed anti-ixekizumab antibodies. The presence of anti-ixekizumab antibodies did not affect response to ixekizumab through week 60 of treatment. Citation60
Brodalumab
Brodalumab (AMG 827, Siliq®, Valeant Pharmaceuticals, Bridgewater Township, NJ) is a fully human IgG2 anti-IL-17RA monoclonal antibody. It binds with high affinity to human IL-17RA, which leads to a disruption in the IL-17 pathway by blocking the activity of IL-17A, IL-17F, IL-17A/F heterodimer, IL-17C, and IL-25 molcules. Citation61
Dosing
Brodalumab is available as 210 mg/1.5 mL solution in a PFS. It is given at a dose of 210 mg at Weeks 0, 1, and 2 followed by 210 mg every 2 weeks Citation61 ().
Phase I clinical trials
In a phase I, randomized, double-blind, placebo-controlled, dose-ranging trial, 25 patients with moderate-to-severe plaque psoriasis were randomized to receive a single dose of placebo or 140 or 350 mg SC or 700 mg IV of brodalumab. Citation62 In the 350 mg SC arm, 62.5% patients achieved PASI 75 during the study. In the 700 mg IV arm, 88% reached PASI 75 and 38% reached PASI 90 by week 6. Skin biopsy results showed significant reductions in epidermal thickening, keratin 16 (KRT16) levels, and Ki67-expressing cells in patients receiving 350 mg SC and 700 mg IV on day 8 (350 mg SC arm) or day 15 (140 mg SC and 700 mg IV arms). In the 700 mg IV arm, KRT16 protein expression in suprabasal keratinocytes was reduced to the range seen in nonlesional skin by day 43 in 7 of 8 subjects. Citation62 Similar results were seen in another phase I randomized, placebo-controlled trial of 6 patients. Citation63
Phase II clinical trials
In a 16-week dose-ranging, randomized, double-blind, placebo-controlled trial, 188 patients with moderate-to-severe plaque psoriasis were randomized to receive placebo or brodalumab at a dose of 70, 140, or 210 mg SC on day 1 and at weeks 1, 2, 4, 6, 8, and 10, or a dose of 280 mg SC on day 1 and at weeks 4 and 8. Citation64,Citation65 At week 12, mean improvements in the PASI score were significantly greater in the 140, 210, and 280 mg brodalumab-treated groups than in the 70 mg brodalumab-treated group (85.9%, 86.3%, and 76.0%, respectively, vs. 45.0%; p < 0.001) and placebo (16.0%; p < 0.001). In the OLE study, a total of 181 patients continued to receive brodalumab at a dose of 210 or 140 mg. Citation66 sPGA 0/1 was achieved by 90% of patients at week 12 and 72% at week 120. PASI and sPGA improvements were similar for patients who received 210 and 140 mg. Another phase II trial with the same study designed showed similar results. Citation67,Citation68 At week 52, PASI 75 was maintained in 94.4% and 78.1%, and PASI 90 in 87.5% and 71.2% of patients receiving brodalumab 210 and 140 mg, respectively. Citation69
Phase III clinical trials
AMAGINE-1 was a Phase III, randomized, double-blind, placebo-controlled trial. This trial consisted for a 12-week induction phase followed by a withdrawal-retreatment period up to 52 weeks. Citation70,Citation71,Citation72 A total of 661 patients were randomized in 1:1:1 ratio to biweekly injections of 210 mg, 140 mg, or placebo for 12 weeks. Re-randomization occurred at week 12 for patients with sPGA 0/1 in the 210 and 140 mg treated groups to either continue their current dose or switch to placebo. Those re-randomized to placebo and subsequently lost disease control were restarted on the original dose. At week 12, PASI 75 was achieved by 83.3%, 60.3%, and 2.7%, of patients in the 210 mg, 140 mg, and placebo groups, respectively. Efficacy was maintained through week 52. The majority of patients re-randomized to placebo during the withdrawal-retreatment phase lost disease control, but the majority of those patients recaptured their response, most within 12 weeks of retreatment. Citation70,Citation71,Citation72
AMAGINE-2 and AMAGINE-3 were 2 large double-blind, placebo-controlled, active comparator-controlled Phase III clinical trials. Citation73 During the 12-week induction phase, patients were randomized in 2:2:1:1 ratio to receive placebo, brodalumab 210 or 140 mg biweekly, or ustekinumab. In the 40-week maintenance phase, patients who received brodalumab during the induction phase underwent a repeat randomization in 2:2:2:1 ratio to receive brodalumab 210 mg biweekly, 140 mg biweekly, 140 mg every 4 weeks, or 140 mg every 8 weeks. Patients receiving placebo during the induction phase were started on brodalumab 210 mg biweekly. Patients in the ustekinumab group continued to receive ustekinumab every 12 weeks until week 52. At week 12, brodalumab 210 mg was superior to ustekinumab (PASI 90, AMAGINE-2: 69.9% vs. 47%; AMAGINE-3:68.9% vs 49%; PASI 75, AMAGINE-2: 86.3% vs .70%; AMAGINE-3: 85.1% vs. 69.3%, p < 0.001 for all). However, brodalumab 140 mg was superior to ustekinumab only in AMAGINE-3 (p < 0.007). The median time to PASI 75 of brodalumab 210 mg was 4 weeks vs. 8 weeks for ustekinumab. After re-randomization at week 12, patients on 210 mg or 140 mg of brodalumab biweekly maintained or achieved a sPGA 0/1 at a higher rate than 140 mg every 4 or 8 weeks (p < 0.001). Citation73
Safety and tolerability
The most common AEs included nasopharyngitis, headache, URTI, and arthralgia, which were all mild to moderate in severity. Citation72,Citation73,Citation74 Cases of transient, self-resolving neutropenia without associated infection were also reported. Psychiatric AEs including depression and suicide ideation and behavior (SIB) were also reported. Citation75 Three patients out of 4,464 completed suicide. While there is currently no evidence that suggests a causal association between brodalumab and depression or SIB, there is a warning label and Risk Evaluation and Mitigation Strategy (REMS) for SIB per the US FDA. Citation61
Immunogenicity
Approximately 3% of patients treated with brodalumab developed antibodies to the drug through the 52-week treatment period. None of the antibodies to brodalumab were classified as neutralizing. Citation61
IL-23 inhibitors
The role of IL-23 in psoriasis
IL-23 is upregulated in psoriatic lesions and is though to be the major regulator of the Th17 pathway involved in the pathogenesis of psoriasis. Citation76 IL-23 is primarily produced by antigen-presenting cells and induces and maintains differentiation of Th17 and Th22 cells, which produce proinflammatory cytokines such as IL-17 and IL-22 that mediate the inflammation and epidermal hyperplasia of psoriasis. Citation77 IL-23 is composed of p19 and p40 subunits that bind to the IL-23 receptor (IL-23R) and IL-12 receptor b1 (IL-12Rb1), which results in activation of pro-inflammatory Janus kinase 2 (JAK2), tyrosine kinase 2 (TYK2), and signal transducer and activator of transcription (STAT) signaling molecules. Citation78 IL-23 antagonism blocks downstream effector cytokines observed in psoriasis such as IL-17A, IL-17F, IL-22 and TNF secreted by T cells, natural killer cells, type 3 innate lymphoid cells, neutrophils, and mast cells. Citation79,Citation80,Citation81
Guselkumab
Guselkumab (CNTO1959; Janssen Research & Development LLC, Spring House, PA) is a fully human IgG1 lambda monoclonal antibody that binds to the p19 subunit of IL-23 and inhibits the IL-23-specific intracellular and downstream signaling.
Phase I clinical trials
In a randomized, double-blind, placebo-controlled, proof-of-concept study, 24 patients with moderate-to-severe plaque psoriasis were randomized to receive a single dose of placebo or 10, 30, 100, or 300 mg of guselkumab. At week 12, 50% (10 mg), 60% (30 and 100 mg), and 100% (300 mg) of guselkumab-treated patients achieved PASI 75. Citation82 Improvements in PASI scores were generally maintained through week 24 in guselkumab-treated patients. Analysis of lesional and nonlesional skin biopsy specimens demonstrated decreases in epidermal thickness and T-cell and dendritic cell expression in guselkumab-treated patients compared with placebo-treated patients. At week 12, significant reductions in psoriasis gene expression and serum IL-17A levels were observed in guselkumab-treated patients. Citation82
Phase II clinical trials
In a 52-week, dose-ranging, randomized, double-blind, placebo-controlled, active-comparator trial, guselkumab was compared with adalimumab in patients with moderate-to-severe plaque psoriasis. Citation83 A total of 293 patients were randomized to receive guselkumab, placebo, or adalimumab. Guselkumab was given at a dose of 5 mg at weeks 0 and 4 and every 12 weeks thereafter, 15 mg every 8 weeks, 50 mg at weeks 0 and 4 and every 12 weeks thereafter, 100 mg every 8 weeks, or 200 mg at weeks 0 and 4 and every 12 weeks thereafter through week 40. At week 16, patients in the placebo group crossed over to receive guselkumab at a dose of 100 mg every 8 weeks. At week 16, the rate of patients achieving PASI 75 was higher in each guselkumab group than in the placebo group (P < 0.001 for all comparisons). At week 40, the proportion of patients with a PGA 0/1 remained significantly higher in the guselkumab 50 mg, 100 mg, and 200 mg groups than in the adalimumab group (71%, 77%, and 81% vs. 49%, P < 0.05 for all comparisons). Citation84
Phase III clinical trials
Data from 2 Phase III, multicenter, randomized, double blind, placebo- and comparator- controlled clinical trials, VOYAGE 1 and VOYAGE 2, evaluated efficacy and safety of guselkumab compared with placebo and adalimumab in patients with moderate-to-severe psoriasis over 48 weeks. Citation84,Citation85 VOYAGE 2 included a randomized withdrawal and retreatment period to evaluate the effect of interrupted treatment on the safety and efficacy of guselkumab.
In VOYAGE 1, a significantly higher proportion of patient receiving guselkumab achieved PASI 90 (2.9% vs. 73.3%) compared with placebo at week 16. Similarly, significantly higher proportion of subjects in the guselkumab vs. adalimumab group achieved PASI 90 (73.3% vs 49.7%) and PASI 75 (91.2% vs 73.1%) at week 16. These findings were maintained through weeks 24 and 48. In VOYAGE 2, a significantly higher proportion of subjects taking guselkumab achieved PASI 90 (2.4% vs. 70.0%) compared with placebo at week 16. Similarly, significantly higher proportion of subjects in the guselkumab vs. adalimumab group achieved PASI 90 (70.0% vs. 46.8%) and PASI 75 (86.3% vs. 68.5%) at week 16. In the randomized withdrawal and re-treatment phase, the median time to loss of PASI 90 response for patients in the withdrawal group was 15.2 weeks (23 weeks after last guselkumab dose). Through week 48, PASI 90 was maintained in 88.6% of patients in the maintenance group vs. 36.8% of those in the withdrawal group (P < .001). In the 112 adalimumab non-responders who initiated guselkumab at week 28 (5 weeks after the last adalimumab dose), PASI 90 and PASI 100 rates increased from baseline after switching, reaching 66.1% and 28.6%, respectively, at week 48. Citation85
Safety and tolerability
In the Phase I, II, and III clinical trials for guselkumab, the rates of AEs were comparable between guselkumab, placebo, and adalimumab (phase II and III only) groups throughout the durations of the trials. Citation82,Citation83,Citation84,Citation85 There was no evidence of a relationship between guselkumab dose and the rate of AEs. Citation83 In Phase III trials, the most common AEs in patients treated with guselkumab were nasopharyngitis, headache, and URTI. Citation84,Citation85 Serious infection, malignancy, and MACE did not appear to be increased in patients treated with guselkumab compared with placebo and adalimumab. ISR were more common in patients treated with adalimumab compared with guselkumab. There were 5 cases of NMSC, 4 of which were in the guselkumab group (2 BCCs and 3 SCCs) and the other in the adalimumab group.
Immunogenicity
Antibodies to guselkumab were detected in 5.3% (VOYAGE 1) and 6.6% (VOYAGE 2) of patients through week 48. Titers were generally low (81% ≤ 1:320) and no association was observed between antibody development and reduced efficacy or ISR occurrence. Citation84,Citation85
Tildrakizumab
Tildrakizumab (MK-3222 or SCH 900222; MERCK/Sun Pharma, Kenilworth, NJ) is a high-affinity humanized IgG1/κ monoclonal antibody that binds to the p19 subunit of human IL-23.
Phase I clinical trials
A randomized, placebo-controlled, sequential, rising multiple-dose, proof-of-concept phase I study was conducted to evaluate tildrakizumab for the treatment of moderate-to-severe psoriasis. Citation86 Seventy-seven patients underwent a 3-part study. In part 1, patients were randomized to intravenous injections of placebo (n = 6) or 0.1 mg/kg (n = 3), 0.5 mg/kg (n = 3), 3 mg/kg (n = 6) or 10 mg/kg (n = 6) of tildrakizumab on days 0, 56 and 84. In part 2, patients were randomized to placebo (n = 11) or 3 mg/kg (n = 15) or 10 mg/kg (n = 14) of tildrakizumab on days 1, 28 and 56. In part 3, patients received placebo (n = 3) or 0.05 mg/kg (n = 6) or 0.1 mg/kg (n = 3) of tildrakizumab on days 1, 56 and 84. Tildrakizumab at a dose of 0.05 to10 mg/kg resulted in a mean reduction in PASI score of 50–80% on day 112 with a sustained response at day 196. In part 2, mean decrease in PASI score of 50% was observed on study day 308, which was 36 weeks after the last administered dose. All patients who received 3 and 10 mg/kg of tildrakizumab achieved PASI 75 in part 1 by day 196 and a majority achieved PASI 75 in part 2 by day 112. Citation86
Phase II clinical trials
A 3-part, randomized, double-blind, Phase IIb dose-finding trial was conducted in 355 adults with moderate-to-severe chronic plaque psoriasis. Patients were randomized to receive subcutaneous tildrakizumab (5, 25, 100, 200 mg) or placebo at weeks 0 and 4 (part 1) and every 12 weeks thereafter until week 52 (part 2). Study drug was discontinued at week 52 and participants were followed through week 72 (part 3). Citation88 At week 16, PASI 75 was achieved in 33.3%, 64.4%, 66.3%, 74.4% and 4.4% in the 5, 25, 100, and 200 mg tildrakizumab and placebo groups, respectively (P ≤ 0.001 for all comparisons to placebo). PASI 75 was generally maintained through week 52. During part 2, more than 90% of PASI 75 responders at week 16 who continued to receive doses of 100 or 200 mg tildrakizumab maintained PASI 75 at week 52 vs. 70% of those who received a reduction in dose from 100 to 25 mg. For PASI 75 non-responders at week 16 who received an escalated dose of tildrakizumab (from 100 to 200 mg), PASI 75 tended to increase over time. Relapse was seen in only 8 of 222 participants up to week 72 who achieved PASI 75 at week 52 and continued to part 3. Citation87
Phase III clinical trials
Phase III trials for Tildrakizumab are currently underway. NCT01722331 (reSURFACE 1) is a 64-week, randomized, placebo-controlled, parallel-design study to evaluate the efficacy and safety/tolerability of subcutaneous tildrakizumab, followed by an optional long-term safety extension study. Citation88 Patients are randomized to tildrakizumab 100 or 200 mg SC at week 0, 4, and then every 12 weeks or placebo at week 0 and 4, followed by tildrakizumab starting at week 12. The study completion date is August 2019. Citation89 NCT01729754 (reSURFACE 2) is a 52-week, randomized, active (tildrakizumab)-comparator (etanercept) and placebo-controlled study. Citation89,Citation90
Preliminary results for reSURFACE 1 and reSURFACE 2 are available. Citation91 At weeks 12 and 28, 63% and 77% of patients receiving tildrakizumab achieved PASI 75, respectively. IGA 0/1 was achieved in 57% and 66% of patients with the 100 mg dose at weeks 12 and 28, respectively. Of those receiving the 200 mg dose, IGA 0/1 was attained in 59% and 69% at weeks 12 and 28, respectively. PASI 90 and PASI 100 were observed in 37% (100 mg dose) and 36% (200 mg dose) of patients at week 12, and 54% (100 mg dose) and 59% (200 mg dose) at week 28, respectively. A higher proportion of patients on tildrakizumab achieved PASI 90 and 100 compared with placebo and etanercept. Citation91,Citation92
Safety and tolerability
Safety data are available from Phase I and II studies. The overall incidence of AEs was generally similar for all treatment arms and did not differ from placebo. Citation86,Citation87 No dose-related increase in AEs was observed with tildrakizumab. In the Phase I trial, the most common AEs included headache, nasopharyngitis, URTI, and cough. Citation86 In Phase II trial, the most frequent AEs were nasopharyngitis and headache, which occurred with similar frequency in all treatment groups. Citation87
Immunogenicity
In the Phase I trial, of the 56 tildrakizumab-treated subjects, 51 were pre-treatment negative for ADA. Citation86 Nine of these (18%) developed ADA and 5 of these 9 showed lower tildrakizumab exposure than ADA negative patients. ADA-positive patients did not differ in their PASI response or adverse effect profile compared with ADA-negative patients. In the Phase II trial, 46 of the 355 participants developed ADAs. Citation87 There was no apparent correlation between development of ADAs and AEs.
Risankizumab
Risankizumab (BI 655066, Abbvie, North Chicago, IL) is another fully human fully human IgG1 monoclonal antibody specific for the IL-23 p19 subunit.
Phase I clinical trials
A single-rising-dose, multicenter, randomized, double-blind, placebo-controlled, within-dose cohort phase I trial has been conducted to assess safety of risankizumab. Citation93 Thirty nine patients received risankizumab at a dose of 0.01, 0.05, 0.25, 1, 3, or 5 mg/kg intravenously (n = 18), 0.25 (n = 13) or 1 mg/kg (n = 8) subcutaneously, or matched placebo. At week 12, PASI 75, PASI 90, and PASI 100 were observed in 87% (27/31, P < .001 vs. placebo), 58% (18/31, P = .007 vs. placebo), and 16% (5/31, P = .590 vs. placebo) of patients receiving a single dose of risankizumab at any dose, respectively, and improvement was observed as early as week 2. Risankizumab treatment resulted in reduced expression of lesional skin genes associated with IL-23/IL-17 signaling pathways and normalization of psoriatic lesion gene expression profiles to a profile approaching that of nonlesional skin. Significant correlation between treatment-associated molecular changes and PASI scores was observed. Citation93
Phase II clinical trials
In a 48-week, multicenter, randomized, dose-ranging, head-to-head Phase II trial, a total of 166 patients received subcutaneous injections of risankizumab (a single 18 mg dose at week 0 or 90 or 180 mg doses at weeks 0, 4, and 16) or ustekinumab (45 or 90 mg, according to body weight, at weeks 0,4, and 16). Citation94 At week 12, PASI 90 was achieved in 77% (64/83) of patients receiving risankizumab (90 and 180 mg groups, pooled) as compared with 40% (16/40) of patients receiving ustekinumab (P < 0.001). PASI 100 was observed in 45% in the risankizumab group (pooled 90 and 180 mg) compared with 18% in the ustekinumab group. Efficacy was generally maintained up to 20 weeks after the final dose of 90 mg or 180 mg of risankizumab. Complete clearing was maintained in 29% and 26% of the patients 32 weeks following the last dose of risankizumab in the 90 and 180 mg groups, respectively.
Safety and tolerability
Over the 24 weeks after treatment in the single-dose Phase I trial, 65% (20/31) of patients receiving risankizumab administered intravenously or subcutaneously experienced an AE compared with 88% (7/8) of patients receiving placebo. Citation93 The most frequently reported AEs were URTI, nasopharyngitis, and headache. The severity of the AEs did not appear related to the dose of drug. There were no SAEs considered related to risankizumab. In the Phase II trial, the most common AE (< 10% of patients) was nasopharyngitis. Citation94 BCC developed in 2 patients who were treated with risankizumab and one patient had a major adverse cardiac event.
Discussion
Recent developments in monoclonal antibodies blocking IL-12, -23, and -17 for the treatment of moderate-to-severe psoriasis show promising efficacy in clinical trials. Compared to traditional TNFα inhibitors such as etanercept, Citation95 adalimumab, Citation96 and infliximab, Citation97 Phase III trials of newer biologic agents, specifically secukinumab, ixekizumab, brodalumab, and guselkumab show higher rates of PASI 75 and PASI 90 (). These monoclonal antibodies also have favorable side effect profiles. Compared to TNFα inhibitors, which carry US FDA boxed warnings for serious infection (including tuberculosis activation) and malignancy, Citation98,Citation99,Citation100 ustekinumab, secukinumab, and ixekizumab have no such boxed warnings. Citation16,Citation37,Citation51 Patients should still be screened for TB before starting any biologic agent. In addition, brodalumab carries a boxed warning for suicidal ideation and behavior Citation62 and all 3 IL-17 agents carry a unique concern for activation or exacerbation of IBD. Citation37,Citation52,Citation62 Summary of safety profiles of the TNFα inhibitors compared with the newer monoclonal antibody agents are shown in . Lastly, the rates of ADA formation vary greatly among agents (< 1% in secukinumab to 22% in ixekizumab) but appear not to be neutralizing and did not affect safety or efficacy of the drug in Phase III trials. On the other hand, studies have shown that ADA to TNFα inhibitors appear to be neutralizing and may be related to reduced drug plasma levels and efficacy. Citation101
Table 2. PASI 75 and PASI 90 rates from Phase III trials of TNFα inhibitors vs. newer agents targeting IL-12, -23, and -17 for the treatment of psoriasis.
Table 3. The most common side effects of TNFα inhibitors vs. newer agents targeting IL-12, -23, and -17 for the treatment of psoriasis.
Expert opinion
Given the widely available options for biologic agents for the treatment of moderate-to-severe psoriasis today, it can be a difficult task for the clinician to choose “the best” agent. The authors feel that there is no “one right answer” when choosing a treatment of a particular patient but several considerations unique to each individual patient should be considered:
-
Efficacy: infliximab and the IL-17 agents appear to have the highest PASI 75 and 90 rates in clinical trials.
-
Speed of onset: patients who are erythrodermic or have other urgent circumstances may also consider infliximab or IL-17 agents which have rapid onset of action.
-
Safety: Although there are warnings regarding serious infection and malignancy, the traditional TNFα inhibitors have close to 20 y of long-term safety data vs. newer agents.
-
Past medical history: some medical problems may preclude the patient from certain agents (i.e. heart failure and TNFα inhibitors, demyelinating diseases and TNFα inhibitors, IBD and IL-17 agents, active depression and brodalumab, etc.).
-
Presence or absence of psoriatic arthritis: some biologic agents are more effective for psoriatic arthritis than others.
-
Patient convenience and compliance: i.e., infliximab requires infusion vs. subcutaneous self-administration of all other agents; needle-phobic patients or those who travel frequently may benefit from less frequent dosing schedules, etc.
-
Insurance or cost issues
-
The authors recommend that for each patient, a fine balance all of the above relevant factors can help to reach a decision that is agreeable to both the clinician and the patient.
Conclusion
Although long-term data and real-world experiences of these biologic agents are needed to further assess their therapeutic implications, the available data to date show an extremely promising future for deepening our knowledge of psoriasis immunopathogenesis and treatment.
Disclosure of potential conflicts of interest
Dr. Bhutani conducts research for AbbVie, Janssen, Merck/Sun Pharmaceutical Industries, Mela, and Novartis. Mr. Jeon, Dr. Sekhon, Ms. Yan, Ms. Afifi, and Dr. Nakamura have no conflicts of interest to disclose.
References
- Rachakonda T , Schupp C , Armstrong A. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512-6. doi:10.1016/j.jaad.2013.11.013. PMID:24388724
- Takeshita J , Grewal S , Langan SM , Mehta NN , Ogdie A , Van Voorhees AS , Gelfand JM . Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol. 2017;76(3):377-90. doi:10.1016/j.jaad.2016.07.064. PMID:28212759
- Rapp SR , Exum ML , Reboussin DM , Feldman SR , Fleischer A , Clark A . The physical, psychological and social impact of psoriasis. J Health Psychol. 1997;2(4):525-37. doi:10.1177/135910539700200409. PMID:22013093
- Rapp SR , Feldman SR , Exum ML , Fleischer AB , Reboussin DM . Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 Pt 1):401-7. doi:10.1016/S0190-9622(99)70112-X. PMID:10459113
- Pearce DJ , Singh S , Balkrishnan R , Kulkarni A , Fleischer AB , Feldman SR . The negative impact of psoriasis on the workplace. J Dermatolog Treat. 2006;17(1):24-8. doi:10.1080/09546630500482886. PMID:16467020
- Tonel G , Conrad C . Interplay between keratinocytes and immune cells –recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol. 2009;41(5):963-8. doi:10.1016/j.biocel.2008.10.022. PMID:19027868
- Kim J , Krueger JG . The immunopathogenesis of psoriasis. Dermatol Clin. 2015;33(1):13-23. doi:10.1016/j.det.2014.09.002. PMID:25412780
- Lowes MA , Kikuchi T , Fuentes-Duculan J , Cardinale I , Zaba LC , Haider AS , Bowman EP , Krueger JG . Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Investig Dermatol. 2008;128:1207-11. doi:10.1038/sj.jid.5701213. PMID:18200064
- Menter A , Gottlieb A , Feldman SR , Van Voorhees AS , Leonardi CL , Gordon KB , Lebwohl M , Koo JY , Elmets CA , Korman NJ , et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826-50. doi:10.1016/j.jaad.2008.02.039. PMID:18423260
- Chan JR , Blumenschein W , Murphy E , Diveu C , Wiekowski M , Abbondanzo S , Lucian L , Geissler R , Brodie S , Kimball AB , et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577-87. doi:10.1084/jem.20060244. PMID:17074928
- Hong K , Chu A , Ludviksson BR , Berg EL , Ehrhardt RO . IL-12, independently of IFN-gamma, plays a crucial role in the pathogenesis of a murine psoriasis-like skin disorder. I Immuno. 1999;162(12):7480-91. PMID:10358203
- Presky DH , Yang H , Minetti LJ , Chua AO , Nabavi N , Wu CY , Gately MK , Gubler U . A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Nat Acad Sci. 1996;93(24):14002-7. doi:10.1073/pnas.93.24.14002. PMID:8943050
- Parham C , Chirica M , Timans J , Vaisberg E , Travis M , Cheung J , Pflanz S , Zhang R , Singh KP , Vega F , et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168(11):5699-708. doi:10.4049/jimmunol.168.11.5699. PMID:12023369
- Wilson NJ , Boniface K , Chan JR , McKenzie BS , Blumenschein WM , Mattson JD , Basham B , Smith K , Chen T , Morel F , et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature Immunol. 2007;8(9):950-7. doi:10.1038/ni1497. PMID:17676044
- Lee E , Trepicchio WL , Oestreicher JL , Pittman D , Wang F , Chamian F , Dhodapkar M , Krueger JG . Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199(1):125-30. doi:10.1084/jem.20030451. PMID:14707118
- Ustekinumab [package insert] . Horsham (PA): Janssen Pharmaceuticals; 2016
- Gottlieb AB , Cooper KD , McCormick TS , Toichi E , Everitt DE , Frederick B , Zhu Y , Pendley CE , Graham MA , Mascelli MA . A phase 1, double-blind, placebo-controlled study evaluating single subcutaneous administrations of a human interleukin-12/23 monoclonal antibody in subjects with plaque psoriasis. Curr Med Res Opinion. 2007;23(5):1081-92. doi:10.1185/030079907X182112. PMID:17519075
- Kauffman CL , Aria N , Toichi E , McCormick TS , Cooper KD , Gottlieb AB , Everitt DE , Frederick B , Zhu Y , Graham MA , et al. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J Invest Dermatol. 2004;123(6):1037-44. doi:10.1111/j.0022-202X.2004.23448.x. PMID:15610511
- Krueger GG , Langley RG , Leonardi C , Yeilding N , Guzzo C , Wang Y , Dooley LT , Lebwohl M , CNTO 1275 Psoriasis Study Group . A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. New Eng J Med. 2007;356(6):580-92. doi:10.1056/NEJMoa062382. PMID:17287478
- Leonardi CL , Kimball AB , Papp KA , Yeilding N , Guzzo C , Wang Y , Li S , Dooley LT , Gordon KB; PHOENIX 1 study investigators . Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665-74. doi:10.1016/S0140-6736(08)60725-4. PMID:18486739
- Kimball AB , Gordon KB , Fakharzadeh S , Yeilding N , Szapary PO , Schenkel B , Guzzo C , Li S , Papp KA . Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: Results from the PHOENIX 1 trial through up to 3 years. Br J Dermatol. 2012;166(4):861-72. doi:10.1111/j.1365-2133.2012.10901.x. PMID:22356258
- Kimball AB , Papp KA , Wasfi Y , Chan D , Bissonnette R , Sofen H , Yeilding N , Li S , Szapary P , Gordon KB , et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27(12):1535-45. doi:10.1111/jdv.12046. PMID:23279003
- Papp KA , Langley RG , Lebwohl M , Krueger GG , Szapary P , Yeilding N , Guzzo C , Hsu MC , Wang Y , Li S , et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675-84. doi:10.1016/S0140-6736(08)60726-6. PMID:18486740
- Tsai TF , Ho JC , Song M , Szapary P , Guzzo C , Shen YK , Li S , Kim KJ , Kim TY , Choi JH , et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: A phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63(3):154-63. doi:10.1016/j.jdermsci.2011.05.005. PMID:21741220
- Zhu X , Zheng M , Song M , Shen YK , Chan D , Szapary PO , Wang B , LOTUS Investigators . Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: Results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol. 2013;12(2):166-74. PMID:23377389
- Paul C , Puig L , Kragballe K , Luger T , Lambert J , Chimenti S , Girolomoni G , Nicolas JF , Rizova E , Lavie F , et al. Transition to ustekinumab in patients with moderate-to-severe psoriasis and inadequate response to methotrexate: A randomized clinical trial (TRANSIT). Br J Dermatol. 2014;170(2):425-34. doi:10.1111/bjd.12646. PMID:24116959
- Reich K , Puig L , Paul C , Kragballe K , Luger T , Lambert J , Chimenti S , Girolomoni G , Nicolas JF , Rizova E , et al. One-year safety and efficacy of ustekinumab and results of dose adjustment after switching from inadequate methotrexate treatment: The TRANSIT randomized trial in moderate-to-severe plaque psoriasis. Br J Dermatol. 2014;170(2):435-44. doi:10.1111/bjd.12643. PMID:24116868
- Griffiths CE , Strober BE , van de Kerkhof P , Ho V , Fidelus-Gort R , Yeilding N , Guzzo C , Xia Y , Zhou B , Li S , Dooley LT , et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010 Jan 14;362(2):118-28. 27. doi:10.1056/NEJMoa0810652. PMID:20071701
- Landells I , Marano C , Hsu MC , Li S , Zhu Y , Eichenfield LF , Hoeger PH , Menter A , Paller AS , Taieb A , et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: Results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73(4):594-603. doi:10.1016/j.jaad.2015.07.002. PMID:26259989
- Lebwohl M , Leonardi C , Griffiths CE , Prinz JC , Szapary PO , Yeilding N , Guzzo C , Li S , Hsu MC , Strober B . Long-term safety experience of ustekinumab in patients with moderate-to-severe psoriasis (Part I of II): Results from analyses of general safety parameters from pooled Phase 2 and 3 clinical trials. J Am Acad Dermatol. 2012;66(5):731-41. doi:10.1016/j.jaad.2011.06.011. PMID:21930328
- Gordon KB , Papp KA , Langley RG , Ho V , Kimball AB , Guzzo C , Yeilding N , Szapary PO , Fakharzadeh S , Li S , et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (Part II of II): Results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66(5):742-51. doi:10.1016/j.jaad.2011.06.041. PMID:21978572
- Strober BE , Bissonnette R , Fiorentino D , Kimball AB , Naldi L , Shear NH , Goyal K , Fakharzadeh S , Calabro S , Langholff W , et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: Results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016 May; 74(5):851-61.e4. doi:10.1016/j.jaad.2015.12.017. PMID:26853180
- Papp K , Gottlieb AB , Naldi L , Pariser D , Ho V , Goyal K , Fakharzadeh S , Chevrier M , Calabro S , Langholff W , et al. Safety Surveillance for ustekinumab and other psoriasis treatments from the psoriasis longitudinal assessment and registry (PSOLAR). J Drugs Dermatol. 2015;14(7):706-14. doi:10.4049/jimmunol.1201505. PMID:26151787
- Yamauchi PS , Bagel J . Next-generation biologics in the management of plaque psoriasis: A literature review of IL-17 inhibition. J Drugs Dermatol. 2015;14(3):244-53. PMID:25738846
- Johnston A , Fritz Y , Dawes SM , Diaconu D , Al-Attar PM , Guzman AM , Chen CS , Fu W , Gudjonsson JE , McCormick TS , et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol. 2013;190:2252-62. doi:10.4049/jimmunol.1201505. PMID:23359500
- Secukinumab [package insert] . East Hanover (NJ): Novartis Pharmaceuticals; 2016
- Hueber W , Patel DD , Dryja T , Wright AM , Koroleva I , Bruin G , Antoni C , Draelos Z , Gold MH , Psoriasis Study Group, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72. doi:10.1126/scitranslmed.3001107. PMID:20926833
- Papp KA , Langley RG , Sigurgeirsson B , Abe M , Baker DR , Konno P , Haemmerle S , Thurston HJ , Papavassilis C , Richards HB . Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168(2):412-21. doi:10.1111/bjd.12110. PMID:23106107
- Rich P , Sigurgeirsson B , Thaci D , Ortonne JP , Paul C , Schopf RE , Morita A , Roseau K , Harfst E , Guettner A , et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168(2):402-11. doi:10.1111/bjd.12070. PMID:23362969
- Langley RG , Elewski BE , Lebwohl M , Reich K , Griffiths CE , Papp K , Puig L , Nakagawa H , Spelman L , Sigurgeirsson B , et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. New Engl J Med. 2014;371(4):326-38. doi:10.1056/NEJMoa1314258. PMID:25007392
- Paul C , Lacour JP , Tedremets L , Kreutzer K , Jazayeri S , Adams S , Guindon C , You R , Papavassilis C , JUNCTURE study group . Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: A randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29(6):1082-1090. doi:10.1111/jdv.12751. PMID:25243910
- Blauvelt A , Prinz JC , Gottlieb AB , Kingo K , Sofen H , Ruer-Mulard M , Singh V , Pathan R , Papavassilis C , Cooper S , et al. Secukinumab administration by pre-filled syringe: Efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172(2):484-93. doi:10.1111/bjd.13348. PMID:25132411
- Mrowietz U , Leonardi CL , Girolomoni G , Toth D , Morita A , Balki SA , Szepietowski JC , Regnault P , Thurston H , Papavassilis C , et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: A randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015;73(1):27-36.e21. doi:10.1016/j.jaad.2015.04.011. PMID:25982539
- Thaci D , Humeniuk J , Frambach Y , Bissonnette R , Goodman JJ , Shevade S , Gong Y , Papavassilis C , STATURE study group . Secukinumab in psoriasis: Randomized, controlled phase 3 trial results assessing the potential to improve treatment response in partial responders (STATURE). Br J Dermatol. 2015;173(3):777-87. doi:10.1111/bjd.13814. PMID:25823958
- Abrouk M, Gandy J, Nakamura M, Lee K, Brodsky M, Singh R, Zhu H, Farahnik B, Bhutani T, Koo J . Secukinumab in the treatment of psoriasis and psoriatic arthritis: A review of the literature. Skin Therapy Lett. 2017;22(4):1-6.
- van de Kerkhof PC , Griffiths CE , Reich K , Leonardi CL , Blauvelt A , Tsai TF , Gong Y , Huang J , Papavassilis C , Fox T . Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(1):83-98.e84. doi:10.1016/j.jaad.2016.03.024. PMID:27180926
- López-Ferrer A, Vilarrasa E, Puig L . Secukinumab (AIN457) for the treatment of psoriasis. Expert Rev Clin Immunol. 2015;11(11):1177-88. doi:10.1586/1744666X.2015.1095092.
- Hueber W , Sands BE , Lewitzky S , Vandemeulebroecke M , Reinisch W , Higgins PD , Wehkamp J , Feagan BG , Yao MD , Karczewski M , et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693-700. doi:10.1136/gutjnl-2011-301668. PMID:22595313
- Karle A , Spindeldreher S , Kolbinger F . Secukinumab, a novel anti-IL-17A antibody, shows low immunogenicity potential in human in vitro assays comparable to other marketed biotherapeutics with low clinical immunogenicity. mAbs. 2016;8(3):536-50. doi:10.1080/19420862.2015.1136761. PMID:26817498
- Taltz [package insert] . Indianapolis (IN): Eli Lilly and Company; 2016
- Krueger JG , Fretzin S , Suárez-fariñas M , Haslett PA , Phipps KM , Cameron GS , McColm J , Katcherian A , Cueto I , White T , et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130(1):145-54.e9. doi:10.1016/j.jaci.2012.04.024. PMID:22677045
- Leonardi C , Matheson R , Zachariae C , Cameron G , Li L , Edson-Heredia E , Braun D , Banerjee S . Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190-9. doi:10.1056/NEJMoa1109997. PMID:22455413
- Gordon KB , Leonardi CL , Lebwohl M , Blauvelt A , Cameron GS , Braun D , Erickson J , Heffernan M . A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2014;71(6):1176-82. doi:10.1016/j.jaad.2014.07.048. PMID:25242558
- Gordon KB , Blauvelt A , Papp KA , Langley RG , Luger T , Ohtsuki M , Reich K , Amato D , Ball SG , Braun DK , et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345-56. doi:10.1056/NEJMoa1512711. PMID:27299809
- Griffiths CE , Reich K , Lebwohl M , van de Kerkhof P , Paul C , Menter A , Cameron GS , Erickson J , Zhang L , Secrest RJ , et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials. Lancet. 2015;386(9993):541-51. doi:10.1016/S0140-6736(15)60125-8. PMID:26072109
- Syed YY . Ixekizumab: A review in moderate to severe plaque psoriasis. Am J Clin Dermatol. 2017;18(1):147-158. doi:10.1007/s40257-017-0254-4. PMID:28138946
- Strober B , Leonardi C , Papp KA , Mrowietz U , Ohtsuki M , Bissonnette R , Ferris LK , Paul C , Lebwohl M , Braun DK , et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: Etanercept comparisons and integrated data. J Am Acad Dermatol. 2017;76(3):432-440.e17. doi:10.1016/j.jaad.2016.09.026. PMID:27889292
- Kimball AB , Schenfeld J , Accortt NA , Anthony MS , Rothman KJ , Pariser D . Incidence rates of malignancies and hospitalized infectious events in patients with psoriasis with or without treatment and a general population in the U.S.A.: 2005-09. Br J Dermatol. 2014;170(2):366-73. doi:10.1111/bjd.12744. PMID:24251402
- Reich K , Leonardi C , Langley RG , Warren RB , Bachelez H , Romiti R , Ohtsuki M , Xu W , Acharya N , Solotkin K , et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: A presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J Am Acad Dermatol. 2017;76(3):441-448.e2. doi:10.1016/j.jaad.2016.10.027. PMID:28027825
- Blauvelt A , Cameron G , Gordon K , et al. Ixekizumab, a novel anti-IL-17A antibody, exhibits low immunogenicity during long-term treatment in patients with psoriasis [abstract no. 3232]. J Am Acad Dermatol. 2016;74(5 Suppl 1):AB258
- SiliqTM [prescribing information] . Bridgewater (NJ): Valeant Pharmaceuticals North America LLC; 2017
- Papp KA , Reid C , Foley P , Sinclair R , Salinger DH , Williams G , Dong H , Krueger JG , Russell CB , Martin DA . Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: Results from a phase i, randomized, placebo-controlled trial. J Invest Dermatol. 2012;132(10):2466-9. doi:10.1038/jid.2012.163. PMID:22622425
- Osamu N , Hirotaka N , Koji S , Kenji T . Clinical pharmacology of the anti-IL-17 receptor antibody brodalumab (KHK4827) in Japanese normal healthy volunteers and Japanese subjects with moderate to severe psoriasis: A randomized, dose-escalation, placebo controlled study. J Dermatol Sci. 2014;75(3):201-4. doi:10.1016/j.jdermsci.2014.05.007. PMID:24957501
- Papp KA , Leonardi C , Menter A , Ortonne JP , Krueger JG , Kricorian G , Aras G , Li J , Russell CB , Thompson EH , et al. Brodalumab, an anti interleukin-17–receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181-9. doi:10.1056/NEJMoa1109017. PMID:22455412
- Papp KA , Reid C , Foley P , Sinclair R , Salinger DH , Williams G , Dong H , Krueger JG , Russell CB , Martin DA . Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: Results from a phase i, randomized, placebo-controlled trial. J Invest Dermatol. 2012;132(10):2466-9. doi:10.1038/jid.2012.163. PMID:22622425
- Papp K , Leonardi C , Menter A , Thompson EH , Milmont CE , Kricorian G , Nirula A , Klekotka P . Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71(6):1183-90. doi:10.1016/j.jaad.2014.08.039. PMID:25313095
- Nakagawa H , Niirob H , Ootaki K , Japanese brodalumab study group . Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: Efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81(1):44-52. doi:10.1016/j.jdermsci.2015.10.009. PMID:26547109
- Papp K , Menter A , Strober B , Kricorian G , Thompson EH , Milmont CE , Nirula A , Klekotka P . Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to severe plaque psoriasis. J Am Acad Dermatol. 2015;72(3):436-9. doi:10.1016/j.jaad.2014.10.026. PMID:25553889
- Umezawa Y , Nakagawa H , Niiro H , Ootaki K , Japanese Brodalumab Study Group . Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30(11):1957-60. doi:10.1111/jdv.13785. PMID:27358210
- Papp K , Reich K , Leonardi C , et al. AMAGINE-1: A phase 3, randomized, doubleblind, placebo-controlled study of brodalumab in subjects with psoriasis. Br J Dermatol. 2014;171(6):e119-20
- Papp K , Reich K , Leonardi C , et al. Efficacy and safety of brodalumab in patients with moderate to severe plaque psoriasis: Results of AMAGINE-1, a phase 3, randomized, double-blind, placebo-controlled study through week 12. J Am Acad Dermatol. 2015;72(5 Suppl 1):AB233
- Papp KA , Reich K , Paul C , Blauvelt A , Baran W , Bolduc C , Toth D , Langley RG , Cather J , Gottlieb AB , et al. A prospective phase III, randomized, doubleblind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273-86. doi:10.1111/bjd.14493. PMID:26914406
- Lebwohl M , Strober B , Menter A , Gordon K , Weglowska J , Puig L , Papp K , Spelman L , Toth D , Kerdel F , et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318-28. doi:10.1056/NEJMoa1503824. PMID:26422722
- Farahnik B , Beroukhim K , Abrouk M , Nakamura M , Zhu TH , Singh R , Lee K , Bhutani T , Koo J . Brodalumab for the treatment of psoriasis: A review of phase III trials. Dermatol Ther. 2016;6(2):111-24. doi:10.1007/s13555-016-0121-x. PMID:27221323
- Marcus KA . Overview of the July 19, 2016 DODAC meeting. Dermatologic and Ophthalmic Drugs Advisory Committee Meeting. 19 July 2015. https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/dermatologicandophthalmicdrugsadvisorycommittee/ucm511357.pdf (accessed 15 May 2017)
- Toichi E , Torres G , McCormick TS , Chang T , Mascelli MA , Kauffman CL , Aria N , Gottlieb AB , Everitt DE , Frederick B , et al. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J Immunol. 2006;177(7):4917-26. doi:10.4049/jimmunol.177.7.4917. PMID:16982934
- Puig L . The role of IL 23 in the treatment of psoriasis. Expert Rev Clin Immunol. 2017;13(6):525-34. doi:10.1080/1744666X.2017.1292137. PMID:28165883
- Teng MW , Bowman EP , McElwee JJ , Smyth MJ , Casanova JL , Cooper AM , Cua DJ . IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21(7):719-29. doi:10.1038/nm.3895. PMID:26121196
- Lowes MA , Suárez-Fariñas M , Krueger JG . Immunology of psoriasis. Annu Rev Immunol. 2014;32:227-55. doi:10.1146/annurev-immunol-032713-120225. PMID:24655295
- Villanova F , Flutter B , Tosi I , Grys K , Sreeneebus H , Perera GK , Chapman A , Smith CH , Di Meglio P , Nestle FO . Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol. 2014;134:984-91. doi:10.1038/jid.2013.477. PMID:24352038
- Keijsers RR , van der Velden HM , van Erp PE , de Boer-van Huizen RT , Joosten I , Koenen HJ , van de Kerkhof PC . Balance of Treg vs. T-helper cells in the transition from symptomless to lesional psoriatic skin. Br J Dermatol. 2013;168:1294-302. doi:10.1111/bjd.12236. PMID:23330679
- Sofen H , Smith S , Matheson RT , Leonardi CL , Calderon C , Brodmerkel C , Li K , Campbell K , Marciniak SJ Jr , Wasfi Y , et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. Allergy Clin Immunol. 2014;133(4):1032-40. doi:10.1016/j.jaci.2014.01.025. PMID:24679469
- Gordon KB , Duffin KC , Bissonnette R , Prinz JC , Wasfi Y , Li S , Shen YK , Szapary P , Randazzo B , Reich K . A Phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373(2):136-44. doi:10.1056/NEJMoa1501646. PMID:26154787
- Blauvelt A , Papp KA , Griffiths CE , Randazzo B , Wasfi Y , Shen YK , Li S , Kimball AB . Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405-17. doi:10.1016/j.jaad.2016.11.041. PMID:28057360
- Reich K , Armstrong AW , Foley P , Song M , Wasfi Y , Randazzo B , Li S , Shen YK , Gordon KB . Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017 Mar; 76(3):418-31. doi:10.1016/j.jaad.2016.11.042. PMID:28057361
- Kopp T , Riedl E , Bangert C , Bowman EP , Greisenegger E , Horowitz A , Kittler H , Blumenschein WM , McClanahan TK , Marbury T , et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature. 2015;521(7551):222-6. doi:10.1038/nature14175. PMID:25754330
- Papp K , Thaçi D , Reich K , Riedl E , Langley RG , Krueger JG , Gottlieb AB , Nakagawa H , Bowman EP , Mehta A , et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173(4):930-9. doi:10.1111/bjd.13932. PMID:26042589
- ClinicalTrials.gov . A 64-week, phase 3, randomised, placebo-controlled, parallel design study to evaluate the efficacy and safety/tolerability of subcutaneous tildrakizumab (SCH 900222/MK-3222), followed by an optional long-term safety extension study, in subjects with moderate-to-severe chronic plaque psoriasis (protocol no. MK-3222 010). https://clinicaltrials.gov/ct2/show/NCT01722331
- ClinicalTrials.gov . A study to evaluate the efficacy and safety/tolerability of subcutaneous MK-3222 in participants with moderate-to-severe chronic plaque psoriasis (MK-3222-012). http://www.clinicaltrials.gov/ct2/show/NCT01936688
- ClinicalTrials.gov . A 52-week, phase 3, randomised, active comparator and placebo-controlled, parallel design study to evaluate the efficacy and safety/tolerability of subcutaneous SCH 9000222/MK3222, followed by an optional long-term safety extension study, in subjects with moderate to severe chronic plaque psoriasis. https://clinicaltrials.gov/ct2/show/NCT01729754
- Reich K , Papp K , Blauvelt A , et al. Tildrakizumab, a selective IL-23p19 antibody, in the treatment of chronic plaque psoriasis: Results from two randomized, controlled, phase 3 trials (reSURFACE 1 and reSURFACE 2). Poster presented at: the European Academy of Dermatology and Venereology Congress; October 1, 2016; Vienna, Austria.
- Galluzzo M , D'adamio S , Bianchi L , Talamonti M . Tildrakizumab for treating psoriasis. Expert Opin Biol Ther. 2017;17(5):645-57. doi:10.1080/14712598.2017.1304537. PMID:28271735
- Krueger JG , Ferris LK , Menter A , Wagner F , White A , Visvanathan S , Lalovic B , Aslanyan S , Wang EE , Hall D , et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136(1):116-124.e7. doi:10.1016/j.jaci.2015.01.018. PMID:25769911
- Papp KA , Blauvelt A , Bukhalo M , Gooderham M , Krueger JG , Lacour JP , Menter A , Philipp S , Sofen H , Tyring S , et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376(16):1551-60. doi:10.1056/NEJMoa1607017. PMID:28423301
- Papp KA , Tyring S , Lahfa M , Prinz J , Griffiths CE , Nakanishi AM , Zitnik R , van de Kerkhof PC , Melvin L , Etanercept Psoriasis Study Group, et al. A global phase III randomized controlled trial of etanercept in psoriasis: Safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152(6):1304-12. doi:10.1111/j.1365-2133.2005.06688.x. PMID:15948997
- Menter A , Tyring SK , Gordon K , Kimball AB , Leonardi CL , Langley RG , Strober BE , Kaul M , Gu Y , Okun M , et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106-15. doi:10.1016/j.jaad.2007.09.010. PMID:17936411
- Reich K , Nestle FO , Papp K , Ortonne JP , Evans R , Guzzo C , Li S , Dooley LT , Griffiths CE , EXPRESS study investigators . Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: A phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367-74. doi:10.1016/S0140-6736(05)67566-6. PMID:16226614
- Enbrel [prescribing information] . Thousand Oaks, (CA): Amgen; 1998
- Humira [prescribing information] . North Chicago (IL): Abbvie Inc; 2002
- Remicade [prescribing information] . Horsham (PA): Janssen Biotech Inc; 1998
- Kui R , Gál B , Gaál M , Kiss M , Kemény L , Gyulai R . Presence of antidrug antibodies correlates inversely with the plasma tumor necrosis factor (TNF)-α level and the efficacy of TNF-inhibitor therapy in psoriasis. J Dermatol. 2016;43(9):1018-23. doi:10.1111/1346-8138.13301. PMID:26892625
