ABSTRACT
In this study, purified GM-CSF and LMP2A mRNAs were amplified by PCR. Then, the GM-CSF and LMP2A sequences were connected by the polypeptide linker (Gly4Ser)3 using gene splicing by overlap extension. The constructed fusion gene GC2A was inserted into the adenovirus vector. Then the recombinant vector was introduced into HEK 293T cells by calcium phosphate transfection to package the adenovirus. The levels of antibodies against the GM-CSF and LMP2Afusion proteins were measured by ELISA, and the CTL activity of the mouse splenic lymphocytes was determined by lactate dehydrogenase (LDH) release assay. Immunotherapy of mouse tumor (EBV-positive epithelial tumor cell line (GT39)) tissues was performed, and their morphologies were assessed. Finally, the data of each group were analyzed using SPSS 11.5 statistical software. The recombinant adenovirus could replicate in HEK 293Tcells and induce humoral and cellular immune responses in the mice. The maximum dose resulted in an antibody titer of 18500 (184.5 ± 8.7 pg/ml). At an effector: target ratio of 40:1, maximum specific lysis was observed which was approximately three times that detected in the control immunized mice. The tumor inhibition rate was approximately 76% compared with the control groups, indicating the presence of significant differences among the groups. Tumor-infiltrating lymphocytes were detected by hematoxylin-eosin (HE) staining. The recombinant adenovirus induced humoral and cellular immune responses and inhibited tumor growth in mice. It provided a theoretical basis and candidate vaccine for further preclinical trials.
Introduction
Epstein-Barr virus (EBV) is a γ-herpes virus with B lymphocyte tropism. It was been found by Epstein and Barr when they researched the malignant lymphoma of african children in 1964 andits full-length genes is 184 kb. EBV can cause two different types of infection, one is a proliferative infection, the other is a non proliferative infection under certain conditions. Due to some induced factors, Latent EBV genome can be activated and transformed to the proliferation of infection. In addition, the EBV infected and transformed host cell may lead to abnormal chromosome translocation, cell proliferation and malignant transformation. Citation1 EBV encodes a viral protein that causes “immortality” and continuous cell growth, eventually resulting in the development of cancer in some individuals. Citation2 EBV affects p53 regulation and causes tumor formation. Citation3 So EBV infection causes of a number of human malignancies, including nasopharyngeal carcinoma, Burkitt's lymphoma, Hodgkin's lymphoma and stomachcancer. Therefore, it is urgent to develop a safe and effective EB vaccine to prevent many diseases and tumors caused by the virus. Because of the potential carcinogenicity of EBV, EBV live attenuated vaccine is not good for prevention of human health, vaccine research carried out based on EBV subunit vaccine has become a hot research, but there is not EB virus subunit vaccine reported. Citation4
The LMP2A gene encodes the EBV latent membrane protein. LMP2A contains epitopes that are recognized specifically by virus-specific CTLs. This protein mediates the activity of cytotoxic T cells and is an ideal target antigen. Citation5 Recent studies have examined LMP2A gene expression in vivo and EBV latent infection of B cells in vitro. Other studies have confirmed that approximately 50% of EBV-associated gastric cancer cases are LMP2A-positive and that LMP2A is expressed in nasopharyngeal carcinoma and lymphoma. Other types of tumor cells that stably express one of the few conservative EBV antigens have been demonstrated to activate T cell epitopes and to be mediated by cytotoxic T cells. Citation6 Redchenko and Rickinson Citation7 used the LMP2A epitope peptide to load DCs and demonstrated the induction of strong, specific CTL immune responses, indicating that LMP2A is an ideal target antigen for immunotherapy of EBV-associated tumors. Therefore, we hypothesized that if the LMP2A gene is inserted into an expression vector, alone or in combination with another gene, the cells may express this target antigen and induce the production of specific cellular or humoral immunity, preventing EBV infection and killing EBV-positive tumor cells.
GM-CSF is currently a focus of anti-cancer research. This glycoprotein cytokine is 144 aa in length, and its molecular weight is approximately 22 kDa. It strongly regulates dendritic cells (DCs), promoting their proliferation and differentiation. It also plays a very important regulatory role in maintaining DC viability and distribution and antigen presentation. The transfer of GM-CSF into tumor cells could greatly enhance their immunogenicity and effectively improve the human anti-tumor response. Citation8 GM-CSF not only promotes DC proliferation, differentiation, and viability but also plays an important regulatory role in DC distribution in vivo and in antigen presentation. The transfer of GM-CSF into tumor cells could greatly enhance their immunogenicity, improving the organism-specific anti-tumor response. GM-CSF promotes expression of MHC-I molecules on DCs and CD8+ Tcells in tumor-bearing animals in vivo and significantly increases activity of the immune co-stimulatory molecule B7-1, greatly enhancing the tumor-specific CTL killing ability of mouse splenic cells. GM-CSF recombinant adenovirus also effectively induces anti-tumor immunity; intratumoral injection of a GM-CSF expression vector cotransformation could enhance the body's anti-tumor response. Citation8,Citation9 GM-CSF expression activates or attracts antigen-presenting cells to the tumor site, and it is involved in the anti-tumor immune response.
In this study, we constructed a fusion gene formed from latent membrane protein 2A of EBV and granulocyte-macrophage colony stimulating factor (GM-CSF) for insertion into an adenoviral expression vector to develop a new method for the targeted killing of tumor cells. The aim was to express GM-CSF and LMP2A proteins at the same time and the fusin gene vaccine could take a synergistic, collaborative and promoting effect in the human body.
Results
Construction and identification of recombinant adenovirus vector
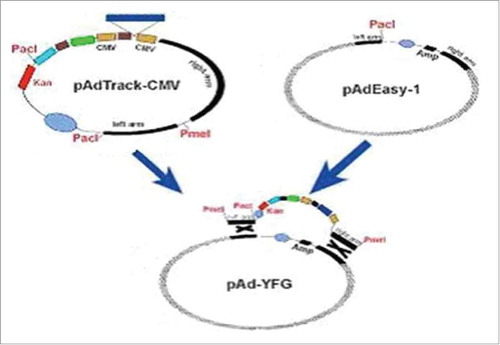
The PCR amplification products showed identical theoretical values. The target genes GM-CSF and LMP2A, which were amplified by RT-PCR, were approximately 470 bp and 1545 bp in size, as determined by 1% agarose gel electrophoresis. The sizes were consistent with the expected results (). The fusion gene was amplified by PCR and analyzed by 1% agarose gelelectrophoresis. The results were consistent with the expected results (). Sequence analysis of the GC2A fusion gene revealed that itwas1961 bp, which is consistent with the theoretical value (461 +1545-45 bp = 1961 bp). Bg1II and EcoRV were used to digest GC2A, and then GC2A was cloned into pAdTrack-CMV to construct pAdTrack-CMV-GC2A. Bg1II and EcoRV were used to analyze the plasmid DNA, and electrophoresis was performed, which revealed the presence of approximately 1961 bp and 9200 bp bands (). Restriction enzyme digestion cut the recombinant adenovirus vector into fragments of approximately 33 kb and 4.5kb in size, indicating that recombination occurred in the ori and right arm of pAdTrack-CMV GC2A and pAdEasy-1. The results are shown in ).
Figure 1. A. Electrophoresis results for GM-CSF and LMP2A. Lane 1 = DNA DL2000 Marker; lanes 2 and 3 = GM-CSF gene; lane 4 = DNA DL2000 Marker; lane 5 = LMP2A gene. B. Electrophoresis results for the fusion gene GC2A. Lane 1 = DNA DL2000 Marker; lanes 2 and 3 = GC2A genes. C. Identification of pAdTrack-CMV-GC2A. Lane 1 = enzyme analysis after Bg1II and EcoRV digest; lane 2 = DNA DL2000 Marker; lane 3 = pAdTrack-CMV-GC2A. D. Restriction enzyme digestion for pAd-GC2A analysis and identification. Lane 1 = DNA DL15000 Marker; lane 2 = PacI enzyme digestion of recombinant vector.
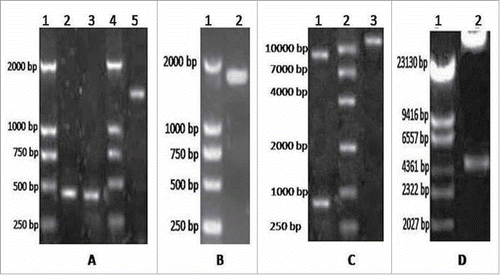
Figure 2. Delection of the fusion gene GM-CSF and LMP2A expression in recombinant adenovirus. A. The 293 cells transfected by recombinant adenovirus vectors (200 ×,56 h). B. Western blotting analysis of expressed (Lane 1 = western blotting with GM-CSF of Adenovirus 5 control; lane 2 = western blotting with GM-CSF of vAd-GC2A; lane 3 = Protein Markers; lane 4 = western blotting with LMP2A of Adenovirus 5 control; lane 5 = western blotting with LMP2A of vAd-GC2A; lane 6 = protein markers).
Adenovirus vector packaging
After PacI digestion was performed to linearize pAd-GC2A, the calcium phosphate method was used to transfect HEK 293T cells. At 56 h after transfection, green fluorescent cells were observed under a fluorescence microscope, indicating that the transfected eukaryotic cells expressed exogenous genes (). Protein was isolated from the infected HEK 293T cells and analyzed by SDS-PAGE and Western blotting. The results revealed that the protein offusion gene from GM-SCF and LMP2A was expressed in vAd-GC2A (). Molecular mass of the fusion protein was about 72KDa. This was consistent with the theoretical values (GM-CSF approximately 22KDa, LMP2A approximately 50KDa).
Analysis of antibody levels in peripheral blood of mice immunized with recombinant adenovirus
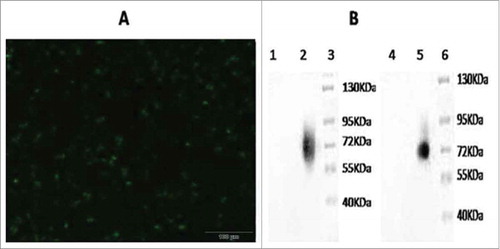
The mice were immunized 7 times at 2-week intervals. The tail blood of the mice was collected once every 2 weeks. Serum was isolated from the blood samples, the LMP2A (GM-CSF) recombinant antigen was prepared and antibody detection was performed by enzyme-linked immune sorbent assay (ELISA). At low doses of recombinant adenovirus, fewer antibodies were produced compared with high doses of the immune antigen. Antibodies were produced relatively rapidly at high doses, resulting in relatively high antibody levels. Specifically, the antibody titers in the blood of mice at 1 week before immunization were close to zero; at 1 week after immunization, the antibody titer at the maximum dose was 7500 (74.8 ± 8.4 pg/mL), and that at the secondary dose was 5500 (55.5 ± 8.2 pg/mL). The mice immunized with wild-type control strains and PBS had antibody titers of close to zero. With extension of the immunization time, the recombinant adenovirus stimulated the mice to increase the rate of antibody production. At 6 weeks after immunization, the antibody titer at the maximum dose was 18500 (184.5 ± 8.7 pg/mL), and that at the secondary dose was 12,500 (126 ± 7.2 pg/mL). At 7 weeks after immunization, no increase in the antibody titer was observed in the experimental group. In contrast, the two control groups did not display increased antibody titers at any time point during the experiment. This difference was statistically significant (P ≤ 0.05) ().
Figure 3. Specific IgG antibody response of mice immunized with recombinant adenovirus by intraperitoneal injection. Recombinant adenovirus maximum dose was 5 × 108 virus particles/100 μl, recombinant adenovirus secondary dose was 5 × 107 virus particles/100 μl, and PBS control dose was 100 μl. The recombinant adenovirus maximum dose exerted the most proliferative effect, antibody titers peaked on week 7.
CTL activity in mouse spleen after recombinant adenovirus immunization
In this study, the experimental mice were divided into a recombinant adenovirus group, (adenovirus 5+ hGM-CSF) group, (adenovirus 5+ LMP2A) group, (adenovirus 5+ plasmid) group, adenovirus type 5 wild-type strain group and a PBS control group, with six mice in each group. After the mice were immunized, their spleens were collected, and splenic cells were isolated. EB virus-positive tumor cells were harvested as the target cells and the effector/target ratios (E/T) were set to 10:1, 20:1, and 40:1, 60:1. The absorbance was measured after cells from each group were incubated. The CTL killing rate of EBV-positive tumor cells(GT39) was calculated using the following formula: cell killing rate (%) = [(average value A of the experimental group- control group value A of naturally released target cells-the control group value A of naturally released effector cells) / (average value A of the maximum release of target cells - average value A of naturally released target cells)] × 100%. The lactate dehydrogenase assay results showed that the CTL activity of the mice immunized with recombinant adenovirus was significantly higher compared with the mice in the adenovirus type 5 and PBS groups (P < 0.01), suggesting that infection of the mouse lymphocytes with the recombinant virus vAd-GC2A played a role in antigen presentation and effectively stimulated CTL responses (). The specific cytotoxic effects of the spleen cells from the (Adenovirus 5+ hGM-CSF) group were also increased. But it was lower than the effects induced by the recombinant adenovirus group (P < 0.05).
Table 1. Specific CTL killing activity of immunized mice (%).
The statistical results showed that the recombinant adenovirus-mediated activation of CTLs was obvious. It shows the CTL activities induced by different stimuli, presented as EBV-positive tumor cell killing rates (%). The rates were 66.7 ± 6.9% in the recombinant adenovirus experimental group and32.4 ± 3.1% and 24.3 ± 2.5% in the adenovirus 5 wild-type and PBS groups, respectively. This difference was statistically significant (P ≤ 0.05).
Effect of recombinant adenovirus vaccine on EBV-positive tumors in mice
The mice were sacrificed. Fat and blood were removed and tumor volumes were measured. Comparison of the adenovirus vaccine treatment group with the adenovirus 5 wild-type control group revealed that both groups of mice had tumors, but that the tumor formation ability of the vaccine group was significantly reduced compared to that in the adenovirus 5 wild-type control group. Furthermore, the tumor volume of the vaccine group was significantly lower than that of the adenovirus 5 control group (306.23 ± 108.2 mm3 and 1274.0 ± 115 mm3, respectively). Comparison of the tumor volumes between the two groups revealed that their distributions met the normality assumption but that they did not display homogeneity of variance, indicating that the difference in tumor volume between the two groups was statistically significant (P < 0.01) ( and ).
Figure 4. Tumor size. The adenovirus vaccine group was injected with 2 × 108 plaque-forming unit recombinant adenovirus, other groups were injected with 2 × 108 plaque-forming unit (adenovirus 5+ LMP2A), (adenovirus 5+ hGM-CSF), (adenovirus 5+ plasmid) (soluble in 250 μL PBS)/mouse, respectively. The adenovirus 5 control group was injected with 2 × 108 plaque-forming unit adenovirus 5 (soluble in 250 μL PBS)/mouse, the PBS control group was injected with 250 μL PBS/mouse.
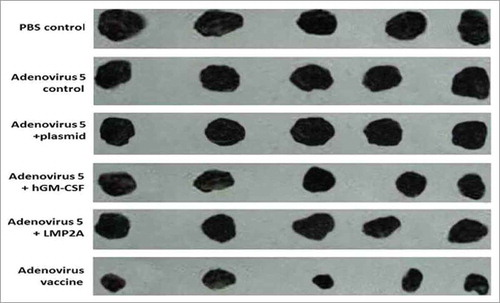
Table 2. Subcutaneous tumor model results (P < 0.01).
The results showed that the adenovirus vaccine effectively inhibited tumor growth and induced specific tumor rejection while protecting the animals against EBV-mediated transformation of cells to malignant tumors, clearing tumor cells from the mouse bodies.
Morphological observation of tumor tissue
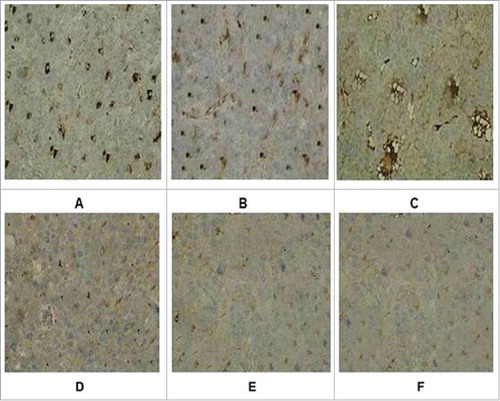
The tumor tissue was completely stripped and immediately fixed with formalin solution, and then stained with HE and immunohistochemistry (immunohistochemical staining with Rabit anti-CD4 antibody). The tumor tissues were infiltrated by local lymphocytes. The recombinant adenovirus produced strong cellular immunity that resulted in the killing of tumor cells and restriction of tumor growth. Under microscopy, the cells of CD4+ in the tumor tissues were stained with brown (). The numbers of CD4+ in recombinant adenovirus 5 group were (23.873+ 1.09/ high-power visual field), (adenovirus 5+ LMP2A) group were (13.39+ 2.16/ high-power visual field), (adenovirus 5+ hGM-CSF) group were (10.54 + 2.41/ high-power visual field), significantly higher than adenovirus 5 group (2.37+ 2.05/ high-power visual field) and PBS blank control group (5.278+ 2.18/ high-power visual field).
Figure 5. Immunohistochemical staining of tumor tissue in groups. (A) Recombinant adenovirus 5 group. (B)(Adenovirus 5+ LMP2A) group. (C) (Adenovirus 5+ hGM-CSF) group. (D) (Adenovirus 5+ plasmid) group. (E) Adenovirus 5. (F) PBS. The tumor cells in the recombinant adenovirus 5 group compared with the control group, the CD4 cells were more, and in a large number of CD4 in tumor tissue; Adenovirus 5(LMP2A) and Adenovirus 5 (hGM-CSF) group can also be seen with a certain extent part of tumor tissue necrosis and some of CD4 cells, but CD4 cells were less than recombinant adenovirus 5 group, there is no significant difference in the staining results on adenovirus 5 and (adenovirus 5+ plasmid) group. PBS had no tumor necrosis and CD4 cells.
Discussion
EBV is strongly associated with the formation of many types of tumors, including large-cell lymphoma, which often occurs in immune compromised patients. However, the available treatments for EBV-associated tumors are not effective. With advances in tumor molecular biology, immunology methods used to prevent cancer are useful complements to the current preventative and treatment methods. Thus, these immunology methods have attracted attention in this field. Citation5 In recent years, a large number of in-depth studies of a variety of cytokines and antigen-presenting cells have been conducted to determine their anti-tumor effects.
Intratumoral injection of recombinant adenovirus expressing the GM-CSF gene has been shown to effectively induce the human anti-tumor immune response. Citation8 The LMP2A protein effectively triggers the CTL response, and many researchers have attempted to induce LMP2A CTLs for the treatment of EBV-positive tumors. The LMP2A gene encodes the EBV latent membrane protein, which stimulates the production of antibodies. Citation10,Citation11 Insertion of the LMP2A and GM-CSF genes into an adenoviral expression vector would result in the expression of both antigens. This expression would promote the cellular and humoral immune responses in the body, aiding in resistance to EBV infection and EBV-positive cell production by tumors.
It has been found that the adenovirus vector carrying various antigens can stimulate the body to produce strong humoral immunity and cellular immunity. In addition, the adenovirus vector can infect the respiratory tract and intestinal cells, which can be conveniently used to immunize the mucosa and induce the mucosal and systemic immune responses. Citation12 Adenovirus vectors have a wide range of hosts, are able to infect cells in the dividing, resting, and terminal phases, and are safe for use in humans. They are typically used as carriers in genetic engineering, Citation13,Citation14 and are considered good vectors for tumor vaccine development that can be used to express other genes. Many previous studies Citation15,Citation16 have shown that recombinant adenovirus produces strong cellular immunity, which can result in the killing of tumor cells and restriction of tumor growth.
In EBV related tumors, the presence of EBV provides a good target for immunotherapy. Application of EBV's especial CTL in the treatment and prevention of EBV inducing lymphoproliferative disorders have achieved success. In the United States, Rooney Citation17 matched allogeneic CTL treatment of EBV inducing B lymphocyte proliferation. This was the first successful use of EBV's special HLA, the clinical effect was very significant, strategies for the use of specific CTL to treat and prevent EBV related diseases and tumors have been developed. Using LMP2 antigen determinant is very important in the current treatment of EB virus relevant malignant diseases. However, it is limited in the field of using single gene therapy, and the effect on the EB virus positive tumor is limited and there is no report on the application more than 2 genes. Although the research of EB vaccine has been reported, but there is not effective vaccine for clinical application. Citation4
Cytokine recombinant fusion protein is using a kind of genetic engineering method to connect the encoded cytokine and other specific protein gene sequences to express the corresponding protein fusion products. Each structural feature of the fusion protein is the functional domain of cytokines and be fused with other active domains and can play a synergistic role. In this study, a recombinant adenovirus was successfully constructed. The fusion gene GC2A, through joint action, has been shown to stimulate the body's immune system to kill tumor cells. The underlying mechanism of this process remains to be determined, which would provide a foundation for the development of genetic preventative and therapeutic methods. In this experiment, the recombinant adenovirus could replicate in HEK 293T cells and induce humoral and cellular immune responses in the mice. The results revealed that at low doses of immune antigen, the rate of antibody production was relatively low. In contrast, at high doses of recombinant adenovirus, the rate of antibody production was high, and relatively high antibody levels were observed. The maximum dose resulted in an antibody titer of 18500. It showed the recombinant adenovirus induced significant humoral immune responses. LMP2A is the surface antigen of EB virus, if LMP2A antibody could bind and neutralize the free EB virus, EB virus would not cause tumor.
At an effector: target ratio of 40:1, maximum specific lysis was observed in the immunized mice. When the effector: target ratio ≥10:1, the effector: target ratio does not influence the extent of specific lysis. But if it was compared with control, it was very significant. These showed that recombinant adenovirus induced significant CTL immune responses. Tumor-infiltrating lymphocytes were detected by hematoxylin-eosin (HE) staining. It showed the recombinant adenovirus induced obvious cellular immune responses. Mice bearing recombinant vAd-GC2A-infected lymphocytes exhibited antigen presentation and effective stimulation of the CTL response. The results of this study have demonstrated that recombinant adenovirus expressing a fusion gene formed from GM-CSF andLMP2A can take advantage of LMP2A to induce specific CTL generation and immune enhancement of GM-CSF, which synergistically functions toil EBV-positive tumor cells. The tumor inhibition rate of the recombinant adenovirus vaccine was approximately 76% compared with the control groups, indicating the presence of significant differences among the groups.
In this study, the research method is innovative. Using splicing overlap extension technology, the fusion gene of DNA was obtained by connecting the GM-CSF gene with LMP2A gene through the polypeptide linker (Gly4Ser)3 sequence. The polypeptide linker is composed of a flexible amino acid sequence without affecting the activity of GM-CSF and LMP2A. We used adenovirus expression vector to express the fusion protein GC2A, So GM-CSF andLMP2A proteins can play a better role in activity simultaneously. The results showed that LMP2A and hGM-CSF fusion gene had a synergistic effect of the fusion product and postulated humoral and cellular immune response, which could cause the amount of CD4+ and CTL increasing, we will do further study in antitumor experiments by flow cytometryn to quantify and characterize the CD3+ and CD8+ cell in tumor infiltrating tissue. The research provided a theoretical basis and candidate vaccine for further preclinical trials. It can be used in children and adolescents for the prevention of EB virus infection and also be used for the treatment and prevention of EB virus positive tumors.
Material and methods
Reagents
Taq DNA polymerase and T4 DNA ligase were purchased from Promega Corporation (Madison, WI, USA). DNA marker, EcoRI, SalI, BamHI, BglII, EcoRV, PmeI and PacI were provided by Boya Company (Shanghai, China). DNA transfection kits were acquired from Invitrogen (Carlsbad, CA, USA). GM-CSF and LMP2A antibodies were obtained from Abcam (Cambridge, UK). Primers were synthesized by Sangon Biotech Co. (Shanghai, China). pAdTrack-CMV and a skeleton plasmid, pAdEasy-1, were purchased from Puruting Biotechnology Company (Beijing, China). Healthy BALB/c(SPF) female mice, 2 weeks of age were purchased from Vital River Co.(Beijing, China).
Methods
The following pair of polymerase chain reaction (PCR) primers was designed for LMP2A and GM-CSF:
F1: 5′-GAA GAT CTA TGC ACC CGC CCG CTC GCC CAG-3′
Downstream overlapping primer F2:
5′-GCT GCC GCC ACC GCC GCT TCC GCC ACC GCC GCT TCC ACC GCCACC CTC CTG GAC TGG CTC CCA-3′
The BglII restriction site and protective bases GA were introduced at the 5′ end of the GM-CSF primer and the stop codon TGA was removed from the end of the downstream primer. The linker was introduced into the 5′ downstream LMP2A primer at the EcoRV restriction sites and retained bases GC, and the upstream primer was located at the 3′ end of the introduced linker. The polypeptide DNA sequence of the linker (Gly4Ser)3, encoding 15 amino acids, was as follows: 5′-GGT GGC GGT GGA AGC GGC GGTGGC GGA AGC GGC GGT GGC GGC AGC-3′. In addition, the primer sequences were as follows: LMP2A upstream overlapping primer F3: 5′-GGT GGC GGA GGT AGCGGC GGT GGC GGA AGC GGC GGT GGC GGC AGC ATG GGG TCC CTA GAAATG GTG-3′; downstream primer F3: 5′-GC GAT ATC TAC AGT GTT GCG ATATGG GGT-3′; GM-CSF downstream primer F5: 5′-GC GAT ATC TCA CTC CTGGAC TGG CTC-3′; and LMP2A upstream primer F6: 5′-GG AGA TCT ATG GGG TCCCTA GAA ATG GTG-3′. The purified PCR amplification products were sequenced using the upstream promoter sequences as sequencing primers.
Construction and identification of recombinant adenovirus vector
Cells were extracted from human bone marrow. Citation16 GT39 gastric cancer cells (EB-positive gastric cancer cells) were a gift from Professor Xilianshigang of the Medical Department of Tottori University, Japan. RNA was reverse-transcribed using a reverse transcription kit. Next, PCR was performed using primers F1 and F5 to amplify GM-CSF in human bone marrow cells, and it was also conducted using primers F6 and F4 to amplify LMP2A in GT39 cells. GM-CSF and LMP2A were separately amplified using the upstream and downstream primer pairs F1 and F5 and F6 and F4, respectively. Using the overlap extension method, the GM-CSF and LMP2A genes were connected to (Gly4Ser)3 DNA fragments. GM-CSF and LMP2A cDNAs were used as templates for PCR using the upstream and downstream primer pairs F1 and F2 as well as F3 and F4, respectively. Next, linkers with sequences complementary to the GM-CSF and LMP2A gene fragments were obtained. The gene fragments were used as templates for overlap extension PCR using the upstream and downstream primers F1 and F4, respectively, to obtain the GC2A fusion gene fragment. After the sequencing of GC2A to verify its sequence, BglII and EcoRV restriction enzymes were used to cut the pMD18-T-GC2A plasmid and pAdTrack-CMV vector (pAdTrack-CMV contains PmeI restriction sites, kanamycin resistance gene, the GFP reporter gene and PacI restriction sites). The GC2A gene was then cloned into the pAdTrack-CMV vector, and competent Escherichia coli DH10B cells were transformed with pAdTrack-CMV-GC2A. White colonies were selected, and plasmid DNA was extracted. The plasmid DNA was cut with the restriction enzymes Bg1II and EcoRV and then analyzed by 1% agarose gel electrophoresis. The recombinant plasmid was named as pAd-CMV-GC2A. Competent E. coli BJ5183cells were cotransformed with 2 μl (0.1 μg) dephosphorylated pAdEasy-1 and pAdTrack-CMV-GC2A linearized plasmids. The cells were cultured at 37°C for 0.5 h at 300 rpm. Next, bacteria were spread on LB plates containing 35 μg/ml kanamycin and cultured at 37°C for 16–20 h. Typical colonies were selected and cultured for 16 h in LB liquid medium containing 35 μg/ml kanamycin. The plasmid was extracted and electrophoresed. The recombinant plasmid, which was larger than pAdEasy-1, was identified by PacI digestion. The positive plasmid was named pAd-GC2A.
Kanamycin resistance was assessed to select the recombinant plasmid pAdTrack-CMV-GC2A, which was then digested with PmeI and introduced into E.coli BJ5183 cells. Supercoiled pAdEasy-1 and linear pAdTrack-CMV-GC2Arecombined in the BJ5183 cells, and the foreign gene integrated into the E1 region of the adenovirus vector. The pAdTrack-CMV and pAdEasy-1 right arms containing viral genomic sequences were approximately 3534∼5790 bp. The pAdTrack-CMV and pAdEasy-1 left arms contained the other ends of the genomic sequences, and the two plasmids could recombine in this region (). In addition, the pAdTrack-CMV and pAdEasy-1 right arms contained the same origins of replication; thus, restructuring could also occur in these regions. If the left arm and right arm underwent homologous recombination after reorganization of the plasmid, the PacI enzyme could cut the plasmid to obtain an approximately 3.0 kb fragment. If the origin of replication and right arm recombined, we would obtain a 4.5-kb fragment after digestion of the plasmid.
Transfection of HEK 293T cells with recombinant adenovirus
The recombinant adenoviral vector pAd-GC2A was extracted using a plasmid extraction kit (Sangon) according to the manufacturer instructions. pAd-GC2A was digested with the restriction enzyme PacI, and then the DNA was dissolved in 80 μL sterile water. Next, 5 μL recombinant plasmid and 50 μL CaCl2 were mixed, and cotransformation antibiotic-free, serum-free Dulbecco's modified Eagle medium was added to a total volume of 200 μL and incubated for 40 min at 37°C. HEK 293T cells were inoculated when they reached 60–75% confluence. When the DNA-CaPi mixture turned milky white, it was immediately dropped onto the culture plate, which was gently shaken, placed in an incubator, and cultured at 37°C for 5h. The culture supernatant was then removed, and 8 mL Dulbecco's modified Eagle medium supplemented with antibiotics was added after 40–50 h. An inverted fluorescence microscope was used to determine whether green fluorescent cells appeared after 56 h. The cells were harvested after 5 d of transfection. The precipitate was suspended in 1 mL 1 mol/L phosphate-buffered saline (PBS) and frozen/thawed at 37°C/−70°C, respectively, 5 times. After the cells were centrifuged at 5000 g for 10 min, the sedimented cell debris was discarded, and the supernatant (including the virus) was stored at −80°C. The virus was named as vAd-GC2A. Control viral vectors were also established using this method.
Detection of GC2A gene expression by western blot analysis
vAd-GC2A was used to infect HEK 293T cells, and the cells were harvested after 96 h. Next, 2 mL lysis buffer was added to the cells, and the cells were frozen/ thawed 4 times at 37°C −80°C, respectively. The cells were then centrifuged at 4°C and 12000 rpm (10 cm centrifugal radius) for 5 min, and the supernatant protein was collected. The collected supernatant was analyzed by Western blot hybridization with LMP2A or GM-CSF antibody at 4°C (TaKaRa Bio Co., Shiga, Japan).
Detection of antibody levels in peripheral blood
Specific pathogen-free, 5-week-old BALB/c female mice were randomly divided into the following 4 groups, with 10 mice in each group: group 1, each mouse was injected with 100 μL recombinant adenovirus particles (5 × 108)/100μL; group 2, each mouse was injected with 100 μL recombinant adenovirus particles(5 × 107)/100 μL; group 3, each mouse was injected with 100 μL adenovirus 5 control virus particles (1 × 108)/100 μL; and group 4, each mouse was injected with 100 μL sterile PBS. The mice were immunized 7 times at 2-week intervals. The tail blood of the mice was collected once every 2 weeks as well at week 21 after immunization. Serum was isolated from the blood samples, and antibody detection was performed by enzyme-linked immune sorbent assay (ELISA). The LMP2A (GM-CSF) recombinant antigen was prepared and incubated on a 96-well plate at 0.5 μg/well. Next, serum samples (at different dilutions) were added to the wells and incubated with a goat anti-mouse IgG antibody. The substrate tetramethylbenzidine (3, 3′, 5, 5′-tetramethylbenzidine, TMB) was added. Finally, 2 mol/L of an H2SO4 solution was used to terminate the reaction. The samples were analyzed using a Thermo Multiskan MK3 Microplate Reader (Thermo Fisher Scientific, Waltham, MA, USA). The ELISA results were statistically analyzed.
CTL activity of splenic lymphocytes in mice
Mouse splenic lymphocytes were separated and then the cells were suspended in RPMI 1640 medium containing 3 μg/mL Con A, 20 U/mL interleukin-2 and 10% calf serum. The cells were cultured for 2 days at 37°C in a 5% CO2 incubator. Recombinant adenovirus at a multiplicity of 1000 was added to 500 μL RPMI 1640 cell culture. After 120 min of infection at 37°C, the mouse splenic lymphocytes were continuously cultured for 48 h. Next, the cells were collected and washed twice in culture medium, and the remaining virus was removed. Six-week-old mice were injected subcutaneously with recombinant adenovirus-infected splenic lymphocytes by subcutaneous injection, adenovirus 5+ LMP2A, adenovirus 5+ hGM-CSF, wild-type strains and PBS were used as controls. The mice were re-immunized on the third and fifth weeks. After the seventh week, the mice were killed, and splenic lymphocytes were obtained. Cells were cultured in 100 μL culture supernatant per well for 24 h, and then lactate dehydrogenase release assays (Promega) were performed according to the manufacturer instructions. The optical density (A450) of each well was determined, and the CTL killing rate of EBV-positive tumor cells (GT39) was calculated.
Immunotherapy
GT39 cells were cultured to approximately 80% confluence, and the number of cells was counted under a microscope. The cell concentration was adjusted to 1 × 108/mL, and try pan blue staining was performed to determine the number of living cells. The cells were transported to the animal room and used for subsequent experiments. After the right ventral ribs of female BALB/c mice were disinfected with iodine, they were subcutaneously injected with GT39 cells (2 × 106/ mouse). Ten days after the mice were injected, 25 mice with growing tumors (5 mm diameters) were divided into five groups. The experimental group was injected with 2 × 108 plaque-forming unit recombinant adenovirus, (soluble in 250 μL PBS, pH 7.4) /mouse, and the control group was injected with 2 × 108 plaque-forming unit adenovirus 5 (soluble in 250 μL PBS) /mouse. Each mouse was subcutaneously inoculated with a 250 µL volume under sterile conditions via a multipoint injection in the neck. The mice were injected again following the same dosing strategy after ten days of the first immunization. The mice were sacrificed at 70 days after the first injection. Tumors were obtained and kept as intact as possible, and fat and blood were removed. Then, tumor volumes were measured.
Morphology of tumor tissues in mice
After the mice were sacrificed, the obtained tumors from the immunized mice were embedded in paraffin and sectioned. the tumor tissue was completely stripped and immediately fixed with formalin solution, Hematoxylin-eosin (HE) staining was performed to observe and analyze the morphological changes that occurred in the tumor tissues and to determine whether tumor-infiltrating lymphocytes appeared after immunization, immunohistochemistry (immunhistochemical staining with rabit anti-CD4 antibody) was carried out. Under microscope, visions were selected randomly, and the number of CD4 in tumor tissue was counted under microscope.
Statistical methods
The data of each group are reported as means ± standard deviation and the t-test was performed using SPSS 11.5 statistical software (t = 5.87, P = 0.018, P < 0.05) (SPSS, Inc., Chicago, IL, USA). P ≤ 0.05was considered statistically significant.
Abbreviations
| CTL | = |
cytotoxic lymphocyte |
| EBV | = |
Epstein-Barr virus |
| ELISA | = |
enzyme linked immunosorbent assay |
| GC2A | = |
GM-CSF and LMP2A fusion gene |
| GM-CSF | = |
granulocyte/macrophage colony-stimulating factor |
| GT39 | = |
EBV-positive epithelial tumor cell line |
| HEK | = |
human emborynic kidney |
| HE | = |
hematoxylin-eosin |
| LMP2A | = |
Epstein-Barr virus latent membrane protein |
| PCR | = |
Polymerase Chain Reaction |
| PBS | = |
phosphate-buffered saline |
| pAdTrack-CMV | = |
adenovirus shuttle vector |
| pAdEasy-1 | = |
adenoviral skeleton plasmid |
| RT-PCR | = |
reverse transcription polymerase chain reaction |
| SPSS | = |
Statistical Product and Service Solutions |
| SDS-PAGE | = |
sodium dodecyl sulfate polyacrylamide gel electropheresis |
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Authors' contributions
Ting Chen designed and conceived the study. Qing-jie Xue designed and performed the experiments. Chun-Mei Wang and Chun-qing Yang integrated the data and wrote the manuscript. All authors read and approved the final manuscript. All authors have been involved in revising the manuscript critically.
Acknowledgments
Hui Wang and Shuang Wang are thanked for their excellent technical assistance.
Funding
Research supported by NSFC cultivation project of Jining Medical University(2016), the Natural Science Foundation of Shandong Province (#ZR2012HM037,#ZR2014HL040), the Science and Technology Project of Colleges in Shangdong Province (#J12LK56,#J13LK54), and the National Natural Science Foundation of China (# 31500056, #30971081, #31271243, and # 81070961).
References
- Gaur N , Gandhi J , Robertson ES , Verma SC , Kaul R . Epstein–Barr virus latent antigens EBNA3C and EBNA1 modulate epithelial to mesenchymal transition of cancer cells associated with tumor metastasis. Tumor Biol. 2015;36(4):3051-60. doi:10.1007/s13277-014-2941-6
- Borza CM , Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med. 2002;8:594-9. doi:10.1038/nm0602-594. PMID:12042810
- Liu MT , Chang YT , Chen SC , Chuang YC , Chen YR , Lin CS , Chen JY . Epstein-Barr virus latent membrane protein 1 represses p53-mediated DNA repair and transcriptional activity. Oncogene. 2005;24:2635-42. doi:10.1038/sj.onc.1208319. PMID:15829976
- Smith C , Khanna R . The development of prophylactic and therapeutic EBV vaccines. Curr Top Microbiol Immunol. 2015;391:455-73. doi:10.1007/978-3-319-22834-1_16. PMID:26428385
- Lee SP , Tierney RJ , Thomas WA , Brooks JM , Rickinson AB . Conserved CTL epitopes within EBV latent membrane protein 2: A potential target for CTL-based tumor therapy. J Immunol. 1997;158:3325-34. doi:10.1128/JVI.77.1.105-114.2003. PMID:9120290
- Portis T , Longneeker R . Epstein-Barr virus LMP2A interferes with g1obal transcription factor regulation when expressed during B-lymphocyte development. J Virol. 2003;77(1):105-14. PMID:12477815
- Redchenko IV , Rickinson AB . Accessing Epstein- Barr virus specific T-cell memory with peptide loaded dendritic cells. J Virol. 1999;73:334-42. doi:10.1111/j.1048-891X.2004.014206.x. PMID:9847337
- Cui H , Chang XH , Liu B , Feng J , Li Y , Ye X , Cao SJ , Fu TY , Yao Y , Li HQ , et al. The anti-tumor immune responses induced by a fusion protein of ovarian carcinoma anti idiotypic antibody 6BllScFv and murine GM-CSF in BALB/c mice. Int J Gynecol Cancer. 2004;14:234-41. PMID:15086722
- Liao WH , Yang LF , Liu XY , Zhou GF , Jiang WZ , Hou BL , Sun LQ , Cao Y , Wang XY . DCE-MRI assessment of the effect of Epstein-Barrvirus-encoded latent membrane protein-1 targeted DNA zyme on tumor vasculature in patients with nasopharyngeal carcinomas. BMC Cancer. 2014;14:835. doi:10.1186/1471-2407-14-835. PMID:25407966
- Sun H , Yao K , Chen Y , Zhou F . Induction of T-cell immunity against Epstein-Barrvirus-associated tumors by means of adenovirally transduced dendritic cells. Chin Med J (Engl). 2004;117(10):1558-63. doi:10.1002/jmv.23901. PMID:15498383
- Xue QJ , Dai J , Li XZ , Zhu W , Si CP , Chen T . Construction of a recombinant-BCG containing the LMP2A and BZLF1 genes and its significance in the Epstein-Barr virus positive gastric carcinoma. J Med Virol. 2014;86:1780-7. PMID:24699993
- Tan Q , Ma S , Hu J , Chen X . Adenovirus vector harboring the HBcAg and tripeptidyl peptidase II genes induces potent cellular immune responses in vivo. Cell Physiol Biochem. 2017;41(2):423-38. doi:10.1159/000456579. PMID:28214886
- Liu GY , Li ZJ , Li QL , Jin Y , Zhu YH , Wang YH , Liu MY , Li YG , Li Y . Enhanced growth suppression of TERT-positive tumor cells by oncolytic adenovirus armed with CCL20 and CD40L. Int Immunopharmacol. 2015;28:487-93. doi:10.1016/j.intimp.2015.07.005. PMID:26208317
- Inturi R , Kamel W , Akusjvi G , Punga T . Complementation of the human adenovirus type 5 VA RNAI defect by the vaccinia virus E3L protein and serotype-specific VA RNAIs. Virology. 2015;485:25-35. doi:10.1016/j.virol.2015.07.002. PMID:26196231
- Hilleman MR , Stallones RA , Gauld RL , Warfield MS , Anderson SA . Prevention of acute respiratory illness in recruits by adenovirus (RI-APC-ARD) vaccine. Proc Soc Exp Biol Med. 1956;92:377-83. doi:10.3181/00379727-92-22484. PMID:13350354
- Al-Nbaheen M , Vishnubalaji R , Ali D , Bouslimi A , Al-Jassir F , Megges M , Prigione A , Adjaye J , Kassem M , Aldahmash A . Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. 2013;9:32-43. doi:10.1007/s12015-012-9365-8. PMID:22529014
- Rooney CM , Smith CA , Ng CY , Loftin S , Li C , Krance RA , Brenner MK , Heslop HE . Use of gene-modified virus-spefic T lymphocytes to control Epstein-Barr-virus-related lympholiferation. Lancet. 1995;345(8941):9-13. doi:10.1016/S0140-6736(95)91150-2. PMID:7799740