 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Although clinical trials of the pentavalent rotavirus vaccine (RotaTeq®, RV5) have demonstrated efficacy against RV gastroenteritis (RGE) in low and high-income settings, a clear correlate of protection or a measure of immune response that could predict efficacy has yet to be identified. This is the first time that immunogenicity data with both serum neutralized antibody (SNA) titers and anti-RV IgA titers from several clinical efficacy trials were pooled to provide a unique context for evaluating the correlation between immunogenicity and RGE risk or efficacy of RV5. The correlation between immunogenicity and RGE risk is evaluated with data at the individual subject level. The analyses show that higher Postdose 3 (PD3) G1 SNA titers are associated with lower odds of contracting any RGE. The correlation between immunogenicity and efficacy is assessed using aggregated population level data, which shows higher efficacy associated with higher PD3 G1 SNA geometric mean titer (GMT) ratio (between RV5 and placebo) and PD3 serum anti-RV IgA GMT ratio. Among high-income countries, efficacy plateaus over the range of PD3 G1 SNA GMT ratios and PD3 serum anti-RV IgA GMT ratios. From both individual- and population-level analyses, PD3 G1 SNA titers correlated most closely with the RGE risk or efficacy for RV5.
Introduction
Rotavirus (RV) is the leading cause of severe gastroenteritis in children <5 y globally. Citation1 Almost all children are infected by 5 y of age regardless of their country of residence or socioeconomic status. Citation2 The annual number of childhood deaths due to RV is estimated to be 215,000 with approximately 37% of all hospitalizations due to diarrhea being positive for RV. Citation1,Citation2,Citation3
The pentavalent RV vaccine, RotaTeq® (Merck & Co., Inc., Kenilworth, NJ, USA. RV5), contains 5 human-bovine reassortant RVs consisting of a bovine (WC3) backbone with human RV surface proteins representative of the most common G (G1, G2, G3, G4) or P (P1A) types worldwide. As of March 2017, RV5 has been licensed in over 120 countries with ∼200 million doses distributed worldwide. Although vaccine efficacy against RV gastroenteritis (RGE) has been demonstrated in low and high-income settings, Citation4 a clear correlate of protection or a measure of immune response that could predict efficacy has yet to be identified.
Measurements of serum-neutralizing antibody (SNA) and serum anti-RV immunoglobulin A (IgA) are current standards for assessing immune responses following RV vaccination and wild-type infection. However, immunity to RV is not completely understood. Citation5-Citation9 Studies of wild-type RGE have shown conflicting results. Citation10,Citation11 Previous vaccine studies showed no clear relationship between antibody titers and protection against RGE. Citation12,Citation13 Some data suggests that serum anti-RV IgA can serve as a relative correlate for RV vaccines in clinical trialsbased on meta-analytic approaches, Citation14,Citation15 and on individual subject data. Citation16
This is the first time that immunogenicity data with both SNA (G1-G4 and P1A; the serotypes contained in the vaccine) and serum anti-RV IgA geometric mean titers (GMT) from 5 clinical efficacy trials (see ) for RV5 were pooled and are being presented to provide a unique context for evaluating the correlation between immunogenicity and RGE risk or efficacy, at both the individual and aggregated population level.
Results
Individual subject data
In the hypothesis generation analysis using data from P005, a statistically significant association was seen only for the PD3 G1 SNA titer (p-value = 0.033) and PD3 P1A SNA titer (p-value = 0.020). However, only the PD3 G1 SNA titer was confirmed in the combined data from Phase III studies (P006 and P007). summarizes the results from Phase II (P005) and Phase III studies (P006 and P007) for the PD3 G1 SNA titer. It is noted that the G1 SNA GMT values for P005 were much lower than that in Phase III studies because of the inclusion of the low potency pentavalent vaccine arm, as well as the monovalent vaccine arm, which did not contain the G1 reassortant.
In both hypothesis generation using P005 and the confirmatory analysis using Phase III studies, the estimated odds ratio for one unit increase in PD3 G1 SNA titer was very close to 1, which implies that the efficacy, against any severity, is relatively robust and insensitive (i.e., has little change) over the range of PD3 G1 SNA titers observed in these Phase II and III studies.
In P005, a PD3 G1 SNA titer > 51 was significantly associated with lower odds for RGE; however, this dichotomized endpoint was not confirmed in the Phase III study data. The evaluations were based on sensitivity, which measures the probability of correctly predicting RGE with the cutpoint, and specificity, which measures the probability of correctly predicting non-RV gastroenteritis. Overall, there was no single cut-point for PD3 G1 SNA titer identified that provides a high degree of both sensitivity and specificity to predict efficacy.
Aggregated data
shows scatter plots of aggregated efficacy values against GMT ratios for SNA G1, G2, G3, G4, P1A, and serum anti-RV IgA. Visual evaluation of the scatter plots with smooth spline curves indicates that each of the GMT ratios for SNA G1, G2, G3, G4, P1A, and serum anti-RV IgA showed some association with efficacy. A forward variable selection analysis was conducted with leave-one-out cross validation to minimize the prediction error. The analysis used re-scaled logit-transformed efficacy as a dependent variable and GMT ratios for SNA G1, G2, G3, G4, P1A, and serum anti-RV IgA as independent variables. The analysis showed that the 2 significant variables selected were the SNA G1 GMT ratio and the IgA GMT ratio. The proportion of variance in efficacy explained by the SNA G1 GMT ratio alone (R-Squared value) is 0.80. The R-squared value increased to 0.86 after the addition of the IgA GMT ratio in the regression model. The weighted linear regression analyses were then conducted for these 2 GMT ratios on the re-scaled logit-transformed efficacy. The results showed that the association between efficacy against any RGE and the PD3 G1 SNA GMT ratio or PD3 serum anti-RV IgA GMT ratio was statistically significant (p-value <0.05).
Figure 1. Scatter plot of efficacy on GMT ratios of SNA for G1 – G4, P1A, and serum anti-rotavirus IgA (aggregated data from P005, P006, P007, P015 and P024). Note: 5L, 5M, 5H = P005 low, middle, and high potency, 5Mo = P005 monovalent, 5Q = P005 quadrivalent, 6F = P006 (Finland), 6U = P006 (US), 7 = P007, 15Af = P015 (Africa), 15A = P015 (Asia), 24 = P024 (China). Dotted lines are smooth spline curves.
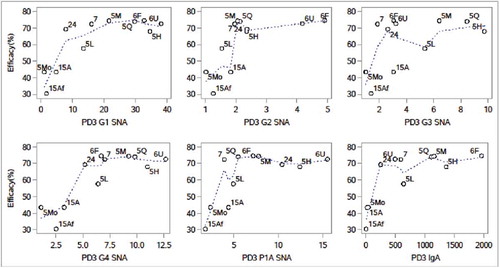
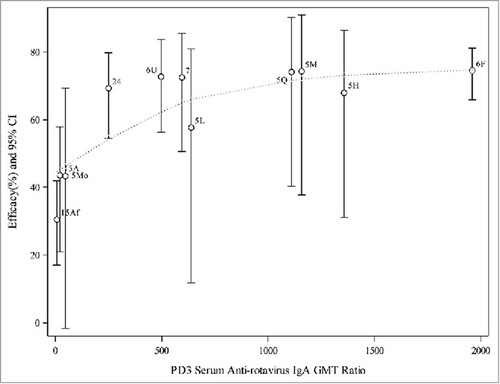
and show scatter plots of aggregated efficacy values against GMT ratios for PD3 G1 SNA and serum anti-RV IgA, along with the line representing the weighted regression. The confidence intervals were also included for each estimated efficacy to show the variation. The observed efficacy values were similar across studies conducted in higher-income countries, i.e., in Phase II (P005) and Phase III studies (P006 and P007), except that the efficacy was lower for the low potency pentavalent vaccine and monovalent vaccine arms in P005. The confidence intervals for P005 groups were wider due to the small number of subjects in this Phase II study. The efficacy values and their associated PD3 G1 SNA GMT ratios or serum anti-RV IgA GMT ratios were lower for both African and Asian subjects enrolled in P015.
Figure 2. Weighted regression analyses of efficacy on PD3 G1 SNA GMT ratio (aggregated data from P005, P006, P007, P015 and P024). Note: 5L, 5M, 5H = P005 low, middle, and high potency, 5Mo = P005 monovalent, 5Q = P005 quadrivalent, 6F = P006 (Finland), 6U = P006 (US), 7 = P007, 15Af = P015 (Africa), 15A = P015 (Asia), 24 = P024 (China). Dotted line represents the weighted regression line (p-value = 0.0002).
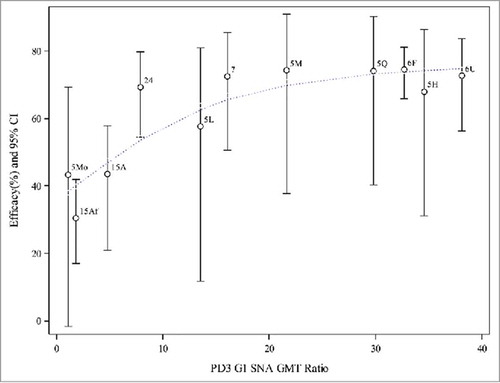
Figure 3. Weighted regression analyses of efficacy on PD3 serum anti-rotavirus IgA GMT ratio (aggregated data from P005, P006, P007, P015 and P024). Note: 5L, 5M, 5H = P005 low, middle, and high potency, 5Mo = P005 monovalent, 5Q = P005 quadrivalent, 6F = P006 (Finland), 6U = P006 (US), 7 = P007, 15Af = P015 (Africa), 15A = P015 (Asia), 24 = P024 (China). Dotted line represents the weighted regression line (p-value = 0.0002).
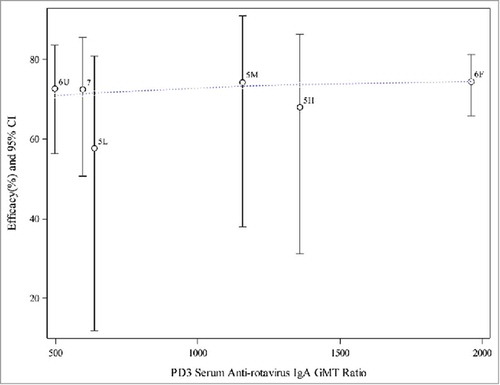
The scatter plots and weighted regression model analyses were repeated after excluding P015, P024, and the non-final formulation arms (monovalent and quadrivalent vaccine) in P005. Results are shown in and . It appears that the observed efficacy from these 3 higher-income setting trials may be similar and on a plateau. The weighted logistic regression model did not have a significant association for efficacy with PD3 G1 SNA GMT ratios or serum anti-RV IgA GMT ratios.
Figure 4. Weighted regression analyses of efficacy on PD3 G1 SNA GMT ratio (aggregated data from final formulation in P005, P006, and P007). Note: 5L = P005 low potency, 5M = P005 middle potency, 5H = P005 high potency, 6F = P006 (Finland), 6U = P006 (US), 7 = P007. Dotted line represents the weighted regression line (p-value = 0.3958).
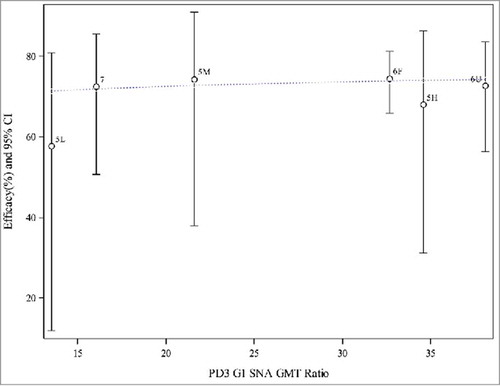
Figure 5. Weighted regression analyses of efficacy on PD3 anti-rotavirus IgA GMT ratio (aggregated data from final formulation in P005, P006, and P007). Note: 5L = P005 low potency, 5m = P005 middle potency, 5H = P005 high potency, 6F = P006 (Finland), 6U = P006 (US), 7 = P007. Dotted line represents the weighted logit regression line (p-value = 0.0890).
Discussion
Based on the analyses of data from 5 RV5 clinical trials, PD3 G1 SNA titers appear to be the best available correlate of protection for RV5. Evaluating individual subjects with immunogenicity measures and case status from Phase II and Phase III studies of RV5 confirm that higher PD3 G1 SNA titers are associated with lower odds of contracting any RGE. Aggregated data analyses from Phase II and III studies show that higher efficacy is associated with higher PD3 G1 SNA GMT ratios and higher PD3 serum anti-RV IgA GMT ratios. In addition, among studies in higher-income settings, efficacy appears to be in a plateau over the range of PD3 G1 SNA and PD3 serum anti-RV IgA GMT ratios. The predicted efficacy values from the logit regression analysis were fairly similar over these GMT ratios, and no GMT ratio value was identified as a threshold for differentiating the efficacy of the vaccine.
The aggregated data analysis investigated the association between the efficacy and the GMT ratios between RV5 and placebo groups. We also looked at the analysis using PD3 GMTs but found that the GMT ratios that accounted for responses in placebo recipients had better association with efficacy. As shown in , in the developed world studies (P005, P006, P007) the placebo subjects had no immune response following vaccination and therefore very low GMT values for G1 SNA or serum anti-RV IgA. Yet in the Asia and Africa trial (P015) both the RV5 and placebo groups had higher baseline G1 GMT values than those observed in the developed world studies; this may reflect the presence of maternal antibody or early environmental exposure to RV. In addition, the potentially high exposure to wild-type RV during the course of the developing world studies would likely boost the immune response for the placebo group whilte at the same time may reduce the relative vaccine effect. The concomitant administration of OPV in some subjects in the developing world studies may also impact the response of RV5. Citation17 Thus, the RV5 group had relatively low GMT values post-vaccination (especially in Africa) which resulted in a lower GMT ratio and consequently a lower efficacy observed in P015. In comparison, the placebo group in another efficacy trial conducted in rural China (P024) had similar baseline G1 GMT values as in P015 but the RV5 group had a better immune response (PD3 G1 GMT value was similar to that in the P007 and P005 middle dose group), which resulted in a relatively higher GMT ratio than in the P015 study. Correspondingly, the efficacy for P024 was higher than in the developing world study and consistent with that of the developed world studies (P005, P006, P007). One possibility is that the environment exposure level to wide-type RV in China was lower than that in Africa during the conduct of the trial, although the exact reason remains to be determined.
It should be noted that in P005 monovalent group, the vaccine did not contain the G1 reassortant so the G1 GMT ratio was very low and close to 1.0. The efficacy of approximately 43% was likely a result of cross protection from the included P1A reassortant because the majority of G1 serotypes are serotypes associated with P1A. Therefore, the G1 SNA may be a non-mechanistic correlate of protection per the nomenclature of Plotkin and Gilbert. Citation18 The subjects enrolled in the phase II study P005 were also relatively older than those in the phase III trials (median age of 5 months in P005 versus 2 – 2.5 months in phase III trials). Nevertheless, the efficacy observed in the middle and high potency groups in P005 was comparable to that of the phase III studies (P006, P007) in the developed world.
These analyses provide a unique perspective for a potential immune correlate for a RV vaccine and is one of the most in-depth evaluations conducted to date. The 5 RV5 clinical trials covered both high and low-income settings worldwide, which is impactful as there was a relatively large range of efficacy estimates available across these studies (from ∼30% to ∼74%). The analyses were conducted at both an individual subject level and an aggregated population level. In addition to the typically evaluated serum anti-RV IgA, these analyses also investigated the SNAs for the serotypes contained in the vaccine as a potential immune correlate of protection.
Previous studies have mostly been focused on the serum anti-RV IgA as a correlate of protection. Citation14-Citation16 One study, with aggregated clinical trial data, showed that there are higher efficacy estimates with serum anti-RV IgA GMTs > 90. Citation15 Evaluation of routine use of RV5 in Finland concluded that both wild-type RV infections and administration of vaccine resulted in similar levels of serum anti-RV IgA antibodies, which persisted over several years. Citation19 An additional study assessed the serum anti-RV IgA as a surrogate for vaccine efficacy for another RV vaccine (RV1, Rotarix™ by GSK), but found that only a low proportion of vaccine effect was accounted for by IgA seropositivity. Citation16 It should note that the RV5 is a live reassortant RV vaccine containing G1, G2, G3, G4 and P1A Citation8 serotypes. The RV1 contains only attenuated human G1P Citation8 serotype. This difference may contribute to the findings of G1 SNA being the best correlate of protection for RV5 as compared with the anti-RV IgA as a correlate of protection for RV1 in previous studies.
Although a single cut-off value, which provides a high degree of both sensitivity and specificity, was not identified, the PD3 G1 SNA titer shows good correlation with efficacy against RGE based on individual and aggregated data analyses. This result is consistent with a recent publication where statistical methods for identifying biomarkers as trial level general surrogates Citation20 were applied to the RV5 clinical trial data, and demonstrated that both SNA G1 and serum anti-RV IgA have some value as trial level general surrogates for vaccine efficacy against RGE of any severity, and SNA G1 is a slightly better trial level general surrogate than IgA.
The analysis on individual subject level data follows the framework as described in Citation25 to assess the correlation of immunogenicity and RGE risk. Because subjects in the placebo group of the developed world studies (P005, P006, and P007) had no immune response and no subjects in placebo group were vaccinated at the end of the studies, the Prentice criteria and principal stratification analyses cannot be performed. Some other limitations of these analyses include: 1) The aggregated analyses were based on summary statistics at the population level; therefore, the results may not be interpreted as association at an individual subject level. 2) The immunogenicity results in the aggregated data analyses were from a subset of subjects in the efficacy studies. The analysis assumed that the GMT ratios from the subsets could represent the overall immunogenicity for the study population used in the efficacy calculation for each of the aggregate data point. The subset contained several subjects enrolled into some pre-specified sites that might not be completely random in the study population. 3) The analyses were also post-hoc and exploratory as not all the original trials were designed to investigate the correlate of protection. Although significant correlation was seen between efficacy and G1 SNA, but not for other vaccine serotypes, this may be due to as the fact that the majority of the RGE cases were G1. 4) The individual subject data analysis has been focused on investigating the association of immunogenicity with the probability of RGE without considering other baseline factors such as age at first dose and gender, which may also affect efficacy. Citation21
In conclusion, this study adds to the information currently available regarding the correlation between immunogenicity and efficacy of RV vaccines. Although serum anti-RV IgA has been proposed as surrogate for efficacy in trials of RV vaccines, the data generated in these analyses demonstrates that this may not be the most robust correlate for RV5. From both individual- and population-level analyses, the PD3 G1 SNA titer correlated most closely with efficacy for RV5.
Methods
There are 5 completed efficacy trials for RV5, which collected both efficacy and immunogenicity data, used for these analyses. These studies are summarized in and brief descriptions are provided in the supplementary appendix.
Table 1. Summary of phase II and III efficacy clinical trials of RV5.
Table 2. Summary and logistic regressions for RGE cases on PD3 G1 SNA titer based on data from individual subjects.
Table 3. GMT at baseline, PD3, and PD3 GMT ratio for G1 SNA and serum antirotavirus IgA by study/group.
For all these studies, the case definition for RGE required subjects to meet both of the following criteria: (1) ≥ 3 watery or looser-than-normal stools within a 24-hour period and/or forceful vomiting, and (2) RV detected by enzyme-linked immunosorbent assay (ELISA) in a stool specimen taken within 14 d of the onset of symptoms. The primary analysis of efficacy included only cases caused by naturally occurring RV as confirmed by reverse-transcriptase polymerase chain reaction (RT-PCR) occurring at least 14 d after the third dose of RV5 or placebo. In each of these studies, serum samples were collected for all or a subset of subjects before vaccination (baseline) and approximately 2 to 6 weeks after the third dose. For all studies, a central laboratory at the Division of Infectious Diseases of Cincinnati Children's Hospital Medical Center was used to evaluate the serological response to the vaccine including SNA against each of human RV serotypes G1, G2, G3, G4 and P1A, and serum anti-RV IgA. Citation22-Citation24
To identify a correlation between the magnitude of RV-associated antibody responses and any breakthrough RGE cases in subjects who received RV5, 2 analyses were conducted: 1) Based on data from individual subjects in one Phase II study (P005) and 2 Phase III studies (P006 and P007), which were conducted in higher-income settings. These studies were pre-planned to collect adequate individual subject level immunogenicity and RGE cases for investigating the correlate of RGE risk; 2) Based on aggregated data from all 5 efficacy trials. The analyses follow the framework as described in Qin et al. Citation25 Because all these analyses were exploratory, a p-value of less than 0.05 was considered as statistically significant without consideration of multiplicity.
Analysis based on data from individual subjects
One of the exploratory objectives in the Phase II study (P005) was to investigate the correlate of protection for RV5, where serum samples were collected and tested for all subjects in the study. In the 2 Phase III studies conducted in higher-income countries, a large cohort of more than 1300 subjects were included in a sub-study to demonstrate that RV5 can be administrated concomitantly with other routine infant vaccines. Thus, immunogenicity results for RV5 recipients who were also RGE cases were available. In addition, another cohort of more than 1200 subjects was selected to evaluate the immunogenicity responses to RV5. Both immunogenicity results and RGE case status were available for these 2 cohorts, which provided adequate information to conduct the correlate of protection analysis using individual subject data. The other 2 studies (P015 and P024) were excluded from the individual subject level analyses due to very few RGE cases in subjects with immunogenicity data.
As pre-specified in the statistical analysis plan for P005, logistic regressions were used to model the log odds of RGE cases as a function of PD3 immunogenicity measures. Models included factors for baseline titers, PD3 titers, and days of follow-up (within the first season). Data from all 5 treatment groups in P005 were used in the analyses. Subjects who received placebo were not included in these analyses, because there was generally no immune response following receipt of placebo. The analyses were done separately for each of the immunogenicity endpoints, including SNA for G1, G2, G3, G4, P1A, and serum anti-RV IgA. The immunogenicity endpoints that were significantly associated with the RGE risk from P005 were used to generate hypotheses. The data from Phase III studies (P006 and P007) were then used to test and confirm the hypotheses to identify the immunological correlates.
For the confirmed immunogenicity endpoints from Phase III studies, additional analyses were performed to determine if an acceptable titer level cutpoint exists that correlates with a presence or absence of RGE based on sensitivity (the probability of correctly predicting RGE if titer levels are below the cutpoint) and specificity (the probability of correctly predicting non-RV gastroenteritis if titer levels are above the cutpoint) criteria using Receiver Operating Characteristic (ROC) methodology.
Analyses based on aggregated data
In the aggregated data analyses, the association between each of the SNA and anti-RV IgA geometric mean titer ratios (GMT ratios between RV5 and placebo groups) and efficacy from all 5 studies was evaluated. A total of 11 efficacy and aggregated immunogenicity data points were created from these studies. Specifically, for P005, there were 5 aggregated data points (one for each vaccine treatment arm: pentavalent high, middle, and low potency, quadrivalent and monovalent). Considering the 2 different 3-dose administration schedules used in P006, we also separately calculated aggregated data points in 2 subsets: one in Finland, which primarily used a 2–3–4 month schedule and one in the US, which primarily used a 2–4–6 month schedule. For P015, aggregated data points were obtained by region (Africa and Asia) to reflect potential differences in host populations, environments, and associated health conditions.
PD3 GMT ratios for SNA G1, G2, G3, G4, P1A, and serum anti-RV IgA were considered in the analysis. Because only a subset of subjects were tested for immunogenicity for all studies except for P005, we assumed that the immunogenicity measures obtained from the subset of subjects would represent the immunogenicity response for the corresponding overall study population where the efficacy measure was obtained. In P006, P007, P015 and P024, the subsets for immunogenicity measures were selected based on several first enrolled subjects into pre-specified sites for the studies. The similarity of baseline characteristics between the subjects in the immunogenicity subset and that in the whole study is evaluated as supportive evidence for this assumption. The following linear regression model is considered to assess the association of logit-transformed efficacy with each of the PD3 GMT ratios, that is,
Considering the different population and study conditions between higher and lower income countries/regions, the weighted regression analyses were also done after excluding the 3 data points from P015 and P024. The data points for monovalent and quadrivalent vaccine treatment arms from P005 were also excluded because the vaccine formulation was different from the final formulation of RV5.
Disclosure of potential conflicts of interest
This study was funded by Merck & Co., Inc., Kenilworth, NJ, USA (sponsor). This study was designed, executed, and analyzed by the sponsor. All co-authors approved the final version of the manuscript.
Employees of the sponsor (indicated on the title page) may own stock or stock options in the company.
Supplemental_Material.docx
Download MS Word (84.5 KB)Funding
Funding for this research was provided by Merck & Co., Inc., Kenilworth, NJ, USA.
References
- Tate JE , Burton AH , Boschi-Pinto C , Parashar UD , World Health Organization–Coordinated Global Rotavirus Surveillance Network . Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62(Suppl 2):S96-S105. doi:10.1093/cid/civ1013. PMID:27059362
- Staat MA , Azimi PH , Berke T , Roberts N , Bernstein DI , Ward RL , Pickering LK , Matson DO. Clinical presentations of rotavirus infection among hospitalized children. Pediatr Infect Dis J. 2002;21:221-7. doi:10.1097/00006454-200203000-00012. PMID:12005086
- Parashar UD , Burton A , Lanata C , Boschi-Pinto C , Shibuya K , Steele D , Birmingham M , Glass RI . Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200(Suppl 1):S9-S15. doi:10.1086/605025. PMID:19817620
- Tate JE , Patel MM , Steele AD , Gentsch JR , Payne DC , Cortese MM , Nakagomi O , Cunliffe NA , Jiang B , Neuzil KM , et al. Global impact of rotavirus vaccines. Expert Rev Vaccines. 2010;9(4):395-407. doi:10.1586/erv.10.17. PMID:20370550
- Kapikian AZ , et al. Rotavirus. In: Kripe DM , editor(s). Fields virology. Vol 2. 4th ed. Lippincott Williams & Wilkins; 2001. p. 1787-825.
- Franco MA , Angel J , Greenberg HB . Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24(15):2718-31. doi:10.1016/j.vaccine.2005.12.048. PMID:16446014
- Desselberger U , Huppertz H . Immune responses to rotavirus infection and vaccination and associated correlates of protection. JID. 2011;203:188-94. doi:10.1093/infdis/jiq031. PMID:21288818
- Angel J , Franco MA , Greenberg HB . Rotavirus immune responses and correlates of protection. Curr Opin Virol. 2012;2(4):419-25. doi:10.1016/j.coviro.2012.05.003. PMID:22677178
- Angel J , Steele AD , Franco MA . Correlates of protection for rotavirus vaccines: Possible alternative trial endpoints, opportunities, and challenges. Hum Vaccin Immunother. 2014;10:3659-71. doi:10.4161/hv.34361. PMID:25483685
- Velázquez FR , Matson DO , Guerrero ML , Shults J , Calva JJ , Morrow AL , Glass RI , Pickering LK , Ruiz-Palacios GM . Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000;182(6):1602-9. doi:10.1086/317619. PMID:11069230
- Hjelt K , Grauballe PC , Paerregaard A , Nielsen OH , Krasilnikoff PA . Protective effect of preexisting rotavirus-specific immunoglobulin A against naturally acquired rotavirus infection in children. J Med Virol. 1987;21(1):39-47. doi:10.1002/jmv.1890210106. PMID:3025356
- Ciarlet M1 , Schödel F . Development of a rotavirus vaccine: Clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, PRV. Vaccine. 2009;27(Suppl 6):G72-81. doi:10.1016/j.vaccine.2009.09.107. PMID:20006144
- Ward RL , Bernstein DI . Lack of correlation between serum rotavirus antibody titers and protection following vaccination with reassortant RRV vaccines. US Rotavirus Vaccine Efficacy Group. Vaccine. 1995;13(13):1226-32. doi:10.1016/0264-410X(95)00060-E. PMID:8578808
- Ward RL , Knowlton DR , Zito ET , Davidson BL , Rappaport R , Mack ME . Serologic correlates of immunity in a tetravalent reassortant rotavirus vaccine trial. US Rotavirus Vaccine Efficacy Group. J Infect Dis. 1997;176(3):570-7. doi:10.1086/514076. PMID:9291301
- Patel M , Glass RI , Jiang B , Santosham M , Lopman B , Parashar U . A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis. 2013;208(2):284-94. doi:10.1093/infdis/jit166. PMID:23596320
- Cheuvart B , Neuzil K , Steele A , Cunliffe N , Madhi S , Karkada N , Han H , Vinals C . Association of serum anti-rotavirus immunoglobulin A antibody seropositivity and protection against severe rotavirus gastroenteritis. Hum Vaccin Immunother. 2014;10:505-11. doi:10.4161/hv.27097. PMID:24240068
- Ciarlet M , Sani-Grosso R , Yuan G , Liu G , Heaton P , Gottesdiener K , Arredondo J , Schodel F . Concomitant use of the oral pentavalent human-bovine reassortant rotavirus vaccine and oral poliovirus vaccine. Pediatr Infect Dis J. 2008;27:874-80. doi:10.1097/INF.0b013e3181782780. PMID:18756184
- Plotkin SA , Gilbert PB . Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis. 2012;54:1615-7. doi:10.1093/cid/cis238. PMID:22437237
- Lappalainen S , Blazevic V , Malm M , Vesikari T . Rotavirus vaccination and infection induce VP6-specific IgA responses. J Med Virol. 2017;89:239-45. doi:10.1002/jmv.24636. PMID:27431308
- Gabriel EE , Daniels MJ , Halloran ME . Comparing biomarkers as trial level general surrogates. Biometrics. 2016;72:1046-54. doi:10.1111/biom.12513. PMID:27038302
- Gruber JF , Hille DA , Liu GF , Kaplan SS , Nelson M , Goveia MG , Mast TC . Heterogeneity of rotavirus vaccine efficacy among infants in developing countries. Pediatr Infect Dis J. 2016;36:72-78. https://doi.org/10.1097/INF.0000000000001362
- Knowlton DR , Spector DM , Ward RL . Development of an improved method for measuring neutralizating antibody for rotavirus. J Virol Methods. 1991;33:127-34. doi:10.1016/0166-0934(91)90013-P. PMID:1658027
- Ward RL , Kapikian AZ , Goldberg KM , Knowlton DR , Watson MW , Rappaport R . Serum rotavirus neutralizing-antibody titers compared by plaque reduction and enzyme-linked immunosorbent assay-based neutralization assays. J Clin Microbiol. 1996;34:983-5. doi:10.1093/infdis/159.1.79. PMID:8815124
- Ward RL , Bernstein DI , Shukla R , Young EC , Sherwood JR , McNeal MM , Walker MC , Schiff GM . Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989;159:79-88. PMID:2535868
- Qin L , Gilbert PB , Corey L , McElrath MJ , Self SG . A framework for assessing immunological correlates of protection in vaccine trials. J Infect Dis. 2007;196:1304-12. doi:10.1086/522428. PMID:17922394
- Vesikari T , Clark HF , Offit PA , Dallas MJ , DiStefano DJ , Goveia MG , Ward RL , Schödel F , Karvonen A , Drummond JE , et al. Effects of the potency and composition of the multivalent human-bovine (WC3) reassortant rotavirus vaccine on efficacy, safety and immunogenicity in healthy infants. Vaccine. 2006;24(22):4821-9. doi:10.1016/j.vaccine.2006.03.025. PMID:16621194
- Vesikari T1 , Matson DO , Dennehy P , Van Damme P , Santosham M , Rodriguez Z , Dallas MJ , Heyse JF , Goveia MG , Black SB , et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23-33. doi:10.1056/NEJMoa052664. PMID:16394299
- Block SL1 , Vesikari T , Goveia MG , Rivers SB , Adeyi BA , Dallas MJ , Bauder J , Boslego JW , Heaton PM , Pentavalent Rotavirus Vaccine Dose Confirmation Efficacy Study Group . Efficacy, immunogenicity, and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics. 2007;119(1):11-8. doi:10.1542/peds.2006-2058. PMID:17200266
- Armah GE , Sow SO , Breiman RF , Dallas MJ , Tapia MD , Feikin DR , Binka FN , Steele AD , Laserson KF , Ansah NA , et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: A randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606-14. doi:10.1016/S0140-6736(10)60889-6. PMID:20692030
- Zaman K , Dang DA , Victor JC , Shin S , Yunus M , Dallas MJ , Podder G , Vu DT , Le TP , Luby SP , et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: A randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615-23. doi:10.1016/S0140-6736(10)60755-6. PMID:20692031
- Mo Z , Mo Y , Li M , Tao J , Yang X , Diao D , Wei D , Fu B , Liao X , Chu J , et al. Efficacy and safety of a pentavalent live human-bovine reassortant rotavirus vaccine (RV5) in health Chinese infants: A randomized, double-blind, placebo-controlled trial. In: Program and Abstracts of the 12th International Rotavirus Symposium; 2016 Sep 7–9 ; Melbourne, Australia ; Abstract 42
- Liao X , Mo Z , Ma X , Luo P , Mo Y , Kaplan S , Shou A , Zheng M , Hille D , Nelson M , et al. Immunogenicity of pentavalent rotavirus vaccine in Chinese infants: A randomized, double-blind, placebo-controlled trial. In: Program and Abstracts of 8th Asian Congress of Pediatric Infectious Diseases; 2016 Nov 7–10 ; Bangkok, Thailand ; Abstract EP-079
