ABSTRACT
Hepatobiliary and pancreatic cancers along with other gastrointestinal malignancies remain the leading cause of cancer-related deaths worldwide. Strategies developed in the recent years on immunotherapy and cancer vaccines in the setting of primary liver cancer as well as in pancreatic cancer are the scope of this review. Significance of orthotopic and autochthonous animal models which mimic and/or closely reflect human malignancies allowing for a prompt and trustworthy analysis of new therapeutics is underlined. Combinational approaches that on one hand, specifically target a defined cancer-driving pathway, and on the other hand, restore the functions of immune cells, which effector functions are often suppressed by a tumor milieu, are shown to have the strongest perspectives and future directions. Among combinational immunotherapeutic approaches a personalized- and individual cancer case-based therapy is of special importance.
Introduction
Hepatobiliary and pancreatic cancers together with other gastrointestinal malignancies are the leading cause of cancer-related deaths worldwide. Among 8.8 million cancer deaths per year 788,000 and approximately 360,000Citation1 cancer-related deaths occurred due to a hepatobiliary and pancreatic cancer, respectively, as estimated in the latest World Cancer Report (WHO)Citation2 and by GLOBOCAN.Citation3
Hepatobiliary or primary liver cancer comprises roughly 80% of hepatocellular carcinoma (HCC) and 20% of cholangiocarcinoma (CCA), a biliary tract cancer (BTC). Primary liver cancer is reported to have a growing incidence with increasing predictions by the year 2030.Citation4 Pancreatic ductal adenocarcinoma (PDAC) is the most common tumor type of pancreas, accounting for 95% of pancreatic cancer cases.Citation5 Its incidence and mortality rates have been increasing year by year worldwide.Citation1
All 3, HCC, CCA and PDAC, are found to be immunogenic, meaning that cells of immune system are able to recognize tumor cells and several tumor-associated antigens (TAAs) are reported for these malignancies. Clinical observations point out on the improved prognosis if tumor-specific cells are found infiltrating tumor samples. Based on above mentioned, strategies, implicating immunotherapeutic approaches and vaccines able to stimulate the responses against HCC, CCA and PDAC, have strong perspectives in the prediction and treatment of those malignancies.
Here, we review current studies on immunotherapy-based approaches investigated in mouse and human since the year 2000 until March 2017 in HCC, CCA and PDAC and give an overview on ongoing research embedded into the current clinical studies. Data were collected using MEDLINE/PubMed database as well as an open source www.ClinicalTrials.gov. Search keyword criteria included “HCC and immunotherapy,” “CCA and immunotherapy,” “BTC and immunotherapy” and “PDAC and immunotherapy.”
Hepatocellular carcinoma (HCC)
HCC is the fifth most common malignancy in the world.Citation6 In 80% of the cases HCC is associated with cirrhosis or advanced fibrosisCitation7 caused by viral hepatitis,Citation8 toxins, such as alcohol or tobacco,Citation9,10 non-alcoholic steatohepatitisCitation11 or other metabolic disorders, e.g. hemochromatosis.Citation12 Other major risk factors involve aflatoxin exposure,Citation13 genetic disorders such as tyrosinosis or anabolic steroids.Citation7 Caused by the constant inflammatory response, tumor development is promoted in a multistep process.
HCC treatment strategies depend basically on Barcelona Clinic Liver Cancer (BCLC) algorithm, consisting of curative treatments (ablation, resection, transplantation) or palliative treatment (transarterial chemoembolization (TACE), selective internal radiation therapy (SIRT), multikinase inhibitor sorafenib).Citation14
A meta-analysis showed that the survival of HCC patients depends on early diagnosis as these patients profit from possibly curative therapies. However, approximately 60% of the patients are diagnosed at intermediate or advanced stage of the disease.Citation15-17 For these patients after the first line systemic treatment with sorafenib, therapeutic possibilities are limited and therefore the prognosis is most often very poor. The same is true for currently evaluated in phase III studies on multiple kinase inhibitors as reported in ref. Citation18. The failure of therapeutic approaches is mainly caused by resistance to common therapeutic regimens causing an increase in mortalityCitation6,19 that is mediated by tumor-initiating cells which possess stem cell characteristics.Citation20
Several immunotherapeutic approaches are under investigation/development and seem to have promising perspective in the treatment of HCC as reviewed below.
Cytokine-based immunotherapy in HCC
Cytokines, as important mediators of the immune system, have long been within the focus of immunotherapy and their ability to modulate the immune system has been widely used as therapeutic approach. Currently, several ongoing studies analyze the cytokine profiles as well as polymorphisms to better understand the influence of cytokines on HCC disease progression and susceptibility (Supplementary Table 1).
In HCCs the identification of cytokines suitable as biomarkers for diagnosis, prognosis and therapeutic approaches is going on (also reviewed recently by T. GretenCitation21). Screening of 43 Chinese patients with HCC for a Hepatitis B Virus (HBV)-caused HCC-related cytokine profile allowed the identification of 2 potentially diagnostic markers, the macrophage-derived chemokine (MDC) and macrophage-stimulating protein α (MSPα), allowing to construct a HCC diagnosis model, which increased sensitivity from 60% to 73%.Citation22 Furthermore, increased interleukin 17 (IL-17) and IL-17 receptor E (IL-17RE) levels have been associated with poor prognosis.Citation23 Elevated levels of IL-8 correlated with chemoresistance and disease prognosis.Citation24 In line with above mentioned our recent understanding of the role of cytokines shows, that cytokine functions are far more reaching then solely regulation of the immune system.Citation25,26 Cytokines directly influence the survival and resistance of tumor cells with stem cell characteristics.Citation27 It has been shown that increase in certain cytokine levels correlates with increased chemotherapy resistanceCitation28-30 and metastasisCitation31 as well as self-renewal.Citation32 Our study (Kossatz laboratory) for example showed that blockade of IL-8 signaling pathways using the mTORC1 inhibitor Radd001 in combination with doxorubicin inhibited growth of tumor-initiating cells and was also able to significantly block tumor growth in vivo using xenografts.Citation29 Therefore, tumor-specific ablation of IL-8 by for example neutralizing antibodies or as mentioned blockade of signaling pathways could represent a sufficient immunotherapeutic approach in HCC/chemotherapy settings.
Additionally, IL-6Citation33 and IL-10 have been suggested as suitable biomarkers discriminating cirrhotic from cancerous patients.Citation34 Other cytokine profiles have been shown to predict recurrence of HCCCitation35 and to induce immunosurveillance.Citation36
Interferon-γ (IFN-γ) is associated with recurrence-free survival after curative treatmentsCitation37 and induction of autophagy and cell death in HCC cells.Citation36 However, there are reports where IFN-γ expression is associated with hepatic dysfunction in fibrosis and cirrhosis as well as with HCC.Citation38
IFN-α has been shown to have anti-proliferative effects,Citation39 to induce apoptosis in HCC cell linesCitation40 and to prevent neo-plastic growth in a HCC rat model.Citation41 However, as reviewed by Makarova-Rusher et al.Citation42 and Prieto et al.Citation43 the use of IFN-α showed a very low efficacy in clinical trials and revealed a mixed response with regard to recurrence-free survival.
Similarly, mixed results are obtained for IL-12. IL-12 has antitumor effects,Citation44 which are mediated by activation of IFN-γ secretion from T and natural killer (NK) cells,Citation45 activation of cytotoxic lymphocytes (CTL)Citation45 and NK cells.Citation45-47 Overexpression of IL-12 in tumor tissue of several mouse models has been shown to have beneficial effects and prevent metastasis formation caused by sorafenib treatment.Citation48 A study, in which the restoration of IL-12 expression mediated by zoledronic acid was performed, confirmed these results and showed a decrease in metastasis formation.Citation48 Early clinical studies of hematologic malignancies with IL-12 however were disappointing. Care has to be taken as unexpected side effects due to different treatment protocols may arise.Citation47 Overexpression of IL-12 mediated by for example adenoviral delivery in in vivo HCC mouse models showed to be beneficial and prolonged survival.Citation46,49,50 Several other preclinical studies follow different approaches, e.g., by determining the different effect of IL-27 and IL-23, which are the members of the IL-12 family. Their balance is important in carcinogenesis and it has been shown in preclinical studies that both cytokines are attractive candidate agents in anti-cancer therapy.Citation51,52
An important interplay of cytokines/chemokines has been recently observed by us in precancerous livers, when we studied overexpression of oncogenic NrasG12V directly in vivo in hepatocytes.Citation53,54 Precancerous (senescent) hepatocytes were producing numerous secreted factors, so called senescence-associated secretory phenotype (SASP) that led to a massive attraction of various immune cells toward precancerous livers. Recruited immune cells mediated the clearance/killing of precancerous cells thereby protecting from liver cancer development, the mechanism was called senescence surveillance.Citation53,54 Importantly, when the interplay of cytokines/chemokines and receptors thereof on immune cells was dysregulated, precancerous cells were not cleared and resulted in induction of full blown HCC.Citation53,55 A combination of so called M1 cytokines, IL-1α, IL-12/IL-23 and TNF-α, has been shown to be crucial for senescence surveillance.Citation53,54 Currently, one clinical study is recruiting participants to study human telomerase reverse transcriptase (hTERT) immunotherapy in combination with IL-12 DNA electroporation. Studies by Prieto et al. determined the therapeutic use of adenoviral-delivered hIL-12 in HCC patients showing an increased tumor infiltration by effector immune cells in 4/10 patients (2/4 HCC).Citation43
NK cell-based immunotherapy in HCC
On the basis that NK cells kill tumor cells independent on antigen recognition and that the number of NK cells has been correlated with the prognosis of patients, these cells became important effectors in cell-based immunotherapeutic approaches.Citation56 Tumor growth in the background of NK cell function is believed to be caused by NK cell exhaustion, escape of tumor cells from NK recognition, by expression of inhibitory receptors and by secretion of immunosuppressive factors such as TGF-ß (transforming growth factor β), IL-10, PGK2 (phosphoglycerate kinase 2) etc.Citation57,58
Clinical studies that have been completed to focus primarily on the safety of NK-based immunotherapies revealed that application of NK cells is without side effects (NCT01147380). Activation and infiltration of NK cells has been shown to be beneficial for patients with HCC.Citation59 Several other safety studies are under investigation (Supplementary Table 1) determining the safety and toxicity of NK cells. The importance of NK cell-mediated immunotherapy has been underlined in a study showing that activated NK cells extracted from healthy donors treated with poly(I:C) inhibited growth of liver metastasis.Citation60
In current preclinical studies on NK cells, new approaches aiming to activate NK cells to specifically target tumor cells are under investigation. These include cytokine-modified NK cells.Citation61 Blockade of tumor growth in vivo using mouse models was also achieved by hIL-15-modified NKCitation62 and B7-H3 immunogen therapyCitation63 as well as approaches to re-activate NK cell function using Stat3 blockadeCitation64 and miR-182.Citation65
DC-based immunotherapy in HCC
Dendritic cells (DCs), professional antigen-presenting cells, which are able to present tumor antigens to T lymphocytes, opened up a new field in immunobiology and later in immunotherapy.Citation66 For hepatobiliary and pancreatic cancers a retrospective analysis of DC-based vaccination showed mild adverse effects and an activation of the immune system able to target tumor cells.Citation67 For DC-based immunotherapy in HCC 3 clinical trials have been completed recently (NCT01828762, NCT00027534, NCT00004604). Two of these used TRICOM, a triad of co-stimulatory molecules that enhance T cell response,Citation68 in combination with TAA. An often used TAA is the cardioembryonic antigen (CEA), which is overexpressed in many tumors.Citation69,70 The studies are based on previous findings, showing that CEA is present in HCC and that low frequencies of CEA-specific T cell responses have been proven in some patients.Citation68,71
A combination of DCs with granulocyte macrophage colony-stimulating factor (GM-CSF) might improve therapeutic outcome (NCT00027534) as GM-CSF induces rapid maturation of monocytes into DCs, capable to present antigens.Citation72
Preclinical studies also used other sources of T cell antigens, such as tumor-derived autophagosomal peptides used to pulse DCs, which are subsequently applied for immunization.Citation73 A similar approach uses tumor cell derived exosomes.Citation74 Both approaches have the advantage that several tumor antigens are used to enhance T cell immune response. The fusion of tumor cells with DCs follows the same aim, namely that several tumor antigens are used to activate the specific T cell responses.Citation75 Two clinical studies followed this approach (NCT02026362, NCT00327496).
Several other TAAs have been analyzed in preclinical and clinical studies. The usage of the Glypican 3 has been described previously by Greten et al..Citation21 Further HCC linked biomarkers and antigens include the aspartate dehydrogenaseCitation76 which expression is associated with the clinical outcome in patients with HCCCitation77 and α-fetoprotein (AFP). Although AFP is widely used for diagnosis and prognosis of HCC, it has been shown to have a low sensitivity in the diagnosis and prognostic value of HCC.Citation78 Nevertheless, studies with AFP in clinical and preclinical studies are under investigation.Citation79-81
T cell-based immunotherapy in HCC
Antigen-specific tumor-specific T helper (Th)- and T killer (CTLs) cells immune responses play indispensable role in protection against cancer. For instance, as reported in our studies using autochthonous mouse models of premalignant liver disease, antigen-specific CD4+ Th1 cells are the main players in protection against HCC development.Citation53,54
However, in the HCC settings the effector functions of antigen-specific T cells are strongly suppressed by numerous mechanisms that tumor exploits. To (re-)activate and expand HCC-specific Th cells and CTLs different strategies can be followed. Immunomodulatory cytokines and/or expression of costimulatory signals are one possibility.Citation82 Several clinical trials focus on this approach. A phase III study showed very promising results in which cytokine-induced killer cells (CIK), comprising CD3+/CD56+ and CD3+/CD56− T cells and CD3−/CD56+ NK cells, were created by incubation of patients' peripheral blood mononuclear cells (PBMC) with IL-2 and an antibody against CD3. Patients treated with CIK and curative therapy showed increased recurrence free survival (NCT00699816).Citation83 However, other similar approaches did not show these promising effects. A phase I and II study is currently recruiting patients, using CIK cells in combination with TACE or decitabine. However, preliminary results indicate that especially in HCC no response can be monitored or that adverse side effects are detected.Citation84 Other studies unfortunately have not yet published their results.
Modulation of the immune system can also be achieved by vaccination with one or several tumor antigens that are applied either by means of peptide- or DNA vaccination (as described in further sections below). DC-based immunotherapy are using whole cell lysates to achieve a broad anti-tumor response.Citation82 In several animal models these approaches have been tested and validated and showed promising results.Citation85,86 Many clinical studies also target several tumor antigens to induce CIKs (NCT03067493, NCT02858232, NCT02239861, NCT02026362, NCT02678013, NCT02709070), but data are not yet available.
Besides, whole cell lysates-specific tumor-initiating cells that are characterized by expression of certain surface molecules, have been tested as targets for cytotoxic T cell-based therapy and this approach resulted in significant tumor reduction in mouse models.Citation87 So far, this approach is not followed in clinical studies. Improvement of HCC-specific T cell responses can also be driven by bacterial vectors (e.g., Listeria monocytogenes), that have great potential to induce tumor-specific immune responses.Citation88 This approach was already tested using subcutaneous (s.c.) mouse models showing also a synergistic effect of DCs and L. monocytogenes-expressing tumor antigens for the protection against HCC development.Citation89
HEPAVAC, a promising large multicenter European study, is currently under way, aiming to induce T cell responses by vaccination using novel RNA-based adjuvant immunotherapies. It combines multi-target, multi-peptide and multi-HLA (human leukocyte antigen) immunotherapy with personalized treatment approaches.Citation90,91 Enrollment of first patients is expected in 2017.
Checkpoint inhibitors in HCC
Current strategies targeting the immune checkpoint inhibitors programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), or cytotoxic T lymphocyte-associated protein 4 (CTLA-4) in the settings of HCC have been recently reviewed by M. Kudo.Citation92
Based on our search, there are several clinical ongoing or recruiting studies which are testing safety and feasibility of the anti-PD-1 approach in combination with sorafenib (NCT02576509, phase III) and apatinib (NCT02942329, phase I/II), both multikinase inhibitors. Other combinational approaches investigate CC-122, a pleiotropic pathway modifier that mimics an interferon response. The pathway modifier promotes the degradation of transcriptional regulators.Citation93 These approaches test different antibodies targeting and blocking PD-1 such as Nivolumab and SHR-1210. A study combining sorafenib and both, Nivolumab and Ipilimumab, antibodies targeting PD-1 and CTLA-4 respectively, is also currently recruiting patients (NCT01658878).
Recently using orthotopic (grafted and genetically engineered) models of HCC, Chen at al. presented a mechanism showing that anti-PD-1 antibody treatment had additional anti-tumor activity only when combined with sorafenib and AMD3100 (an inhibitor of CXCR4, described below), and not when combined with sorafenib alone.Citation94 Sorafenib-based therapy promoted tumor hypoxia, led to increased expression of a stromal cell-derived 1 α (SDF1α) and its receptor (CXCR4) and recruitment of myeloid-derived suppressor cells (MDSCs) promoting tumor vascularization.Citation94 Blockade of both, SDF1α receptor and PD-1, prevented immunosuppression in HCC tumors, led to increased immune cell tumor penetration as well as activation and subsequently resulted in delayed progression of HCC.Citation94 Therefore, based on these murine data, in addition to sorafenib and anti-PD-1, other strategies e.g., targeting SDF1α/CXCR4 axis and/or tumor vascularization pathways might be required to achieve efficient HCC treatment in human. It´s important to mention that immune-competent orthotopic and autochthonous HCC models strongly contribute to the understanding of mechanism of action of immunotherapies, also to the assessment of their combination regimens and help therefore to predict their translational efficacy.Citation95
Antibody-based immunotherapy in HCC
Apart from antibody-based therapies that target immune checkpoints, several other cellular receptors/molecules on tumor cells have been identified as suitable targets for antibody-based therapies. These targets are mediators of tumor resistance, i.e. tumor-initiating cells. Trop-2 is an antigen which is overexpressed in tumors and characterizes a subpopulation of tumor cells with stem cell characteristics.Citation96 A clinical study is now evaluating the safety of IMMU-132, an antibody targeting Trop-2 in epithelial cancers. Licartin (Iodine-131-Labeled Metuximab) is another monoclonal antibody (mAb) that directly targets tumor cells by binding to an antigen that is overexpressed on HCC cells as well as on fibroblasts (CD147). CD147 receptor is upregulated in early phases of starvation and is an important regulator of glucose metabolism.Citation97,98 Its overexpression has been associated with HCC progression and increased adhesion, invasion and metastasis.Citation99 The study on Licartin is completed, however, with unpublished results (NCT00819650). Mapatumab is an antibody targeting TRAIL1, a receptor that mediates apoptosis. Agonistic antibodies have been shown to promote tumor regression in mouse models of other tumor entities.Citation100 Results of the clinical approach have also not been published. However, several studies indicate that HCC cells are resistant to TRAIL-induced apoptosis.Citation101,102
Other therapies target growth factors, among them the insulin-like growth factor (IGF), which is often found overexpressed in HCC. Both IGF I and IGF II are single chain polypeptides that are produced by several tissues including the liver. Both bind to insulin growth receptors and particularly to insulin-like growth factor I receptor α (IGFIR-α) and both are upregulated in tumors.Citation103 Expression of IGF and activation of associated pathways have been identified in a subclass of HCC among 21% of early HCCs.Citation104 Their overexpression in multiple cancer types is a potential mechanism for resistance to IGFIR-targeting.Citation105
Medi573 and BIIB022, 2 human antibodies neutralizing IGF I/ II are under clinical investigation. IMC-A12 also targets IGF and single-agent activity has been observed. However, its activity was mostly efficient when combined with cytotoxic agents or other targeted therapeutics. The results suggest that it may be an effective therapeutic in a diverse array of oncologic indications (NCT00906373).Citation106
A recent phase III study (REACH) investigating the recombinant IgG1 monoclonal antibody VEGF receptor-2 antagonist, Ramucirumab, missed its primary end point with no significant difference in the overall survival compared with placebo. However, a subgroup of patients with baseline AFP ≥ 400ng/ml showed a significant longer overall survival.Citation107 Thus, Ramucirumab is currently tested in a new phase III trial after sorafenib (NCT02435433). Other approaches that interfere with the ability of the tumor to promote vascularization are under investigationCitation108 (NCT02572687, NCT02024087).
DNA/peptide-based immunotherapy in HCC
DNA and peptide-based immunotherapy induce either a T- or B cell specific immune response or a combined cellular and humoral immune response. The delivery of peptides/DNA is taking place via vaccination using adjuvants, DCs or modified viral vectors.Citation109,110
AFP as a target has been discussed above and it has been mentioned that it is expressed in the majority of HCC and correlative with patients outcome.Citation111 AFP positive cells have been described to define novel prognostic subtypes of HCC.Citation112 Clinical trials using AFP-based peptide immunization have been completed in 2010 and 2012, however with unpublished results. One clinical trial was terminated due to poor accrual and limited target patient population. Comparable studies were performed before, showing that AFP immunization resulted in expanded AFP-specific T cell populations using 4 different AFP peptides that were presented by DCs. Although only 60% of patients responded to the therapy by increased specific T cell activity, the authors concluded that clinical beneficial immune responses need CD4 and CD8 positive T cell responses.Citation113 Other studies also showed AFP-specific T cell responses.Citation114,115 Studies in mouse models showed that optimized AFP epitopes completely protected mice from carcinogen induced HCC or impaired tumor growth.Citation116-119 However, due to the lack of beneficial clinical resultsCitation120 and the comparability of mouse models to the human situation, the importance of AFP as a solely target has been questioned.Citation121,122
Further approaches use intratumoral B7-H3-based DNA vaccines. B7-H3 is upregulated in HCC and correlates with the aggressiveness of the tumor and its postoperative recurrence.Citation123 Expression of B7-H3 is also important for metastasis as it promotes epithelial to mesenchymal transition.Citation124 The usage of B7-H3 as immunotherapeutic adjuvant alone or in combination has been shown to induce CD8+ activation and enhancement of NK cells.Citation63,125
Cholangiocarcinoma (CCA)
CCA is a heterogeneous group of malignant epithelial tumors arising from the biliary epithelium.Citation126 The majority of CCAs are adenocarcinomas.Citation126 CCA can be classified into intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (including hilar and distal bile duct).Citation127 The incidence and mortality of ICC has been increasing over the past 30 years.Citation128 Although CCA is rare in the Western world (incidence 2 to 6 cases per 100,000 people), it is together with HCC the second most common primary liver tumor with increasing incidence worldwide.Citation129
CCA is a very aggressive cancer with high metastatic potential. In patients with advanced CCA median survival is less than 2 years.Citation130 CCA is usually diagnosed in a late stage with a particularly poor prognosis.Citation126 Risk factors for CCA include hepatobiliary flukes, biliary tract cysts, hepatolithiasis, toxins and primary sclerosing cholangitis (PSC).Citation126 Surgical resection of early CCA tumors is so far the only effective therapeutic strategy. Treatment options for patients with advanced or metastatic CCA are limited with short survival times.Citation126,131 Systemic conventional chemotherapy with gemcitabine and cisplatin as current standard of care in advanced or metastatic diseaseCitation132 has only modest gains, with various locoregional treatment options and targeted therapies being under investigation.Citation129 There is just a limited number of genetically engineered mouse models reported for CCA,Citation133 that decelerates the research and translational implementation of treatments of this type of malignancy.
Taking into account that CCA is immunogenic and several TAAs have been identified, immunotherapy has a strong perspective in the treatment of CCA, which will be described in further details below in this review.
Peptide-based immunotherapy in CCA
Several CCA-associated antigens have been recently reported, among them are cancer-testis antigens (CTA),Citation134 TCC52,Citation135 melanoma antigen (MAGE),Citation136,137 Forkhead box M1 (FOXM1)Citation138 and NY-ESO-1139, with high expression rates in CCA suggesting that these antigens can represent promising targets. Therefore, specific immunotherapy targeting CTAs might be a novel treatment option for ICC patients.Citation137,139 Mesothelin is also reported to be overexpressed in one-third of human CCAs making it also a suitable target.Citation140 However, it seems that the vaccination has to be performed using correct adjuvants, because a crude extract was unable to induce antitumor response as reported elsewhere.Citation141 Recently, Rammensee and colleagues have reported a novel therapeutic approach, a case study, in a patient with metastatic ICC who underwent a very successful personalized multi-peptide vaccination. The vaccine was designed based on whole exome sequencing (WES), whole transcriptome sequencing (WTS) and HLA ligandome analysis of the tumors, administered emulsified in adjuvant montanide and reported to induce long-term functional vaccine-specific T cells. The treatment was remarkably successful, the patient is reported to be tumor-free with vaccination continued.Citation142
DC-based immunotherapy in CCA
Using an orthotopic rat model of ICC a DC-based vaccine has been tested.Citation143 DC were loaded with a highly expressed tumor-associated cell surface protein, aspartate-β-hydroxylase (ASPH), and stimulated with GM-CSF, interleukin-4, CD40L, and IFN-γ to generate Th1 responses. Immunization with ASPH-loaded DCs generated cytotoxicity against CCA cells in vitro and significantly suppressed intrahepatic tumor growth and metastasis in vivo. Moreover, the vaccination was associated with increased CD3+ lymphocyte infiltration into the tumors.Citation143
In a retrospective human study with 65 patients who had nonresectable, recurrent or metastatic BTCs the safety and efficacy of DC-based immunotherapy was evaluated. DCs were pulsed with BTC-associated tumor antigens, synthesized peptides: Wilm´s tumor 1 (WT1) and Mucin 1 (MUC1). DC-based immunotherapy for BTCs was safe and produced a clinical response for the patients who underwent chemotherapy and maintained a good nutrition status.Citation144
Two phase I clinical studies based on just DC vaccine have been completed (Supplementary Table 2, NCT00004604, NCT00027534). In the first one autologous cultured DCs were pulsed with CEA RNA. In the second phase I study (as it has been already described for HCC) autologous DCs have been infected with fowlpox-CEA-TRICOM, mixed either with a cytomegalovirus (CMV) pp65 peptide or with tetanus toxoid and administered to patients,Citation145 but data on gallbladder cancer and pancreatic cancer are not available. Combination therapy comprising both DCs and T cells are described below.
T cell-based immunotherapy in CCA
A high potential of T cell-based therapy in CCA is well reflected in several human case reports. In the first study an analysis of pleural effusions collected from a 47-year-old man with recurrent CCA and malignant effusion revealed a presence of tumor-infiltrating lymphocytes (TILs). Preparing several co-culture experiments the researchers have shown that the expression of T cell-related antigens, but not B cell- or NK cell-related antigens, on effusion-associated lymphocytes was blocked temporarily by the malignant pleural effusion. This was the first report describing the existence of a large quantity of unclassified lymphocytes in which the T cell-related antigens were reversibly masked in the malignant pleural effusion.Citation146 In another human study using a WES-based approach the researchers were able to demonstrate that TILs from a patient harboring metastatic CCA contained CD4+ Th1 cells recognizing a mutation in erbb2 interacting protein (ERBB2IP) expressed by the tumor. Adoptive transfer of such TILs into the patient has been shown to lead to a significant decrease in tumor lesions and to a prolonged stabilization of disease. Upon disease progression, the patient was retreated with a >95% pure population of mutation-reactive Th1 cells and again experienced tumor regression.Citation147
In a most recent case report the potential of chimeric antigen receptor-modified T cell (CAR T) immunotherapy in treating advanced unresectable/metastatic CCA has been analyzed. In this case, a patient with advanced CCA and resistant to chemo- and radiotherapy has been treated with CAR T cocktail immunotherapy, composed of successive infusions of CAR T cells targeting epidermal growth factor receptor (EGFR) and CD133, respectively. This resulted in a partial response, however, several infusion-related toxicities especially the epidermal/endothelial damages have also been detected and require further investigation.Citation148 A clinical trial based on the EGFR-targeting CAR T (NCT01869166) is still ongoing.
It is important to mention, that not just CD4+ or CD8+ T lymphocytes are currently tested in cell therapy approaches. Modified autologous CIK cells showed also perspectives in preclinical phase (in SCID mice) of human CCA treatment and a clinical trial based on CD3+CD56+ CIKs (NCT01868490) is currently ongoing.
Treatment approaches based on a combination of both, DC and T lymphocytes possess even stronger perspectives. ICC with lymph node metastasis has been successfully treated in a patient after surgery using immunotherapy consisted of CD3-activated T cells and tumor lysate- or peptide-pulsed DC. The patient is alive without recurrence 3.5 y since the surgery.Citation149 In 2 other ICC studies the combination therapy of adoptive transfer of anti-CD3 activated T cells and autologous tumor lysate-pulsed DCs vaccine therapy (DC-CAT therapy) after surgery was studied. Interestingly, patients who had skin reaction during DC-CAT had a better prognosis with increased numbers of lymphocyte, especially CD8+ T cells and reduced regulatory Foxp3+ T cells.Citation150 Moreover, the patients whose skin reactions were 3 cm or more at the vaccine site showed a strong increase in the 5-year progression-free survival (PFS) and overall survival (OS), making a postoperative combinatorial DC/T cell adjuvant treatment a feasible and effective treatment of preventing recurrence and achieving long-term survival in ICC patients.Citation151
Checkpoint inhibitors in CCA
An encouraging retrospective study performed in 27 patients with ICC showed that ICC patients mount a T cell immune response against their own tumors. TILs found in the tumors possess PD-1+ phenotype and ICC cells have been shown to be PD-L1+. Moreover, the authors claim that an immune escape mechanism is based on defects in HLA class I antigen expression in combination with PD-L1 expression by ICC cells. These promising study results justify the implementation of immunotherapy with checkpoint molecule-specific mAbs in patients bearing ICC tumors without defects in HLA class I antigen expression.Citation152 The soluble form of PD-L1 (sPD-L1) possesses immunosuppressive activity. Therefore, Korean researchers measured the serum level of sPD-L1 and evaluated its prognostic implication in BTC. Patients with high sPD-L1 have been shown to have worse OS.Citation153 In line with this, a recent review on 61 patients with different malignancies including CCA concluded that the use of immune checkpoint inhibitors is effective in cancers that express microsatellite instability.Citation154 Therefore, a phase II trial will be conducted enrolling patients with intrahepatic and extrahepatic CCAs as well as pancreatic cancer. In this phase II trial Nivolumab, an anti-PD-1 mAb will be combined with Ipilimumab, an anti-CTLA-4 inhibitor (Supplementary Table 2).
Another combination immunotherapy study based on Pembrolizumab (checkpoint inhibitor, anti-PD-1 mAb), supplemented with pegIFN-α-2b in patients with advanced CCA is also currently performed/recruiting (NCT02982720). The idea of this phase II study is based on a retrospective study of 375 patients with CCA describing impact of TILSs on BTC as well as 17% response rate of Pembrolizumab in patients with advanced CCA. IFN-α-2b has been shown to increase TILs. Therefore, it is hypothesized that effect of Pembrolizumab will be further enhanced by pegIFN-α-2b.
One more phase II clinical trial (NCT01174121) based on a combination of Pembrolizumab and short-term cultured autologous TILs as well as a lymphocyte-depleting regimen and high-dose Aldesleukin (IL-2) is currently ongoing (Supplementary Table 2) for patients with metastatic cancer including metastatic CCA, HCC and pancreatic cancer.
mAb-based immunotherapy in CCA
An approach based on mAb targeting particular tumor-associated receptors on tumor cells is widely used in immunotherapy (all PD-L1-targeting antibodies for CCA are described in the checkpoint inhibitor section). For example, a successful mAb-mediated targeting of L1 cell adhesion molecule (L1CAM) that is aberrantly expressed in malignant tumors and plays an important role in tumor progression, has been reported in a human CCA xenograft nude mouse model. L1CAM targeting by high affinity Ab417 significantly inhibited tumor growth and did not induce any adverse effect in in vivo study, as reported and suggested for the clinical trials.Citation155 A successful case study trial in a patient with a metastatic EGFR+ bile duct cancer has been reported using anti-EGFR mAb, Cetuximab (Cmab). It is important to mention that diagnostic of EGFR positivity was indispensable for this treatment. The patient was reported to be still healthy 7 y after the onset of peritoneal recurrence (5.5 y after the initiation of Cmab therapy).Citation156 However, there are also unsuccessful reports using mAb. As such, targeting of carbonic anhydrase 9 that is overexpressed on biliary cancers and recognized by chimeric radiolabeled mAb cG250 has been reported to be inefficient and not suitable for biliary cancer targeting as confirmed in a small clinical study with 3 patients harboring biliary cancer.Citation157
Currently, a phase II study compares Ramucirumab with Merestinib, a small molecule that has been shown in vitro to be a reversible type II ATP-competitive inhibitor of MET, or placebo plus the standard palliative regimen cisplatin and gemcitabine as first-line treatment in patients with advanced or metastatic BTC (NCT02711553).
Stem cell transplantation in CCA
Allogeneic haematopoietic stem cell transplantation (HSCT) has been shown to lead to an antitumor effect, based on allogeneic graft-versus-leukemia induction, which recently resulted in the expansion of HSCT for the treatment of solid cancers.Citation158 Several single case studies with various success have been reported on stem cell transplantation in CCA.Citation159-162 In a recent study performed in 17 patients with CCA who received a combination of liver transplantation and HSCT, only 1 out of 17 patients had a long-term survival. Interestingly, this patient rejected the HSCT graft, demonstrating an antitumor effect from allograft rejection.Citation163 HSCT in CCA still requires more investigation and HSCT seems to be more efficient in patients with pancreatic cancer (described below).
Chemo/immunotherapy in CCA
Adjuvant therapy (chemotherapeutic regimens applied after tumor surgery) may improve the outcome after a complete surgical tumor resection (R0) for treatment of CCA. Although postoperative adjuvant chemotherapy for resectable CCA is widely used in clinic, its role is still poorly defined. In a recently developed resectable orthotopic ICC mouse model, the effect of adjuvant gemcitabine chemotherapy after the resection of primary tumor was studied. The adjuvant therapy resulted in significantly improved median survival of treated animals reflecting the treatment outcome in human. Importantly, the efficacy of chemotherapy might be connected with induction of therapy-induced senescence and senescence surveillanceCitation53,54 resulting in the clearance of senescent tumor cells and subsequent prolongation of survival, however it remains to be elucidated. We believe that such immune-competent orthotopic models will therefore contribute in the future to the understanding of the mechanism of adjuvant chemoimmunotherapy.Citation164
A smaller prospective single center clinical study (phase I/II) was investigating adjuvant treatment with cisplatin plus gemcitabine after curative resection of CCA (NCT01073839), but data are not yet available. Currently, a phase III trial (ACTICCA-1) is assessing the clinical performance of gemcitabine with cisplatin and observation vs. observation alone in patients after curative intent resection of CCA and muscle invasive gall bladder carcinoma, (NCT02170090). As it has been already mentioned, it will be important to investigate therapy-induced senescence and senescence surveillance in above mentioned human studies.
Pancreatic ductal adenocarcinoma (PDAC)
Cancer of the exocrine pancreas is a highly lethal disease, with an overall 5-year survival rate of around 5%.Citation165 The most common tumor type among pancreatic cancers is PDAC.Citation166 Major risk factors for exocrine pancreatic cancer are cigarette smoking, obesity, nonhereditary chronic pancreatitis, pancreatic cysts and hereditary risk factors.Citation167 Surgical resection is the only potentially curative treatment,Citation167-170 but only in 20% of the patients surgical resection can be undertaken for curative intend. Adjuvant chemotherapy is given after surgery, but the overall 5-year survival rate is still only 20% to 30%.Citation171 However, neoadjuvant and adjuvant chemotherapy with FOLFIRINOX (fluorouracil [5-FU], leucovorin, irinotecan, oxaliplatin) is still subject to clinical trials. With the introduction of the chemotherapeutic regimens FOLFIRINOX and gemcitabine plus nanoparticle albumin-bound paclitaxel (nab-paclitaxel) for patients with metastatic pancreatic cancer and with good performance status, some improvements of the median and 1- and 2-year survival have been made.Citation167-170
Based on available murine PDAC models and on identification of PDAC-specific TAAs (e.g., mesothelin), several immunotherapeutic approaches have been tested, mechanistically characterized and translated into clinical phases. Some of them show strong perspectives in the treatment of PDAC as described below.
Peptide-based immunotherapy in PDAC
Several studies have been performed to define immunogenic peptides in PDAC. Such, to identify tumor antigens useful for diagnosis and immunotherapy of patients with PDAC, researchers from Japan performed a serological identification of antigens by recombinant expression cloning (SEREX) on PDAC specimen/cells. Among the isolated total 32 genes, they were able to detect 2 immunogenic antigens: Homo sapiens mutS homolog 2 (hMSH2) and Homo sapiens postmeiotic segregation increased 1 (hPMS1) were overexpressed in PDAC compared with normal pancreatic ducts.Citation172 In a follow-up study they immunoscreened a testis cDNA library and detected an immunogenic antigen KU-CT-1 as a new cancer testis antigen that is expressed in pancreatic and also other cancers.Citation173 In another study Inami et al. detected a frequent expression of C-ERC/mesothelin in human pancreatic cancer cell lines and in human PDAC tissues, suggesting C-ERC/mesothelin as a useful target for immunotherapy.Citation174 In other work the researchers detected a new TAA for PDAC, Mucin 5AC (MUC5AC), and established MUC5AC-specific CTLs.Citation175
Several clinical studies are ongoing to define and to implement immunogenic antigens in PDAC settings. A prospective study is dedicated to a comprehensive molecular characterization of advanced PDAC for better treatment selection. Molecular profiling and whole genome sequencing will be performed on collected PDAC samples. The purpose of this study is to see how useful it is to look for changes and characteristics in genes and compare them to genes within the tumor (NCT02750657, observational study, recruiting). In another multicenter, randomized phase II clinical trial for patients with metastatic pancreatic adenocarcinoma, patients will undergo a tumoral biopsy to perform exome sequencing, bioinformatic report and avatar mouse models for drug testing. Subsequently these patients will be administered with a personalized therapy (NCT02795650, recruiting).
A phase I pilot study has been completed and determined whether an autologous vaccine tumor-derived heat shock protein gp96 (NCT00003025) could be purified from completely resected pancreas adenocarcinomas and induce clinical outcomes in vaccinated patients. Patients received additionally neither adjuvant chemotherapy nor radiation. Three of 10 vaccinated patients have been reported to be disease-free and alive at 2.6, 2.7, and 5.0 y follow-up. There has not been observed correlation between immune response and prognosis.Citation176 Subsequently a phase I/II study on immunotherapy of tumor with gp96 in treatment of liver cancer and pancreatic adenocarcinoma (NCT02133079) has been initiated.
Researchers presented data from a clinical phase I/II trial (NCT02261714) involving patients with adenocarcinoma of the pancreas vaccinated by intradermal (i.d.) injection of GM-CSF in combination with synthetic mutant ras peptides (mutated KrasG12V or KrasG12D is one of the most frequent oncogenes in PDAC). Vaccinated patients showed evidence of induction of long-lived immunological memory responses against the ras mutations and ras-specific T cells have been shown to selectively accumulate in the tumor. Despite harboring advanced cancer, patients developed an immune response to the peptide-based vaccine and showed prolonged survival.Citation177 In the follow-up publication the researchers reported a 10-year survival reached in 20% of patients vaccinated with ras-peptide-based vaccine following surgical resection.Citation178
An exploratory trial on combination of immuno-/chemo-/radiotherapy for locally advanced pancreatic adenocarcinoma using vaccination with a telomerase peptide vaccine (GV1001) and GM-CSF along with gemcitabine followed by concurrent radiation therapy is currently ongoing (NCT01342224). In a recent report on this study the researchers aimed to define the optimal dose and scheduling of radiation for combination with gemcitabine and peptide-based immunotherapy and analyzed the effect of fractionated and hypofractionated chemoradiation on immune cells in patients with locally advanced and borderline resectable pancreatic adenocarcinoma. They detected that standard fractionated chemoradiation resulted in a significant and extended loss of lymphocytes whereas treatment with hypofractionated radiation therapy avoided the loss of lymphocytes associated with conventional fractionation.Citation179 A phase I trial of chemoimmunotherapy and hypofractionated radiation therapy for borderline resectable and locally advanced pancreatic adenocarcinoma evaluating the safety of combination treatment that includes chemotherapy, radiation therapy, and immunotherapy (tadalafil, an oral phosphodiesterase-5 inhibitor) in patients with pancreatic cancer has been initiated (NCT01903083).
Cytokines-based immunotherapy in PDAC
Studies implementing GM-CSF-based treatment are reported later in the section on “GVAX” and here we review few studies reported on interleukins. In a case study performed in 65-year-old man with pancreatic adenocarcinoma preoperative recombinant IL-2 (rIL-2) has been administered inducing high intratumoral infiltration for CD1a+ cells and mild infiltration for CD3+ cells.Citation180 A clinical study performed in patients with resectable pancreatic adenocarcinoma using pre- and postoperative rIL-2 treatment showed that preoperative high dose rIL-2 administration is able to counteract surgery-induced deficiency of NK cells and eosinophils in peripheral blood.Citation181 IL-2-activated human NK cells are able to kill PDAC cells and are further boosted when PDAC cells are infected with an oncolytic parvovirus H-1PV.Citation182
In a phase I first-in-human study AM0010 (pegylated recombinant human IL-10) in combination with chemotherapy and checkpoint inhibitors in patients with advanced solid tumors including pancreatic carcinoma will be evaluated (NCT02009449).
NK cell-based immunotherapy in PDAC
NK cells play a fundamental role in tumor immune surveillance. Several clinical trials on combinational therapy involving NK cells have been started, as reviewed inCitation183 and one of the studies based on chimeric antigen receptor-modified NK (CAR NK) cells is recruiting patients with MUC1+ solid tumors, including pancreatic cancer.
DC-based immunotherapy in PDAC
Several DC-related studies are reported using s.c. and orthotopic murine models of PDAC. To increase the numbers of antigen-presenting cells in pancreatic tumors, syngeneic, bone marrow-derived DCs were pulsed with tumor RNA, derived from the pancreatic cell line PANC02 and injected intratumorally into orthotopic PANC02 tumors. Intratumoral administration of such antigen-pulsed DCs induced significantly more potent protective immunity than s.c. or intravenous (i.v.) administration. The antitumor effect in this case was caused by induction of antigen-specific T lymphocytes.Citation184 In another study using a PANC02-based s.c. tumor model, an intratumoral vaccination with α-galactosylceramide (α-GalCer)-loaded DCs resulted in a significant expansion of IFN-γ-producing natural killer T cells (NKT) cells which also correlated with decrease in tumor growth in vivo.Citation185 DC cells loaded with pancreatic tumor-specific glycoepitope C-ter-J28+ were shown to skew CD3+ T cells toward Th1 polarization, to reduce ectopic PANC02 tumors in s.c. and orthotopic murine models and to provide long-lasting protection against PDAC. These studies showed that intratumoral DC-injection was more effective than s.c. DC injection.Citation186
Using a combinational approach Konduri et al. treated orthotopically established KrasG12D/p53−/− PDAC tumors with gemcitabine and mRNA-/tumor lysate-pulsed DCs manipulated to be able to induce Th1 immune response. This has been shown to be highly efficient in eliminating tumor, preventing metastasis and recurrence, and significantly enhancing OS, whereas single therapies resulted in relapse and tumor progress. The protective effect of combinational treatment was dependent on CD8+IFN-γ+CCR7+NK1.1+ T cells.Citation187 Murine models of PDAC underlined the success of DC-based immunotherapeutic approaches and linked mechanisms in pancreatic cancer. Therefore several studies have been initiated in clinic.
A very successful combinational approach has been reported in a case study: a patient with PDAC underwent radical surgery, gemcitabine treatment (that had to be however terminated due to neutropenia), and subsequently the patient received vaccination with DCs loaded with hTERT mRNA for 3 y. The patient developed an immune response against several hTERT-derived CD4- and CD8 epitopes and the complete remission has been achieved.Citation188
A phase I trial has been recently reported in PDAC patients using a combination of gemcitabine treatment and a vaccination based on DCs pulsed with WT1 peptides. The study clearly showed that inclusion of both MHC class I- and II–restricted epitopes may be associated with the long-term survival of patients with PDAC, pointing that both tumor-specific CD4+ and CD8+ T cell immune responses are required for protection against the tumor. Interestingly, delayed-type hypersensitivity (DTH) developed in 3 vaccinated patients correlated significantly with increased OS and PFS.Citation189 Interestingly, decreased levels of IL-6/-8 observed throughout long-term vaccination with WT1-pulsed DCs in combination with chemotherapy were associated with WT1-specific DTH reactions and long-term OS.Citation190
Two phase I clinical trials based on just DC vaccine have been recently completed (as it has been already described for HCC and CCA, NCT00004604 and NCT00027534, Supplementary Table 1, 2, 3) and were based on autologous, cultured, DCs pulsed either with CEA RNA or infected with fowlpox-CEA-TRICOM respectively. In both trials patients were harboring CEA-expressing cancers. The data on PDAC from both trials are still unavailable. Combinatorial therapy comprising both DCs and T cells are described below.
GVAX and beyond as immunotherapy in PDAC
In search of new adjuvant approaches in the treatment of PDAC several studies have been performed using GM-CSF-based immunotherapy administered in patients with resected pancreatic adenocarcinoma. The first clinical evaluation of an irradiated, allogeneic GM-CSF–transduced cancer vaccine composed of 2 allogeneic GM-CSF–secreting pancreatic tumor cell lines (PANC 10.05 and PANC 6.03) has been conducted in a phase I trial of 14 patients with stage 1, 2, or 3 of pancreatic adenocarcinoma to assess the safety and the induction of systemic antitumor immune responses. Several patients also underwent subsequently an adjuvant radiation and chemotherapy. This combinational approach has been reported to be safe. Vaccination induced increased dose-dependent systemic antitumor immunity. DTH responses induced toward autologous tumor cells correlated with increased disease-free survival (DFS) time.Citation191
A follow up phase II study has been performed in 60 patients with resected pancreatic adenocarcinoma, who underwent a combination of surgery, GM-CSF-based immunotherapy (as described above) and 5-FU-based chemoradiation. An immunotherapy approach integrated with chemoradiation was safe. The study observed a survival benefit from vaccine over chemoradiation in the first 2 y after surgery. Additionally, it showed that postimmunotherapeutic induction of mesothelin-specific CD8+ T cells correlated with improved disease-free survival. Numerous numbers of regulatory T cells (Tregs) have been observed present in PDAC specimen.Citation192
In a continuous study both a neoadjuvant and adjuvant clinical trial has been performed evaluating the usage of irradiated, GM-CSF–secreting, allogeneic PDAC vaccine (GVAX) given in combination with cyclophosphamide to deplete Tregs. Examination of resected PDACs revealed the formation of intratumoral tertiary lymphoid aggregates (TLAs, regulatory structures of adaptive immunity) in more than 80% of patients. Suppressed Tregs and enhanced Th17 responses within the vaccine-induced intratumoral lymphoid aggregates correlated with increased mesothelin-specific T cell responses and longer patient survival. Moreover, there has been observed the upregulation of immunosuppressive regulatory mechanisms, suggesting that patients with vaccine-primed PDAC could be better candidates for checkpoint inhibitor therapy.Citation193 Similar results on increased mesothelin-specific CD8+ T cell responses have been reported using GM-CSF–secreting pancreatic cancer cell lines (CG8020/CG2505) as immunotherapy administered in combination with cyclophosphamide in patients with advanced pancreatic cancer.Citation194 Importantly, if an additional blockade of TGF-ß pathway (TGF-β blocking antibody) is combined with GVAX vaccine, it results in significant infiltration of anti-tumor-specific IFN-γ producing CD8+ T cells and intratumoral depletion of Tregs as well as survival advantage.Citation195 The mechanism of action of TGF-β blocking therapy in combination with GVAX vaccination is described in 195, using a metastatic murine model of pancreatic cancer.
A phase II trial of allogeneic pancreatic tumor cell vaccine transfected with the GM-CSF (similar to GVAX) also in combination with cyclophosphamide for the treatment of patients with surgically resected pancreatic adenocarcinoma is currently recruiting patients (NCT01088789). Another phase II clinical trial based on combination comprising allogeneic tumor cell vaccine treated with IFN-α plus radiation therapy and cyclophosphamide plus GM-CSF has been completed in patients with advanced pancreatic cancer. The data are still unavailable (NCT00002773, Supplementary Table 3), however such radiation- or chemotherapy-based pretreatment of cells and their subjection to IFN-α makes the tumor cells susceptible to NK cells. IFN-α also induces the immunoproteasome with impact on the immunogenicity of pancreatic carcinoma cells, as reported.Citation196
A very important approach has been developed using attenuated facultative intracellular bacterium L. monocytogenes as a cancer vaccine in the settings of PDAC. The approach is based on a recombinant live-attenuated L. monocytogenes vaccine strain that is rapidly cleared from mice after immunization and induced potent and durable effector and memory T cell responses with no measurable liver toxicity.Citation197 This strain has been engineered to express human mesothelin giving rise to the vaccine strain CRS-207. Phase I study has been performed using CRS-207 vaccine strain in patients with mesothelin-expressing cancers, showing the development of listeriolysin O (an important virulence factor of L. monocytogenes) and mesothelin-specific T cell responses upon vaccination.Citation198
A very important study has been performed using a combination of GVAX and cyclophosphamide and CRS-207, in prime/boost vaccination regime in 90 patients with pancreatic adenocarcinoma. This combinational approach resulted in extended survival for patients with pancreatic cancer and minimal toxicity.Citation199 There has recently been completed a phase IIb trial to assess the safety of this approach (Supplementary Table 3, NCT02004262). In another phase II clinical trial that is going to be started, an orally available immunomodulator Epacadostat, Pembrolizumab, CRS-207 as well as GVAX and cyclophosphamide will be used in combination in patients with metastatic pancreatic cancer (Supplementary Table 3, NCT03006302).
T cell-based immunotherapy in PDAC
Antigen-specific T cell responses are indispensable in fighting against cancer. In a recent animal study several T cell receptors (TCRs), which are highly reactive to the mutated human KrasG12V/KrasG12D, have been defined and isolated using transgenic mice. TCRs have been subsequently introduced into peripheral blood lymphocytes (PBLs). Transgenic PBLs harboring KrasG12V/KrasG12D –specific TCRs have been shown to recognize multiple human pancreatic tumor lines bearing the appropriate Kras mutations. In a xenograft model of large established tumors, adoptive transfer of these transgenic PBLs significantly reduced the tumor growth.Citation200
However, PDAC develops multiple mechanisms of immune suppression, which was confirmed by many studies using available orthotopic and autochthonous murine models as well as by analysis of human tumor samples. For instance, in contrast to s.c. injected PDAC tumors, an impaired infiltration of CD8+ T cells in spontaneous (autochthonous) pancreatic tumors has been reported.Citation201 In line with above mentioned, the lack of intratumoral infiltration of T effector cells strongly correlated with the presence of intratumoral MDSCs as has been observed in another study using a genetically defined autochthonous mouse model of PDAC. The study also demonstrated an early immunosuppression in PDAC settings.Citation202 In line with the previous study, using an autochthonous PDAC model, the researchers observed that the engineered mesothelin-specific CAR T cells preferentially accumulated in PDAC, however, these tumor-infiltrating T cells became progressively dysfunctional.Citation203 A recent work reported that loss or inhibition of CXC-motif-chemokine receptor 2 (CXCR2) improved T cell entry. Therefore, to restore both, intratumoral infiltration- and function of T cells, the researchers combined inhibition of CXCR2 and PD-1 in mice with PDAC. Animals treated with this combinational therapy approach showed significantly extended survival.Citation204
Several attempts on generation of CAR T cells have been reported using human samples. CAR T cells are able to target a prostate stem cell antigen (PSCA), a TAA that is frequently expressed by pancreatic cancer cells, which have been generated using a retroviral vector encoding a CAR targeting PSCA. These cells were able to specifically kill PSCA-expressing pancreatic cancer cell lines.Citation205 In another study a protocol for generation of TILs from patients with PDAC has been established. Lymphocytes obtained from patient samples were stimulated with several cytokines (IL-2, IL-15, and IL-21) to expand TILs, following by a stimulation with an anti-CD3 antibody and irradiated allogeneic PBMCs. TILs showed reactivity against TAAs (mesothelin and NY-ESO-1).Citation206
A combinational therapy comprising both DCs pulsed with MUC1 peptide (MUC1-DC) and CTLs has been reported by Kondo et al. in 20 patients with unresectable or recurrent pancreatic cancer. Upon this combinational therapy one patient had a complete response and 5 patients have been reported to have stable disease.Citation207
Several clinical trials using T cell-based therapy and combinations thereof are currently recruiting patients. Phase I/II study on autologous lentivirus-modified CAR T cells able to target epithelial cell adhesion molecule (EpCAM) for the treatment of patients with EpCAM positive cancers (NCT03013712) is ongoing. Another phase I/II study based on immunotherapeutic combination comprising invariant NKT (iNKT) cells and CD8+ T cells is recruiting patients with advanced tumors, including HCC and pancreatic cancer (NCT03093688). Patients with metastatic cancers are currently recruited for a phase II study on a combinational therapy consisting of short-term cultured and autologous TILs, Aldesleukin (IL-2), followed by lymphocyte depleting regimen using cyclophosphamide or fludarabine, and subsequent, at time of progression, administration of Pembrolizumab (Supplementary Table 3, NCT01174121). An early phase I study on CD8+NKG2D+ AKT cells and adjuvant gemcitabine in pancreatic cancer patients (NCT02929797), as well as a phase I/II study on combination of iAPA-DC/CTL and gemcitabine therapy on advanced pancreatic cancer (NCT02529579) are ongoing.
Checkpoint inhibitors in PDAC
Importantly, in the phase II clinical trial on checkpoint inhibitor Ipilimumab in patients with locally advanced or metastatic pancreas adenocarcinoma, the therapy has been shown to be ineffective for the treatment of advanced pancreatic cancer. However, a significant delayed response in one subject of this trial suggested that immunotherapeutic approaches to pancreas cancer deserve further exploration.Citation208
A research work from Douglas Fearon group found a mechanism why immunotherapy might be ineffective using an autochthonous model of PDAC. Although tumor-specific CD8+ T cells were found present, still like in human patients, no response could be detected toward 2 immunological checkpoint antagonists: α-CTLA-4- and α-PD-L1 antibodies. However, by removing the carcinoma-associated fibroblast (CAF) expressing fibroblast activation protein (FAP) from tumors permitted immune control of tumor growth and uncovered the efficacy of α-CTLA-4/ α-PD-L1 antibodies. FAP+ CAFs were found to be the only tumoral source of chemokine (C-X-C motif) ligand 12 (CXCL12, also known as SDF1α), and administration of AMD3100, an inhibitor of CXCL12/SDF1α receptor, CXCR4, revealed the anti-tumor effects of checkpoint inhibitors and greatly diminished cancer cells.Citation209 Interestingly, AMD3100 has been also reported to increase the efficiency of anti-PD-1 treatment in HCC after sorafenib treatment.Citation94 In another recent study performed in p48-Cre/LSL-KrasG12D/p53Flox/+ (KPC) mouse model of human PDAC a hyperactivated focal adhesion kinase (FAK) activity in neoplastic PDAC cells has been identified as a significant regulator of the fibrotic and immunosuppressive tumor microenvironment. Inhibition of FAK using the selective FAK inhibitor VS-4718 significantly limited tumor progression and rendered the previously unresponsive KPC mouse model responsive to T cell immunotherapy and PD-1 antagonists.Citation210 Based on the above described, it is important to mention that autochthonous PDAC models strongly contributed to a mechanistic characterization of immunotherapies, which implement checkpoint inhibitors.
A limited efficacy of immune checkpoint inhibitors in PDAC has prompted studies using combinational therapies. Such, a treatment of mice harboring PDACs with GVAX resulted in significant upregulation of PD-L1 membranous expression and combination therapy based on GVAX and PD-1 antibody blockade improved murine survival compared with PD-1 antibody monotherapy or GVAX therapy alone. Furthermore, immunosuppressive pathways, including Tregs and CTLA-4 expression on T cells were overcome by the addition of GVAX and cyclophosphamide to PD-1 blockade.Citation211 A study recently performed in mice revealed that a combinational approach also has perspectives, as the blockade of both, IL-6 and PD-L1, resulted in increased OS of animals with aggressive PDAC.Citation212
Several clinical trials are ongoing at the moment. A phase II clinical trial is in preparation for testing of Ipilimumab and Nivolumab as decribed also for CCA (NCT02923934). A phase Ib trial on Ipilimumab (BMS-734016) and GVAX in patients with locally advanced, unresectable or metastatic pancreatic adenocarcinoma (NCT00836407) is completed. A phase II, multicenter study of FOLFIRINOX followed by Ipilimumab in combination with GVAX in the treatment of metastatic pancreatic cancer (NCT01896869) is suspended. A phase II study on combination of GVAX/cyclophosphamide, Pembrolizumab and stereotactic body radiation therapy (SBRT) in patients with locally advanced pancreatic adenocarcinoma (NCT02648282) is recruiting patients. A pilot study testing a combination of GVAX, cyclophosphamide, SBRT and FOLFIRINOX chemotherapy in patients with resected pancreatic adenocarcinoma (NCT01595321) is ongoing. A multi-center, phase I study on a combination therapy of ABBV 927 and Nivolumab in patients with advanced solid tumors including pancreatic cancer, (NCT02988960) is recruiting patients. A phase II study on irreversible electroporation of locally advanced pancreatic adenocarcinoma in combination with Nivolumab post-operatively in patients with locally advanced pancreatic cancer (NCT03080974) is not yet recruiting patients. A phase Ib and II multi-center study of MEDI4736 in different combinations using chemotherapy (nab-paclitaxel and gemcitabine) and novel anticancer agents (AZD5069) in patients with metastatic PDAC is also recruiting patients (NCT02583477).
mAb-based immunotherapy in PDAC
Several studies based on mAbs that are able to specifically target PDAC-related antigens have been reported. Such, targeting of AXL receptor tyrosine kinase that is implicated in proliferation and invasion of many cancers including PDAC, resulted in significant tumor growth reduction using s.c. and orthotopic pancreatic tumor xenografts.Citation213
In another study human PMAb83 targeting PAUF, a pancreatic adenocarcinoma upregulated factor, that plays an important role in tumor progression and metastasis, has been evaluated. In combination with gemcitabine, PMAb83 therapy resulted in survival of xenografted mice by about twofold compared with gemcitabine alone. PMAb83 treatment decreased the aggressiveness of carcinoma cells and suppressed tumor vascularization.Citation214
A very interesting mAb-based approach has been reported by Oberg et al. who have tested a bispecific antibody that binds simultaneously to both: to CD3 or Vγ9 on γδ T cells and to Her2/neu (ERBB2) expressed by pancreatic tumor cells. Adoptive transfer of γδ T cells with the Her2/Vγ9 antibody reduced growth of pancreatic tumors grafted into SCID-beige immunocompromised mice.Citation215
A combination therapy of gemcitabine, the CHK1 inhibitor PF-477736 and Lutetium-177 (177Lu)–labeled anti-EGFR antibody has been recently investigated in fresh patient-derived xenograft murine models. This triple combination therapy induced extensive DNA damage, apoptosis, and tumor degeneration in patient-derived xenograft.Citation216
Several clinical trials are reported using mAb in PDAC settings. Data from the first-in-human phase I study on the ASG-5ME, an antibody-drug conjugate (ADC), targeting SLC44A4 a novel cell surface protein which is expressed on most pancreatic and gastric cancers has been very recently reported and demonstrating that ASG-5ME treatment was generally well tolerated but limited evidence of antitumor activity has been observedCitation217 (NCT01166490).
A combinational approach based on fractionated radioimmunotherapy and gemcitabine has been studied. Radioimmunotherapy consisted of yttrium-90-labeled humanized Clivatuzumab tetraxetan antibody (90Y-hPAM4) that selectively targets PDAC. Obtained initial results appeared promising: a median OS for all patients of 7.7 months and, when considering only the 10 patients with stage IV disease, a median OS of 10.7 months was achieved,Citation218 (NCT00603863).
A phase II study of neoadjuvant chemotherapy and immunotherapy to CA125 (Oregovomab) followed by SBRT and concurrent HIV protease inhibitor nelfinavir in patients with locally advanced pancreatic cancer is recruiting patients, (NCT01959672). Another phase II trial of in situ tumor vaccination using Durvalumab and “booster” radiation therapy in patients with metastatic pancreatic adenocarcinoma (NCT02885727) is not yet recruiting.
DNA vaccines in PDAC
There are just few reports on DNA-based vaccines in PDAC. Enolase (ENO1) is a glycolytic enzyme and ENO1-specific T cells inhibit the growth of human pancreatic xenograft tumors in mice. Moreover antibodies against ENO1 are detected in more than 60% of patients with PDAC. Italian researchers investigated an ENO1 DNA vaccine administered via electroporation in mice that spontaneously develop autochthonous, lethal pancreatic carcinomas (KPC model). ENO1 vaccine induced antibody and a cellular response, reduced numbers of MDSC and Tregs, increased Th1 and Th17 responses and strongly increased the survival.Citation219 Also an important role of anti-ENO1 antibodies, which affected MDSC functions/recruitment and therefore helped to increase T cell responses, has been observed by the researchers in a follow-up study.Citation220
A phase I study on hTERT immunotherapy alone or in combination with IL-12 DNA followed by electroporation in adults with solid tumors (including pancreatic cancer, HCC and others) at high risk of relapse post definitive surgery and standard therapy is currently recruiting (NCT02960594).
Stem cell transplantation and other cell-based immunotherapy in PDAC
HSCT has been reported to be a promising therapeutic approach in PDAC. All 3 patients who underwent HSCT are alive 2.5- and more than 8 years after HSCT.Citation163 In another study researchers claim that mesenchymal stem cells (MSC) can represent a new tool for delivery of therapeutic agents to cancer sites because of their strong tropism toward tumors and they suggest that MSCs producing IL-15 might represent an innovative strategy for therapy of pancreatic tumor. They have demonstrated significant inhibition of tumor growth and prolonged survival in syngeneic mice bearing Pan02 pancreatic tumors after systemic administration of MSC-IL-15. Mice developed strong immune responses and have been shown to become resistant against tumor rechallenge.Citation221
PDA cells generated to stably express MHC class II transactivator (CIITA) and used as a syngeneic vaccine have been shown to induce the recruitment of T effector cells into the tumor area and long-lasting immune memory against PDAC.Citation222
Chemoimmunotherapy in PDAC
Recently, we have developed a resectable orthotopic model of PDAC based on electroporation of transposable elements expressing oncogenic KrasG12D/Akt2 directly in situ into pancreas at p53-/- background, allowing for a development of a singular tumor nodule that can be subsequently resected. Pancreatic tumors became widely metastatic, reflecting the aggressive clinical features of PDAC in patients. Despite early tumor resection, recurring and distant tumors still occurred and adjuvant gemcitabine treatment after tumor resection prolonged the overall survival. Importantly, therapy-induced senescence has been detected in satellite tumors and also in distal metastases by senescence-associated ß-galactosidase positive staining in a gemcitabine-treated group (unpublished observation). The protective mechanism of the gemcitabine treatment was associated with inhibition of MDSC accumulation and increased NK cell numbers. Furthermore, the data obtained on gemcitabine treatment in this resectable orthotopic mouse model of PDAC allowed to define a mechanism demonstrating that NK cells and not T cells were required for gemcitabine-mediated antitumor responses.Citation223
In another study a combination of gemcitabine and rosiglitazone, an FDA-approved drug for the treatment of type 2 diabetes mellitus, has been tested in an immunocompetent transplantable s.c. mouse model of pancreatic cancer. Combinational treatment resulted in significant tumor reduction, extended OS compared with monotherapy. Rosiglitazone has been reported to affect early MDSC accumulation and intratumoral Tregs. Combination therapy increased intratumoral CD4+ and CD8+ T cells while limiting Tregs.Citation224
In a recent study the researchers showed very promising results using a DNA demethylating drug 5-aza-20-deoxycytidine (DAC) as a treatment of aggressive mouse model of stromal rich PDAC (KPC-Brca1 mice) and suggested implementation of hypomethylating agents for future in combination with immunotherapy.Citation225 It is important to mention that the loss of nuclear DNA methylation leads to growth arrest or nonviable cells,Citation226 and effects of successful therapy using DAC could therefore be also connected with senescenceCitation227 and induced senescence surveillance,Citation53,54 however senescence surveillance upon DAC treatment of pancreatic tumors remains to be investigated.
Perspectives and future directions in the treatment of HCC, CCA AND PDAC
Within the scope of this review we have described currently ongoing research studies and clinical trials in 3 types of gastrointestinal cancers: primary liver (HCC and CCA) and pancreatic (PDAC) cancers. All 3 cancers and, especially liver cancer with its tolerogenic environment, imply numerous complex mechanisms to avoid immune surveillance. There is a clear observation that not a single immunotherapeutic approach, but, in particular, a combinational therapy has the most promising results in the treatment of those malignancies.
Thus, combinational approaches which will:
| i) | stimulate and/or boost tumor-specific immune response against multiple TAAs using different vaccination approaches; | ||||
| ii) | re-activate the function of innate and adaptive immune cells, that are suppressed by the tumor milieu using e.g., checkpoint inhibitors; | ||||
| iii) | target tumor-specific pathway and angiogenesis specifically and locally, | ||||
have altogether the strongest perspectives to treat HCC, CCA and PDAC malignancies.
The most chances in (i) belong to personalized/individual case-related TAAs and tumor epitopes and their efficient delivery for presentation by potent DCs, which will induce strong tumor-specific Th1/CTL response. Several comprehensive studies involving whole genome- and exome sequencing in human tumor samples as reported for CCA,Citation142 PDAC (NCT02750657, NCT02795650) and also HEPAVACCitation90,91 for HCC are ongoing. The results will help to implement personalized therapies leading to a DFS as for example it has been shown in the encouraging case study in CCA.Citation142 Alternative strategies based on autologous live-irradiated tumor cells secreting cytokines (e.g., GM-CSF) that strongly promote immune responses have been shown to be very promising in PDAC patients (GVAX- and similar studies).Citation195,228 Taking into account very successful studies using a safe vaccine strain of L. monocytogenes efficiently delivering a tumor antigen and inducing potent tumor-specific responses, as shown in PDAC,Citation199 this approach could be implemented in HCC and CCA as well. Several research studies using s.c. mouse models have been already performed in this direction in HCC, however use of autochthonous and orthotopic models has to become an absolute priority in immunotherapy studies. Especially taking into account the experience from PDAC models, showing strong differences in terms of T effector cell infiltration in s.c. models vs. autochthonous or spontaneously developing tumors. Noteworthy, in highly heterogenic HCC conditions, a multi-peptide vaccination approach will be required. Importantly both CD4+ and CD8+ T cell responses are necessary for sufficient antitumor responses,Citation113,188,189,229 therefore both MHCI- and MHCII peptides have to be present in vaccine formulations for all 3 types of cancer.
Application of checkpoint inhibitors for (ii), is a very hot topic in immunotherapy. Several trials are ongoing in all 3 HCC, CCA, PDAC cancers (Supplementary Fig. 1, 2, 3). Importantly, an additional combination with inhibition of immune-suppressive cells, such as MDSCs and/or Tregs,Citation195,211 by means of gemcitabine or low doses of cyclophosphamide, respectively will improve the treatment outcome. Several studies implement such combinations already (NCT03006302, NCT01174121).
As first trials using checkpoint blockade failed in PDAC, the researchers and clinicians throve the issue of combinational therapies. Thus, among HCC, CCA and PDAC, PDAC has the leading position in trials implementing 4 and more combinational strategies (Supplementary Table 3). This probably has to become an obligation for HCC as well. Despite so many ongoing HCC human studies () just singular strategies are mostly implemented in the recent phase I studies for HCC (Supplementary Table 1). Furthermore, HCC is especially complicated to treat due its high heterogeneity. In 2000, Saeki et al. published the investigation of intratumoral heterogeneity analyzed by Restriction Landmark Genomic Scanning in tumor nodules.Citation230 Another study analyzed synchronous HCC and showed that tumor nodules which are located in close proximity are very heterogenous.Citation231 Further studies confirmed the heterogeneity and helped to better classify the tumors.Citation232 Based on gene expression profiling, Thorgeirsson and colleaguesCitation233 classified the HCC in more detail and concluded that “the biologic differences identified in the HCC subclasses should provide an attractive source for the development of therapeutic targets (e.g., HIF1a) for selective treatment of HCC patients.” Therefore, combination of immunotherapies and therapies that will target HCC heterogenic tumor cell populations and pathways, will be the most promising.
Figure 1. Total numbers of clinical studies. Summary of total numbers of clinical studies in HCC, CCA and PDAC, found using the defined keywords in www.ClinicalTrials.gov and in MEDLINE/PubMed database.
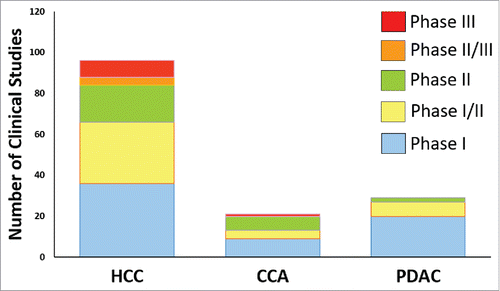
For sufficient targeting in iii) fat diagnostic aiming to identify the cancer pathway and tumor cell receptors in every individual case has to be performed (personalized medicine). Specific targeting of such defined tumor receptors or tumor pathwayCitation216,218 or of angiogenesisCitation107 by mAbs have been described and show promising results. Importantly, local targeting of tumor using radio- and/or chemotherapy has many advantages over a systemic application regimes, especially knowing that standard fractionated chemoradiation results in a significant and extended loss of lymphocytes.Citation179 Therefore, targeted delivery of chemo- and radiotherapy by lyposomal- or nanoparticles linked to antibodies that specifically bind tumor cells, thereby not systemically affecting the function of immune cells, appears to be very important in immunotherapy. Similar approaches are recognized and already implemented into trials (NCT02583477).
Role of therapy-induced senescence and use of power of senescence-associated immune surveillance is an important issue that remains to be elucidated in CCA and PDAC upon chemo/radiotherapy treatment. Moreover, positive results achieved with live-irradiated tumor cells probably also are connected with radiation-induced senescence and SASP-mediated activation of immune responses. Importantly, senescent cells have to be cleared/removed by immune system.Citation53-55
Although several recent epidemiological studies have shown an increasing incidence of CCA worldwide, it is still referred to as an ‘orphane’ cancer. The limited number of CCA mouse models and the rarity and heterogeneity of the disease have made it difficult to push forward new translational therapeutic approaches and to conduct randomized clinical studies (). Therefore, the value of recently developed resectable orthotopic murine models of ICC and PDAC that reflect the human disease outcome are of special importance.Citation164,223 Noteworthy, the research studies on HCC, CCA and PDAC in general have to be performed in immune-competent orthotopic and autochthonous models, which strongly contribute to the understanding of mechanism of action of immunotherapies. These models will further allow an assessment of combination of therapeutic regimens and will help to closer predict a future translational efficacy of selected immunotherapies.Citation95
Importantly, the preventive approaches and preventive vaccines are very poorly represented in reports on HCC, CCA and PDAC and the development of such vaccines in these malignancies would be of advantage.
In conclusion, HCC, CCA and PDAC together with other gastrointestinal cancers still remain a leading cause of cancer-related deaths. However, recent development of and implementation of several immunotherapeutic approaches into clinical trials, described within the scope of this review, point out at a positive progression attempts toward the future treatment of HCC, CCA and PDAC using combinational immunotherapeutic approaches.
Abbreviations
| ADC | = | Antibody-Drug Conjugate |
| AFP | = | α-Fetoprotein |
| ASPH | = | Aspartate-β-Hydroxylase |
| BTC | = | Biliary Tract Cancers |
| CAF | = | Carcinoma-Associated Fibroblast |
| CART | = | Chimeric Antigen Receptor-Modified T Cell |
| CCA | = | Cholangiocarcinoma |
| CEA | = | Cardioembryonic Antigen |
| CIK | = | Cytokine-Induced Killer Cells |
| Cmab | = | Cetuximab |
| CMV | = | Cytomegalovirus |
| CSF1R | = | Colony Stimulating Factor 1 Receptor |
| CTA | = | Cancer-Testis Antigens |
| CTL | = | Cytotoxic T Lymphocytes |
| CTLA-4 | = | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| CXCR | = | C-X-C-Motif-Chemokine Receptor |
| DAC | = | 5-aza-20-deoxycytidine |
| DC | = | Dendritic Cells |
| DNA | = | Desoxyribonucleic Acid |
| DTH | = | Delayed-type Hypersensitivity |
| EGFR | = | Epidermal Growth Factor Receptor |
| ENO1 | = | Enolase 1 |
| EpCAM | = | Epithelial Cell Adhesion Molecule |
| ERBB2IP | = | Erbb2 Interacting Protein |
| FAK | = | Focal Adhesion Kinase |
| FAP | = | Fibroblast Activation Protein |
| FDA | = | Food and Drug Administration |
| FOXM1 | = | Forkhead Box M1 |
| GM-CSF | = | Granulocyte Macrophage Colony-Stimulating Factor |
| H-1PV | = | Human Papillomavirus |
| HBV | = | Hepatitis B Virus |
| HCC | = | Hepatocellular Carcinoma |
| HLA | = | Human Leukocyte Antigen |
| hMSH2 | = | Homo sapiens mutS Homolog 2 |
| HSCT | = | Haematopoietic Stem Cell Transplantation |
| hTERT | = | Human Telomerase Reverse Transcriptase |
| i.d. | = | Intradermal |
| i.v. | = | Intravenous |
| ICC | = | Intrahepatic Cholangiocarcinoma |
| IFN-γ | = | Interferon-γ |
| IGF | = | Insulin-Like Growth Factor |
| IGFIR-α | = | Insulin-Like Growth Factor I Receptor α |
| IL | = | Interleukin |
| IL-17RE | = | IL-17 Receptor E |
| iNKT | = | Invariant Natural Killer Cells |
| L1CAM | = | L1 Cell Adhesion Molecule |
| mAb | = | Monoclonal Antibody |
| MDC | = | Macrophage-Derived Chemokine |
| MDSC | = | Myeloid-Derived Suppressor Cells |
| MSC | = | Mesenchymal Stem Cells |
| MSPα | = | Macrophage-Stimulating Protein α |
| MUC1 | = | Mucin 1 |
| NASH | = | Non-Alcoholic Steatohepatitis |
| NK | = | Natural Killer Cells |
| NKT | = | Natural Killer T Cells |
| OS | = | Overall Survival |
| PAUF | = | Pancreatic Adenocarcinoma Up-Regulated Factor |
| PBL | = | Peripheral Blood Lymphocytes |
| PBMC | = | Peripheral Blood Mononuclear Cells |
| PD-1 | = | Programmed Cell Death 1 |
| PDAC | = | Pancreatic ductal adenocarcinoma |
| PD-L1 | = | Programmed Cell Death Ligand 1 |
| PFS | = | Progression-Free Survival |
| PGK2 | = | Phosphoglycerate Kinase 2 |
| PSC | = | Primary Sclerosing Cholangitis |
| PSCA | = | Prostate Stem Cell Antigen |
| rIL | = | Recombinant Interleukin |
| RNA | = | Ribonucleic acid |
| s.c. | = | Subcutaneous |
| SASP | = | Senescence-Associated Secretory Phenotype |
| SBRT | = | Stereotactic Body Radiation Therapy |
| SDF1α | = | Stromal Cell-Derived 1 α |
| SEREX | = | Serological Identification of Antigens by Recombinant Expression Cloning |
| TAA | = | Tumor-Associated Antigen |
| TACE | = | Transarterial Chemoembolization |
| TCR | = | T Cell Receptor |
| TGF-β | = | Transforming Growth Factor β |
| Th | = | T Helper Cell |
| TIL | = | Tumor-Infiltrating Lymphocyte |
| TLA | = | Tertiary Lymphoid Aggregates |
| Treg | = | Regulatory T Cells |
| WES | = | Whole Exome Sequencing |
| WHO | = | World Health Organization |
| WT1 | = | Wilm´s Tumor 1 |
| WTS | = | Whole Transcriptome Sequencing |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Material.zip
Download Zip (56.3 KB)Funding
This work was supported by the German Research Foundation, DFG (YE 151/2–1 to T.Y.), by the Fritz Thyssen Foundation (Ref.10.16.1031 MN to T.Y.), by the Young Academy Program of the Hannover Medical School (to T.Y.) and by the Wilhelm Sander Foundation (Nr. 2013.107.1 to T.Y. and M.M.)
References
- Zhang Q, Zeng L, Chen Y, Lian G, Qian C, Chen S, Li J, Huang K. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract. 2016;2016:8962321. doi:10.1155/2016/8962321. PMID:26941789.
- Organization, W.H.O. Cancer. http://www.who.int/cancer/en/.
- GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalance worldwide in 2012. http://globocan.iarc.fr/Default.aspx.
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi:10.1158/0008-5472.CAN-14-0155. PMID:24840647.
- Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: Risk factors, screening, and early detection. World J Gastroenterol. 2014;20:11182–98. doi:10.3748/wjg.v20.i32.11182. PMID:25170203.
- Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist. 2010;15 Suppl 4:5–13. doi:10.1634/theoncologist.2010-S4-05. PMID:21115576.
- Severi T, van Malenstein H, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: Risk factors, classification, and therapeutic targets. Acta pharmacologica Sinica. 2010;31:1409–20. doi:10.1038/aps.2010.142. PMID:20953207.
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi:10.1038/nrdp.2016.18. PMID:27158749.
- Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, Trichopoulos D, Stuver SO. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498–502. doi:10.1002/(SICI)1097-0215(20000215)85:4%3c498::AID-IJC9%3e3.0.CO;2-F. PMID:10699921.
- Ogimoto I, Shibata A, Kurozawa Y, Nose T, Yoshimura T, Suzuki H, Iwai N, Sakata R, Fujita Y, Ichikawa S, et al. Risk of death due to hepatocellular carcinoma among drinkers and ex-drinkers. Univariate analysis of JACC study data. Kurume Med J. 2004;51:59–70. doi:10.2739/kurumemedj.51.59. PMID:15150901.
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907–17. doi:10.1016/S0140-6736(03)14964-1. PMID:14667750.
- Dragani TA. Risk of HCC: Genetic heterogeneity and complex genetics. J Hepatol. 2010;52:252–7. doi:10.1016/j.jhep.2009.11.015. PMID:20022654.
- Groopman JD, Scholl P, Wang JS. Epidemiology of human aflatoxin exposures and their relationship to liver cancer. Prog Clin Biol Res. 1996;395:211–22. PMID:8895991.
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi:10.1016/S0140-6736(11)61347-0. PMID:22353262.
- Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014;11:e1001624. doi:10.1371/journal.pmed.1001624. PMID:24691105.
- Edenvik P, Davidsdottir L, Oksanen A, Isaksson B, Hultcrantz R, Stål P. Application of hepatocellular carcinoma surveillance in a European setting. What can we learn from clinical practice? Liver Int. 2015;35:1862–71. doi:10.1111/liv.12764. PMID:25524812.
- Salgia R, Singal AG. Hepatocellular carcinoma and other liver lesions. Med Clin North Am. 2014;98:103–18. doi:10.1016/j.mcna.2013.09.003. PMID:24266917.
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi:10.1016/S0140-6736(16)32453-9. PMID:27932229.
- Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408–24. doi:10.1038/nrclinonc.2015.103. PMID:26054909.
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–91. doi:10.1016/j.stem.2014.02.006. PMID:24607403.
- Greten TF, Duffy AG, Korangy F. Hepatocellular carcinoma from an immunologic perspective. Clin Cancer Res. 2013;19:6678–85. doi:10.1158/1078-0432.CCR-13-1721. PMID:24030702.
- Liu T, Xue R, Dong L, Wu H, Zhang D, Shen X. Rapid determination of serological cytokine biomarkers for hepatitis B virus-related hepatocellular carcinoma using antibody microarrays. Acta Biochim Biophys Sin (Shanghai). 2011;43:45–51. doi:10.1093/abbs/gmq111. PMID:21138899.
- Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J, et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3. doi:10.1186/1756-9966-32-3. PMID:23305119.
- Park SY, Han J, Kim JB, Yang MG, Kim YJ, Lim HJ, An SY, Kim JH. Interleukin-8 is related to poor chemotherapeutic response and tumourigenicity in hepatocellular carcinoma. Eur J Cancer. 2014;50:341–50. doi:10.1016/j.ejca.2013.09.021. PMID:24161763.
- Moretta A. Natural killer cells and dendritic cells: Rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–64. doi:10.1038/nri956. PMID:12461568.
- Ferlazzo G, Moretta L. Dendritic cell editing by natural killer cells. Crit Rev Oncog. 2014;19:67–75. doi:10.1615/CritRevOncog.2014010827. PMID:24941374.
- Sell S, Leffert HL. Liver cancer stem cells. J Clin Oncol. 2008;26:2800–5. doi:10.1200/JCO.2007.15.5945. PMID:18539957.
- Jones VS, Huang RY, Chen LP, Chen ZS, Fu L, Huang RP. Cytokines in cancer drug resistance: Cues to new therapeutic strategies. Biochim Biophys Acta. 2016;1865:255–65. PMID:26993403.
- Wolf B, Krieg K, Falk C, Breuhahn K, Keppeler H, Biedermann T, Schmid E, Warmann S, Fuchs J, Vetter S, et al. Inducing differentiation of premalignant hepatic cells as a novel therapeutic strategy in hepatocarcinoma. Cancer Res. 2016;76:5550–61. doi:10.1158/0008-5472.CAN-15-3453. PMID:27488521.
- Giusy E, Poupak F. Hepatocellular carcinoma and CXCR3 chemokines: A narrative review. Clin Ter. 2017;168:e37–e41. PMID:28240761.
- Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi:10.1038/nrc1252. PMID:14708024.
- Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, Ng IO, Man K, To KF, Lai PB. et al. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55:807–20. doi:10.1002/hep.24739. PMID:21994122.
- Porta C, De Amici M, Quaglini S, Paglino C, Tagliani F, Boncimino A, Moratti R, Corazza GR. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19:353–8. doi:10.1093/annonc/mdm448. PMID:17962206.
- Othman MS, Aref AM, Mohamed AA, Ibrahim WA. Serum levels of interleukin-6 and Interleukin-10 as biomarkers for hepatocellular carcinoma in egyptian patients. ISRN Hepatol. 2013;2013:412317. doi:10.1155/2013/412317. PMID:27335826.
- Chen ZY, Wei W, Guo ZX, Peng LX, Shi M, Li SH, Xiao CZ, Zhong C, Qian CN, Guo RP. Using multiple cytokines to predict hepatocellular carcinoma recurrence in two patient cohorts. Br J Cancer. 2014;110:733–40. doi:10.1038/bjc.2013.781. PMID:24495874.
- Nagao M, Nakajima Y, Kanehiro H, Hisanaga M, Aomatsu Y, Ko S, Tatekawa Y, Ikeda N, Kanokogi H, Urizono Y. et al. The impact of interferon gamma receptor expression on the mechanism of escape from host immune surveillance in hepatocellular carcinoma. Hepatology. 2000;32:491–500. doi:10.1053/jhep.2000.16470. PMID:10960440.
- Lee IC, Huang YH, Chau GY, Huo TI, Su CW, Wu JC, Lin HC. Serum interferon gamma level predicts recurrence in hepatocellular carcinoma patients after curative treatments. Int J Cancer. 2013;133:2895–902. PMID:23749461.
- Attallah AM, El-Far M, Zahran F, Shiha GE, Farid K, Omran MM, Abdelrazek MA, Attallah AA, El-Beh AA, El-Hosiny RM, et al. Interferon-gamma is associated with hepatic dysfunction in fibrosis, cirrhosis, and hepatocellular carcinoma. J Immunoassay Immunochem. 2016;37:597–610. doi:10.1080/15321819.2016.1179646. PMID:27093468.
- Lim R, Knight B, Patel K, McHutchison JG, Yeoh GC, Olynyk JK. Antiproliferative effects of interferon alpha on hepatic progenitor cells in vitro and in vivo. Hepatology. 2006;43:1074–83. doi:10.1002/hep.21170. PMID:16628647.
- Herzer K, Hofmann TG, Teufel A, Schimanski CC, Moehler M, Kanzler S, Schulze-Bergkamen H, Galle PR. IFN-alpha-induced apoptosis in hepatocellular carcinoma involves promyelocytic leukemia protein and TRAIL independently of p53. Cancer Res. 2009;69:855–62. doi:10.1158/0008-5472.CAN-08-2831. PMID:19141642.
- Nakaji M, Yano Y, Ninomiya T, Seo Y, Hamano K, Yoon S, Kasuga M, Teramoto T, Hayashi Y, Yokozaki H. IFN-alpha prevents the growth of pre-neoplastic lesions and inhibits the development of hepatocellular carcinoma in the rat. Carcinogenesis. 2004;25:389–97. doi:10.1093/carcin/bgh028. PMID:14633663.
- Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol. 2015;62:1420–9. doi:10.1016/j.jhep.2015.02.038. PMID:25733155.
- Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. doi:10.1038/nrgastro.2015.173. PMID:26484443.
- Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: Mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665–70. doi:10.4049/jimmunol.165.5.2665. PMID:10946296.
- Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, Kulig P, Becher B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22:237–46. doi:10.1038/cdd.2014.134. PMID:25190142.
- Kayashima H, Toshima T, Okano S, Taketomi A, Harada N, Yamashita Y, Tomita Y, Shirabe K, Maehara Y. Intratumoral neoadjuvant immunotherapy using IL-12 and dendritic cells is an effective strategy to control recurrence of murine hepatocellular carcinoma in immunosuppressed mice. J Immunol. 2010;185:698–708. doi:10.4049/jimmunol.0900187. PMID:20498356.
- Lasek W, Zagozdzon R, Jakobisiak M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother 2014;63:419–35. doi:10.1007/s00262-014-1523-1. PMID:24514955.
- Zhu XD, Sun HC, Xu HX, Kong LQ, Chai ZT, Lu L, Zhang JB, Gao DM, Wang WQ, Zhang W. et al. Antiangiogenic therapy promoted metastasis of hepatocellular carcinoma by suppressing host-derived interleukin-12b in mouse models. Angiogenesis. 2013;16:809–20. doi:10.1007/s10456-013-9357-6. PMID:23716000.
- Harada N, Shimada M, Okano S, Suehiro T, Soejima Y, Tomita Y, Maehara Y. IL-12 gene therapy is an effective therapeutic strategy for hepatocellular carcinoma in immunosuppressed mice. J Immunol. 2004;173:6635–44. doi:10.4049/jimmunol.173.11.6635. PMID:15557154.
- Lo CH, Chang CM, Tang SW, Pan WY, Fang CC, Chen Y, Wu PY, Chen KY, Ma HI, Xiao X. et al. Differential antitumor effect of interleukin-12 family cytokines on orthotopic hepatocellular carcinoma. J Gene Med. 2010;12:423–34. doi:10.1002/jgm.1452. PMID:20440753.
- Ngiow SF, Teng MW, Smyth MJ. A balance of interleukin-12 and -23 in cancer. Trends Immunol. 2013;34:548–55. doi:10.1016/j.it.2013.07.004. PMID:23954142.
- Hu P, Hu HD, Chen M, Peng ML, Tang L, Tang KF, Matsui M, Belladonna ML, Yoshimoto T, Zhang DZ. Expression of interleukins-23 and 27 leads to successful gene therapy of hepatocellular carcinoma. Mol Immunol. 2009;46:1654–62. doi:10.1016/j.molimm.2009.02.025. PMID:19299021.
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–51. doi:10.1038/nature10599. PMID:22080947.
- Yevsa T, Kang TW, Zender L. Immune surveillance of pre-cancerous senescent hepatocytes limits hepatocellular carcinoma development. Oncoimmunology. 2012;1:398–9. doi:10.4161/onci.19128. PMID:22737629.
- Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, Medina-Echeverz J, Longerich T, Forgues M, Reisinger F. et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell. 2016;30:533–47. doi:10.1016/j.ccell.2016.09.003. PMID:27728804.
- Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57:1654–62. doi:10.1002/hep.26115. PMID:23111952.
- Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin. 2015;36:1191–9. doi:10.1038/aps.2015.41. PMID:26073325.
- Sun C, Xu J, Huang Q, Huang M, Wen H, Zhang C, Wang J, Song J, Zheng M, Sun H, et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology. 2017;6:e1264562. doi:10.1080/2162402X.2016.1264562. PMID:28197391.
- Chew V, Tow C, Huang C, Bard-Chapeau E, Copeland NG, Jenkins NA, Weber A, Lim KH, Toh HC, Heikenwalder M, et al. Toll-like receptor 3 expressing tumor parenchyma and infiltrating natural killer cells in hepatocellular carcinoma patients. J Natl Cancer Inst. 2012;104:1796–807. doi:10.1093/jnci/djs436. PMID:23197495.
- Ohira M, Ohdan H, Mitsuta H, Ishiyama K, Tanaka Y, Igarashi Y, Asahara T. Adoptive transfer of TRAIL-expressing natural killer cells prevents recurrence of hepatocellular carcinoma after partial hepatectomy. Transplantation. 2006;82:1712–9. doi:10.1097/01.tp.0000250935.41034.2d. PMID:17198265.
- Uehara K, Ichida T, Sugahara S, Ishikawa T, Yamagiwa S, Yoshida Y, Nomoto M, Katoh M, Satoh H, Watanabe H, et al. Systemic administration of liposome-encapsulated OK-432 prolongs the survival of rats with hepatocellular carcinoma through the induction of IFN-gamma-producing hepatic lymphocytes. J Gastroenterol Hepatol. 2002;17:81–90. doi:10.1046/j.1440-1746.2002.02675.x. PMID:11895558.
- Jiang W, Zhang C, Tian Z, Zhang J. hIL-15 gene-modified human natural killer cells (NKL-IL15) augments the anti-human hepatocellular carcinoma effect in vivo. Immunobiology. 2014;219:547–53. doi:10.1016/j.imbio.2014.03.007. PMID:24721706.
- Ma L, Luo L, Qiao H, Dong X, Pan S, Jiang H, Krissansen GW, Sun X. Complete eradication of hepatocellular carcinomas by combined vasostatin gene therapy and B7H3-mediated immunotherapy. J Hepatol. 2007;46:98–106. doi:10.1016/j.jhep.2006.07.031. PMID:17109987.
- Sui Q, Zhang J, Sun X, Zhang C, Han Q, Tian Z. NK cells are the crucial antitumor mediators when STAT3-mediated immunosuppression is blocked in hepatocellular carcinoma. J Immunol. 2014;193:2016–23. doi:10.4049/jimmunol.1302389. PMID:25015826.
- Abdelrahman MM, Fawzy IO, Bassiouni AA, Gomaa AI, Esmat G, Waked I, Abdelaziz AI. Enhancing NK cell cytotoxicity by miR-182 in hepatocellular carcinoma. Hum Immunol. 2016;77:667–73. doi:10.1016/j.humimm.2016.04.020. PMID:27262453.
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77. doi:10.1038/nrc3258. PMID:22437871.
- Zhang L, Zhu W, Li J, Yang X, Ren Y, Niu J, Pang Y. Clinical outcome of immunotherapy with dendritic cell vaccine and cytokine-induced killer cell therapy in hepatobiliary and pancreatic cancer. Mol Clin Oncol. 2016;4:129–133. PMID:26870371.
- Morse MA, Niedzwiecki D, Marshall JL, Garrett C, Chang DZ, Aklilu M, Crocenzi TS, Cole DJ, Dessureault S, Hobeika AC, et al. A randomized phase II study of immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg. 2013;258:879–86. doi:10.1097/SLA.0b013e318292919e. PMID:23657083.
- Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–43. doi:10.1016/j.lungcan.2011.11.012. PMID:22153832.
- Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: Is it clinically useful? Clin Chem. 2001;47:624–30. PMID:11274010.
- Garnett CT, Greiner JW, Tsang KY, Kudo-Saito C, Grosenbach DW, Chakraborty M, Gulley JL, Arlen PM, Schlom J, Hodge JW. TRICOM vector based cancer vaccines. Curr Pharm Des. 2006;12:351–61. doi:10.2174/138161206775201929. PMID:16454749.
- Song J. Cancer Immunotherapy: Mechanisms of Cancer Immunity, Engineering Immune-Based Therapies and Developing Clinical Trials. Frontiers in Cancer Immunology Vol. 1, 2015.
- Su S, Zhou H, Xue M, Liu JY, Ding L, Cao M, Zhou ZX, Hu HM, Wang LX. Anti-tumor efficacy of a hepatocellular carcinoma vaccine based on dendritic cells combined with tumor-derived autophagosomes in murine models. Asian Pac J Cancer Prev. 2013;14:3109–16. doi:10.7314/APJCP.2013.14.5.3109. PMID:23803088.
- Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du Z, Yin H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64:456–72. doi:10.1002/hep.28549. PMID:26990897.
- Homma S, Toda G, Gong J, Kufe D, Ohno T. Preventive antitumor activity against hepatocellular carcinoma (HCC) induced by immunization with fusions of dendritic cells and HCC cells in mice. J Gastroenterol. 2001;36:764–71. doi:10.1007/s005350170019. PMID:11757749.
- Lavaissiere L, Jia S, Nishiyama M, de la Monte S, Stern AM, Wands JR, Friedman PA. Overexpression of human aspartyl(asparaginyl)beta-hydroxylase in hepatocellular carcinoma and cholangiocarcinoma. J Clin Invest. 1996;98:1313–23. doi:10.1172/JCI118918. PMID:8823296.
- Wang K, Liu J, Yan ZL, Li J, Shi LH, Cong WM, Xia Y, Zou QF, Xi T, Shen F, et al. Overexpression of aspartyl-(asparaginyl)-beta-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology. 2010;52:164–73. doi:10.1002/hep.23650. PMID:20578260.
- Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnù L, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: Both or neither? Am J Gastroenterol. 2006;101:524–32. doi:10.1111/j.1572-0241.2006.00443.x. PMID:16542289.
- Butterfield LH, Ribas A, Potter DM, Economou JS. Spontaneous and vaccine induced AFP-specific T cell phenotypes in subjects with AFP-positive hepatocellular cancer. Cancer Immunol Immunother. 2007;56:1931–43. doi:10.1007/s00262-007-0337-9. PMID:17522860.
- Mizejewski GJ. Alpha-fetoprotein (AFP)-derived peptides as epitopes for hepatoma immunotherapy: A commentary. Cancer Immunol Immunother. 2009;58:159–70. doi:10.1007/s00262-008-0548-8. PMID:18612637.
- Greten TF, Ormandy LA, Fikuart A, Höchst B, Henschen S, Hörning M, Manns MP, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33:211–8. doi:10.1097/CJI.0b013e3181bb499f. PMID:20139774.
- Maher J, Davies ET. Targeting cytotoxic T lymphocytes for cancer immunotherapy. Br J Cancer. 2004;91:817–21. PMID:15266309.
- Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–91 e6. PMID:25747273.
- Fan H, Lu X, Wang X, Liu Y, Guo B, Zhang Y, Zhang W, Nie J, Feng K, Chen M, et al. Low-dose decitabine-based chemoimmunotherapy for patients with refractory advanced solid tumors: A phase I/II report. J Immunol Res. 2014;2014:371087. doi:10.1155/2014/371087. PMID:24963497.
- Zhang H, Zheng SS, Jiang GP, Wu LH, Zhu F, Yang ZL. Antitumor effect of immunization with fusion of dendritic cells and hepatocellular carcinoma cells in mice. Hepatobiliary Pancreat Dis Int. 2004;3:235–40. PMID:15138117.
- Kong J, Diao Z, Deng X, Zhong H, Yao W, Hu X. Anti-tumor effects of immunotherapeutic peptide on the treatment of hepatocellular carcinoma with HBc carrier. Oncol Rep. 2007;18:279–85. PMID:17549380.
- Sun JC, Pan K, Chen MS, Wang QJ, Wang H, Ma HQ, Li YQ, Liang XT, Li JJ, Zhao JJ, et al. Dendritic cells-mediated CTLs targeting hepatocellular carcinoma stem cells. Cancer Biol Ther. 2010;10:368–75. doi:10.4161/cbt.10.4.12440. PMID:20581468.
- Chen Y, Yang D, Li S, Gao Y, Jiang R, Deng L, Frankel FR, Sun B. Development of a Listeria monocytogenes-based vaccine against hepatocellular carcinoma. Oncogene. 2012;31:2140–52. doi:10.1038/onc.2011.395. PMID:21927025.
- Wan X, Cheng C, Lin Z, Jiang R, Zhao W, Yan X, Tang J, Yao K, Sun B, Chen Y. The attenuated hepatocellular carcinoma-specific Listeria vaccine Lmdd-MPFG prevents tumor occurrence through immune regulation of dendritic cells. Oncotarget. 2015;6:8822–38. doi:10.18632/oncotarget.3558. PMID:25826093.
- Circelli L, Petrizzo A, Tagliamonte M, Heidenreich R, Tornesello ML, Buonaguro FM, Buonaguro L. Immunological effects of a novel RNA-based adjuvant in liver cancer patients. Cancer Immunol Immunother. 2017;66:103–12. doi:10.1007/s00262-016-1923-5. PMID:27832318.
- Tagliamonte M, Petrizzo A, Tornesello ML, Buonaguro FM, Buonaguro L. Antigen-specific vaccines for cancer treatment. Hum Vaccin Immunother. 2014;10:3332–46. doi:10.4161/21645515.2014.973317. PMID:25483639.
- Kudo M. Immune checkpoint inhibition in hepatocellular carcinoma: Basics and ongoing clinical trials. Oncology. 2017;92 Suppl 1:50–62. doi:10.1159/000451016. PMID:28147363.
- Hagner PR, Man HW, Fontanillo C, Wang M, Couto S, Breider M, Bjorklund C, Havens CG, Lu G, Rychak E, et al. CC-122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood. 2015;126:779–89. doi:10.1182/blood-2015-02-628669. PMID:26002965.
- Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-PD-1 immunotherapy in sorafenib-treated HCC in mice. Hepatology (Baltimore, Md.). 2015;61:1591–602. doi:10.1002/hep.27665. PMID:25529917.
- Singh M, Ferrara N. Modeling and predicting clinical efficacy for drugs targeting the tumor milieu. Nat Biotech. 2012;30:648–57. doi:10.1038/nbt.2286.
- Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, Lattanzio R, de Lange R, Weidle UH, Piantelli M, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32:222–33. doi:10.1038/onc.2012.36. PMID:22349828.
- Huang Q, Li J, Xing J, Li W, Li H, Ke X, Zhang J, Ren T, Shang Y, Yang H, et al. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J Hepatol. 2014;61:859–66. doi:10.1016/j.jhep.2014.04.035. PMID:24801417.
- Gou X, Tang X, Kong DK, He X, Gao X, Guo N, Hu Z, Zhao Z, Chen Y. CD147 is increased in HCC cells under starvation and reduces cell death through upregulating p-mTOR in vitro. Apoptosis. 2016;21:110–9. doi:10.1007/s10495-015-1189-y. PMID:26496775.
- Peng F, Li H, You Q, Li H, Wu D, Jiang C, Deng G, Li Y, Li Y, Wu Y. CD147 as a novel prognostic biomarker for hepatocellular carcinoma: A meta-analysis. Biomed Res Int. 2017;2017:5019367. doi:10.1155/2017/5019367. PMID:28386553.
- Pukac L, Kanakaraj P, Humphreys R, Alderson R, Bloom M, Sung C, Riccobene T, Johnson R, Fiscella M, Mahoney A, et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer. 2005;92:1430–41. doi:10.1038/sj.bjc.6602487. PMID:15846298.
- Koehler BC, Urbanik T, Vick B, Boger RJ, Heeger S, Galle PR, Schuchmann M, Schulze-Bergkamen H. TRAIL-induced apoptosis of hepatocellular carcinoma cells is augmented by targeted therapies. World J Gastroenterol. 2009;15:5924–35. doi:10.3748/wjg.15.5924. PMID:20014456.
- Shin EC, Seong YR, Kim CH, Kim H, Ahn YS, Kim K, Kim SJ, Hong SS, Park JH. Human hepatocellular carcinoma cells resist to TRAIL-induced apoptosis, and the resistance is abolished by cisplatin. Exp Mol Med. 2002;34:114–22. doi:10.1038/emm.2002.17. PMID:12085986.
- Enguita-German M, Fortes P. Targeting the insulin-like growth factor pathway in hepatocellular carcinoma. World J Hepatol. 2014;6:716–37. doi:10.4254/wjh.v6.i10.716. PMID:25349643.
- Tovar V, Alsinet C, Villanueva A, Hoshida Y, Chiang DY, Solé M, Thung S, Moyano S, Toffanin S, Mínguez B, et al. IGF activation in a molecular subclass of hepatocellular carcinoma and pre-clinical efficacy of IGF-1R blockage. J Hepatol. 2010;52:550–9. doi:10.1016/j.jhep.2010.01.015. PMID:20206398.
- Zhong, H, Fazenbaker C, Breen S, Chen C, Huang J, Morehouse C, Yao Y, Hollingsworth RE. MEDI-573, alone or in combination with mammalian target of rapamycin inhibitors, targets the insulin-like growth factor pathway in sarcomas. Mol Cancer Ther. 2014;13:2662–73. doi:10.1158/1535-7163.MCT-14-0144. PMID:25193511.
- Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549s–55s. doi:10.1158/1078-0432.CCR-07-1109. PMID:17875788.
- Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE. et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–70. doi:10.1016/S1470-2045(15)00050-9. PMID:26095784.
- Hillen F, Griffioen AW. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007;26:489–502. doi:10.1007/s10555-007-9094-7. PMID:17717633.
- Leitner WW, Ying H, Restifo NP. DNA and RNA-based vaccines: Principles, progress and prospects. Vaccine. 1999;18:765–77. doi:10.1016/S0264-410X(99)00271-6. PMID:10580187.
- Bot A, Bona CA. Genetic immunization. US: Springer; 2013.
- Kim JU, Shariff MI, Crossey MM, Gomez-Romero M, Holmes E, Cox IJ, Fye HK, Njie R, Taylor-Robinson SD. Hepatocellular carcinoma: Review of disease and tumor biomarkers. World J Hepatol. 2016;8:471–84. doi:10.4254/wjh.v8.i10.471. PMID:27057305.
- Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, Budhu A, Zanetti KA, Chen Y, Qin LX, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451–61. doi:10.1158/0008-5472.CAN-07-6013. PMID:18316609.
- Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter DM, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817–25. doi:10.1158/1078-0432.CCR-05-2856. PMID:16675576.
- Behboudi S, Alisa A, Boswell S, Anastassiou J, Pathan AA, Williams R. Expansion of anti-AFP Th1 and Tc1 responses in hepatocellular carcinoma occur in different stages of disease. Br J Cancer. 2010;102:748–53. doi:10.1038/sj.bjc.6605526. PMID:20087354.
- Evdokimova VN, Liu Y, Potter DM, Butterfield LH. AFP-specific CD4+ helper T-cell responses in healthy donors and HCC patients. J Immunother. 2007;30:425–37. doi:10.1097/CJI.0b013e31802fd8e2. PMID:17457217.
- Hong Y, Peng Y, Guo ZS, Guevara-Patino J, Pang J, Butterfield LH, Mivechi NF, Munn DH, Bartlett DL, He Y. Epitope-optimized alpha-fetoprotein genetic vaccines prevent carcinogen-induced murine autochthonous hepatocellular carcinoma. Hepatology. 2014;59:1448–58. doi:10.1002/hep.26893. PMID:24122861.
- Cany J, Barteau B, Tran L, Gauttier V, Archambeaud I, Couty JP, Turlin B, Pitard B, Vassaux G, Ferry N. et al. AFP-specific immunotherapy impairs growth of autochthonous hepatocellular carcinoma in mice. J Hepatol. 2011;54:115–21. doi:10.1016/j.jhep.2010.06.027. PMID:20961645.
- Hanke P, Serwe M, Dombrowski F, Sauerbruch T, Caselmann WH. DNA vaccination with AFP-encoding plasmid DNA prevents growth of subcutaneous AFP-expressing tumors and does not interfere with liver regeneration in mice. Cancer Gene Ther. 2002;9:346–55. doi:10.1038/sj.cgt.7700445. PMID:11960285.
- Meng WS, Butterfield LH, Ribas A, Heller JB, Dissette VB, Glaspy JA, McBride WH, Economou JS. Fine specificity analysis of an HLA-A2.1-restricted immunodominant T cell epitope derived from human alpha-fetoprotein. Mol Immunol. 2000;37:943–50. doi:10.1016/S0161-5890(01)00017-7. PMID:11395133.
- Bei R. Are alpha-fetoprotein based-vaccines potential tools for liver cancer therapy? J Liver. 2013;2:e103. doi:10.4172/2167-0889.1000e103. https://www.omicsonline.org/open-access/are-alphafetoprotein-basedvaccines-potential-tools-for-liver-cancer-therapy-2167-0889-2-e103.pdf
- Ngiow SF, Loi S, Thomas D, Smyth MJ. Mouse models of tumor immunotherapy. Adv Immunol. 2016;130:1–24. doi:10.1016/bs.ai.2015.12.004. PMID:26922998.
- Ostrand-Rosenberg S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr Opin Immunol. 2004;16:143–50. doi:10.1016/j.coi.2004.01.003. PMID:15023405.
- Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi Y, Shi JY, Xu YF, Shi YH, Song K. et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61:2171–82. doi:10.1007/s00262-012-1278-5. PMID:22729558.
- Kang FB, Wang L, Jia HC, Li D, Li HJ, Zhang YG, Sun DX. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. doi:10.1186/s12935-015-0195-z. PMID:25908926.
- Luo L, Qiao H, Meng F, Dong X, Zhou B, Jiang H, Kanwar JR, Krissansen GW, Sun X. Arsenic trioxide synergizes with B7H3-mediated immunotherapy to eradicate hepatocellular carcinomas. Int J Cancer. 2006;118:1823–30. doi:10.1002/ijc.21557. PMID:16217749.
- Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–89. doi:10.1016/j.jhep.2014.01.021. PMID:24681130.
- Lafaro KJ, Cosgrove D, Geschwind JF, Kamel I, Herman JM, Pawlik TM. Multidisciplinary care of patients with intrahepatic cholangiocarcinoma: Updates in management. Gastroenterol Res Pract. 2015;2015:860861. doi:10.1155/2015/860861. PMID:26089873.
- Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–29. doi:10.1053/j.gastro.2013.10.013. PMID:24140396.
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261–80. doi:10.1038/nrgastro.2016.51. PMID:27095655.
- Rizvi S, Gores GJ. Emerging molecular therapeutic targets for cholangiocarcinoma. J Hepatol. 2017;67(3):632–44. doi:10.1016/j.jhep.2017.03.026. PMID:28389139.
- Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28–37. doi:10.1093/annonc/mdw324. PMID:27664259.
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. New Engl J Med. 2010;362:1273–81. doi:10.1056/NEJMoa0908721. PMID:20375404.
- Ikenoue T, Terakado Y, Nakagawa H, Hikiba Y, Fujii T, Matsubara D, Noguchi R, Zhu C, Yamamoto K, Kudo Y, et al. A novel mouse model of intrahepatic cholangiocarcinoma induced by liver-specific Kras activation and Pten deletion. Sci Rep. 2016;6:23899. doi:10.1038/srep23899. PMID:27032374.
- Utsunomiya T, Inoue H, Tanaka F, Yamaguchi H, Ohta M, Okamoto M, Mimori K, Mori M. Expression of cancer-testis antigen (CTA) genes in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2004;11:934–40. doi:10.1245/ASO.2004.01.029. PMID:15466353.
- Li S, Hu X, Cui S, He D. Novel centrosome protein, TCC52, is a cancer-testis antigen. Cancer Sci. 2008;99:2274–9. doi:10.1111/j.1349-7006.2008.00937.x. PMID:18957058.
- Lu X, Zhao HT, Sang XT, Mao YL, Chen RR, Zhong SX, Huang JF. [Expression of melanoma antigen-1, 3 genes in human intrahepatic cholangiocarcinoma and its clinical significance]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2008;30:197–200. PMID:18505125.
- Tsuneyama K, Sasaki M, Shimonishi T, Nakanuma Y. Expression of MAGE-A3 in intrahepatic cholangiocarcinoma and its precursor lesions. Pathol Int. 2004;54:181–6. doi:10.1111/j.1440-1827.2003.01605.x. PMID:14989741.
- Yokomine K, Senju S, Nakatsura T, Irie A, Hayashida Y, Ikuta Y, Harao M, Imai K, Baba H, Iwase H, et al. The forkhead box M1 transcription factor as a candidate of target for anti-cancer immunotherapy. Int J Cancer. 2010;126:2153–63. PMID:19688828.
- Zhou JX, Li Y, Chen SX, Deng AM. Expression and prognostic significance of cancer-testis antigens (CTA) in intrahepatic cholagiocarcinoma. J Exp Clin Cancer Res. 2011;30:2. doi:10.1186/1756-9966-30-2. PMID:21211023.
- Yu L, Feng M, Kim H, Phung Y, Kleiner DE, Gores GJ, Qian M, Wang XW, Ho M. Mesothelin as a potential therapeutic target in human cholangiocarcinoma. J Cancer. 2010;1:141–9. doi:10.7150/jca.1.141. PMID:20922056.
- Tesana S, Takahashi Y, Sithithaworn P, Ando K, Sakakura T, Yutanawiboonchai W, Pairojkul C, Ruangjirachuporn W. Ultrastructural and immunohistochemical analysis of cholangiocarcinoma in immunized Syrian golden hamsters infected with Opisthorchis viverrini and administered with dimethylnitrosamine. Parasitol Int. 2000;49:239–51. doi:10.1016/S1383-5769(00)00052-0. PMID:11426579.
- Loffler MW, Chandran PA, Laske K, Schroeder C, Bonzheim I, Walzer M, Hilke FJ, Trautwein N, Kowalewski DJ, Schuster H, et al. Personalized peptide vaccine-induced immune response associated with long-term survival of a metastatic cholangiocarcinoma patient. J Hepatol. 2016;65:849–55. doi:10.1016/j.jhep.2016.06.027. PMID:27397612.
- Noda T, Shimoda M, Ortiz V, Sirica AE, Wands JR. Immunization with aspartate-beta-hydroxylase-loaded dendritic cells produces antitumor effects in a rat model of intrahepatic cholangiocarcinoma. Hepatology. 2012;55:86–97. doi:10.1002/hep.24629. PMID:21898484.
- Kobayashi M, Sakabe T, Abe H, Tanii M, Takahashi H, Chiba A, Yanagida E, Shibamoto Y, Ogasawara M, Tsujitani S, et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg. 2013;17:1609–17. doi:10.1007/s11605-013-2286-2. PMID:23877328.
- Morse MA, Clay TM, Hobeika AC, Osada T, Khan S, Chui S, Niedzwiecki D, Panicali D, Schlom J, Lyerly HK. Phase I Study of Immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clinical Cancer Research. 2005;11:3017–24. doi:10.1158/1078-0432.CCR-04-2172. PMID:15837756.
- Onishi H, Morisaki T, Kuga H, Katano M, Doi F, Uchiyama A, Sugitani A, Wada J, Chijiiwa K, Tanaka M. A large quantity of CD3-/CD19-/CD16- lymphocytes in malignant pleural effusion from a patient with recurrent cholangio cell carcinoma. Immunol Invest. 2002;31:121–35. doi:10.1081/IMM-120004803. PMID:12148948.
- Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5. doi:10.1126/science.1251102. PMID:24812403.
- Feng KC, Guo YL, Liu Y, Dai HR, Wang Y, Lv HY, Huang JH, Yang QM, Han WD. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10:4. doi:10.1186/s13045-016-0378-7. PMID:28057014.
- Higuchi R, Yamamoto M, Hatori T, Shimizu K, Imai K, Takasaki K. Intrahepatic cholangiocarcinoma with lymph node metastasis successfully treated by immunotherapy with CD3-activated T cells and dendritic cells after surgery: Report of a case. Surg Today. 2006;36:559–62. doi:10.1007/s00595-006-3201-1. PMID:16715430.
- Kotera Y, Kougen Y, Aruga A, Yamamoto M. [Dendritic cell vaccine for intrahepatic cholangio cellular carcinoma–a study of relationship between immuno-reaction and clinical outcome]. Gan To Kagaku Ryoho. 2009;36:1964–6. PMID:20037292.
- Shimizu K, Kotera Y, Aruga A, Takeshita N, Takasaki K, Yamamoto M. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:171–8. doi:10.1007/s00534-011-0437-y. PMID:21874278.
- Sabbatino F, Villani V, Yearley JH, Deshpande V, Cai L, Konstantinidis IT, Moon C, Nota S, Wang Y, Al-Sukaini A, et al. PD-L1 and HLA Class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22:470–8. doi:10.1158/1078-0432.CCR-15-0715. PMID:26373575.
- Ha H, Nam AR, Bang JH, Park JE, Kim TY, Lee KH, Han SW, Im SA, Kim TY, Bang YJ, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016;7:76604–12. PMID:27780932.
- Naboush A, Roman CA, Shapira I. Immune checkpoint inhibitors in malignancies with mismatch repair deficiency: A review of the state of the current knowledge. J Investig Med. 2017;65:754–58. doi:10.1136/jim-2016-000342. PMID:28104820.
- Cho S, Park I, Kim H, Jeong MS, Lim M, Lee ES, Kim JH, Kim S, Hong HJ. Generation, characterization and preclinical studies of a human anti-L1CAM monoclonal antibody that cross-reacts with rodent L1CAM. MAbs. 2016;8:414–25. doi:10.1080/19420862.2015.1125067. PMID:26785809.
- Kan N, Yoshikawa K, Matsushita N, Fujii T. [The case of a patient with peritoneal metastasis from cholangiocarcinoma who responded to adoptive immunotherapy and cetuximab]. Gan To Kagaku Ryoho. 2013;40:1759–61. PMID:24393913.
- Hendrickx BW, Punt CJ, Boerman OC, Postema EJ, Oosterwijk E, Mavridu A, Corstens FH, Oyen WJ. Targeting of biliary cancer with radiolabeled chimeric monoclonal antibody CG250. Cancer Biother Radiopharm. 2006;21:263–8. doi:10.1089/cbr.2006.21.263. PMID:16918303.
- Karadurmus N, Sahin U, Basgoz BB, Arpaci F, Demirer T. Review of allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in solid tumors excluding breast cancer. World J Transplant. 2016;6:675–81. doi:10.5500/wjt.v6.i4.675. PMID:28058217.
- Takaue Y. Mini-transplantation strategy for solid tumors. Int J Hematol. 2002;76 Suppl 2:13–4. doi:10.1007/BF03165080. PMID:12430894.
- Soderdahl G, Barkholt L, Hentschke P, Mattsson J, Uzunel M, Ericzon BG, Ringdén O. Liver transplantation followed by adjuvant nonmyeloablative hemopoietic stem cell transplantation for advanced primary liver cancer in humans. Transplantation. 2003;75:1061–6. doi:10.1097/01.TP.0000058515.02300.5E. PMID:12698103.
- Hentschke P, Barkholt L, Uzunel M, Mattsson J, Wersäll P, Pisa P, Martola J, Albiin N, Wernerson A, Söderberg M, et al. Low-intensity conditioning and hematopoietic stem cell transplantation in patients with renal and colon carcinoma. Bone Marrow Transplant. 2003;31:253–61. doi:10.1038/sj.bmt.1703811. PMID:12621459.
- Donckier V, Troisi R, Toungouz M, Colle I, Van Vlierberghe H, Jacquy C, Martiat P, Stordeur P, Zhou L, Boon N, et al. Donor stem cell infusion after non-myeloablative conditioning for tolerance induction to HLA mismatched adult living-donor liver graft. Transpl Immunol. 2004;13:139–46. doi:10.1016/j.trim.2004.05.004. PMID:15380544.
- Omazic B, Remberger M, Barkholt L, Söderdahl G, Potácová Z, Wersäll P, Ericzon BG, Mattsson J, Ringdén O. Long-term follow-up of allogeneic hematopoietic stem cell transplantation for solid cancer. Biol Blood Marrow Transplant. 2016;22:676–81. doi:10.1016/j.bbmt.2015.12.017. PMID:26740375.
- Gurlevik, E, Fleischmann-Mundt B, Armbrecht N, Longerich T, Woller N, Kloos A, Hoffmann D, Schambach A, Wirth TC, Manns MP, et al. Adjuvant gemcitabine therapy improves survival in a locally induced, R0-resectable model of metastatic intrahepatic cholangiocarcinoma. Hepatology. 2013;58:1031–41. doi:10.1002/hep.26468. PMID:23686746.
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi:10.1016/S0140-6736(16)00141-0. PMID:26830752.
- Kloeppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. Histologic typing of tumours of the exocrine pancreas. Berlin: Springer-Verlag; 1996; p. 15. doi:10.1007/978-3-642-61024-0.
- Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56–68. doi:10.1093/annonc/mdv295. PMID:26314780.
- Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, Javle MM, Eads JR, Allen P, Ko AH, et al. Locally advanced, unresectable pancreatic cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34:2654–68. doi:10.1200/JCO.2016.67.5561. PMID:27247216.
- Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, Mohile SG, Mumber M, Schulick R, Shapiro M, et al. Potentially curable pancreatic cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:2324–28. doi:10.1200/JCO.2017.72.4948. PMID:28398845.
- Sohal DP, Mangu PB, Khorana AA, Shah MA, Philip PA, O'Reilly EM, Uronis HE, Ramanathan RK, Crane CH, Engebretson A, et al. Metastatic pancreatic cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34:2784–96. doi:10.1200/JCO.2016.67.1412. PMID:27247222.
- Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide V, He J, et al. Multi-institutional validation study of the american joint commission on cancer (8th Edition) Changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265:185–91. doi:10.1097/SLA.0000000000001763. PMID:27163957.
- Okada T, Noji S, Goto Y, Iwata T, Fujita T, Okada T, Matsuzaki Y, Kuwana M, Hirakata M, Horii A, et al. Immune responses to DNA mismatch repair enzymes hMSH2 and hPMS1 in patients with pancreatic cancer, dermatomyositis and polymyositis. Int J Cancer. 2005;116:925–33. doi:10.1002/ijc.21118. PMID:15856462.
- Okada T, Akada M, Fujita T, Iwata T, Goto Y, Kido K, Okada T, Matsuzaki Y, Kobayashi K, Matsuno S, et al. A novel cancer testis antigen that is frequently expressed in pancreatic, lung, and endometrial cancers. Clin Cancer Res. 2006;12:191–7. doi:10.1158/1078-0432.CCR-05-1206. PMID:16397042.
- Inami K, Kajino K, Abe M, Hagiwara Y, Maeda M, Suyama M, Watanabe S, Hino O. Secretion of N-ERC/mesothelin and expression of C-ERC/mesothelin in human pancreatic ductal carcinoma. Oncol Rep. 2008;20:1375–80. PMID:19020717.
- Yamazoe S, Tanaka H, Iwauchi T, Yoshii M, Ito G, Amano R, Yamada N, Sawada T, Ohira M, Hirakawa K. Identification of HLA-A*0201- and A*2402-restricted epitopes of mucin 5AC expressed in advanced pancreatic cancer. Pancreas. 2011;40:896–904. doi:10.1097/MPA.0b013e31821ad8d1. PMID:21697763.
- Maki RG, Livingston PO, Lewis JJ, Janetzki S, Klimstra D, Desantis D, Srivastava PK, Brennan MF. A Phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci. 2007;52:1964–72. doi:10.1007/s10620-006-9205-2. PMID:17420942.
- Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Søreide O, Eriksen JA, Møller M, Baksaas I, Lothe RA, et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer. 2001;92:441–50. doi:10.1002/ijc.1205. PMID:11291084.
- Wedén S, Klemp M, Gladhaug IP, Møller M, Eriksen JA, Gaudernack G, Buanes T. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer. 2011;128:1120–8. doi:10.1002/ijc.25449. PMID:20473937.
- Crocenzi T, Cottam B, Newell P, Wolf RF, Hansen PD, Hammill C, Solhjem MC, To YY, Greathouse A, Tormoen G, et al. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J ImmunoTher Cancer. 2016;4:45. doi:10.1186/s40425-016-0149-6. PMID:27532020.
- Nobili C, Degrate L, Caprotti R, Franciosi C, Leone BE, Trezzi R, Romano F, Uggeri F, Uggeri F. Prolonged survival of a patient affected by pancreatic adenocarcinoma with massive lymphocyte and dendritic cell infiltration after interleukin-2 immunotherapy. Report of a case. Tumori. 2008;94:426–30. PMID:18705415.
- Degrate L, Nobili C, Franciosi C, Caprotti R, Brivio F, Romano F, Leone BE, Trezzi R, Uggeri F. Interleukin-2 immunotherapy action on innate immunity cells in peripheral blood and tumoral tissue of pancreatic adenocarcinoma patients. Langenbecks Arch Surg. 2009;394:115–21. doi:10.1007/s00423-008-0393-4. PMID:18670745.
- Bhat R, Dempe S, Dinsart C, Rommelaere J. Enhancement of NK cell antitumor responses using an oncolytic parvovirus. Int J Cancer. 2011;128:908–19. doi:10.1002/ijc.25415. PMID:20473905.
- Del Zotto G, Marcenaro E, Vacca P, Sivori S, Pende D, Della Chiesa M, Moretta F, Ingegnere T, Mingari MC, Moretta A, et al. Markers and function of human NK cells in normal and pathological conditions. Cytometry Part B Clin Cytom. 2017;92:100–14. doi:10.1002/cyto.b.21508.
- Schmidt T, Ziske C, Märten A, Endres S, Tiemann K, Schmitz V, Gorschlüter M, Schneider C, Sauerbruch T, Schmidt-Wolf IG. Intratumoral immunization with tumor RNA-pulsed dendritic cells confers antitumor immunity in a C57BL/6 pancreatic murine tumor model. Cancer Res. 2003;63:8962–7. PMID:14695214.
- Nagaraj S, Ziske C, Strehl J, Messmer D, Sauerbruch T, Schmidt-Wolf IG. Dendritic cells pulsed with alpha-galactosylceramide induce anti-tumor immunity against pancreatic cancer in vivo. Int Immunol. 2006;18:1279–83. doi:10.1093/intimm/dxl059. PMID:16772371.
- Collignon A, Perles-Barbacaru AT, Robert S, Silvy F, Martinez E, Crenon I, Germain S, Garcia S, Viola A, Lombardo D, et al. A pancreatic tumor-specific biomarker characterized in humans and mice as an immunogenic onco-glycoprotein is efficient in dendritic cell vaccination. Oncotarget. 2015;6:23462–79. doi:10.18632/oncotarget.4359. PMID:26405163.
- Konduri V, Li D, Halpert MM, Liang D, Liang Z, Chen Y, Fisher WE, Paust S, Levitt JM, Yao QC, et al. Chemo-immunotherapy mediates durable cure of orthotopic KrasG12D/p53-/- pancreatic ductal adenocarcinoma. Oncoimmunology. 2016;5:e1213933. doi:10.1080/2162402X.2016.1213933. PMID:27757308.
- Suso EM, Dueland S, Rasmussen AM, Vetrhus T, Aamdal S, Kvalheim G, Gaudernack G. hTERT mRNA dendritic cell vaccination: Complete response in a pancreatic cancer patient associated with response against several hTERT epitopes. Cancer Immunol Immunother. 2011;60:809–18. doi:10.1007/s00262-011-0991-9. PMID:21365467.
- Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms' tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20:4228–39. doi:10.1158/1078-0432.CCR-14-0314. PMID:25056373.
- Tsukinaga S, Kajihara M, Takakura K, Ito Z, Kanai T, Saito K, Takami S, Kobayashi H, Matsumoto Y, Odahara S, et al. Prognostic significance of plasma interleukin-6/-8 in pancreatic cancer patients receiving chemoimmunotherapy. World J Gastroenterol. 2015;21:11168–78. doi:10.3748/wjg.v21.i39.11168. PMID:26494971.
- Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor–secreting tumor vaccine for pancreatic cancer: A Phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–56. doi:10.1200/JCO.2001.19.1.145. PMID:11134207.
- Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma: A Phase II trial of safety, efficacy, and immune activation. Ann surg. 2011;253:328–35. doi:10.1097/SLA.0b013e3181fd271c. PMID:21217520.
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–31. doi:10.1158/2326-6066.CIR-14-0027. PMID:24942756.
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, et al. Allogeneic granulocyte macrophage colony-stimulating factor–secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: A pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455. doi:10.1158/1078-0432.CCR-07-0371. PMID:18316569.
- Soares KC, Rucki AA, Kim V, Foley K, Solt S, Wolfgang CL, Jaffee EM, Zheng L, et al. TGF-beta blockade depletes T regulatory cells from metastatic pancreatic tumors in a vaccine dependent manner. Oncotarget. 2015;6:43005–15. doi:10.18632/oncotarget.5656. PMID:26515728.
- Schmidt J, Patrut EM, Ma J, Jäger D, Knaebel HP, Büchler MW, Märten A. Immunomodulatory impact of interferon-alpha in combination with chemoradiation of pancreatic adenocarcinoma (CapRI). Cancer Immunol Immunother. 2006;55:1396–405. doi:10.1007/s00262-006-0140-z. PMID:16485127.
- Brockstedt DG, Giedlin MA, Leong ML, BahjatKS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW Jr. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101:13832–7. doi:10.1073/pnas.0406035101. PMID:15365184.
- Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, Sterman DH, Hassan R, Lutz E, Moyer B, et al. A live-attenuated listeria vaccine (ANZ-100) and a live-attenuated listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–68. doi:10.1158/1078-0432.CCR-11-2121. PMID:22147941.
- Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, et al. Safety and Survival With GVAX pancreas prime and listeria monocytogenes–expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–33. doi:10.1200/JCO.2014.57.4244. PMID:25584002.
- Wang QJ, Yu Z, Griffith K, Hanada K, Restifo NP, Yang JC. Identification of T-cell receptors targeting KRAS-mutated human tumors. Cancer Immunol Res. 2016;4:204–14. doi:10.1158/2326-6066.CIR-15-0188. PMID:26701267.
- Garbe AI, Vermeer B, Gamrekelashvili J, von Wasielewski R, Greten FR, Westendorf AM, Buer J, Schmid RM, Manns MP, Korangy F, et al. Genetically induced pancreatic adenocarcinoma is highly immunogenic and causes spontaneous tumor-specific immune responses. Cancer Res. 2006;66:508–16. doi:10.1158/0008-5472.CAN-05-2383. PMID:16397267.
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–27. doi:10.1158/0008-5472.CAN-07-0175. PMID:17909062.
- Stromnes IM, Schmitt TM, Hulbert A, Brockenbrough JS, Nguyen H, Cuevas C, Dotson AM, Tan X, Hotes JL, Greenberg PD, et al. T cells engineered against a native antigen can surmount immunologic and physical barriers to treat pancreatic ductal adenocarcinoma. Cancer Cell. 2015;28:638–52. doi:10.1016/j.ccell.2015.09.022. PMID:26525103.
- Steele CW, Karim SA, Leach JD, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29:832–45. doi:10.1016/j.ccell.2016.04.014. PMID:27265504.
- Katari UL, Keirnan JM, Worth AC, Hodges SE, Leen AM, Fisher WE, Vera JF. Engineered T cells for pancreatic cancer treatment. HPB (Oxford). 2011;13:643–50. doi:10.1111/j.1477-2574.2011.00344.x. PMID:21843265.
- Meng Q, Liu Z, Rangelova E, Poiret T, Ambati A, Rane L, Xie S, Verbeke C, Dodoo E, Del Chiaro M, et al. Expansion of tumor-reactive T cells from patients with pancreatic cancer. J Immunother. 2016;39:81–9. doi:10.1097/CJI.0000000000000111. PMID:26849077.
- Kondo H, Hazama S, Kawaoka T, Yoshino S, Yoshida S, Tokuno K, Takashima M, Ueno T, Hinoda Y, Oka M. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28:379–87. PMID:18383873.
- Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi:10.1097/CJI.0b013e3181eec14c. PMID:20842054.
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–7. doi:10.1073/pnas.1320318110. PMID:24277834.
- Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851–60. doi:10.1038/nm.4123. PMID:27376576.
- Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38:1–11. doi:10.1097/CJI.0000000000000062. PMID:25415283.
- Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, Nordquist E, Cruz-Monserrate Z, Yu L, Young G, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2016. doi:10.1136/gutjnl-2016-311585. PMID:27797936.
- Leconet W, Larbouret C, Chardès T, Thomas G, Neiveyans M, Busson M, Jarlier M, Radosevic-Robin N, Pugnière M, Bernex F, et al. Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy. Oncogene. 2014;33:5405–14. doi:10.1038/onc.2013.487. PMID:24240689.
- Kim SJ, Chang S, Lee Y, Kim NY, Hwang Y, Min HJ, Yoo KS, Park EH, Kim S, Chung YH, et al. A PAUF-neutralizing antibody targets both carcinoma and endothelial cells to impede pancreatic tumor progression and metastasis. Biochem Biophys Res Commun. 2014;454:144–50. doi:10.1016/j.bbrc.2014.10.056. PMID:25450371.
- Oberg HH, Peipp M, Kellner C, Sebens S, Krause S, Petrick D, Adam-Klages S, Röcken C, Becker T, Vogel I, et al. Novel bispecific antibodies increase gammadelta T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 2014;74:1349–60. doi:10.1158/0008-5472.CAN-13-0675. PMID:24448235.
- Al-Ejeh F, Pajic M, Shi W, Kalimutho M, Miranda M, Nagrial AM, Chou A, Biankin AV, Grimmond SM; Australian Pancreatic Cancer Genome Initiative, et al. Gemcitabine and CHK1 inhibition potentiate EGFR-directed radioimmunotherapy against pancreatic ductal adenocarcinoma. Clin Cancer Res. 2014;20:3187–97. doi:10.1158/1078-0432.CCR-14-0048. PMID:24838526.
- Coveler AL, Ko AH, Catenacci DV, Von Hoff D, Becerra C, Whiting NC, Yang J, Wolpin B. A phase 1 clinical trial of ASG-5ME, a novel drug-antibody conjugate targeting SLC44A4, in patients with advanced pancreatic and gastric cancers. Invest New Drugs. 2016;34:319–28. doi:10.1007/s10637-016-0343-x. PMID:26994014.
- Ocean AJ, Pennington KL, Guarino MJ, Sheikh A, Bekaii-Saab T, Serafini AN, Lee D, Sung MW, Gulec SA, Goldsmith SJ, et al. Fractionated radioimmunotherapy with (90) Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial. Cancer. 2012;118:5497–506. doi:10.1002/cncr.27592. PMID:22569804.
- Cappello P, Rolla S, Chiarle R, Principe M, Cavallo F, Perconti G, Feo S, Giovarelli M, Novelli F. Vaccination with ENO1 DNA prolongs survival of genetically engineered mice with pancreatic cancer. Gastroenterology. 2013;144:1098–106. doi:10.1053/j.gastro.2013.01.020. PMID:23333712.
- Cappello P, Tonoli E, Curto R, Giordano D, Giovarelli M, Novelli F, et al. Anti-alpha-enolase antibody limits the invasion of myeloid-derived suppressor cells and attenuates their restraining effector T cell response. Oncoimmunology. 2016;5:e1112940. doi:10.1080/2162402X.2015.1112940. PMID:27467915.
- Jing W, Chen Y, Lu L, Hu X, Shao C, Zhang Y, Zhou X, Zhou Y, Wu L, Liu R, et al. Human umbilical cord blood-derived mesenchymal stem cells producing IL15 eradicate established pancreatic tumor in syngeneic mice. Mol Cancer Ther. 2014;13:2127–37. doi:10.1158/1535-7163.MCT-14-0175. PMID:24928851.
- Ekkirala CR, Cappello P, Accolla RS, Giovarelli M, Romero I, Garrido C, Garcia-Lora AM, Novelli F. Class II transactivator-induced MHC class II expression in pancreatic cancer cells leads to tumor rejection and a specific antitumor memory response. Pancreas. 2014;43:1066–72. doi:10.1097/MPA.0000000000000160. PMID:24987872.
- Gurlevik E, Fleischmann-Mundt B, Brooks J, Demir IE, Steiger K, Ribback S, Yevsa T, Woller N, Kloos A, Ostroumov D, et al. Administration of gemcitabine after pancreatic tumor resection in mice induces an antitumor immune response mediated by natural killer cells. Gastroenterology. 2016;151:338–50 e7. doi:10.1053/j.gastro.2016.05.004.
- Bunt SK, Mohr AM, Bailey JM, Grandgenett PM, Hollingsworth MA. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother. 2013;62:225–36. doi:10.1007/s00262-012-1324-3. PMID:22864396.
- Shakya R, Gonda T, Quante M, Salas M, Kim S, Brooks J, Hirsch S, Davies J, Cullo A, Olive K, et al. Hypomethylating therapy in an aggressive stroma-rich model of pancreatic carcinoma. Cancer Res. 2013;73:885–96. doi:10.1158/0008-5472.CAN-12-1880. PMID:23204224.
- Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–26. doi:10.1016/0092-8674(92)90611-F. PMID:1606615.
- Clozel T, Yang S, Elstrom RL, Tam W, Martin P, Kormaksson M, Banerjee S, Vasanthakumar A, Culjkovic B, Scott DW, et al. Mechanism-based epigenetic chemosensitization therapy of diffuse large B-cell lymphoma. Cancer Discov. 2013;3:1002–19. doi:10.1158/2159-8290.CD-13-0117. PMID:23955273.
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, et al. Allogeneic GM-CSF secreting tumor immunotherapy (GVAX(®)) alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: A pilot study of safety, feasibility and immune activation. Clin Cancer Res. 2008;14:1455–63. doi:10.1158/1078-0432.CCR-07-0371. PMID:18316569.
- Circelli L, Petrizzo A, Tagliamonte M, Heidenreich R, Tornesello ML, Buonaguro FM, Buonaguro L. Immunological effects of a novel RNA-based adjuvant in liver cancer patients. Cancer Immunol Immunother. 2017;66:103–12. doi:10.1007/s00262-016-1923-5. PMID:27832318.
- Saeki R, Nagai H, Kaneko S, Unoura M, Yamanaka N, Okamoto E, Kobayashi K, Matsubara K. Intratumoral genomic heterogeneity in human hepatocellular carcinoma detected by restriction landmark genomic scanning. J Hepatol. 2000;33:99–105. doi:10.1016/S0168-8278(00)80165-8. PMID:10905592.
- Sirivatanauksorn Y, Sirivatanauksorn V, Bhattacharya S, Davidson BR, Dhillon AP, Kakkar AK, Williamson RC, Lemoine NR. Genomic heterogeneity in synchronous hepatocellular carcinomas. Gut. 1999;45:761–5. doi:10.1136/gut.45.5.761. PMID:10517917.
- Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti J, Franco D, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi:10.1002/hep.21467. PMID:17187432.
- Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, Durnez A, Demetris AJ, Thorgeirsson SS. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–76. doi:10.1002/hep.20375. PMID:15349906.
