ABSTRACT
Respiratory syncytial virus (RSV) fusion (F) protein is suggested to be a protective vaccine target although its efficacy and safety concerns remain not well understood. We investigated immunogenicity, efficacy, and safety of F proteins in a soluble form or on virus-like particle (F-VLP). F VLP preferentially elicited IgG2a antibody and T helper type 1 (Th1) immune responses whereas F protein induced IgG1 isotype and Th2 responses. Despite lung viral clearance after prime or prime-boost and then RSV challenge, F protein immune mice displayed weight loss and lung histopathology and high mucus production and eosinophils. In contrast, prime or prime-boost vaccination of F VLP induced effective protection, prevented infiltration of eosinophils and vaccine- enhanced disease after challenge. This study provides insight into developing an effective and safe RSV vaccine candidate.
Introduction
Human respiratory syncytial virus (RSV) is an enveloped negative-sense RNA virus in the Pneumoviridae family. RSV is a major pathogen causing severe lower respiratory tract infections responsible for recurrent hospitalizations over 3 million admissions and mortality between 66,000 and 190,000 in children < 5 y old.Citation1,2 Older adults are also highly susceptible to significant disease and mortality due to RSV infection.Citation3 Formalin-inactivated RSV vaccine (FI-RSV) was evaluated in young children in 1960s.Citation4–6 Unfortunately, FI-RSV vaccination did not confer protection against RSV but rather caused 80% hospitalizations and 2 deaths,Citation6 which is termed vaccine-enhanced disease. Palivizumab is an RSV F specific humanized mouse monoclonal antibody and licensed to prevent RSV in preterm babies and infants at high risk.Citation7 Purified RSV F, attachment (G), or chimeric FG glycoprotein vaccines were demonstrated to be immunogenic and effective in controlling lung viral titers.Citation8–10 RSV F glycoprotein is believed to be the most important vaccine antigen. Additional studies reported that these RSV protein vaccines develop enhanced pulmonary pathology after several months of vaccination after RSV challenge in cotton rats.Citation9,11 Therefore, RSV vaccine-enhanced disease is a major concern in the development of an effective and safe RSV vaccine.
Virus-like particle (VLP) is non-replicating and noninfectious but can represent a morphological and structural similarity of an enveloped virus such as RSV. Newcastle disease virus (NDV) structural proteins (NP, M) were co-expressed to produce VLP vaccines containing the ectodomains of RSV F and G proteins in a chimeric fusion to the transmembrane and cytoplasmic tail of the NDV membrane proteins.Citation12,13 NDV VLP vaccines containing RSV F and G ectodomains provided protection against RSV by inducing long-lived RSV neutralizing antibodies without lung inflammation upon RSV challenge in mice,Citation12,14,15 and cotton rats.Citation16 Our previous studies demonstrated the immunogenicity and efficacy of RSV F VLP produced in insect cells by co-expressing the influenza virus M1 matrix protein. RSV F VLP was found to be immunogenic and confer protection against RSV.Citation17,18 F VLP vaccine is unique in conferring protection against RSV without causing lung inflammation after RSV challenge.Citation19
It remains unknown whether prime only vaccination with F VLP would confer protection against RSV and how F protein vaccines presented in VLP or soluble platforms result in different outcomes in terms of preventing vaccine-enhanced pulmonary histopathology after RSV challenge. This study has focused on addressing immunological mechanisms underlying these important questions.
Result
RSV F VLP vaccination induces a distinct pattern of IgG isotypes compared with F proteins
RSV F protein is a common platform of RSV subunit vaccines that are being explored. The F VLP and soluble F protein vaccines used in this study were found to be highly reactive to post-fusion F specific monoclonal antibody (131–2a) recognizing the post fusion F conformation but low or no reactivity to pre-fusion F specific 5C4 monoclonal antibody (data not shown). We determined immunogenicity and efficacy of RSV F in a soluble protein form or in a particulate VLP after prime or prime-boost vaccination. Groups of mice were intramuscularly immunized with F-VLP (10 μg total proteins, no adjuvant), or F proteins (0.1 μg or 0.3 μg) with alum adjuvant. Both 0.1 μg and 0.3 μg dose RSV F protein immunization groups induced significantly higher levels of RSV F specific IgG1 isotype antibody than F VLP immunized group (). In contrast, F VLP induced IgG2a isotype dominant antibody responses (). Meanwhile, F protein groups showed low or background levels of IgG2a (). Boost dose vaccination induced moderate increases in IgG2a and IgG1 isotype antibodies in the F VLP and F protein groups respectively. The F VLP group showed significantly higher ratios of IgG2a/IgG1 compared with those in F protein groups ().
Figure 1. RSV-specific IgG isotype antibody levels and neutralizing activity after prime or prime-boost vaccination with F VLP or F protein vaccine. BALB/c mice (N = 5) were immunized with F VLP (10 μg) or F protein (0.1 or 0.3 μg) with alum adjuvant (50 μg). Sera were collected 3 weeks after prime or prime-boost. (A-C) The IgG isotype levels were determined using F protein as antigen for antibody detection. (D) Ratios of IgG2a/IgG1 isotypes. (E) Neutralizing antibody titers. RSV neutralizing activity was determined by an immune-plaque reduction assay and titers were presented as dilution factors resulting in 50% reduction in plaque numbers. Results are representative out of 2 independent experiments and presented as mean ± SEM. Statistical significances were performed by one-way ANOVA in GraphPad Prism; ***p < 0.001, **p < 0.01, *p < 0.05 comparing F VLP and F protein groups. Prime; prime only immunization, Boost; prime and boost immunization, PBS; unimmunized mice, F VLP; F VLP immunized mice, Fp(0.1) or Fp(0.3); 0.1 μg or 0.3 μg RSV F proteins with alum adjuvant.
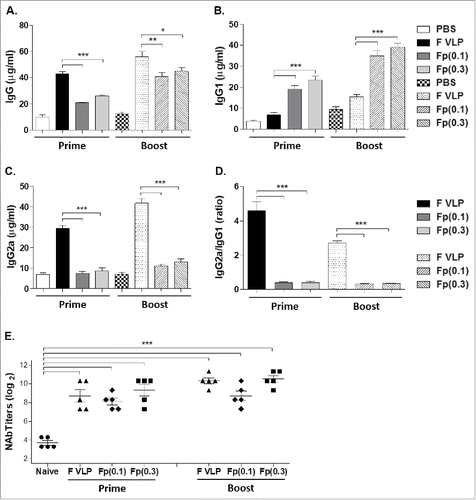
Neutralizing antibody is considered a major correlate of protection against RSV. F protein and F VLP prime immune sera showed similar neutralizing activities (∼9 log2) which were significantly higher than those in naïve sera exhibiting the background neutralizing activity of approximately 4 log2 (). This neutralizing assay of 50% plaque reduction titers resulted in significant increases in a range of 16- to 64-fold higher titers in immunized sera than naïve sera, which are statistically significant. Previous studies reported similar ranges of the background neutralizing activity in sera from the naïve mice,Citation13,17,18,20–22 suggesting a limitation in this assay. Moderate increases in neutralizing titers (∼10 log2) were observed after boost vaccination with F VLP or 0.3 μg F protein ().
Mice with RSV F protein vaccination induce protection against viral replication but display disease phenotypes
Immunized mice were challenged with RSV (5 × 105 PFU) and body weight changes were monitored (). The naïve infection and F protein (0.3 μg) groups displayed approximately 10% loss of body weight whereas the low dose (0.1 μg) F protein group showed 5–7% body weight loss. The F-VLP group exhibited only a slight change in body weight after RSV challenge (, ). There were no significant differences in body weight between the prime and prime-boost groups after challenge.
Figure 2. Body weight changes and lung viral clearance in vaccinated BALB/(C)mice after RSV challenge. Naïve (PBS only) and vaccinated mice were i.n. challenged with RSV A2 (5 105 PFU) at 4 weeks after prime or prime-boost immunization. (A) Body weight changes in prime immunized mice after RSV challenge. (B) Body weight changes in prime-boost immunized mice after RSV challenge. (C) Lung RSV titers after prime immunization. RSV titers were determined in individual lungs collected at 5 d after RSV challenge. (D) Lung RSV titers after prime-boost immunization. Individual lungs were collected 5 d after RSV challenge. The dotted line represents the detection limit. Results were reproducible in 2 independent sets of animal studies with each set n = 5 and presented as mean ± SEM. Statistical significances were performed by one-way ANOVA in GraphPad Prism; ***P < 0.001(C, D), **p < 0.01(D), *P < 0.05(D). Naïve R.; Naïve mice + RSV challenge, F VLP; F VLP vaccinated mice + RSV challenge, Fp (0.1); F protein 0.1 μg + RSV challenge, Fp (0.3); F protein 0.3 μg + RSV challenge.
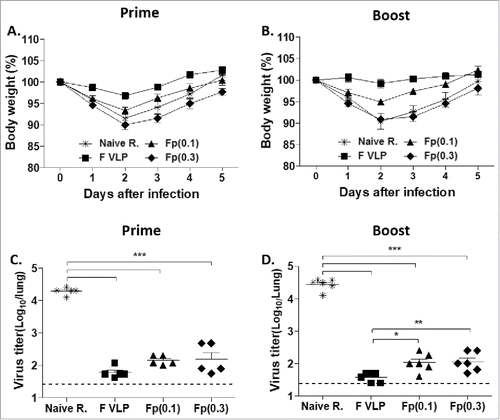
Lung tissues were collected from individual mouse after 5 days (d) of challenge and viral loads were evaluated with an immunoplaque assay. The naïve infection group had highest viral loads in the lungs. Prime or prime-boost vaccinated mice showed significantly lower levels of RSV lung viral loads than naïve mice (, ). A better control of viral loads was observed in the boost F VLP group than in the F protein groups (, ).
RSV F protein vaccination induces histopathology upon RSV challenge
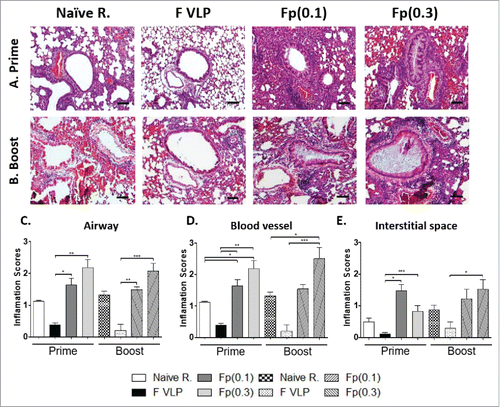
To determine whether RSV F VLP or soluble F protein vaccination induces pulmonary inflammation after RSV challenge, we examined lung histology after staining with hematoxylin and eosin (H&E), and evaluated inflammation on airway, blood vessels and intestinal space in a blind scoring (). The naive control (PBS only) group with RSV infection exhibited moderate levels of lung inflammation around the airways, blood vessels, and interstitial spaces (). The F protein prime or prime-boost (0.1 μg, 0.3 μg) groups showed the highest influx of inflammatory cells, most severe symptoms of alveolitis, and infiltrates in the airways, blood vessels, and interstitial spaces. F VLP prime only or prime-boost immune mice did not display lung histopathology after RSV challenge ().
Figure 3. Lung histopathology in prime or prime-boost vaccinated mice after RSV challenge. Lung tissues collected from individual BALB/c mice (N = 5) were prime or prime-boost vaccinated mice day 5 post challenge. (A, B) Photographs of H&E. Hematoxyline and Eosin (H&E) stained lung tissues were dissected to assess histopathology of peribronchiolar and alveolar pneumonia. Scale bars indicate 100 μm. (C-E) The lungs were scored using a 1–3 scoring system where 1 = minimal pathology and 3 = maximum pathology for the airways (C), interstitial spaces (D), blood vessels (E). Results are presented as mean ± SEM. Statistical significances were performed by one-way ANOVA in GraphPad Prism; ***P < 0.001, **p < 0.01, *p < 0.05. Group labels are the same as described in the .
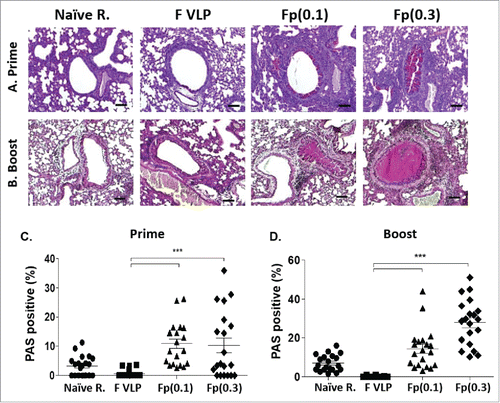
Excessive mucus production contributes to blocking the airways. Lung tissue sections were stained with Periodic acid-Schiff (PAS) to quantify mucus in the randomly selected airways epithelium. Consistent with histopathology, F protein prime or prime-boost mice showed the highest PAS-positive mucus production with an increasing trend in the high dose (0.3 μg) group. Naïve mice with RSV challenge showed moderate to low levels of airway mucus production whereas mucus was not observed in F VLP immune mice ().
Figure 4. Levels of mucus production in the lungs from vaccinated mice after RSV challenge. (A, B) Photographs of PAS. Periodic-Schiff (PAS) stained lung tissue were dissected to assess mucus production in the bronchiolar and alveolar area. Scale bars: 100μm. (C, D) Percentages of PAS positive airway mucus expression. The percentage of PAS positive lung section area in each airway was determined; individual airways are shown per group. Results are presented as mean ± SEM. Statistical significances were performed by one-way ANOVA in GraphPad Prism; ***P < 0.001. Group labels are the same as described in the .
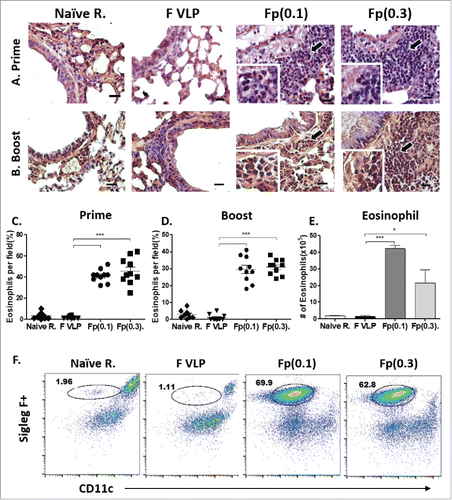
We examined eosinophils infiltration from lung tissue using hematoxylin and congo red (H&CR) staining. Significantly infiltrated eosinophils were detected in the airways of 0.1 μg or 0.3 μg F protein but not F VLP prime or prime-boost immune mice after RSV challenge (). The results of eosinophilic histology were consistent with Siglec F+ eosinophils in BALF from the F protein groups but not from the F VLP group as determined by flow cytometry (, ). Taken together, these results suggest that RSV F soluble protein immunization but not when presented on particulate VLPs causes lung histopathology upon RSV challenge.
Figure 5. Assessment of eosinophils in the lungs from vaccinated mice after RSV challenge. Lung tissues were collected from individual BALB/c mouse (N = 5) after vaccination and RSV challenge. (A, B) Lung tissue histology after staining with Hematoxyline and Congo red (H&CR). Arrows indicate eosinophil granulocytes in the individual airways. Scale bars: 20 μm. (C, D) H&CR positive eosinophils were counted under the microscopic field and presented in percentages in the prime and prime-boost groups. (E, F) Numbers and flow profiles of gated Siglec F+ eosinophils (Siglec F+CD11b+CD11c-F4/80+CD45+) as determined by flow cytometry of BAL fluids. Results are presented as mean ± SEM. Statistical significances were performed by one-way ANOVA in GraphPad Prism; ***P < 0.001, *p < 0.05. Group labels are the same as described in the .
Biased Th2 cytokines are elicited after F protein vaccination and RSV challenge
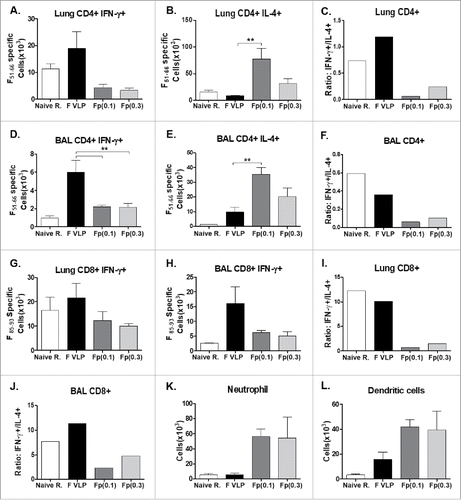
To investigate the Th1 and Th2 immune responses after immunization and RSV challenge, IL-4, IL-5, IL-13 (Th2) and IFN-γ (Th1) cytokines were determined. The F protein (0.1 μg, 0.3 μg) groups showed high levels of Th2 cytokines IL-4, IL-5 and IL-13 in both lung and BALF samples collected at day 5 post challenge (, ). In contrast, high IFN-γ and low Th2 cytokine levels were detected in lung and BALF samples from the F-VLP group, suppressing the induction of Th2 type cytokines ( and ). Next, we determined the profile of IFN-γ or IL-4 secreting CD4 and CD8 T cells in response to stimulation with CD4 (F51–66) and CD8 T (F85–93) cell epitopes respectively (). Higher levels of IFN-γ secreting CD4 and CD8 T cells were observed in lungs (, ) and BAL (, ) samples from F VLP immune mice than those from F protein immune mice. When the ratios of IFN-γ+/IL-4+ CD4 T cells and IFN-γ+/IL-4+ CD8 T cells were compared, naïve infection and F VLP immune mice resulted in significantly higher IFN-γ ratios for both T cells, suggesting dominant Th1 responses (IFN- γ) compared with F protein immune mice (, , , ). Neutrophils (CD11b+Ly6c+F4/80−) and dendritic cells (DCs, CD45+CD11c+MHCII+) were found to be recruited to higher levels in F protein immune mice compared with that in the F VLP group after RSV challenge (, )
Figure 6. Cytokines in the Lung tissue and BALF from prime-boost immune mice after RSV challenge. (A, B) Lung tissue and BALF were collected from individual mice (N = 5) after RSV challenge. Levels of IL-4, IL-5, IL-13 and IFN-γ cytokines were measured in lung and BALF samples by each corresponding cytokine ELISA kits. Results are presented as mean ± SEM. Statistical significances were performed by one-way ANOVA and Tukey's multiple-comparison tests in GraphPad Prism; ***p < 0.001, **P < 0.01, *p < 0.05. Group labels .are the same as described in the .
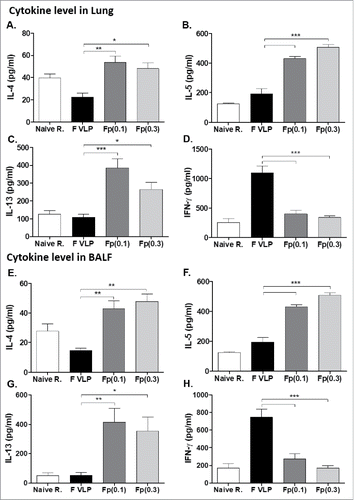
Figure 7. CD4+, CD8+ (T)cells secreting Th1 or Th2 cytokines by intracellular cytokine staining, and airway infiltrating innate immune cells. Lung and BALF cells were collected from prime-boost immunized mice at 5 d after challenge, or subjected to intracellular cytokine staining. (A, B, D, E, G, H) Intracellular cytokine staining of CD4 and CD8 T cells from lungs or. BALF by flow cytometry after in vitro stimulation with CD4 T cell F51−66 epitope and CD8 T cell F85–93 epitope. (C, F, I, J) Ratios of IFN-γ+/IL4+ CD4 or CD8 T cell number averages from intracellular cytokine flow cytometry of lung and BAL samples. (K) Neutrophils (CD11b+Ly6c+F4/80−) and (L) Dendritic Cells (DCs, CD45+CD11c+MHCII+) were analyzed from BALF samples. PBS: Naïve mice with RSV infection. Other groups are vaccinated mice as indicated. The results are presented as averages with standard errors and statistical significance was determined using one-way ANOVA with Tukey's multiple comparison test in GraphPad Prism. ***,p < 0.001, **p < 0.01. Other group labels are the same as described in the .
F VLP induces transient inflammatory responses
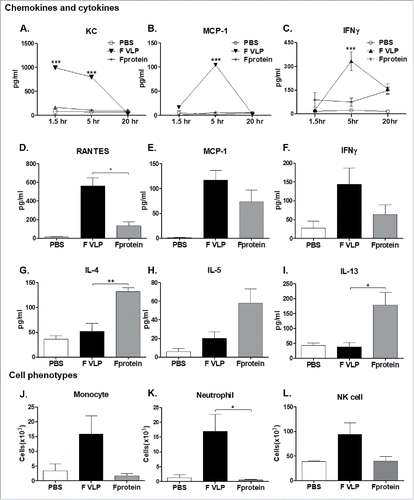
To better understand the mechanisms of inducing a distinct pattern of immune responses between F VLP and F protein vaccination, we determined innate immune responses at the site of injection. Intraperitoneal (IP) injection of F VLP transiently induced chemokines (KC; CXCL1, MCP-1) and IFN-γ in sera within 1.5 hours (h) or 5 h, which rapidly waned after 5 h (). Peritoneal exudates displayed high levels of chemokines (RANTES, MCP-1) and IFN-γ at 24 h post treatment (). In contrast, the levels of Th2 cytokines (IL-4, IL-5, and IL-13) were higher in peritoneal exudates after injection with F protein but not with F VLP (). Flow cytometric determination of cellular phenotypes in peritoneal cavity showed that F VLP is more effective in recruiting monocytes, neutrophils, and natural killer (NK) cells at the site of injection than F protein ().
Figure 8. Acute responses of cytokines, chemokines, and cellular infiltrates at the site of injection with (F)VLP or (F)protein. BALB/c mice (n = 3) were intra-peritoneally injected with PBS, F VLP, or F protein. (A-C) Kinetics of cytokines and chemokine levels in sera from adjuvant injected mice (n = 3) mice per group). ***; p < 0.001 compared with naïve group (PBS only). (D-I) Cytokines and chemokines in peritoneal exudates at 24h after injection. (J) Monocytes: CD11b+Ly6c high F4/80+, (K) Neutrophils; CD11b+Ly6c+F4/80-, (L) NK cells; DX5+CD3-, data were shown as mean ± SEM. Statistical significances were calculated by 1-way ANOVA and Tukey's multiple comparison test. ***; p < 0.001 **; p < 0.01, and *; p < 0.05, as indicated among the groups. Naïve; unimmunized mice, F VLP; F VLP immunized mice, F protein; 0.1 μg RSV F protein with alum adjuvant. Group information of D-I were same as J-L.
Discussion
In this study, we compared the pattern of immune responses to F immunogen in VLP and in a soluble protein, and their resulting efficacy and lung inflammation after RSV challenge. F VLP or F protein vaccination induced IgG2a and IgG1 isotype dominant antibody responses respectively, representing Th1 and Th2 type immune patterns. Prime or prime-boost immunization with F VLP and F protein vaccines showed comparable RSV neutralizing activity and was effective in controlling lung viral loads after RSV challenge, which is consistent with a previous study reporting moderate increases in IgG titers after boost with FI-RSV or purified F protein.Citation9 Nonetheless, there were striking differences in pulmonary histopathology, mucus production, and eosinophilia as well as body weight loss of RSV disease between the F VLP and F protein vaccine groups.
Since RSV vaccine-enhanced disease was first observed during 1960s clinical trials of FI-RSV vaccines,Citation6 numerous efforts have been made to develop RSV vaccines that can avoid vaccine-enhanced pulmonary histopathology. RSV F, G (attachment), and chimeric FG protein subunit vaccines were previously shown to cause vaccine-enhanced pulmonary histopathology in mouse and cotton rat animal models.Citation9,23,24 Bronchiolar histopathology and pulmonary polymorphonucleocytes infiltration appeared to be evident after vaccination with Vac-F and RSV challenge in miceCitation25,26 but less likely in cotton rats,Citation11 suggesting a greater tendency for mice to develop RSV-vaccine enhanced disease. Viral-vectored RSV vaccines are likely to mimic natural infection inducing innate and adaptive responses. Modified vaccinia virus Ankara and simian adenovirus expressing RSV proteins were immunogenic and safe in healthy adults (phase I), warranting further clinical evaluation of efficacy.Citation27,28 The recombinant Sendai virus, a mouse type I parainfluenza virus expressing a full-length RSV F protein, conferred protection against RSV in a monkey model.Citation29 RSV-reinfections appeared to induce pulmonary histopathology in miceCitation30 and in cotton rats.Citation11 A recent study reported that low doses (0.3 −0.03 µg) of post-fusion or pre-fusion F proteins primes histopathology in cotton rats after challenge despite lung viral clearance.Citation31 F VLP and soluble F proteins used in this study were found to be expressed mostly in a post-fusion conformation (data not shown). We observed that mice prime or prime-boost immunization with RSV F protein vaccines displayed severe lung histopathology around the airways, blood vessels, and interstitial spaces as well as mucus production and eosinophil infiltration compared with naïve mice, which is correlating with weight loss disease after RSV challenge. In contrast, F VLP prime only or prime-boost vaccination could prevent pulmonary histopathology lower than that in naïve mice after RSV challenge, which are consistent with the results in cotton rats.Citation32
Comparative studies of F VLP and F protein vaccination might provide insight into underlying mechanisms by which RSV vaccine-enhanced disease could be avoided, which will help in developing a safe and effective RSV vaccine. F VLP raises IgG2a Th1 type dominant antibodies whereas F protein IgG1 Th2 type dominant responses. These results are consistent with cytokine patterns in lung and BALF samples where the F VLP group induced Th1 type (IFN-γ) cytokine and the F protein group elicited Th2 (IL-4, IL-5, IL-13) cytokines. Consistent with histopathology in the F protein groups, high levels of IL-13 and neutrophil infiltrates due to RSV infection were shown to be correlated with mucus production and pathology.Citation33 CD11b+ granulocytes and plasmacytoid DCs infiltrating into the lungs after RSV challenge were suggested to contribute to RSV disease.Citation34,35 Lung and BAL T cell intracellular cytokine staining flow cytometry assay revealed that F VLP vaccination resulted in higher ratios of IFN-γ/IL-4 CD4 and IFN-γ/IL-4 CD8 T cells than F protein vaccination. Naïve mice with live RSV infection also displayed similarly high ratios of IFN-γ/IL-4 CD4 and IFN-γ/IL-4 CD8 T cells although cell numbers of T cells secreting IFN-γ were lower than the F VLP group. F VLP vaccination appears to induce Th1 bias T cell responses. Induction of Th1 responses including the production of IFN-γ may modulate to suppress priming Th2 responses such as IL-4, IL-5, and IL-13 responsible for RSV pathology. The F VLP group induces higher levels of IFN-γ and CD8 T cells expressing IFN-γ compared with F protein vaccination. These humoral and cellular immunity and antiviral IFN-γ cytokines induced by F VLP vaccination might have contributed to a better control of lung viral loads in addition to neutralizing antibodies.
Previous studies explored RSV vaccine formulations with immune-modulating adjuvants and reported improved efficacy and safety of an intranasal virosomal RSV vaccine adjuvanted with monophosphoryl lipid A.Citation36 In contrast, cotton rats immunized with the combination of F protein and oligodeoxynucleotide CpG adjuvant developed enhanced pulmonary histopathology after RSV challenge.Citation24 Also, a squalene-based emulsion adjuvant mixed with RSV F protein vaccine was not able to avoid RSV vaccine-enhanced inflammatory disease and inclusion of glucopyranosyl lipid A (TLR4 agonist) modulated to prime IFN-γ producing T cell responses.Citation21 RSV subunit vaccines of recombinant pre-fusion or post-fusion F proteins at low doses (< 0.3 µg) were shown to prime immune responses enhancing lung histopathology regardless of a Th1- or Th2-biasing adjuvant in cotton rats after challenge.Citation31 Mice vaccinated with Vac-G or FI-RSV and treated with a natural killer T cell activating adjuvant (α-GalCer) or live RSV vaccine plus delta-inulin adjuvant did not diminish lung histopathology after RSV challenge.Citation37,38 Interestingly, addition of F VLP to F protein vaccination was found to improve protective efficacy and to suppress F protein vaccine-enhanced lung histopathology in infant age mice (data not shown). It is important to determine whether F VLP with Th2 bias immune inducing adjuvant will result in vaccine enhanced disease. Therefore, further studies are required to better understand the detail mechanisms of RSV vaccine-enhanced disease and to develop a safe and effective RSV vaccine.
To obtain insight into what reactions are occurring at the site of vaccine injection, we analyzed acute cytokine, chemokine, and cellular phenotypes at day 1 after IP injection. F VLP injection induced transiently chemokines (KC, MCP-1, and RANTES) and IFN-γ. Whereas, F protein injection developed Th2 cytokines (IL-4, IL-5, and IL13). Monocyte chemoattractant protein-1 (MCP-1/CCL2) is known to regulate migration and infiltration of monocytes/macrophages.Citation39 RANTES (regulated on activation normal T cell expressed and secreted), now known to be secreted by haematopoietic and non-haematopoietic cell types including epithelial cells, acts as a chemoattractant for monocytes, natural killer cells, T cells, eosinophils, and dendritic cells as well as for maintaining antiviral CD8 T cells.Citation40 F VLP injection resulted in acutely inducing cellular infiltrates such as monocytes, neutrophils, and natural killer (NK) cells. The levels of these innate acute inflammatory cells appeared to be lower than those reported in previous studies on adjuvants such as oil-in-water emulsion MF59 and combination AS04, alum + monophosphoryl lipid A (MPL, a TLR4 ligand).Citation41 These intrinsic differences between F VLP and F protein in generating inflammatory microenvironment might be contributing to developing differential quality of immunity responsible for enhancing or preventing RSV vaccine-associated disease.
Material and methods
Cells, Virus, F protein, and Virus-like particles containing RSV F (F VLP)
Hep2 and Spodoptera frugiperda 9 (Sf9) insect cells were obtained from American Type Culture Collection (ATCC). Hep2 cells were grown in DMEM supplemented with 5% fetal bovine serum and penicillin/streptomycin antibiotics. The affinity-purified RSV A2 F (fusion) glycoprotein was obtained from Biodefense and Emerging Infections Research Resources (BEI Resources, Manassas, VA). Sf9 insect cells were grown in serum free SF900 II medium from Gibco-BRL and used for amplifying recombinant baculoviruses (rBVs) expressing RSV F proteins and F VLP vaccine production. For RSV F VLP vaccine production, insect cells were co-infected with rBVs expressing RSV F proteins (RSV A2 strain) and influenza virus M1 matrix protein, and F VLP was purified from Sf9 culture supernatants using ultracentrifugation and characterized as described previously.Citation17 Human RSV A2 was kindly provided by Dr. Martin Moore (Emory University, GA). The conformation of F protein and F VLP vaccines was determined by ELISA using post-F specific (131–2a purchased from Millipore) and pre-F specific (5C4) monoclonal antibodies.
Immunization and RSV challenge
BALB/c mice (n = 5, 6–8 weeks old males and females, Charles River Laboratories, Inc.) were intramuscularly (i.m.) primed (a single dose) with 10 µg of F VLP, 0.1 µg or 0.3 µg of F protein in the alum (50 µg) adjuvant, and phosphate buffered saline (PBS, naïve control). Additional sets of the BALB/c groups (n = 5) were prime-boost vaccinated with the same vaccines at a 3-week interval. Blood samples were collected at 3 weeks after each vaccination. Naïve control, F-VLP, F protein immunized mice were intranasally (i.n.) infected with 5 × 105 PFU of RSV A2 under isofluorane anesthesia to determine the efficacy of protection and vaccine-enhanced disease 3 to 4 weeks after vaccination. The body weight changes were monitored upon RSV challenge. Individual lung, bronchiolar alveolar lavage fluids (BALF), mediastinal lymph nodes (MLN), and spleens were collected at 5 d after challenge. All animal experimental procedures were approved and performed by following the guidelines of Georgia State University Institutional Animal Care and Use Committee.
ELISA assay
RSV F-specific isotype antibodies (IgG, IgG1, and IgG2a) were measured in immune sera using enzyme-linked immunosorbent assay (ELISA) and purified RSV F protein as a coating antigen (100ng/ml, BEI resources, Manassas, VA) as described previously.Citation19 RSV F specific IgG isotype antibodies were detected using horseradish peroxidase-conjugated anti-mouse IgG, IgG1 and IgG2a secondary antibodies (Southern Biotechnology). The color-developing substrate (TMB, 3, 3′, 5, 5′-tetramethylbenzidine, Sigma Aldrich) was treated and stopped with 1M H3PO4. Optical densities (O.D) were read at 450nm. The cytokine concentrations in lungs homogenates and bronchoalveolar lavage fluid (BALF) were quantified by using commercially available cytokine ELISA kits of interleukin (IL)-4, IL-5, IL-6, interferon (IFN)-γ, IL-13, and tumor necrosis factor (TNF)-α (eBioscience, SanDiego, CA).
RSV immuno-plaque and neutralizing assays
RSV lung viral titers were measured in individual lung samples day 5 post challenge and neutralizing antibody titers were determined with prime or prime-boost immune sera. RSV plaque-forming units (PFU) were determined using an immuno-plaque assay (IPA) as described previously.Citation17 Immune sera were heat inactivated for 1 h at 56°C and then diluted to mix with RSV A2 strain (200 PFU/well). RSV control, lung homogenates or mixed immune sera with RSV were incubated on HEp-2 cells for 1 h 37°C, 5% CO2. After 4% formalin fixation, the plaques were detected with anti RSV F monoclonal antibody (Millipore).
Flow cytometry
BALF were harvested by infusing 1 ml of PBS into the lungs via the trachea at day 5 post-challenge. Lung tissues were isolated by homogenization and used by percoll gradient (44 and 67%) centrifugation. The frosted microscope glasses were used for harvesting mediastinal lymph nodes (MLN) to suspend the cells. Lung, and BAL cells were stimulated with F peptide (F51–66 CD4 and F85–96 CD8 epitopes of RSV F, 4μg/ml) before staining of intracellular cytokines, and then the cells were fixed and permeabilized according to the manufacturer's instructions (BD Biosciences). Intracellular cytokines and surface markers for T cells or eosinophils were stained with antibodies for IFN-γ, IL-4 (eBioscience), TNF-α (BioLegend), CD3, CD4, CD8, CD11b, CD11c, CD103, F4/80, Ly6c and DX5 or Siglec F (BD Biosciences). For analysis, the Becton-Dickinson LSR-II/Fortessa flow cytometer (BD, San Diego, CA) was used to collect cell populations. Acquired flow cytometry data were analyzed by using Flowjo software (Tree Star Inc.).
Lung histopathology
Lung tissues harvested day 5 post-infection and formalin-fixed, embedded in paraffin blocks. Lung tissue sections were determined by hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), and congo red (C&R) staining and analyzed using light microscopy. For numerical assessment of histopathology and pneumonia in lung tissues, the bronchioles, vessels and interstitial spaces were scored on a scale of 0–3 by blinded observers with the severity scoring system as described previously.Citation30,42 Randomly 20 airways were selected to quantify for PAS positive stain with mucin expression area. The present pulmonary eosinophilia were expressed by H&CR stain with 400x magnified microscope. The scored figures were determined for lymphocytes, polymorphonuclear cells, macrophages and eosinophils in peribronchiolar, perivascular, interstitial, and alveolar spaces.Citation30,43
Intraperitoneal (IP) injection
BALB/c mice (n = 3) were injected with 200 μl of phosphate buffered saline (PBS), F VLP (10 μg), F protein (0.1 μg) with alum (50 μg) adjuvants with intraperitoneally. Collected sera were analyzed to determine the levels of cytokines and chemokines by using ELISA kits (KC, MCP-1, and RANTES; R&D system, IL-4, 5, 13, and IFN-γ; eBioscience) at 1.5, 5, and 20 hours (h) post injection. The elicited peritoneal exudate cells were obtained at 24 h post injection by PBS flushing. The cells were isolated and analyzed for cellularity of infiltrating cells by flow cytometry. Peritoneal exudates were used for detection of cytokines and chemokines.
Statistical analysis
Statistical differences were performed using GraphPad prim version 5 (Graph Pad software Inc., San Diego, CA). Data were analyzed for significance using one-way ANOVA with Tukey's test for multiple comparisons or 2-way ANOVA with Bonferroni posttests. The difference was considered statistically significant when the P value was less than 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The following reagent was obtained through BEI Resources, NIAID, NIH: Purified recombinant fusion glycoprotein (RSV A2) with C-Terminal His tag produced in 293F cells. The authors express appreciation for Dr. Barney S. Graham for kindly providing the 5C4 monoclonal antibody.
Funding
This work was supported by NIH/NIAID grants AI105170 (S.M.K.), AI119366 (S.M.K.), and AI093772 (S.M.K.).
References
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–55. doi:10.1016/S0140-6736(10)60206-1. PMID:20399493
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi:10.1016/S0140-6736(12)60560-1. PMID:22579125
- Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206:56–62. doi:10.1093/infdis/jis309. PMID:22529314
- Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 1969;89:449–63. doi:10.1093/oxfordjournals.aje.a120957. PMID:4305200
- Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 1969;89:435–48. doi:10.1093/oxfordjournals.aje.a120956. PMID:4305199
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969;89:422–34. doi:10.1093/oxfordjournals.aje.a120955. PMID:4305198
- Boeckh M, Berrey MM, Bowden RA, Crawford SW, Balsley J, Corey L. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;184:350–4. doi:10.1086/322043. PMID:11443562
- Murphy BR, Sotnikov A, Paradiso PR, Hildreth SW, Jenson AB, Baggs RB, Lawrence L, Zubak JJ, Chanock RM, Beeler JA, et al. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine. 1989;7:533–40. doi:10.1016/0264-410X(89)90278-8. PMID:2692334
- Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8:497–502. doi:10.1016/0264-410X(90)90253-I. PMID:2251875
- Walsh EE, Hall CB, Briselli M, Brandriss MW, Schlesinger JJ. Immunization with glycoprotein subunits of respiratory syncytial virus to protect cotton rats against viral infection. J Infect Dis. 1987;155:1198–204. doi:10.1093/infdis/155.6.1198. PMID:3553346
- Connors M, Collins PL, Firestone CY, Sotnikov AV, Waitze A, Davis AR, Hung PP, Chanock RM, Murphy BR. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia–RSV recombinants or RSV. Vaccine. 1992;10:475–84. doi:10.1016/0264-410X(92)90397-3. PMID:1609551
- McGinnes LW, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Schmidt MR, Morrison TG. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J Virol. 2011;85:366–77. doi:10.1128/JVI.01861-10. PMID:20980510
- Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Heaton PM, Fraire AE, Morrison TG. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol. 2010;84:1110–23. doi:10.1128/JVI.01709-09. PMID:19889768
- Schmidt MR, McGinnes LW, Kenward SA, Willems KN, Woodland RT, Morrison TG. Long-term and memory immune responses in mice against Newcastle disease virus-like particles containing respiratory syncytial virus glycoprotein ectodomains. J Virol. 2012;86:11654–62. doi:10.1128/JVI.01510-12. PMID:22896618
- Schmidt MR, McGinnes-Cullen LW, Kenward SA, Willems KN, Woodland RT, Morrison TG. Modification of the respiratory syncytial virus f protein in virus-like particles impacts generation of B cell memory. J Virol. 2014;88:10165–76. doi:10.1128/JVI.01250-14. PMID:24965456
- Cullen LM, Blanco JC, Morrison TG. Cotton rat immune responses to virus-like particles containing the pre-fusion form of respiratory syncytial virus fusion protein. J Transl Med. 2015;13:350. doi:10.1186/s12967-015-0705-8. PMID:26541285
- Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, Moore ML, Compans RW. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis. 2011;204:987–95. doi:10.1093/infdis/jir474. PMID:21881112
- Lee S, Quan FS, Kwon Y, Sakamoto K, Kang SM, Compans RW, Moore ML. Additive protection induced by mixed virus-like particles presenting respiratory syncytial virus fusion or attachment glycoproteins. Antiviral Res. 2014;111C:129–35. doi:10.1016/j.antiviral.2014.09.005
- Kim KH, Lee YT, Hwang HS, Kwon YM, Kim MC, Ko EJ, Lee JS, Lee Y, Kang SM. Virus-Like Particle Vaccine Containing the F Protein of Respiratory Syncytial Virus Confers Protection without Pulmonary Disease by Modulating Specific Subsets of Dendritic Cells and Effector T Cells. J Virol. 2015;89:11692–705. PMID:26355098
- Mok H, Lee S, Utley TJ, Shepherd BE, Polosukhin VV, Collier ML, Davis NL, Johnston RE, Crowe JE Jr. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J Virol. 2007;81:13710–22. PMID:17928349
- Lambert SL, Aslam S, Stillman E, MacPhail M, Nelson C, Ro B, Sweetwood R, Lei YM, Woo JC, Tang RS. A novel respiratory syncytial virus (RSV) F subunit vaccine adjuvanted with GLA-SE elicits robust protective TH1-type humoral and cellular immunity in rodent models. PloS One. 2015;10:e0119509. PMID:25793508
- Phan SI, Chen Z, Xu P, Li Z, Gao X, Foster SL, Teng MN, Tripp RA, Sakamoto K, He B. A respiratory syncytial virus (RSV) vaccine based on parainfluenza virus 5 (PIV5). Vaccine. 2014;32:3050–7. PMID:24717150
- Garlapati S, Garg R, Brownlie R, Latimer L, Simko E, Hancock RE, Babiuk LA, Gerdts V, Potter A, van Drunen Littel-van den Hurk S. Enhanced immune responses and protection by vaccination with respiratory syncytial virus fusion protein formulated with CpG oligodeoxynucleotide and innate defense regulator peptide in polyphosphazene microparticles. Vaccine. 2012;30:5206–14. PMID:22713718
- Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol. 2003;77:13156-60. PMID:14645572
- Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol 1992;4:493–500. PMID:1591217
- Stott EJ, Taylor G, Ball LA, Anderson K, Young KK, King AM, Wertz GW. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol 1987;61:3855–61. PMID:3316707
- Green CA, Scarselli E, Voysey M, Capone S, Vitelli A, Nicosia A, Cortese R, Thompson AJ, Sande CS, de Lara C, et al. Safety and immunogenicity of novel respiratory syncytial virus (RSV) vaccines based on the RSV viral proteins F, N and M2-1 encoded by simian adenovirus (PanAd3-RSV) and MVA (MVA-RSV); protocol for an open-label, dose-escalation, single-centre, phase 1 clinical trial in healthy adults. BMJ Open. 2015;5:e008748. PMID:26510727
- Green CA, Scarselli E, Sande CJ, Thompson AJ, de Lara CM, Taylor KS, Haworth K, Del Sorbo M, Angus B, Siani L, et al. Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci Transl Med. 2015;7:300ra126. doi:10.1126/scitranslmed.aac5745
- Jones BG, Sealy RE, Rudraraju R, Traina-Dorge VL, Finneyfrock B, Cook A, Takimoto T, Portner A, Hurwitz JL. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine. 2012;30:959–68. doi:10.1016/j.vaccine.2011.11.046. PMID:22119594
- Hwang HS, Kwon YM, Lee JS, Yoo SE, Lee YN, Ko EJ, Kim MC, Cho MK, Lee YT, Jung YJ, et al. Co-immunization with virus-like particle and DNA vaccines induces protection against respiratory syncytial virus infection and bronchiolitis. Antiviral Res. 2014;110C:115–23. doi:10.1016/j.antiviral.2014.07.016
- Schneider-Ohrum K, Cayatte C, Bennett AS, Rajani GM, McTamney P, Nacel K, Hostetler L, Cheng L, Ren K, O'Day T, et al. Immunization with Low Doses of Recombinant Postfusion or Prefusion Respiratory Syncytial Virus F Primes for Vaccine-Enhanced Disease in the Cotton Rat Model Independently of the Presence of a Th1-Biasing (GLA-SE) or Th2-Biasing (Alum) Adjuvant. J Virol. 2017;91:e02180–16. doi:10.1128/JVI.02180-16.
- Hwang HS, Kim KH, Lee Y, Lee YT, Ko EJ, Park S, Lee JS, Lee BC, Kwon YM, Moore ML, et al. Virus-like particle vaccines containing F or F and G proteins confer protection against respiratory syncytial virus without pulmonary inflammation in cotton rats. Hum Vaccin Immunother. 2017;13:1031–9. doi:10.1080/21645515.2016.1272743. PMID:28129031
- Stokes KL, Currier MG, Sakamoto K, Lee S, Collins PL, Plemper RK, Moore ML. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J Virol. 2013;87:10070–82. doi:10.1128/JVI.01347-13. PMID:23843644
- Ko EJ, Kwon YM, Lee JS, Hwang HS, Yoo SE, Lee YN, Lee YT, Kim MC, Cho MK, Lee YR, et al. Virus-like nanoparticle and DNA vaccination confers protection against respiratory syncytial virus by modulating innate and adaptive immune cells. Nanomedicine: nanotechnology, biology, and medicine. 2015;11:99–108. doi:10.1016/j.nano.2014.07.013. PMID:25109662
- Lee YT, Kim KH, Hwang HS, Lee Y, Kwon YM, Ko EJ, Jung YJ, Lee YN, Kim MC, Kang SM. Innate and adaptive cellular phenotypes contributing to pulmonary disease in mice after respiratory syncytial virus immunization and infection. Virology. 2015;485:36–46. doi:10.1016/j.virol.2015.07.001. PMID:26196232
- Kamphuis T, Shafique M, Meijerhof T, Stegmann T, Wilschut J, de Haan A. Efficacy and safety of an intranasal virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice and cotton rats. Vaccine. 2013;31:2169–76. doi:10.1016/j.vaccine.2013.02.043. PMID:23499594
- Johnson TR, Hong S, Van Kaer L, Koezuka Y, Graham BS. NK T cells contribute to expansion of CD8(+) T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol. 2002;76:4294–303. doi:10.1128/JVI.76.9.4294-4303.2002. PMID:11932395
- Wong TM, Petrovsky N, Bissel SJ, Wiley CA, Ross TM. Delta inulin-derived adjuvants that elicit Th1 phenotype following vaccination reduces respiratory syncytial virus lung titers without a reduction in lung immunopathology. Hum Vaccin Immunother. 2016:1–10.
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi:10.1089/jir.2008.0027. PMID:19441883
- Crawford A, Angelosanto JM, Nadwodny KL, Blackburn SD, Wherry EJ. A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection. PLoS Pathog. 2011;7:e1002098. doi:10.1371/journal.ppat.1002098. PMID:21814510
- Ko EJ, Lee YT, Kim KH, Lee Y, Jung YJ, Kim MC, Lee YN, Kang T, Kang SM. Roles of Aluminum Hydroxide and Monophosphoryl Lipid A Adjuvants in Overcoming CD4+ T Cell Deficiency To Induce Isotype-Switched IgG Antibody Responses and Protection by T-Dependent Influenza Vaccine. J Immunol. 2017. doi:10.4049/jimmunol.1600173
- Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi:10.1038/nm.1894. PMID:19079256
- Meyerholz DK, Griffin MA, Castilow EM, Varga SM. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol Pathol. 2009;37:249–55. doi:10.1177/0192623308329342. PMID:19181630
