ABSTRACT
Extracellular vesicles (EVs) are membrane-derived vesicles that are enriched with RNAs, proteins and other functional molecules. We exploit the unique physical properties of EVs as a promising and advantageous nanoplatform for the delivery of therapeutic drugs and genetic materials. Early successes in the discovery of various disease-related characteristics of EVs have driven a new wave of innovation in developing nanoscale drug-delivery systems (DDSs). Nevertheless, there are several issues that need to be considered during the development of these alternative DDSs, such as standardized isolation and preservation methods, efficient drug encapsulation, mechanisms of drug release and so on. In this mini-review, we summarize the current status and progress of EV-based DDSs as an efficient nanoplatform for therapeutics delivery, followed by a discussion on their challenges and future prospects for clinical translation and applications.
Introduction
Various drug-delivery systems (DDSs) have been extensively explored to improve the efficacy of drugs and reduce their side effects. The development of DDSs involves improving drug solubility,Citation1-4 activity,Citation5,6 bioavailability,Citation4,7-11 targetingCitation12 and dosing regimenCitation13 as well as reducing toxicity.Citation14,15 Conventional DDSs, including liposomes, polymeric nanoparticles, inorganic nanoparticles, etc.,Citation16-18 are widely used. Despite considerable progress in developing advanced DDSs, efforts are urgently needed to develop a clinically adequate therapeutic delivery platform.Citation19 Conventional nanoparticles are multipurpose and have great potential in therapeutic drug delivery applications, but they also have considerable defects (e.g., their xenobiotic origin), which often result in unexpected immune reactions and toxicity in organisms.Citation20-24 Inherent toxicity and side effects of drug nanocarriers are significant obstacles in the development of a high-performance and clinically safe nano-delivery platforms.Citation25-27
In light of the obstacles associated with current nano-DDSs, significant efforts have been made to determine revolutionary nanomaterials with biological origins. Very recently, scientists have been attracted by a group of cell-derived endogenous nanovesicles called extracellular vesicles (EVs).Citation28 EVs are membrane vesicles formed from the endosomal system that are released by nearly all types of cells to the extracellular space and thus play an important role in the intercellular communication.Citation29 The capability of EVs to transport molecules between cells indicates that they might serve as a natural DDS.Citation30 In recent years, EVs have been investigated as promising DDSs to target cells or tissues for nanomedicine.Citation31-33 Compared with conventional DDSs (e.g., liposome), EVs demonstrate attractive advantages and features such as natural biological effects, favorable pharmacokinetics and targeting specificities. However, therapeutic applications of EVs as DDSs are still in early stages of research, and further investigation are expected for their scalable isolation methods, high-efficient encapsulations as well as intrinsic cell targeting properties.
In this review, we summarize the progress in EV-based DDSs with emphasis on the challenges and hurdles in the development of EVs as DDSs. Although EV-based DDSs are not fully optimized for manufacturing scale-up and clinical translation, they provide alternative DDS models for delivering therapeutic drugs.
Biogenesis of EVs
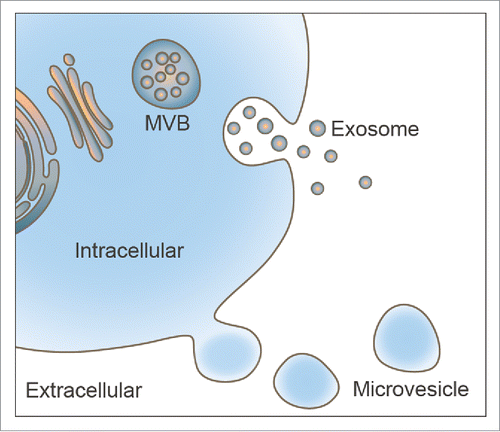
Extracellular vesicles are membrane vesicles released from nearly all cell types in mammalian species, they display versatile physiological functions and are involved in the maintenance of homeostasis, and the regulation of signaling and intercellular communication between different cell types.Citation29 EVs play important roles in many pathological and physiological processes including inflammation,Citation34 angiogenesis,Citation35 immune response,Citation36 autophagy,Citation37 cell survival,Citation38,39 and cancer drug resistance.Citation40,41 Based on their morphology, formation pathway and content, EVs can be classified as exosomes, microvesicles (MVs) and apoptotic bodies.Citation42,43 Exosomes are the smallest membrane-bound vesicles with sizes varying from 40–100 nm,Citation44 and were first reported by Johnstone et al. as small vesicles released by reticulocytes.Citation45 They are produced from multivesicular bodies (MVBs) during endosomal maturation and are secreted via the fusion of MVBs with the cytomembrane. Exosomes are full of various biomolecules including mRNAs, miRNAs, lipid molecules (cholesterol, sphingomyelin, ceramide, etc.)Citation46,47 and proteins such as heat-shock proteins, glyceraldehyde-3-phosphate dehydrogenase (GAPDH),Citation48 endosomal sorting complex required for transport (ESCRT) machineryCitation42,43 and tetraspanin family molecules. In contrast, MVs also referred to as ectosomes, shedding vesicles or microparticles, and are directly formed from the cell membrane outward budding; they are more heterogeneous in diameter compared with exosomes (50–1000 nm).Citation49,50 Besides, microvesicles also contain a large number of biological molecules such as integrins, CD40 ligand, selectins and phosphatidylserine.Citation51,52 However, apoptotic bodies are significantly different from the other two types of EVs, as they are small vesicles formed in cells that suffered from programmed cell death (namely apoptosis) and exhibit a heterogeneous range of sizes and different morphologies (50–5000 nm).Citation53,54
In the intercellular environment, all three types of cellular vesicles—designated “early endosomes,” “late endosomes” and “recycling endosomes”—are successively formed during endosomal maturation. First, incoming cargos are generated from the internalized plasma membrane and then sorted into diverse intracellular destinations by early endosomes.Citation55 When early endosomes transform into late endosomes, intraluminal vesicles (ILVs) are formed. These late endosomes contain ILVs called MVBs. Subsequently, some MVBs fuse with lysosomes by degrading the cargos. Moreover, other MVBs fuse with the plasma membrane, resulting in the formation of exosomes in the extracellular space. Several studies have demonstrated that tetraspanins and endosomal-sorting complexes are essential to the formation of the intraluminal vesicles in the cell, and this special superfamily of membrane proteins noted mentioned is often used as exosome biomarkers.Citation56
Endosomal sorting complexes required for transport (ESCRT) work together with accessory proteins relating to the formation of ILVs, and exosome biogenesis relies on both ESCRT-dependent and ESCRT-independent mechanisms.Citation57 Recently, Colombo et al. analyzed the function of ESCRT components in EVs biogenesis using RNA interference. It was found that various ESCRT components such as vacuolar protein sorting-associated protein 4B and tumor susceptibility gene 101 were related to the composition, size and productivity of secreted EVs.Citation58 The existence of ESCRT-independent mechanisms have been corroborated by Stuffers et al., they demonstrated that CD63-positive EVs were secreted from cells that lack of four subunits of the ESCRT complex.Citation59
Exosome biogenesis occurs in MVBs, and MVs are formed by direct budding from the plasma membrane (). MVs are larger than exosomes and more heterogeneous in size and morphology.Citation43 The activation of MVs varies from cell to cell. For example, MVs are released by endothelial cells and circulating blood cells in response to complement, whereas monocytes, platelets and fibroblasts budding are released in response to bacterial cell wall components, thrombin, and stress relaxation, respectively.Citation60,61 As proteins express procedurally, the production of MVs that occurs throughout the cell cycle and under various culture conditions may not be consistent.Citation41 MVs could reflect the antigenic content of the cells that they originate, providing a new strategy for natural vaccine delivery systems. However, exosomes are known to relate to intercellular communications and offer distinct advantages as highly effective drug carriers for future clinical translation.Citation61
Extracellular vesicles as novel DDSs
The structural characteristics of EVs are analogous to liposomes, which renders EVs attractive for drug delivery.Citation62 Since liposomes are constructed of phospholipids, they are similar to the plasma membranes, and have been widely used for efficient drug delivery.Citation63 Thus far, several commercialized liposome-based products such as DaunoXome (a liposomal for the delivery of daunorubicin (DNR), approved for the management of advanced HIV-associated Kaposi's sarcoma), Myocet (a non-pegylated liposomal doxorubicin, approved for treatment of metastatic breast cancer) and Depocyt (a cytarabine liposome injection, approved for treatment of lymphomatous meningitis) have been put into the market.Citation64 Liposome research lays the foundation for investigations of the physicochemical characteristics, stability and drug loading of EVs.Citation65-67 Moreover, compared with liposomes, EVs are produced by the cells themselves, which make them more advantageous than liposomes in mimicking the cell membrane. These superior properties of EVs indicates the possibility of utilizing EVs from the body's own cells to deliver drugs, even across blood-brain barrier (BBB).Citation68
Extracellular vesicles for cancer treatments
More and more evidence has shown that EVs have splendid prospects in therapeutic delivery of small interfering RNAs and synthetic molecules.Citation69 Several studies have revealed the potential of EVs as therapeutic DDSs in various animal models of diseases.Citation70 Furthermore, EVs are being widely studied as anti-tumor DDSs due to their passive targeting ability to tumor tissues via enhanced permeation and retention effect.Citation71 More importantly, EVs could be genetically engineered as targeted DDSs, offering a versatile platform for delivering drugs to specific targets with significantly promoted improved therapeutic effects. For example, Alvarez-Erviti et al. developed a DDS to deliver siRNA to the central nervous system using modified EVs from self-derived dendritic cells (DCs).Citation68 The DCs were isolated from mice and transfected with a plasmid encoding an exosomal membrane protein lysosome-associated membrane glycoprotein 2b (Lamp2b), genetically fused to a rabies viral glycoprotein (RVG), a peptide that binds to the acetylcholine receptor. The DC-derived EVs loaded with GAPDH siRNA exhibited specific brain-targeting gene knockdown, demonstrating the potential of EVs to act as targeted DDSs. As expected, both proteins and genes could be effectively delivered by EVs, which serve as cell-derived liposome-like nanoplatforms for the treatment of diseases such as cancer.Citation42-46 Interestingly, Bolukbasi et al. demonstrated that a zipcode-like 25-nt sequence promoted package miRNAs into EVs, demonstrating great potential for high-yielding EVs loaded with various RNAs.Citation77 Moreover, Gujrati et al. employed bacterial outer membrane vesicles (OMVs) to deliver siRNA for anticancer treatments. These results revealed that bacteria are a potential producer of biological nanovesicles for drug delivery.Citation78
In addition to delivering biomolecule-based drugs, EVs have also been exploited to deliver chemotherapeutic agents, with the goal of increasing their efficacy and reducing side effects. For example, EVs encapsulated with doxorubicin and curcumin both effectively inhibit the progression and deterioration of colon and mammary cancer.Citation79,80 These research results indicate that EVs have the capability to availably deliver chemotherapeutics drugs to suppress malignant tumors. Tian et al. engineered immature mouse DCs (imDCs) to express Lamp2b-iRGD peptide and used exosomes derived from these cells to deliver the chemotherapeutic drug doxorubicin to αv integrin-positive breast cancer cells in nude mice after i.v. injection.Citation79 These authors found that therapeutic exosomes caused less cardiac damage and more effectively inhibited tumor growth.Moreover, excellent delivery effects of therapeutic chemotherapeutic drugs in EVs have been validated with a variety of tumor models, including hepatocarcinomaCitation81, prostate cancerCitation82, lymphocytic leukemiaCitation83 and pancreatic tumors.Citation84 summarizes some recent studies using EVs as therapeutic delivery tools for cancer treatment.
Table 1. Application of EVs as therapeutic delivery tools for cancer treatments.
Extracellular vesicles for other diseases
Recent studies have suggested that EVs are related to cardiovascular diseases and that EV levels of blood circulates may be associated with the onset and progression of disease severity.Citation88-91 Nevertheless, EVs also exhibit a cardioprotective effect.Citation92 Chen et al. demonstrated protective benefits on the myocardium from serious ischemia-reperfusion injury by treating mice with EVs originating from cardiac progenitor cells.Citation93 Generally, sonic hedgehog (SHH) signaling is critical for neovascularization and angiogenesis, and SHH may be a therapeutic target in the vascular repair process. Fleury et al. demonstrated that SHH enriched EVs originating from T-lymphocytes and that SSH signaling could correct Ang II-induced hypertension and endothelial dysfunction in mouse models.Citation94,96
Cerebral inflammation is the defense reaction of an organism to brain injury. Inflammation is a pathological process of damage and resistance to damage. Macrophages play a significant role in the inflammatory response, producing a variety of cytokines and inflammatory mediators that are the internal mechanism of the inflammatory response and its development. A reasonable strategy to relief the disease is inhibiting the inflammatory factors and decreasing the number of macrophages, which would accordingly inhibit the inflammatory response. Nevertheless, the utilization of traditional medical remedies is restricted by the BBB. Zhuang et al. demonstrated that intranasal administration of curcumin-containing EVs efficaciously delivered curcumin to the brain. These results revealed that curcumin-containing EVs apparently suppressed brain diseases such as experimental autoimmune encephalitis (EAE), LPS-induced brain inflammation and brain tumors, providing a noninvasive treatment option for inflammatory brain disease.Citation97 In another study, Kalani et al. showed that curcumin-primed EVs possess the ability to alleviate endothelial dysfunction in a hyperhomocysteinemia mouse model.Citation98 Similarly, Sun et al. studied EVs as DDSs and found that EVs ensured the stability and concentration of curcumin during delivery, which was relevant to better bioavailability of drugs. The authors administered curcumin-loaded EVs to a mouse model of LPS-induced septic shock, and the results revealed that EVs significantly suppressed inflammation.Citation99 Recently, anti-inflammatory EVs derived from gene-modified DCs have attracted the attention of many researchers. Kim et al. expressed the IL-10 gene in DCs. These authors injected purified EVs secreted from the DCs into mouse models of collagen-induced arthritis. They detected inhibited inflammation progression. It has also been noted that EVs delivered either locally or systemically efficaciously downregulate the immunological reaction via the MHC II-dependent pathway. 100
Extracellular vesicle-based vaccines are potential candidates for infectious disease vaccines. Toxoplasmosis, caused by toxoplasma gondii, is widespread in humans and warm-blooded animals. Recently, Aline et al. demonstrated that EVs originating from DCs loaded with toxoplasma gondii antigens induced a potent protective immune reaction that inhibited toxoplasma gondii infection.Citation101 Severe acute respiratory syndrome (SARS), also called atypical pneumonia, is characterized by progressive respiratory failure and death in approximately 10% of cases. 102 In 2007, Kuate et al. showed that EVs originating from HEK293T expressed SARS proteins and induced high levels of SARS-specific neutralizing antibodies titers in mice.Citation103 Moreover, after vaccinated patients with EV vaccines and adenoviral vector boosters, the serum-neutralizing antibody titer were even higher than that of recovering SARS patients.
Altogether, EVs are promising candidate drug carriers for the delivery of therapeutics (). Specifically, EVs can load with different drugs and maintain their nature biological properties during the encapsulation process for personalized medicine. The researchers mentioned above highlight the wider potential of EVs, beyond biomolecule-based drugs delivery, for the transfer of various other chemotherapeutic cargoes. In addition, EVs are superior biocompatibility with the lowest cytotoxicity, and more compatible with the host immune system than other nano-carriers.
Clinical trials: Progress and challenges
Various EVs have been investigated as therapeutic agents in clinical trials based on their superior performances in preclinical studies ().Citation104,105 Dendritic cell-derived EVs as anti-tumor therapies have been widely investigated in preclinical and clinical trials, and two trials entered phase I clinical trails. One trial focused on melanoma and the other trial focused on non-small cell lung cancer.Citation106,107 In addition, a phase II clinical trial was performed to treat non-small-cell lung carcinoma patients.Citation108 Feasibility and safety were demonstrated and the activation of natural killer (NK) cells was still detectable in later therapy. Following the same method, DC-derived EVs might be effective for treating other diseases such as angiocardiopathy, inflammation, nervous system diseases and infectious diseases. In a phase I clinical trial, EVs from ascites fluid were used for immunotherapy against colorectal cancer.Citation109 Preclinical EV-based antitumor studies have indicated that the therapeutic strategies are promising.
Table 2. Therapeutic applications of EVs in clinical trials.
Outer membrane vesicles have also been studied widely as anti-bacterial vaccines due to they are secreted naturally by bacteria.Citation110,111 Outer membrane vesicle-based vaccines significantly suppressed bacterial infection in a phase I trial.Citation110,112 A phase II trial revealed that OMV combined with a recombinant vaccine displayed a better immunogenicity than recombinant vaccine alone.Citation112 Another phase I trial demonstrated that OMV vaccines from a FetA modified strain conferred protective effect against N. meningitides.Citation113 The phase I clinical trial results showed an excellent immune response and mild side effects induced by OMV-based vaccines. These findings indicate that further exploration is essential before using OMVs as vaccines or DDSs.
Plant-based exosomes (plexosomes) used for DDSs are characterized by more advantages than conventional DDSs due to their being minimally toxic and low in immunogenicity. They are additionally unlimited in source. In two phase I clinical trials, plexosomes were loaded with curcumin for treating mucositis and colon cancers, respectively.Citation114,115 Results showed the possibility to avoided contact between chemoradiation and oral mucosa in the treatment of head and neck cancer. Inspired by the fact that mesenchymal stem cells (MSCs) possess an immunological suppression ability against specific immune and nonspecific immune responses, a phase I clinical trial has been conducted to explore the inhibitive effect of MSC-based EVs for graft-versus-host disease (GVHD).Citation116
However, it should be noted that many issues still need to be addressed to bridge laboratory EVs experimentation with practical clinical settings. For instance, it is very important to select a suitable producer cell type. Besides, the consistency of the quality and quantity of EV production upon scale-up is required for developing an EV-based product, some mammalian primary cells have been widely investigated but are not suitable for large-scale production due to their low EV yield.Citation117 Therefore, it is urgent to find other alternative EV sources. Isolation techniques are key issues that need to be improved, along with the lack of reliable isolation methods, which hinder the translation of EVs into clinical applications.Citation118 At the present, ultracentrifugation is the most commonly used method to isolate EVs, but it is difficult to avoid undesirable co-isolation of contaminants.Citation119 Not only the scheme but the technology requires a breakthrough in non-destructive, contamination-free isolation methods that are characterized by a short processing time. In addition, the optimization of storage conditions of EVs is also of great importance. Such work involves the selection of isotonic buffers, storage temperatures and container materials. However, there are no standard EV storage conditions thus far. The methods for characterization of EVs have much room for improvement, and conventional methods—including flow cytometry, fluorescence microscopy, nanoparticle tracking analysis and transmission electron microscopy—have many limitations.Citation120 Techniques need to be developed to define the degree of heterogeneity of scaled-up EV preparations and the acceptable limits on this variability that does not compromise the safety, efficacy and stability of the product. Deeper fundamental research about the biological and pharmacological functions of EVs is also essential. Dose, immunization route, immune response, cytotoxicity and tumorigenic effects all require intensive study for therapeutic applications. Another major problem for EV translational applications is the lack of an existing legislation for regulating EV-based therapies; it necessary that both adequate infrastructure and quality management systems be developed. Before using EVs in clinics, it is necessary to standardize their isolation, storage and characterization and establish criteria for a quality-control system.
One possible solution is EV-mimetic nanovesicles. Lunavat et al. have generated EV-mimetic vesicles via serial extrusions of cells through filters, and this production method resulted in a 100-fold increase in the yield of naturally produced extracellular vesicles.Citation121 In addition, EV-mimetic nanovesicles loaded with siRNA could effectively inhibit the expression of targeted genes. A similar method to generate EV-mimetic nanovesicles was applied by Jang et al.Citation80 Drug-loaded EV-mimetic nanovesicles were produced by serial extrusion in the presence of doxorubicin. The results revealed that nanovesicles were effectively accumulated within tumor-inhibiting cancer cell growth after intravenous injection in mice, and no side effects were observed. These studies demonstrated that EV-mimetic nanovesicles have a capacity for RNA and chemotherapeutics delivery. Viral antigen-loaded EV-mimetic nanovesicles (i.e., virus-mimetic vesicles), have been recently developed in a bioinspired manner for vaccines by maintaining the natural conformation of epitopes.Citation122 Viral antigen-loaded EV-mimetic nanovesicles can resemble natural viruses in morphology and immunogenicity, and may result in a high level of antibody titers in response to the corresponding antigen.Citation122-124 Such EV-mimetic nanovesicles have significant potential to deliver therapeutics with specific ligands on the surface for targeted drug delivery and therapy.
Conclusions and future perspectives
Medical experts are constantly seeking to develop novel DDSs and improve the targeting and bioavailability of drugs. These professionals are also concerned with reducing drug toxicity and improving therapeutic efficacy. Advantages such as a strong packing capacity, a long half-life time, minimal undesirable immunogenicity and limited side effect are requirements for a perfect drug-delivery platform. In the past decade, thanks to persistent endeavors by researchers, EVs have been exploited as ideal drug-delivery platforms and have been widely used in preclinical studies and clinical trials to efficiently transport biological substances and chemotherapeutics to desired targets. The outstanding advantages of EVs in drug transmission as a DDS lie primarily in their biological origin, which is associated with better biocompatibility with organism tissue. In addition, many studies have shown that EVs as drug carriers possess excellent performance at improving drug stability, prolonging blood circulation time, reducing toxicity to healthy tissues and increasing tumor targeting and tumor inhibition. Utilizing autologous EVs for personalized nanomedicines is a promising therapy.
Although EVs play an important role and have exhibited promise in preclinical studies, there are several issues that need to be considered. These challenges include: the detailed mechanisms of the formation and release of drug remain unarticulated, distinct criteria for classification and nomenclature have not been established, and suitable and standardized isolation, separation, refinement and preservation methods are still urgently needed. In addition, regulative biodistribution to accumulate EVs at desired sites still necessitates further research. Of course, challenges also include production scale-up and characterization of the purified EV product, and the toxicological and ADME profile of the EV product also needs to be defined. Furthermore, EVs from aberrant cells or pathogens may carry tumorigenic and pathogenic potential, this concern may be laid to rest by selection of an appropriate benign cell type as source of EVs. In summary, EVs-based DDSs open up new avenues for the treatment of various diseases. However, it is imperative to fully understand the basic principles involved in the EVs. The future of EV-based DDSs mainly depends on cooperation among biologists, physicists, nanomaterial scientists and clinical specialists. With continuous efforts by multidisciplinary strategies, the use of such nanoplatforms will shed new light on the delivery of therapeutics to cancers and various other diseases.
Abbreviations
| ADME | = | absorption, distribution, metabolism, excretion |
| BBB | = | blood-brain barrier |
| DDSs | = | drug-delivery systems |
| EAE | = | experimental autoimmune encephalitis |
| ESCRT | = | endosomal sorting complex required for transport |
| EVs | = | extracellular vesicles |
| fHBP | = | factor H binding protein |
| GAPDH | = | glyceraldehyde-3-phosphate dehydrogenase |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| GVHD | = | graft-versus-host disease |
| IFN-γ | = | interferon gamma |
| ILVs | = | intraluminal vesicles |
| imDCs | = | immature mouse DCs |
| Lamp2b | = | lysosome-associated membrane glycoprotein 2b |
| MHC | = | major histocompatibility complex |
| MSCs | = | mesenchymal stem cells |
| MVBs | = | multivesicular bodies |
| MVs | = | microvesicles |
| NadA | = | neisserial adhesin A |
| NHBA | = | neisserial heparin binding antigen |
| NK | = | natural killer (NK) cells |
| OMVs | = | outer membrane vesicles |
| plexosomes | = | plant-based exosomes |
| RVG | = | rabies viral glycoprotein |
| SARS | = | severe acute respiratory syndrome |
| SHH | = | sonic hedgehog |
Disclosure of potential conflicts of interest
All authors declare that they have no conflict of interest in relation to this work.
Funding
This study was supported by the Major State Basic Research Development Program of China (973 Program) (Grant Nos. 2017YFA0205201, 2014CB744503, and 2013CB733802), the National Natural Science Foundation of China (NSFC) (Grant Nos. 81422023, 51273165, and U1505221), the Fundamental Research Funds for the Central Universities, China (Grant No. 20720160065 and 20720150141), the International Postdoctoral Exchange Fellowship Program, and the Program for New Century Excellent Talents in University (NCET-13–0502).
References
- Merisko-Liversidge EM, Liversidge GG. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol Pathol. 2008;36:43-8. doi:10.1177/0192623307310946. PMID:18337220
- Nazar MF, Khan AM, Shah SS. Microemulsion system with improved loading of piroxicam: A study of microstructure. AAPS Pharmscitech. 2009;10:1286-94. doi:10.1208/s12249-009-9328-9. PMID:19876741
- Wei T, Liu J, Ma H, Cheng Q, Huang Y, Zhao J, Huo S, Xue X, Liang Z, Liang XJ. Functionalized nanoscale micelles improve drug delivery for cancer therapy in vitro and in vivo. Nano Lett. 2013;13:2528-34. doi:10.1021/nl400586t. PMID:23634882
- Dang YJ, Zhu CY. Oral bioavailability of cantharidin-loaded solid lipid nanoparticles. Chin Med. 2013;8:1. doi:10.1186/1749-8546-8-1. PMID:23298453
- Kiziltepe T, Ashley JD, Stefanick JF, Qi YM, Alves NJ, Handlogten MW, Suckow MA, Navari RM, Bilgicer B. Rationally engineered nanoparticles target multiple myeloma cells, overcome cell-adhesion-mediated drug resistance, and show enhanced efficacy in vivo. Blood Cancer J. 2012;2:e64. doi:10.1038/bcj.2012.10. PMID:22829966
- Azzi J, Tang L, Moore R, Tong R, El HN, Akiyoshi T, Mfarrej B, Yang S, Jurewicz M, Ichimura T, et al. Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. FASEB J. 2010;24:3927-38. doi:10.1096/fj.10-154690. PMID:20547662
- Abd-Allah FI, Dawaba HM, Ahmed AM. Development of a microemulsion-based formulation to improve the availability of poorly water-soluble drug. Drug Discov Ther. 2010;4:257-66. PMID:22491208
- Jesson G, Brisander M, Andersson P, Demirbuker M, Derand H, Lennernas H, Malmsten M. Carbon dioxide-mediated generation of hybrid nanoparticles for improved bioavailability of protein kinase inhibitors. Pharm Res. 2014;31:694-705. doi:10.1007/s11095-013-1191-4. PMID:23990314
- Dwivedi P, Khatik R, Khandelwal K, Taneja I, Raju KS, Wahajuddin, Paliwal SK, Dwivedi AK, Mishra PR. Pharmacokinetics study of arteether loaded solid lipid nanoparticles: An improved oral bioavailability in rats. Int J Pharm. 2014;466:321-7. doi:10.1016/j.ijpharm.2014.03.036. PMID:24657144
- Singh G, Pai RS. Optimized PLGA nanoparticle platform for orally dosed trans-resveratrol with enhanced bioavailability potential. Expert Opin Drug Deliv. 2014;11:647-59. doi:10.1517/17425247.2014.890588. PMID:24661109
- Joshi G, Kumar A, Sawant K. Enhanced bioavailability and intestinal uptake of Gemcitabine HCl loaded PLGA nanoparticles after oral delivery. Eur J Pharm Sci. 2014;60:80-9. doi:10.1016/j.ejps.2014.04.014. PMID:24810394
- Chen JP, Yang PC, Ma YH, Tu SJ, Lu YJ. Targeted delivery of tissue plasminogen activator by binding to silica-coated magnetic nanoparticle. Int J Nanomedicine. 2012;7:5137-49. doi:10.2147/IJN.S36197. PMID:23055726
- Amaral AC, Bocca AL, Ribeiro AM, Nunes J, Peixoto DL, Simioni AR, Primo FL, Lacava ZG, Bentes R, Titze-De-Almeida R, et al. Amphotericin B in poly(lactic-co-glycolic acid) (PLGA) and dimercaptosuccinic acid (DMSA) nanoparticles against paracoccidioidomycosis. J Antimicrob Chemother. 2009;63:526-33. doi:10.1093/jac/dkn539. PMID:19151037
- Kiziltepe T, Ashley JD, Stefanick JF, Qi YM, Alves NJ, Handlogten MW, Suckow MA, Navari RM, Bilgicer B. Rationally engineered nanoparticles target multiple myeloma cells, overcome cell-adhesion-mediated drug resistance, and show enhanced efficacy in vivo. Blood Cancer J. 2012;2:e64. doi:10.1038/bcj.2012.10. PMID:22829966
- Casa DM, Carraro TC, de Camargo LE, Dalmolin LF, Khalil NM, Mainardes RM. Poly(L-lactide) nanoparticles reduce amphotericin B cytotoxicity and maintain its in vitro antifungal activity. J Nanosci Nanotechnol. 2015;15:848-54. doi:10.1166/jnn.2015.9177. PMID:26328449
- Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64:1020-37. doi:10.1016/S1734-1140(12)70901-5. PMID:23238461
- Zhang J, Liang YC, Lin X, Zhu X, Yan L, Li S, Yang X, Zhu G, Rogach AL, Yu PK, et al. Self-monitoring and self-delivery of photosensitizer-doped nanoparticles for highly effective combination cancer therapy in Vitro and in Vivo. ACS Nano. 2015;9:9741-56. doi:10.1021/acsnano.5b02513. PMID:26390118
- Zhang J, Li S, An FF, Liu J, Jin S, Zhang JC, Wang PC, Zhang X, Lee CS, Liang XJ. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale. 2015;7:13503-10. doi:10.1039/C5NR03259H. PMID:26199064
- Bale S, Khurana A, Reddy AS, Singh M, Godugu C. Overview on therapeutic applications of microparticulate drug delivery systems. Crit Rev Ther Drug Carrier Syst. 2016;33:309-61. doi:10.1615/CritRevTherDrugCarrierSyst.2016015798. PMID:27910739
- Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology. 2010;151:458-65. doi:10.1210/en.2009-1082. PMID:20016026
- Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:1-35. doi:10.1080/10590500802708267. PMID:19204862
- Dhawan A, Sharma V. Toxicity assessment of nanomaterials: Methods and challenges. Anal Bioanal Chem. 2010;398:589-605. doi:10.1007/s00216-010-3996-x. PMID:20652549
- Shukla S, Arora V, Jadaun A, Kumar J, Singh N, Jain VK. Magnetic removal of Entamoeba cysts from water using chitosan oligosaccharide-coated iron oxide nanoparticles. Int J Nanomedicine. 2015;10:4901-17. doi:10.2147/IJN.S77675. PMID:26261417
- Kumar V, Qin J, Jiang Y, Duncan RG, Brigham B, Fishman S, Nair JK, Akinc A, Barros SA, Kasperkovitz PV. Shielding of lipid nanoparticles for siRNA delivery: Impact on physicochemical properties, cytokine induction, and efficacy. Mol Ther Nucleic Acids. 2014;3:e210. doi:10.1038/mtna.2014.61. PMID:25405467
- Chan VS. Nanomedicine: An unresolved regulatory issue. Regul Toxicol Pharmacol. 2006;46:218-24. doi:10.1016/j.yrtph.2006.04.009. PMID:17081666
- El-Ansary A, Al-Daihan S. On the toxicity of therapeutically used nanoparticles: An overview. J Toxicol. 2009;2009:754810. doi:10.1155/2009/754810. PMID:20130771
- Mogharabi M, Abdollahi M, Faramarzi MA. Toxicity of nanomaterials; an undermined issue. DARU. 2014;22:59. doi:10.1186/s40199-014-0059-4. PMID:25123555
- Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1-11. doi:10.1007/s11060-013-1084-8. PMID:23456661
- Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836-48. doi:10.1016/j.ccell.2016.10.009. PMID:27960084
- Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: A novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525-41. PMID:22619510
- El AS, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-57. doi:10.1038/nrd3978. PMID:23584393
- Lee J, Lee H, Goh U, Kim J, Jeong M, Lee J, Park JH. Cellular engineering with membrane fusogenic liposomes to produce functionalized extracellular vesicles. ACS Appl Mater Interfaces. 2016;8:6790-5. doi:10.1021/acsami.6b01315. PMID:26954538
- Armstrong JP, Holme MN, Stevens MM. Re-Engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69-83. doi:10.1021/acsnano.6b07607. PMID:28068069
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581-93. doi:10.1038/nri2567. PMID:19498381
- Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: Introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi:10.3390/ijms17020170. PMID:26861301
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006; Chapter 3:3-22.
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-20. PMID:3597417
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244-7. doi:10.1126/science.1153124. PMID:18309083
- Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841:108-20. doi:10.1016/j.bbalip.2013.10.004. PMID:24140720
- Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012;1:24009883. doi:10.3402/jev.v1i0.18374
- van Doormaal FF, Kleinjan A, Di Nisio M, Buller HR, Nieuwland R. Cell-derived microvesicles and cancer. Neth J Med. 2009;67:266-73.
- D'Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: Shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287-99. doi:10.1101/gad.192351.112. PMID:22713869
- Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98-110. PMID:21969178
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009;19:43-51. doi:10.1016/j.tcb.2008.11.003. PMID:19144520
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309-18. doi:10.4049/jimmunol.166.12.7309. PMID:11390481
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-89. doi:10.1146/annurev-cellbio-101512-122326. PMID:25288114
- Wang GJ, Liu Y, Qin A, Shah SV, Deng ZB, Xiang X, Cheng Z, Liu C, Wang J, Zhang L, et al. Thymus exosomes-like particles induce regulatory T cells. J Immunol. 2008;181:5242-8. doi:10.4049/jimmunol.181.8.5242. PMID:18832678
- Fan GC. Hypoxic exosomes promote angiogenesis. Blood. 2014;124:3669-70. doi:10.1182/blood-2014-10-607846. PMID:25498451
- Baragano RA, Suarez-Alvarez B, Lopez-Larrea C. Secretory pathways generating immunosuppressive NKG2D ligands: New targets for therapeutic intervention. OncoImmunology. 2014;3:e28497. doi:10.4161/onci.28497. PMID:25050215
- Baixauli F, Lopez-Otin C, Mittelbrunn M. Exosomes and autophagy: Coordinated mechanisms for the maintenance of cellular fitness. Front Immunol. 2014;5:403. doi:10.3389/fimmu.2014.00403. PMID:25191326
- Corrado C, Raimondo S, Saieva L, Flugy AM, De Leo G, Alessandro R. Exosome-mediated crosstalk between chronic myelogenous leukemia cells and human bone marrow stromal cells triggers an interleukin 8-dependent survival of leukemia cells. Cancer Lett. 2014;348:71-6. doi:10.1016/j.canlet.2014.03.009. PMID:24657661
- Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta. 2015;1852:1-11. doi:10.1016/j.bbadis.2014.10.008. PMID:25463630
- Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707-21. doi:10.1016/j.ccell.2014.09.005. PMID:25446899
- Wang J, Hendrix A, Hernot S, Lemaire M, De Bruyne E, Van Valckenborgh E, Lahoutte T, De Wever O, Vanderkerken K, Menu E. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood. 2014;124:555-66. doi:10.1182/blood-2014-03-562439. PMID:24928860
- Jovic M, Sharma M, Rahajeng J, Caplan S. The early endosome: A busy sorting station for proteins at the crossroads. Histol Histopathol. 2010;25:99-112. PMID:19924646
- Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi:10.3389/fimmu.2014.00442. PMID:25278937
- Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: Regulation of exosome loading. Semin Cancer Biol. 2014;28:3-13. doi:10.1016/j.semcancer.2014.04.009. PMID:24769058
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553-65. doi:10.1242/jcs.128868. PMID:24105262
- Stuffers S, Sem WC, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925-37. doi:10.1111/j.1600-0854.2009.00920.x. PMID:19490536
- Sadallah S, Eken C, Schifferli JA. Ectosomes as modulators of inflammation and immunity. Clin Exp Immunol. 2011;163:26-32. doi:10.1111/j.1365-2249.2010.04271.x. PMID:21039423
- Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396-405. doi:10.1016/j.jconrel.2015.07.030. PMID:26241750
- van der Meel R, Fens MH, Vader P, van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. J Control Release. 2014;195:72-85. doi:10.1016/j.jconrel.2014.07.049. PMID:25094032
- Lee Y, Thompson DH. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1450. doi:10.1002/wnan.1450. PMID:28198148.
- Chang HI, Yeh MK. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012;7:49-60. PMID:22275822
- van Dommelen SM, Vader P, Lakhal S, Kooijmans SA, van Solinge WW, Wood MJ, Schiffelers RM. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J Control Release. 2012;161:635-44. doi:10.1016/j.jconrel.2011.11.021. PMID:22138068
- El AS, Lakhal S, Mager I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391-7. doi:10.1016/j.addr.2012.08.008. PMID:22921840
- Kosaka N, Takeshita F, Yoshioka Y, Hagiwara K, Katsuda T, Ono M, Ochiya T. Exosomal tumor-suppressive microRNAs as novel cancer therapy: “exocure” is another choice for cancer treatment. Adv Drug Deliv Rev. 2013;65:376-82. doi:10.1016/j.addr.2012.07.011. PMID:22841506
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341-5. doi:10.1038/nbt.1807. PMID:21423189
- Jiang XC, Gao JQ. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521:167-75. doi:10.1016/j.ijpharm.2017.02.038. PMID:28216464
- Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63-78. doi:10.1016/j.pharmthera.2017.02.020. PMID:28202367
- Maeda H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J Control Release. 2012;164:138-44. doi:10.1016/j.jconrel.2012.04.038. PMID:22595146
- Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Strobel T, Breakefield XO, Saydam O. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther. 2013;21:101-8. doi:10.1038/mt.2012.161. PMID:22910294
- Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185-91. doi:10.1038/mt.2012.180
- Zhang Y, Li L, Yu J, Zhu D, Zhang Y, Li X, Gu H, Zhang CY, Zen K. Microvesicle-mediated delivery of transforming growth factor beta1 siRNA for the suppression of tumor growth in mice. Biomaterials. 2014;35:4390-400. doi:10.1016/j.biomaterials.2014.02.003. PMID:24565517
- Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F, Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201-4. doi:10.1016/j.canlet.2013.02.019. PMID:23419525
- Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. doi:10.1038/mtna.2013.60. PMID:24084846
- Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Strobel T, Erkan EP, Fan JB, Breakefield XO, Saydam O. miR-1289 and “Zipcode”-like sequence enrich mRNAs in microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. doi:10.1038/mtna.2011.2. PMID:23344721
- Gujrati V, Kim S, Kim SH, Min JJ, Choy HE, Kim SC, Jon S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 2014;8:1525-37. doi:10.1021/nn405724x. PMID:24410085
- Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383-90. doi:10.1016/j.biomaterials.2013.11.083. PMID:24345736
- Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lotvall J, Kim YK, Gho YS. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698-710. doi:10.1021/nn402232g. PMID:24004438
- Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen GX, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. doi:10.1038/ncomms2282. PMID:23250412
- Saari H, Lazaro-Ibanez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727-37. doi:10.1016/j.jconrel.2015.09.031. PMID:26390807
- Guo F, Chang CK, Fan HH, Nie XX, Ren YN, Liu YY, Zhao LH. Anti-tumour effects of exosomes in combination with cyclophosphamide and polyinosinic-polycytidylic acid. J Int Med Res. 2008;36:1342-53. doi:10.1177/147323000803600623. PMID:19094445
- Pascucci L, Cocce V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Vigano L, Locatelli A, Sisto F, Doglia SM, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262-70. doi:10.1016/j.jconrel.2014.07.042. PMID:25084218
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297-303. doi:10.1038/85438. PMID:11231627
- Shimoda A, Ueda K, Nishiumi S, Murata-Kamiya N, Mukai SA, Sawada S, Azuma T, Hatakeyama M, Akiyoshi K. Exosomes as nanocarriers for systemic delivery of the Helicobacter pylori virulence factor CagA. Sci Rep. 2016;6:18346. doi:10.1038/srep18346. PMID:26739388
- Aspe JR, Diaz OC, Jutzy JM, Deshields S, Whang S, Wall NR. Enhancement of Gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the Survivin-T34A mutant. J Extracell Vesicles. 2014;3:24624263. doi:10.3402/jev.v3.23244
- Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356-64. doi:10.1038/nrrheum.2014.19. PMID:24535546
- Candelario KM, Steindler DA. The role of extracellular vesicles in the progression of neurodegenerative disease and cancer. Trends Mol Med. 2014;20:368-74. doi:10.1016/j.molmed.2014.04.003. PMID:24835084
- Gaceb A, Martinez MC, Andriantsitohaina R. Extracellular vesicles: New players in cardiovascular diseases. Int J Biochem Cell Biol. 2014;50:24-8. doi:10.1016/j.biocel.2014.01.018. PMID:24509128
- Fleury A, Martinez MC, Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol. 2014;5:370. doi:10.3389/fimmu.2014.00370. PMID:25136343
- Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259-72. doi:10.1038/nrcardio.2017.7. PMID:28150804
- Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431:566-71. doi:10.1016/j.bbrc.2013.01.015. PMID:23318173
- Benameur T, Soleti R, Porro C, Andriantsitohaina R, Martinez MC. Microparticles carrying Sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS One. 2010;5:e12688. doi:10.1371/journal.pone.0012688. PMID:20856928
- Soleti R, Benameur T, Porro C, Panaro MA, Andriantsitohaina R, Martinez MC. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis. 2009;30:580-8. doi:10.1093/carcin/bgp030. PMID:19168578
- Agouni A, Mostefai HA, Porro C, Carusio N, Favre J, Richard V, Henrion D, Martinez MC, Andriantsitohaina R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007;21:2735-41. doi:10.1096/fj.07-8079com. PMID:17428963
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769-79. doi:10.1038/mt.2011.164. PMID:21915101
- Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci. 2014;107:1-7. doi:10.1016/j.lfs.2014.04.018. PMID:24780320
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606-14. doi:10.1038/mt.2010.105. PMID:20571541
- Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174:6440-8. doi:10.4049/jimmunol.174.10.6440. PMID:15879146
- Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun. 2004;72:4127-37. doi:10.1128/IAI.72.7.4127-4137.2004. PMID:15213158
- Hoheisel G, Luk WK, Winkler J, Gillissen A, Wirtz H, Liebert UG, Hui DS. [Severe acute respiratory syndrome (SARS)]. Med Klin (Munich). 2006;101:957-63. doi:10.1007/s00063-006-1127-4. PMID:17171319
- Kuate S, Cinatl J, Doerr HW, Uberla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362:26-37. doi:10.1016/j.virol.2006.12.011. PMID:17258782
- Tominaga N, Yoshioka Y, Ochiya T. A novel platform for cancer therapy using extracellular vesicles. Adv Drug Deliv Rev. 2015;95:50-5. doi:10.1016/j.addr.2015.10.002. PMID:26482189
- Fais S, O'Driscoll L, Borras FE, Buzas E, Camussi G, Cappello F, Carvalho J, Cordeiro DSA, Del PH, El AS, et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano. 2016;10:3886-99. doi:10.1021/acsnano.5b08015. PMID:26978483
- Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. doi:10.1186/1479-5876-3-10. PMID:15740633
- Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi:10.1186/1479-5876-3-9. PMID:15723705
- Viaud S, Ploix S, Lapierre V, Thery C, Commere PH, Tramalloni D, Gorrichon K, Virault-Rocroy P, Tursz T, Lantz O, et al. Updated technology to produce highly immunogenic dendritic cell-derived exosomes of clinical grade: a critical role of interferon-gamma. J Immunother. 2011;34:65-75. doi:10.1097/CJI.0b013e3181fe535b. PMID:21150714
- Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782-90. doi:10.1038/mt.2008.1. PMID:18362931
- Sandbu S, Feiring B, Oster P, Helland OS, Bakke HS, Naess LM, Aase A, Aaberge IS, Kristoffersen AC, Rydland KM, et al. Immunogenicity and safety of a combination of two serogroup B meningococcal outer membrane vesicle vaccines. Clin Vaccine Immunol. 2007;14:1062-9. doi:10.1128/CVI.00094-07. PMID:17634513
- van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015;10:1689-706. doi:10.1002/biot.201400395. PMID:26912077
- Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant Meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010;51:1127-37. doi:10.1086/656741. PMID:20954968
- Marsay L, Dold C, Green CA, Rollier CS, Norheim G, Sadarangani M, Shanyinde M, Brehony C, Thompson AJ, Sanders H, et al. A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: A phase I clinical trial. J Infect. 2015;71:326-37. doi:10.1016/j.jinf.2015.05.006. PMID:25982025
- James Graham Brown Cancer Center. Study investigating the ability of plant exosomes to deliver curcumin to normal and colon cancer tissue. 2011 Feb 3 [accessed 2017 Feb 6]. https://www.clinicaltrials.gov/ct2/show/study/NCT01294072
- James Graham Brown Cancer Center. Edible plant exosome ability to prevent oral mucositis associated with chemoradiation treatment of head and neck cancer. 2012 Aug 6 [accessed 2017 Feb 6[. https://clinicaltrials.gov/show/NCT01668849
- Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970-3. PMID:24445866
- Leblanc P, Arellano-Anaya ZE, Bernard E, Gallay L, Provansal M, Lehmann S, Schaeffer L, Raposo G, Vilette D. Isolation of exosomes and microvesicles from cell culture systems to study prion transmission. Methods Mol Biol. 2017;1545:153-76. doi:10.1007/978-1-4939-6728-5_11. PMID:27943213
- Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the international society for extracellular vesicles. J Extracell Vesicles. 2014;3:26913. doi:10.3402/jev.v3.26913. PMID:25536934
- Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, Le Pecq JB. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211-26. doi:10.1016/S0022-1759(02)00330-7. PMID:12379326
- Lozano-Ramos I, Bancu I, Oliveira-Tercero A, Armengol MP, Menezes-Neto A, Del PH, Lauzurica-Valdemoros R, Borras FE. Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J Extracell Vesicles. 2015;4:27369. doi:10.3402/jev.v4.27369. PMID:26025625
- Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lasser C, Nilsson JA, Gho YS, Lotvall J. RNAi delivery by exosome-mimetic nanovesicles – Implications for targeting c-Myc in cancer. Biomaterials. 2016;102:231-8. doi:10.1016/j.biomaterials.2016.06.024. PMID:27344366
- Zhang P, Chen Y, Zeng Y, Shen C, Li R, Guo Z, Li S, Zheng Q, Chu C, Wang Z, et al. Virus-mimetic nanovesicles as a versatile antigen-delivery system. Proc Natl Acad Sci U S A. 2015;112:E6129-38. doi:10.1073/pnas.1505799112. PMID:26504197
- Mi P, Zhang P, Liu G. Bio-inspired virus-like nanovesicle for effective vaccination. Hum Vaccin Immunother. 2016;12:2090-1. doi:10.1080/21645515.2016.1157244. PMID:27141919
- Zhang P, Liu G, Chen X. Nanobiotechnology: Cell membrane-based delivery systems. Nano Today. 2017;13:7-9. doi:10.1016/j.nantod.2016.10.008. PMID:28435439
- Thomas Jefferson University Hospita. Pilot immunotherapy trial for recurrent malignant gliomas. 2012 Feb 14 ]accessed 2017 Feb 6]. https://www.clinicaltrials.gov/ct2/show/study/NCT01294072
- Sahel Teaching Hospital. Effect of microvesicles and exosomes therapy on β-cell mass in type I diabetes mellitus (T1DM). 2014 May 12 [accessed 2017 Feb 6]. https://clinicaltrials.gov/show/NCT02138331