ABSTRACT
More than 25% of the global population has IgE mediated allergic diseases. Allergen immunotherapy (AIT) is the only available form of treatment that alters the underlying mechanism of IgE-mediated allergic diseases. AIT is aimed at desensitizing allergic individuals by repeatedly administering disease-causing allergens over a long period of time. Despite its proven efficacy in numerous clinical trials, the effectiveness of AIT still suffers some drawbacks due to the quality of allergens used and in particular the unavailability of efficient allergen delivery systems. Several studies have demonstrated that bacterial ghosts (BG) systems can be used to display and deliver antigens to their targets for the management of diseases. However, there is no report documenting the use of BG systems for immunotherapy of IgE-mediated diseases so far. Thus, in this review, we intend to discuss the potentialities of BG systems for displaying and delivering allergens for future management of IgE-mediated diseases.
Introduction
More than 25% of the world population has IgE-mediated allergy, Citation1 , Citation2 a hypersensitivity disease characterized by production of IgE antibodies against innocuous allergens such as pollen, dander, insect venom, house dust mites etc. In atopic individuals, these allergen specific-IgEs upon production, bind onto the surfaces of mast, eosinophil and basophil cells via FcϵR I. Citation4 Upon second exposure, these same allergens cause cross-linking of adjacent FcϵR I bound IgEs, resulting in the production pro-inflammatory mediators such as histamines, leukotrines, proteases, cytokines as well as activation and recruitment of cells to the mucosa. Citation5 Allergic individuals demonstrate Th2-like immune responses characterized by elevated levels of IL-4, IL-5, IL-13, and an elevated level of IgE antibodies against innocuous allergens. Citation3 Resultant clinical symptoms include urticaria, conjunctivitis, dermatitis, allergic rhinitis and sometimes more severe conditions like asthma and anaphylactic shock. Citation3 On the other hand, non-allergic individuals exhibit a Th1-like immune response in which they produce higher levels of IFN-γ, TNF-α, regulatory IL-10 and specific IgGs to innocuous allergens. Citation3 , Citation4
Allergen immunotherapy (AIT) has been demonstrated in numerous clinical trials to be the only form of treatment that addresses the underlying pathology of IgE-mediated diseases. Citation6–Citation9 It is based on inducing a state of allergen specific unresponsiveness by the administration of an increased dose of allergens (usually an allergen extract) that an individual is sensitive to over a long period of time. Citation10 , Citation11 Although the underlying mechanism of AIT is still unclear, studies investigating patients who received successful AIT indicated a modification of the immune response to allergen exposure at T lymphocytes level. Hence successful AIT is generally associated with a shift in the balance of Th2 (IL-4, IL-5 and IL-13) in favor of a Th1-like cytokine profile and/or an induction of regulatory Treg cells (IL-10). Citation7 , Citation12
Allergen extracts for AIT are prepared from natural sources (pollen, animal dander, house dust mites etc.), hence the quality of these allergens is always compromised as a result of batch-to-batch inconsistencies. Citation13 Natural allergens often show variation in their contents. Citation14 In some cases they lack important allergens, Citation15 often get contaminated with non-target but allergenic substances making patients to become sensitized to allergens which they were not sensitive to before. Citation16
With the advancement of genetic engineering technologies, recombinant allergens and their hypoallergenic variants of high purity, known immunologic and molecular characteristics can now be produced under controlled expression procedures. Citation17 Some of these recombinant allergens or their variants have undergone various stages of clinical trials with some exhibiting promising results. Citation1 , Citation18 , Citation19 However recombinant allergens and other hypoallergenic peptides often lack effective delivery systems to get them to their target tissues and cells thus limiting their applicability in AIT. Citation20 In this review, we discuss strategies of improving AIT by manipulating bacterial ghost (BGs) system to display and deliver allergens of interest.
Bacterial ghost systems
Bacterial ghosts (BGs) are empty bacterial cell envelopes (0.5–3 μm) derived from gram-negative bacteria. They are devoid of nucleic acids, ribosomes or other intracellular constituents but they still possess intact bacterial surface structures and proteins. Citation21 Thus, depending on the delivery mode, when inside the body they are able to induce a strong humoral and cell-mediated immune response. Citation22
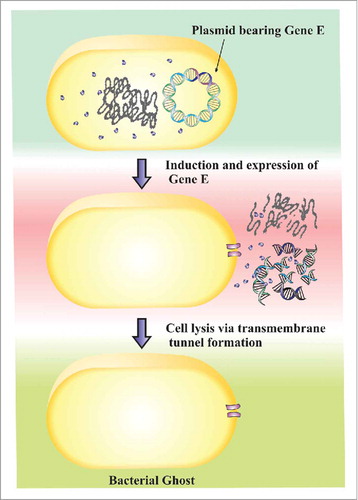
The production of BGs was reviewed in detail by Langemann et al. Citation23 However, in summary, they are produced from gram-negative bacteria by the controlled expression of a cloned lysis gene E, a 91 amino-acid polypeptide from bacteriophage ϕX174. Expression of gene E causes bacterial lysis via the formation of transmembrane tunnels (40–200 nm diameter), which facilitate leakage of bacterial cytoplasmic contents by an osmotic pressure difference between the bacterial cell interior and the outside environment resulting in an empty bacterial ‘shell’. Citation24 This process is so rapid and efficient that BG formation takes only a few milliseconds Citation25 resulting in 96% of bacteria carrying plasmids bearing lysis genes being converted to BGs 10 minutes after lysis induction. Citation44 However, unlike other lytic pathways, protein E does not cause much physical or chemical damage to bacterial surface structures. Rather the channel formation is restricted to only a small part of the total cell surface. This results in empty bacterial shells otherwise lacking cellular components (). Gene E-mediated lysis in principle works in many if not all gram-negative bacterial species as long as the E lysis cassette is successfully introduced into the recipient gram-negative bacterium by an appropriate vector and that there be available a tight repression and induction system for gene E. Production of BGs has been successfully performed in a broad spectrum of bacteria including, Salmonella enteritidis, Klebsiella pneumonia, Vibrio cholera, Salmonella typhimurium, Helicobacter pylori, Pseudomonas aeruginosa, Haemophilus influenza and Escherichia coli K12 strains. Citation55 Cryo-electron tomography of membrane protein complexes has shown that these ‘ghosts’ still retain their antigenic determinants and other surface molecules just like their living counterparts. Citation22 , Citation26 Thus, save for the formation of trans-membrane channels, the morphology of resultant BGs including all cell surface structures remain unchanged as a result of the lysis events. Unlike convectional vaccines that need refrigeration, once produced and purified, BGs can be stored for several years at ambient room temperature as lyophilized products without loosing their potency. Citation23
Benefits of using BGs in allergen immunotherapy
Since they share functional epitopes on their surfaces with their living counterparts, BGs can be used as excellent delivery systems of allergens. Citation27 As previously demonstrated, heterologous proteins can be carried onto the BGs’ outer membranes (OM), the periplasmic space (PPS), inner-membrane (IM) or in the hollow lumen. Citation28 Their ability to bear foreign peptides either on their surfaces or in the interior not only render them as targets for major histocompatibility complex class II antigen processing and presentation pathways by APCs, Citation29 but also enables them to be combined with other display platforms such as the autotransporter systems. Citation30 BGs’ intact pathogen associated molecular patterns (PAMPs) provide them with the original targeting functions from the host organism's pattern recognition receptors (PRRs) as well as enhancing the activation of macrophages and DCs. Citation31 Particulate substances are readily taken up by APCs since allergen uptake is dependent on several properties among them receptor interactions, surface charge, size, shape, hydrophobicity and hydrophilicity, Citation32 , Citation58 Hence due to their inherent particulate nature, BGs tend to interact better with APCs compared with soluble allergens.
Displaying allergens on bacterial ghosts membrane surfaces
Allergen surface display entails the presentation of allergens or their hypoallergenic variants on the surfaces of BG shells. The first report documenting bacterial display of foreign peptides was published in 1986 when a 15 amino acid gene fragment was displayed in an accessible form onto an Escherichia coli (E. coli) surface. Citation33 , Citation34 Following this, short gene fragments were linked onto the genes of gram-negative bacteria outer membrane β–barrel proteins, maltoporin LamB, outer membrane protein A (OmpA) and phosphate-inducible porin (PhoE). The gene fusion products would be accessible on the outer surfaces of bacteria. This break through gave way to new insights into how bacterial cells and their components could be engineered and applied into the field of vaccinology and immunotherapeutics.
Some studies have used extracts from common bacterial species that affect the upper and lower airway tract infections for AIT. Citation35 Co-administration of allergens with killed bacteria yielded good protective results in miceCitation 36 and allergic dogs. Citation37 For example in one randomized double blind, placebo-controlled trial, aiming at evaluating the efficacy and antibody response changes to Dermatophagoides pteronyssinus (Der p) major allergens Der p 1 and Der p 2 in house dust mite allergic patients, it was found that immunotherapy using Der p allergens mixed with bacterial extracts was more effective at reducing allergy symptoms than Der p allergens administered alone. Citation38 Building up from this phenomenon, Bohle et al. demonstrated that a recombinant fusion protein rSbsC-Bet v 1, consisting of Bet v 1 allergen and a bacterial surface protein SbsC exhibited a reduced histamine releasing capacity and a cytokine profile skewed toward a Th1 response. Citation39 In addition, chemically linking Bet v allergens to bacterial proteins was also shown to display strong Th1-promoting capabilities from birch pollen-allergic individuals in vitro Citation40 , Citation41 proving that bacteria or their components could be incorporated in immunotherapy. Citation42
However, in most of these earlier studies, the allergens were produced as recombinant proteins intracellularly and recovery from whole cell extracts is a cumbersome multistep down streaming procedure. On the contrary, displaying of allergens on bacterial surfaces can be an easier and better strategy compatible with continuous culturing. Citation43 , Citation44 Using recombinant DNA technology, allergens can be incorporated onto the bacterial envelope complexes before lysis and made to become elements of the BGs with heterologous proteins’ biophysical and functional properties being retained. Citation45 Further, heterologous surface allergens that are exposed on the surface of bacteria elicit a superior immune response than those present intracellularly. Citation46 , Citation47
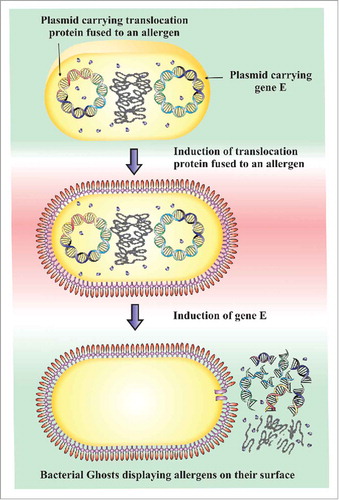
From the earlier studies, it sounds logical to speculate that BGs may also be able to display allergen molecules or their hypoallergenic variants on their surfaces for allergen immunotherapy. Hence, it is possible to design BGs displaying therapeutic allergens for AIT with improved efficacy and safety in a similar manner described by Hjelm et al. Citation48 Using this straightforward approach any allergen can theoretical be fused to proteins that have specific translocation and surface anchoring abilities like the autotransporter or ice nucleating systems. These systems can then be jointly expressed with lysis protein E in gram-negative bacteria such as E. coli. Thus, an allergen gene is fused to a specific translocation and surface anchoring protein gene (for example the hemoglobin protease in case of an autotransporter system) via the C-terminal in a plasmid downstream of an inducible promoter as shown in . The lysis protein E is placed into a different plasmid and the 2 plasmids are then co-transformed into a single E. coli host. The E. coli cells are then induced to express the allergen gene of interest first followed by their processing to BGs as described by Langemann et al. Citation23 Using this platform, foreign peptides/ proteins for AIT (e.g. recombinant whole allergens, hypoallergens and T-cell epitopes) can be inserted into the inner or outer membranes via specific N-, or C-, or N and C- terminal anchor sequences. This novel combination of translocation protein-mediated surface display of allergens and BG formation allows allergens to be expressed on cell surfaces in relatively high quantities to the magnitudes of up to 100 000 copies per cell, making these systems favorable candidates for allergen immunotherapy.
Figure 2. Schematic representation of BG-mediated surface display of allergens on Gram-negative cells. Cells are co-transformed with 2 plasmids, in which one harbors allergens under the control of a suitable promoter while the other plasmid carries protein E. Allergen bearing plasmid is induced to express the allergen/ hypoallergens first followed by the expression of lysis gene E, leading to the formation of BGs displaying allergens.
Loading allergens into BG the lumen
BGs have been shown to have an outstanding loading capacity, Citation31 as such one or more allergens can be loaded into the periplasmic space (PPS) or the hollow lumen. Citation23 , Citation49 , Citation50 For example, one study demonstrated that a mixture of 5 recombinant grass pollen allergens was effective in reducing grass pollen allergy symptoms. Loading this mixture in BG might be beneficial to people who are allergic to grass pollen. Citation51 Pohlit et al. Citation20 suggested that apart from maintaining allergen integrity, entrapping allergens inside carrier systems might also hinder the production of pro-inflammatory mediators by "hiding" allergens away from IgEs bound on mast and basophil cells before engulfment by APCs. Thus if allergens are to be entrapped inside BGs, they would be shielded away from binding with IgE on mast and basophil cells resulting in no effector cell degranulation. Citation52 In addition, BGs’ intact muco-adhesive PAMPs enable them to attach to specific tissues such as the mucosal surfaces of respiratory tract where there is an abundance of macrophages. Citation53 Depending on the route of administration, entrapment of allergens into BGs can also help protect allergens from environmental influences such as unfavorable pH, humidity, and temperature. Citation20 This increases the allergens’ half-life, enabling the lowering of frequency of allergen administrations and ultimately aiding patients’ adherence to therapy. Citation54
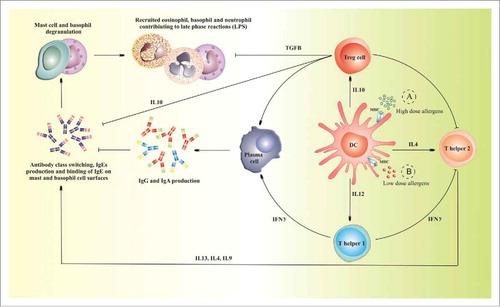
In addition to their inherent immunostimulatory properties, BGs are also excellent targets of APCs, such as macrophages and dendritic cells (DCs). Their efficient uptake, processing and presentation by APC would help reduce the allergen dose or the frequency of immunizations required for AIT. Hence when allergen loaded BGs are taken up by APCs there is an induction of an immune response against the BG proteins and against foreign entrapped allergens as well (As shown in ). One study investigated the uptake of BGs by APCs and the induction of inflammatory mediators in human macrophages, it was found that BGs stimulated a significant increase in the secretion of cytokines INF-γ, TNF-α and IL-12. Demonstrating BGs’ capacity to induce Th1 immune responses. Furthermore, DCs exposed to BGs were shown to have an increased ability to activate T-cells. Citation42 , Citation53
Figure 3. The downregulation mechanisms of allergen immunotherapy using allergens entrapped into BGs When high allergen doses (green dots, A) are taken up by APCs via BG-mediated allergen immunotherapy, DCs in particular produce IL-12 which stimulates naive Th0 cell to differentiate and favor skewing of the immune response from a pro-inflammatory Th2 to a Th1-driven one. This is followed by an increase in the ratio of Th1 cytokine profile as well as an induction of Treg cells and regulatory cytokines (IL-10). Furthermore, BG-mediated allergen immunotherapy leads to the production of allergen-specific IgGs that inhibit IgEs from binding onto mast and basophil cell surfaces. On the other hand, low dose (red dots, B) and repeated exposure to natural allergens via mucosal surfaces leads to a Th2-driven allergic response.
Although several methods of loading allergens into BGs are currently under various stages of development, the most popular approach involves first anchoring BG's inner membranes with streptavidin. Citation55 Following lyophilization, streptavidin-bearing BGs can then be loaded with allergens by re-suspending them in solution containing the desired biotinylated allergen of interest. To prevent leakages, BGs’ transmembrane channels are sealed by inside-out membrane vesicles in the presence of Ca2+ ions. Citation31 However, the process of sealing BGs is also currently under improvement and it can be envisaged that it will be optimized in the near future. It is also important to note that the composition of the target allergen to be packaged into the BG structures is dependent on the allergens’ own physiochemical properties. This then determines if it is beneficial to either loosely package them into the PPS or to attach them to a matrix. Citation28
Conclusion and future pespective
With the continued rise in the global prevalence of IgE-mediated allergic disease, BG systems can constitute a promising platform for loading and delivery of allergens for AIT. Since different types of BG based delivery and display systems have been developed in attempting to combat infectious diseases in recent years, similar approaches can be pursued to bring improvement to allergen immunotherapy. For efficient treatment of IgE-mediated allergy, an efficient allergen delivery platform has to possess some immunomodulatory properties, must be safe and be able to carry foreign allergens. BG system is one such targeting and delivery system, that combines targeting of allergen component to APCs and as well as acting as an adjuvant, Citation56 in a simple, cost-effective manner and with an additional advantage of having to store them for years by freeze-drying without loosing their potency. Citation48
However, the application of BGs as delivery systems is still at an early stage of development. One major problem with using BG platforms is the restriction of efficacy as a result of dose-limiting toxicity of bacterial cells. Citation57 Hence researchers working on AIT vaccines development face an inherent dilemma of maximizing immunogenicity without compromising the vaccines’ safety and tolerability. Citation58 In addition, there is need to come up with quality control procedures that discriminate against non-lysed bacteria and fully formed BGs if the processes are to be scaled up for production of AIT vaccines. Citation44
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
The authors would like to thank Tehran University of Medical Sciences-International Campus for their support.
References
- Marek J , Lothar J , Roland S , Hanns M , Helmut F , Oliver C. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116(3):608–613. doi:10.1016/j.jaci.2005.06.004. PMID:16159631
- Flöistrup H , Swartz J , Bergström A , Alm JS , Scheynius A , Van Hage M , Waser M , Braun-Fahrlander C , Schram-Bijkerk D , Huber M , et al. Allergic disease and sensitization in Steiner school children. J Allergy Clin Immunol. 2005;117(1):59–66. doi:10.1016/j.jaci.2005.09.039. PMID:16387585
- Hansen I , Klimek L , Mösges R , Hörmann K . Mediators of inflammation in the early and the late phase of allergic rhinitis. Curr Opin Allergy Clin Immunol. 2004;4(3):159–63. doi:10.1097/00130832-200406000-00004. PMID:15126935
- Bacharier LB , Geha RS . Molecular mechanisms of IgE regulation. J Allergy Clin Immunol. 2000;105(2 Pt 2):S547–58. doi:10.1016/S0091-6749(00)90059-9. PMID:10669540
- Pawankar R , Mori S , Ozu C , Kimura S . Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011;1(3):157–167. doi:10.5415/apallergy.2011.1.3.157. PMID:22053313
- Burks WA , Calderon MA , Casale T , Cox L , Demoly P , Jutel M , Nelson H , Akdis CA . Update on allergy immunotherapy : American Academy of Allergy, Asthma & Immunology / European Academy of Allergy and Clinical Immunology / PRACTALL consensus report. J Allergy Clin Immunol. 2013;131(5):1288–1296.e3. doi:10.1016/j.jaci.2013.01.049. PMID:23498595
- Durham SR , Till SJ . Immunologic changes associated with allergen immunotherapy. J Allergy Clin Immunol. 1998;102(2):157–64. doi:10.1016/S0091-6749(98)70079-X. PMID:9723654
- Bousquet J , Lockey R , Malling H . Allergen immunotherapy : Therapeutic vaccines for allergic diseases. J Allergy Clin Immunol. 1997;102(4):558–62. doi:10.1016/S0091-6749(98)70271-4
- Makatsori M , Pfaar O , Lleonart R , Calderon MA . Recombinant Allergen Immunotherapy : Clinical Evidence of Efficacy-A Review. Curr Allergy Astma Rep. 2013;13(4):371–80. doi:10.1007/s11882-013-0359-7
- Durham SR , Emminger W , Kapp A , Colombo G , De JGR , Rak S , Scadding GK , Andersen JS , Riis B , Dahl R . Long-term clinical efficacy in grass pollen – induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol. 2010;125(1):131-138.e1-7. doi:10.1016/j.jaci.2009.10.035
- Akdis M , Akdis CA . Therapeutic manipulation of immune tolerance in allergic disease. Nat Reviews Drug Disc. 2009;8:645–660. doi:10.1038/nrd2653
- Jutel M , Akdis CA . Immunological mechanisms of allergen-specific immunotherapy. Allergy. 2011;66(6):725–32. doi:10.1111/j.1398-9995.2011.02589.x. PMID:21466562
- Brunetto B , Tinghino R , Braschi MC , Antonicelli L , Pini C , Iacovacci P . Characterization and comparison of commercially available mite extracts for in vivo diagnosis. Allergy. 2010;65(2):184–90. doi:10.1111/j.1398-9995.2009.02150.x. PMID:19796217
- Curin M , Reininger R , Swoboda I , Focke M , Valenta R , Spitzauer S . Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011;154(3):258–63. doi:10.1159/000321113. PMID:20861648
- Blank S , Seismann H , Michel Y , McIntyre M , Cifuentes L , Braren I , Grunwald T , Darsow U , Ring J , Bredehorst R , et al. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Allergy. 2011;66(10):1322–1329. doi:10.1111/j.1398-9995.2011.02667.x. PMID:21658068
- Focke M , Swoboda I , Marth K , Valenta R . Developments in allergen-specific immunotherapy: From allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin e and T cell reactivity. Clinical and Experimental Allergy. 2010;40:385–97. doi:10.1111/j.1365-2222.2009.03443.x. PMID:20210812
- Chapman MD , Smith AM , Vailes LD , Arruda LK , Dhanaraj V , Pomes A . Recombinant allergens for diagnosis and therapy of allergic diseases. J Allergy Clin Immunol. 2000;106(3):409–18. doi:10.1067/mai.2000.109832. PMID:10984358
- Pauli G , Larsen TH , Rak S , Horak F , Pastorello E , Valenta R , Purohit A , Arvidsson M , Kavina A , Schroeder JW , et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunology. 2008;122(5):951–60. doi:10.1016/j.jaci.2008.09.017
- Akdis CA , Akdis M . Advances in allergen immunotherapy: Aiming for complete tolerance to allergens. Sci Transl Med. 2015;7(280):280–286. doi:10.1126/scitranslmed.aaa7390
- Pohlit H , Holger F , Saloga J . Could allergen-specific immunotherapy benefit from the use of nanocarriers? Nanomedicine. 2016;11(11):1329–31. doi:10.2217/nnm-2016-0111. PMID:27221075
- Witte A , Wanner G , Blasi UDO , Halfmann G , Szostak M , Lubitz W . Endogenous transmembrane tunnel formation mediated by pX174 Lysis protein E. J Bacteriology. 1990;172(7):4109–4114. doi:10.1128/jb.172.7.4109-4114.1990
- Szostak MP , Hensel A , Eko F , Klein R , Auer T , Mader H , Haslberger A , Bunka S , Wanner G , Lubitz W . Bacterial ghosts : Non-living candidate vaccines. J Biotechnol. 1996;44(1–3):161–170. doi:10.1016/0168-1656(95)00123-9. PMID:8717400
- Langemann T , Koller VJ , Muhammad A , Kudela P , Mayr UB , Lubitz W . The bacterial ghost platform system Production and applications. Bioeng Bugs. 2010;1(5):326–36. doi:10.4161/bbug.1.5.12540. PMID:21326832
- Paukner S , Stiedl T , Kudela P , Bizik J , Laham F Al , Lubitz W . Bacterial ghosts as a novel advanced targeting system for drug and DNA delivery. Expert Opin drug Deliv. 2006;3(1):11–22. doi:10.1517/17425247.3.1.11. PMID:16370937
- Witte A , Wanner G , Sulzner M , Lubitz W . Dynamics of PhiX174 protein E-mediated lysis of Escherichia coli . Arch. Microbiolol. 1992;157(4):381–388. doi:10.1007/BF00248685
- Fu X , Himes BA , Ke D , Rice WJ , Ning J , Zhang P . Controlled bacterial lysis for electron tomography of native cell membranes. Structure. 2014;22(12):1875–82. doi:10.1016/j.str.2014.09.017. PMID:25456413
- Macri C , Dumont C , Johnston APR , Mintern JD . Targeting dendritic cells : a promising strategy to improve vaccine effectiveness. Clin Transl Immunol. 2016;5(3):e66–68. doi:10.1038/cti.2016.6
- Jechlinger W , Haller C , Resch S , Hofmann A , Szostak MP , Lubitz W . Comparative immunogenicity of the Hepatitis B virus core 149 antigen displayed on the inner and outer membrane of bacterial ghosts. Vaccine. 2005;23(27):3609–17. doi:10.1016/j.vaccine.2004.11.078. PMID:15855021
- Mayr UB , Walcher P , Azimpour C , Riedmann E , Haller C , Lubitz W , Beate U , Walcher P , Azimpour C , Riedmann E , et al. Bacterial ghosts as antigen delivery vehicles. Adv Drug Deliv Rev. 2005;57(9):1381–91. doi:10.1016/j.addr.2005.01.027. PMID:15878634
- Choi SH , Nam YK , Kim KH . Novel expression system for combined vaccine production in edwardsiella tarda ghost and cadaver cells. Mol Biotechnol. 2010;46(2):127–33. doi:10.1007/s12033-010-9277-2. PMID:20369310
- Paukner S , Kohl G , Jalava K , Lubitz W . Sealed bacterial ghosts — novel targeting vehicles for advanced drug delivery of water-soluble substances. J Drug Target. 2003;11(3):151–61. PMID:13129825
- Bottazzi B , Doni A , Garlanda C , Mantovani A . An integrated view of humoral innate immunity: Pentraxins as a Paradigm. Annu Rev Immunol. 2010;28:157–83. doi:10.1146/annurev-immunol-030409-101305. PMID:19968561
- Charbit A , Boulain JC , Ryterl A , Hofnung M . Probing the topology of a bacterial membrane protein by genetic insertion of a foreign epitope ; expression at the cell surface. EMBO J. 1986;5(11):3029–37. PMID:2431904
- Freudl R , Maclntyre S , Degen M , Henning U . Cell surface exposure of the outer membrane protein OmpA of escherichia coli K-12. J Mol. 1986;188(3):491–4. doi:10.1016/0022-2836(86)90171-3
- Matricardi PM , Bjorksten B , Bonini S , Bousquet J , Djukanovic R , Dreborg S , Gereda J , Malling JH , Popov E , Raz E , et al. Microbial products in allergy prevention and therapy. Allergy. 2003;58(6):461–71. doi:10.1034/j.1398-9995.2003.00175.x. PMID:12757444
- Frick OL , Teuber SS , Buchanan BB , Morigasaki S , Umetsu DT . Allergen immunotherapy with heat-killed Listeria monocytogenes alleviates peanut and food-induced anaphylaxis in dogs. Allergy. 2005;60(2):243–50. doi:10.1111/j.1398-9995.2004.00711.x. PMID:15647048
- Li X , Srivastava K , Grishin A , Huang CH , Schofield B , Burks W , Sampson HA . Food and drug reactions and anaphylaxis Rapid publication Persistent protective effect of heat- killed Escherichia coli producing “ engineered,” recombinant peanut proteins in a murine model of peanut. J Allergy Clin Immunol. 2003;112(1):159–67. doi:10.1067/mai.2003.1622. PMID:12847493
- Guimarães JQM , Silva ODA , Alves R , Fukuhara CH , Soares de Amaral VB , Almeida VB , Ynoue LH , Oliveira R , Camargo SM , Rodrigues GSS , et al. Mite-specific immunotherapy using allergen and / or bacterial extracts in atopic patients in Brazil. J Investig Allergol Clin Immunol. 2008;18(2):84–92. PMID:18447136
- Bohle B , Breitwieser A , Zwölfer B , Jahn-schmid B , Sára M , Sleytr UB , Ebner C . A novel approach to specific allergy treatment: The recombinant fusion protein of a bacterial cell surface (S-Layer) protein and the major birch pollen allergen Bet v 1 (rSbsC-Bet v 1) combines reduced allergenicity with immunomodulating capacity. J Immunol. 2004;172(11):6642–8. doi:10.4049/jimmunol.172.11.6642. PMID:15153479
- Jahn-schmid B , Messner P , Unger FM , Sleytr UB , Scheiner O , Kraft D . Toward selective elicitation of TH1-controlled vaccination responses: Vaccine applications of bacterial surface layer proteins. J Biotechnol. 1996;44(1–3):225–231. doi:10.1016/0168-1656(95)00124-7. PMID:8717408
- Jahn-schmid B , Siemann U , Zenker A , Bohle B , Messner P , Unger FM , Sleytr UB , Scheiner O , Kraft D , Ebner C . Bet v 1, the major birch pollen allergen, conjugated to crystalline bacterial cell surface proteins, expands allergen-specific T cells of the T h 1 / T h 0 phenotype in vitro by induction of IL-12. Intl Immunol. 1997;9(12):1867–1874. doi:10.1093/intimm/9.12.1867
- Haslberger AG , Kohl G , Felnerova D , Mayr UB , Furst-Ladani S , Lubitz W . Activation, stimulation and uptake of bacterial ghosts in antigen presenting cells. J Biotechnol. 2000;83(1–2):57–66. doi:10.1016/S0168-1656(00)00298-4. PMID:11000461
- Langemann T , Mayr UB , Meitz A , Lubitz W , Herwig C . Multi-parameter flow cytometry as a process analytical technology (PAT) approach for the assessment of bacterial ghost production. Appl Microbiol Biotechnol. 2016;100(1):409–418. doi:10.1007/s00253-015-7089-9. PMID:26521248
- Haidinger W , Szostak MP , Jechlinger W , Lubitz W . Online Monitoring of Escherichia coli Ghost Production. Appl Environ Microbiol. 2003;69(1):468–474. doi:10.1128/AEM.69.1.468-474.2003. PMID:12514029
- Kassmannhuber J , Rauscher M , Schöner L , Witte A . Functional display of ice nucleation protein InaZ on the surface of bacterial ghosts. Bioengineered. 2017;1–13. doi:10.1080/21655979.2017.1284712. PMID:28121482
- Hess J , Gentschevt I , Miko D , Welzel M , Ladel C , Goebelt W , Kaufmann SH . Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis. Proc Natl Acad Sci USA. 1996;93(4):1458–1463. doi:10.1073/pnas.93.4.1458. PMID:8643654
- Muralinath M , Kuehn MJ , Roland KL , Curtiss R . Immunization with salmonella enterica serovar typhimurium-derived outer membrane vesicles delivering the pneumococcal protein pspa confers protection against challenge with streptococcus pneumoniae. Infect Immun. 2011;79(2):887–94. doi:10.1128/IAI.00950-10. PMID:21115718
- Hjelm A , Söderström B , Vikström D , Jong WS , Luirink J , De Gier JW . Autotransporter-based antigen display in bacterial ghosts. Appl Environ Microbiol. 2015;81(2):726–735. doi:10.1128/AEM.02733-14. PMID:25398861
- Walcher P , Mayr UB , Azimpour-tabrizi C , Eko FO , Jechlinger W , Mayrhofer P , Alefantis T , Mujer CV , DelVecchio VG , Lubitz W . Antigen discovery and delivery of subunit vaccines by nonliving bacterial ghost vectors. Expert Rev Vaccines. 2004;3(6):681–691. doi:10.1586/14760584.3.6.681. PMID:15606353
- Gangl K , Niederberger V , Valenta R . Multiple grass mixes as opposed to single grasses for allergen immunotherapy in allergic rhinitis. Clin Exp Allergy. 2013;43(11):1202–16. doi:10.1111/cea.12128. PMID:24152153
- Jutel M , Jaeger L , Suck R , Meyer H , Fiebig H , Cromwell O . Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 116(3):608–13. doi:10.1016/j.jaci.2005.06.004. PMID:16159631
- Moghimi SM. Chemical camouflage of nanospheres with a poorly reactive surface: Towards development of stealth and target-specific nanocarriers. Biochim Biophys Acta. 2002;1590(1–3):131–9. doi:10.1016/S0167-4889(02)00204-5. PMID:12063176
- Haslberger AG , Mader HJ , Schmalnauer M , Kohl G , Szostak MP , Messner P , Sleytr UB , Wanner G , Furst-Ladani S , Lubitz W . Bacterial cell envelopes (ghosts) and LPS but not bacterial S-layers induce synthesis of immune-mediators in mouse macrophages involving CD14. Innate Immun. 1997;4(6):431–441.
- Wang T , Li Y , Wang F , Zhou C . Nonadherence to sublingual immunotherapy in allergic rhinitis: a real-life analysis. Int Forum Allergy Rhinol. 2017;7(4):389–92. doi:10.1002/alr.21909. PMID:28151587
- Huter V , Szostak MP , Gampfer J , Prethaler S , Wanner G , Gabor F , Lubitz W . Bacterial ghosts as drug carrier and targeting vehicles. J Control Release.1999;61(1–2):51–63. doi:10.1016/S0168-3659(99)00099-1. PMID:10469902
- Lubitz W. Bacterial ghosts as carrier and targeting system. Expert Opin Ther. 2001;1(5):765–71. doi:10.1517/14712598.1.5.765
- Palffy R , Gardlık R , Hodosy J , Behuliak M , Resko P , Radvansky J , Celec P . Bacteria in gene therapy : bactofection versus alternative gene therapy. 2006;13(2):101–5.
- Bachmann MF , Jennings GT . Vaccine delivery : A matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–96. doi:10.1038/nri2868. PMID:20948547