ABSTRACT
Tuberculosis (TB) remains a main public health concern and 10.4 million new cases occurred in 2015 around the world. BCG is the only approved vaccine against TB, but has variable efficacy and new vaccines are needed. We developed two new mTB vaccine candidates based on the recombinant fusion proteins, rCMX and rECMX formulated with Advax4, a new combination adjuvant combining delta inulin, CpG oligonucleotide and murabutide. BALB/c mice were immunized three times intramuscularly with these vaccine formulations. Injection of Advax4 alone increased the percentage of lymphatic endothelial cells and activated macrophages (F480/CD11b+) in the draining lymph nodes consistent with a chemotactic adjuvant effect. Advax4+CMX and Advax4+ECMX induced the highest levels of IgG1 and IgG2a antibodies against rCMX and rECMX, respectively. Immunized mice challenged with Mycobacterium tuberculosis (Mtb) had increased vaccine-specific Th1 responses in the lungs together with reduced Mtb – associated alveolar damage, although only the Advax4+ECMX vaccine demonstrated significant reduction of lung bacterial load. This study confirmed Advax4+ECMX as a potential TB vaccine candidate, with potential for further optimization and clinical development.
Highlights
| 1. | Advax4+ECMX was well tolerated and was transported to local lymph nodes without associated inflammation. | ||||
| 2. | Advax4 adjuvant resulted in increased lymphatic endothelial cells and activated macrophages in draining lymph nodes. | ||||
| 3. | Advax4+ECMX immunization induced high vaccine-specific IgG2a and IgG1 levels and Th1 T cell response. | ||||
| 4. | Advax4+ECMX reduced mTB-associated inflammatory damage in the lungs together with a reduction in bacterial load. | ||||
Introduction
The Global Report from the World Health Organization estimated there were 10.4 million new cases of tuberculosis (TB) due to infection with Mycobacterium tuberculosis (Mtb) worldwide resulting in 1.8 million deaths in 2015.Citation1 Around one third of the world population is infected with latent Mtb, of which 10% will develop active disease at some point in their life e.Citation2 The only current vaccine against TB, Bacillus Calmette Guerin (BCG), is a live vaccine created over 80 years ago that has variable efficacy in children and does not protect adults.Citation3 Although multiple other vaccines have been clinically tested, none of them have proved more effective than BCG in controlling TB infection.Citation4 Hence there is ongoing need for development of a more effective TB vaccine.
Subunit protein vaccines have the advantage of being safer than live vector vaccines and generate very focused immune responses against the specific protein target.Citation5 CMX is a fusion protein composed of the immunodominant epitopes from Mtb proteins Ag85c and MPT51, that are recognized during active TB, plus the whole HspX protein sequence that is highly expressed during TB latency.Citation6 CMX was shown to be immunogenic when formulated with liposome or when expressed by Mycobacterium smegmatis or BCG live vectors,Citation6–8 resulting in Mtb protection. ESAT-6 is another latency-associated antigen that promotes the generation of Th17 responses that correlate with Mtb protection.Citation9
Advax is an adjuvant derived from delta inulin, a polysaccharide found in the roots of plants of the Compositae family.Citation10,Citation11 Advax adjuvant has shown high levels of efficacy in vaccines against respiratory syncytial virus,Citation11 West Nile virus and Japanese encephalitis virus,Citation12,Citation13 influenza,Citation14–16 severe acute respiratory syndrome coronavirus,Citation17 Listeria,Citation18 onchocercosisCitation19 and anthrax,Citation20 amongst others, consistent with its having broad efficacy across viral, bacterial and parasitic vaccines. An advantage of delta inulin adjuvant is that it enhances both Th1 and Th2 T cell responses. It has also been shown to be safe and effective in pregnant mothers and newborns in addition to the general population.Citation14,Citation16,Citation21–23 This prompted us to test the suitability of Advax adjuvant for formulation with TB subunit vaccines.
We have previously shown that Advax delta inulin adjuvant can be combined with other immunomodulators such as TLR9 agonists (CpG oligonucleotides)Citation17 or NOD2 agonists (murabutide),Citation20 to make potent combination adjuvants. In this study we hypothesized that a new adjuvant formulation designated as Advax4 that combined all three individual immunomodulatory agents would when combined with rCMX or a new fusion protein of CMX and ESAT-6 (rECMX) induce potent cellular and humoral immune responses that might better control TB infection.
Results
rCMX vaccine and Advax4 effects at the local injection site
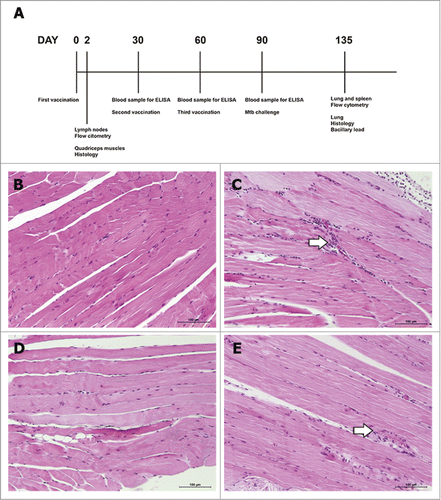
Many cellular adjuvants used in experimental TB vaccines such as Freund's complete adjuvant or DDA/MPL are highly reactogenic, making them unsuitable for routine human use.Citation24,Citation25 To evaluate the reactogenicity of the new Advax4 vaccine formulation, adjuvant alone and combined with CMX protein was injected into the quadriceps muscle and the tissues collected after 2 days (). There was no difference in the striated muscle tissue morphology in the saline group () as compared to group injected with Advax4 alone (). The mice inoculated only with rCMX () or Advax4+CMX () had a small increase in mononuclear cells. These results confirmed the lack of muscle damage in response to injection of Advax4 adjuvant and also showed a capacity of the recombinant rCMX antigen alone or when combined with adjuvant to initiate a chemotactic signal resulting in the recruitment of mononuclear cells to the injection site.
Figure 1. Upper part of the figure: representative schematic time line of vaccinations. (A) Mice were immunized three times with 30 days intervals. Blood samples were collected 30 days after each vaccination. Two days after the first vaccination, mice were euthanized to collect right quadriceps muscle and draining lymph nodes. Thirty days after the last vaccination, mice were infected with Mtb. Forty-five days after Mtb challenge, mice were euthanized to collect lung and spleen for analysis. Lower part of the figure: representative histopathological images of quadriceps muscle tissues of immunized mice. BALB/c mice were immunized in the quadriceps muscle with the different formulations and euthanized after two days for morphological analysis. Saline group (B), recombinant protein alone (C), Advax4 alone (D), Advax4 plus recombinant protein (E). In (E) is possible to observe a leukocyte increase consistent with chemotaxis induced by the vaccine formulation rather than an inflammatory process. The results shown are representative of two independent experiments (N = 6, *p<0.05). H&E staining, x100 magnification. Arrows indicate increase of mononuclear cells.
Advax4+CMX vaccine increases lymphatic endothelial cells and activated macrophages in the draining lymph nodes
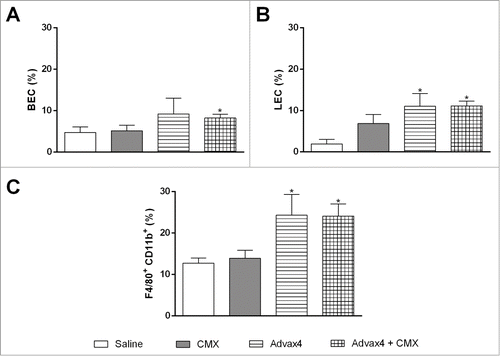
To assess if the Advax4+CMX vaccine could stimulate cells from local lymph nodes (inguinal and popliteal), nodes were collected and processed to analyze cellular profile (). Two days post-immunization with Advax4+CMX the percentages of blood endothelial cells (BECs) were similar to the nodes from control mice (). However, immunization with Advax4+CMX or Advax4 alone increased the percentage of lymphatic endothelial cells (LECs) and activated macrophages (F480/CD11b+) in the lymph nodes ( and ).
Figure 2. Specific rCMX cells present in the draining lymph nodes 2 days after injection. BALB/c mice inoculated with Saline, rCMX, Advax4, or Advax4 + CMX were euthanized after 2 days, and the draining lymph nodes were collected. Cells were stained with gp38, CD31, F4/80, CD11C. (A) Percentage of blood endothelial cells (BEC). (B) Percentage of lymphatic endothelial cells (LEC). (C) Percentage of activated macrophages (F4/80+/CD11b). The results shown are representative of two independent experiments (N = 6, *p < 0.05).
Both Advax4+CMX and Advax4+ECMX vaccines are highly immunogenic
While TB vaccines are postulated to mainly work via induction of T cell immunity, serum antibodies and the IgG2a/IgG1 ratio are a convenient marker to assess vaccine immunogenicity. Sera from immunized mice were therefore analyzed to compare the immunogenicity of CpG DNA+CMX, Advax4+CMX, Advax4+ECMX and control groups (Saline, BCG, CMX, Advax4 and CpG-DNA+CMX). Immunized mice developed vaccine-specific IgG1 (, and ), and IgG2a (, and ). There was no significant difference between the IgG1 and IgG2a levels induced by Advax4+CMX, Advax4+ECMX and CpG-DNA+CMX. The ratio of IgG2a/IgG1 showed a bias to IgG2a responses in the BCG vaccinated group ().
Figure 3. Humoral Immune Response to CMX. Animals were bled 30 days after the first, second and third vaccinations. Antibody levels were measured after sera separation. (A) IgG1 levels induced by CpG-DNA + CMX, Advax4 + CMX and controls. (B) IgG2a levels induced by CpG-DNA + CMX, Advax4 + CMX and controls. (C) IgG1 levels induced by Advax4 + ECMX. (D) IgG2a levels induced by Advax4 + ECMX. (E) IgG2a/IgG1 ratio of all vaccine formulations. All vaccines induced high antibody levels since 30 days after the first vaccination. The results shown are representative of two independent experiments (N = 6; *, # p < 0.05). * Statistical difference between the analyzed group and the same group after the first vaccination. # Statistical difference with the saline group.
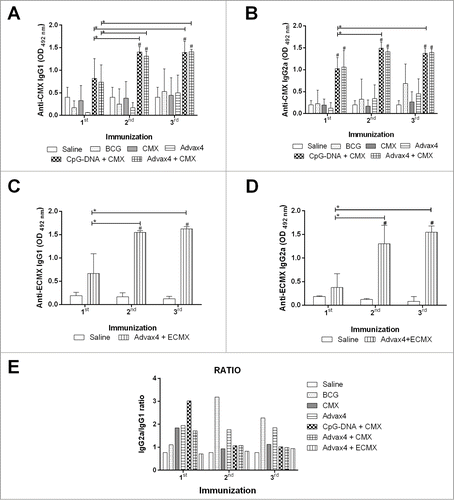
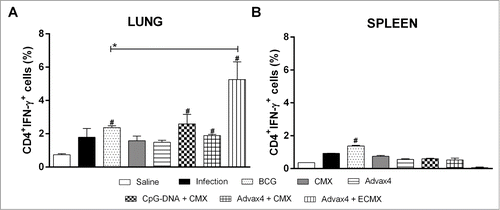
Figure 4. Cellular immune response against rCMX 45 days after challenge. Thirty days after the third immunization, BALB/c mice were challenged with Mtb (105 CFU per mice) and then euthanized after 45 days to evaluate the cellular response. (A) Anti-rCMX or anti-rECMX CD4+IFN-γ+ lung cells 45 days after challenge. (B) Anti-CMX or anti-ECMX CD4+IFN-γ+ splenocytes 45 days after challenge. The results shown are representative of two different experiments (N = 3; *p < 0.05). * Statistical difference between the analyzed group and the BCG group. # Statistical difference with the saline group.
Advax4+CMX induces a Th1 cellular response in lungs and spleen
In order to evaluate if the vaccines were able to induce specific Th1 responses, T cell responses were evaluated in the lungs and spleens 45 days after challenge of the mice with Mtb. Animals immunized with Advax4+ECMX had significantly higher anti-rECMX CD4+IFN-γTh1 cells in the lungs that were 2–3 fold higher than all the other groups including the BCG-immunised group (). Only BCG-immunized animals showed an increase in the frequency of CD4+IFN-γTh1 cells in the spleen when compared to the control group ().
Advax4 formulated vaccines reduce lung inflammatory injury caused by Mtb
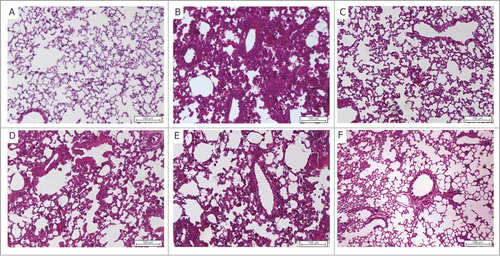
Forty-five days post infection the normal lung alveolar architecture () was extremely altered due to inflammation associated with Mtb infection (). BCG vaccination () partially reduced the Mtb-associated lung damage, but the presence of mononuclear cells at periarteriolar inflammatory areas could still be observed. Notably, both vaccine formulations containing Advax4, namely Advax4+CMX but more specifically Advax4+ECMX, exhibited significantly reduced Mtb-associated alveolar inflammatory damage ( & and ). Immunization with Advax4+CMX or Advax4+ECMX induced a similar mononuclear cell infiltrate but prevented alveolar wall thickening when compared to control infected animals, ( and ).
Table 1. Histopathological lesion score of the lungs from vaccinated mice.
Figure 5. Representative histopathological images of lungs, 45 days post Mtb challenge (A) Saline, (B) infection control, (C) BCG, (D) Advax4, (E) Advax4 + CMX, (F) Advax4 + ECMX. H&E staining, x100 magnification. Inflammatory injury reduction is observed 45 days after challenge in the Advax4 + ECMX group.
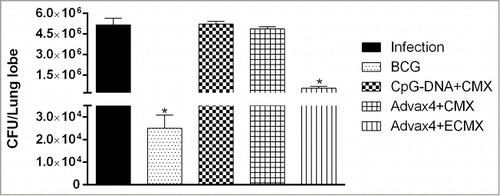
The combination of ESAT-6 and CMX protein when formulated with Advax4 adjuvant reduces lung bacillary load
To evaluate protection induced by the different vaccine formulations, superior and middle lung lobes of immunized animals were collected 45 days after challenge. rCMX formulated with either CpG-DNA or Advax4 adjuvant did not reduce lung bacillary loads when compared to control unimmunized mice. By contrast, immunization with Advax4+ECMX significantly reduced lung bacterial load when compared to unimmunized mice although BCG vaccine induced a greater bacillary reduction than Advax4+ECMX (p < 0.05) ().
Discussion
This study assessed the efficacy and protection of the novel Advax4 delta inulin combination adjuvant with two different recombinant fusion proteins: rCMX and rECMX. Advax4 adjuvanted vaccine formulation increased cellular recruitment to the site of injection, increased the frequency of LEC and activated macrophages in the draining lymph nodes, and induced high antibody levels against both rCMX and rECMX. After Mtb challenge, mice vaccinated with Advax4+CMX and Advax4+ECMX exhibited increased numbers of antigen-specific Th1 cells in the lung to a greater extent even than BCG immunization and this was associated with reduced inflammation as measured by alveolar wall thickening. The absence of protection with rCMX antigen was a little surprising as when the CMX antigen was expressed by a live M. smegmatis vector, a potent and protective response was induced against M. tuberculosis, with this vector vaccine recruiting neutrophils that played a key role in generation of specific Th1 and Th17 cells.Citation28 In particular, the rCMX antigen even when formulated with Advax-4 was non-protective and stimulated poor T-cell responses when compared to rECMX protein that further contains ESAT6. Vaccination using rCMX mainly induced a response against HspX protein, so this might suggest the importance of inclusion of ESAT6 antigen in recombinant protein vaccines against mTB. Notably, only Advax4+ECMX immunization significantly reduced lung bacillary load in addition to reducing pulmonary inflammatory damage, suggesting this is a promising candidate to advance towards human clinical testing.
Advax delta inulin adjuvant has been previously shown to be non-inflammatory,Citation26 and the current study demonstrates that this benefit is maintained even when delta inulin is co-formulated with the innate immune activators, CpG and murabutide. Histology of the injection site in our study showed an equal increase of mononuclear cells when rCMX alone or together with Advax4 adjuvant was injected consistent with the rCMX antigen itself having a chemotactic effect. rCMX protein alone has previously been reported to activate macrophages through TLR-2 and TLR-4 resulting in production of IL-6, a pro-inflammatory cytokine.Citation27 Hence, it is possible that the adjuvant signals induced by Advax4 synergize with independent chemotactic signals induced by the CMX protein, thereby explaining the positive effects of the combined vaccine formulation.Citation27
Advax delta inulin adjuvant alone or combined with either CpG or murabutide innate immune activators has been shown to be effective in numerous animal vaccine models and delta inulin adjuvant itself has been shown to be safe and effective in human subjects.Citation21,Citation22 Rather than imparting immune bias like other adjuvants, it has been suggested that delta inulin works as an immune amplifier or adjuvant of adjuvants, by enhancing immune activation signals contained within the antigen itself. Hence, if the vaccine antigen generates Th1 responses, delta inulin will potentiates this Th1 response, but if the vaccine formula induces Th2 responses, it will potentiate the Th2 response.Citation26 This provides the rationale for co-formulating delta inulin with CpG, which should then result in a balanced Th1/Th2 response, a property borne out in this study where Advax4 adjuvant was associated with a balanced IgG2a/IgG1 response.Citation27 This type of response was also observed using liposome formulation containing CpG-DNA+CMX.Citation6
In the present work, we showed an increase of BEC and LEC populations in the draining lymph nodes 2 days after injection of Advax4 alone or with rCMX. LECs capture foreign antigens and maintain these antigens in lymph nodes for long periods of time, transferring these antigens to local APCs thereby allowing the ongoing activation of antigen-specific T cells, as demonstrated in influenza vaccine models.Citation29 The data suggests that Advax4 stimulates an increase in LECs and activated macrophages in the draining lymph node, either through increased chemotaxis and/or proliferation and expansion, and this may be an important mechanism through which Advax exerts its favourable adjuvant effects. Enhanced antigen presentation in the draining lymph nodes could lead to generation of Th1 T cells that then migrate to the lung in response to mTB infection, thereby explaining the higher levels of specific Th1 cells generated after Mtb challenge in the Advax4 adjuvanted vaccine group. In addition, Advax4 formulated vaccines reduced lung inflammation and alveolar collapse caused by Mtb, with reduced lung Mtb loads seen in Advax4+ECMX immunized animals. Although not shown here, the survival curves of mice vaccinated with Advax4+CMX were superior to those vaccinated with CpG-DNA+CMX. This stresses the potential importance of a balanced Th1/Th2 immune response to overall survival after Mtb challenge. While IFN-γ produced by Th1 cells has a major role in Mtb protection by inducing macrophage activation and the release of reactive nitrogen intermediates, like NO, capable of killing mycobacteria,Citation30 too much Th1-associated inflammation in the lung may be detrimental, hence the importance of a balanced immune response.
Whilst Advax4+ECMX vaccine was associated with the highest frequency of vaccine specific Th1 cells in the lungs post-challenge together with the best lung protection against alveolar damage, nevertheless, immunization with live BCG vaccine provided significantly higher reduction in lung Mtb counts. Differences between BCG and our vaccine formulation include the vaccine type (attenuated vector versus subunit protein) and the type of immune response induced. BCG induces Th1 and Th17 cellular responses associated with protection.Citation32 By contrast, Advax4+ECMX vaccine did not induce measurable Th17 responses (data not shown). Th17 responses can, however, be a double-edged sword in the lung with excess Th17 activation being associated with lung damage and even leading to increased mortality in immunized animals after challenge.Citation33 The lack of IL17 induction by Advax4 may help explain the reduced lung damage despite higher Mtb colony counts in lungs of Advax4+ECMX-immunized animals when compared to BCG-immunized animals.
The addition of ESAT-6 made a major difference in the protection provided by the recombinant protein vaccine. The rCMX protein was previously shown to be protective for mice in other vaccine formulations against Mtb, when expressed by recombinant M. smegmatis.Citation28 However, the contrast with the lack of protection of rCMX protein in the current study may be explained by M. smegmatis being a live vector as opposed to the recombinant rCMX protein used here. However, the combination of recombinant protein with Advax4 has the advantage of being safer than a live vector vaccine. Recently, Liu et al. (2017)Citation34 observed that a BCG recombinant vaccine expressing Ag85B/ESAT-6 induced a long-lasting Th1 response in mice. Also, in adolescents, H1:IC31 (Ag85b-ESAT-6 fusion protein + IC31 adjuvant) induced long-lived TNF-α/IL-2+ CD4 T cells.Citation35 ESAT-6, in complex with CFP-10, inhibits class I-mediated (MHC-I) antigen presentation, by interacting with β2M of the host cell thereby protecting mycobacteria against host defenses.Citation36 ESAT-6 also induces an increase in macrophage glucose uptake and dysregulation of enzymes involved in triglyceride formation, which benefits mycobacterial nutrition and survival.Citation37 Hence, inclusion of ESAT-6 might explain the enhanced vaccine protection against Mtb seen with ECMX when compared to CMX vaccine.This study has a number of limitations. Due to the large number of animals needed for challenge testing, comparing the effectiveness of ECMX fusion protein against each individual antigen composing ECMX was not logistically possible. Nevertheless, we were able to compare the ECMX fusion protein results with historical results with the individual antigens. The activation of professional antigen presenting cells such as dendritic cells,, might have played a major role in the effectiveness of the Advax4 adjuvant and in future studies we plan to look further at the mechanism of action of the Advax4 combination adjuvant. An ongoing challenge is to create an Mtb vaccine able to act at the start of infection with enough strength to overcome Mtb's ability to escape and survive within myeloid immune cells. Ideally, a recombinant vaccine would boost the BCG memory response providing the opportunity to better protect adults. An important next step for Advax4+ECMX vaccine development will be to further evaluate its effects on cellular immunity, pre-challenge rather than just post-challenge as presented here. An additional important question that still needs to be addressed is whether Advax4+ECMX could be used as a boost after BCG vaccination. Since Advax4 induces predominantly Th1 cellular responses, improved protection may be obtained by addition of IL-17 or IL-23 to the vaccine composition or formulation of additional adjuvant elements that further stimulate Th17 responses.
In summary, these results demonstrate for the first time protection provided by a recombinant fusion protein ECMX containing epitopes from three major Mtb antigens, when formulated with Advax4, a novel delta inulin combination adjuvant, revealing this as a promising new subunit mTB vaccine strategy.
Materials and methods
Ethics
The experiments using animals were performed in accordance with the guidelines of the Conselho Nacional de Controle de Experimentação Animal (CONCEA- Ministério da Ciência e Tecnologia-Brazil). The protocols used in this study were approved by the Ethical Committee in the use of animal (CEUA) from Federal University of Goiás protocol number: 0153/10. Animals were kept in animal-housing facilities located at Instituto de Patologia Tropical e Saúde Pública. Animal handling were done under veterinarian supervision.
Animals
For this study, we used 86 specific pathogen-free female mice (BALB/c) provided by Unicamp – (Campinas University – São Paulo) animal-housing facilities. They were maintained in micro-isolators containing HEPA filters. Mice were fed with sterilized water and chow diet ad libitium. Paper cylinders were used to reduce stress and occasionally the animals were supplemented with sunflower seeds. Room humidity controlled from 40 to 70% and light/dark cycles of 12 hours. Temperature was maintained at 24ºC.
rCMX and rECMX construction
De Sousa et al. (2012)Citation6 described pET23a/CMX plasmid construction. For the construction of the ECMX fusion gene, the ESAT-6 gene was amplified from Mtb, H37Rv strain and cloned in a pGEM-T easy (Promega) plasmid with concomitant creation of the restriction enzyme sites for NdeI and BamHI enzymes at the amino and carboxyl corresponding regions of the gene. The ESAT-6 gene was removed from the recombinant pGEM-T easy construction by digestion with the above enzymes and inserted in the pET23a/CMX previously digested with the same enzymes. The resulting recombinant plasmid named pET23a/ECMX was confirmed by enzyme-restricted profiles as well as sequencing of the entire construction.
rCMX and rECMX production
Large-scale recombinant CMX (rCMX) or rECMX protein production was achieved by culturing E. coli BL21(DE3) pLysS transformed with each recombinant plasmid separately in liquid LB medium (containing chloramphenicol and ampicillin) for 4–6 hours until OD600 reached 0.6 as described in.Citation6 At this point recombinant protein expression was induced by the addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 1 mM and cultures were further incubated for additional 4–5 hours. Protein purification was done under denaturing conditions with an His-Tag Protein Purification Ni-NTA purification kit (Qiagen). After addition of lysis buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M urea, pH 8), the suspension was centrifuged for 45 minutes, 10,000 x g, at 22ºC. The supernatant was transferred to a purification column and separated according to the kit manufacturer's instructions. rCMX protein has a molecular weight of 30 KDa and the rECMX protein has 36 KDa.
Vaccine formulation
Advax4 adjuvant was supplied by Vaxine Pty Ltd, Adelaide Australia and was a combination adjuvant comprising delta inulin 1mg, CpG oligonucleotide 10 μg and murabutide 10 μg per vaccine dose. The vaccines were formulated immediately before vaccination as follows: 1: Advax4+CMX contained Advax4 (1mg delta inulin/injection) plus the rCMX fusion protein (200 μg/ml) (20 μg/injection); 2: Advax4+ECMX contained Advax4 (1mg delta inulin/injection) with the rECMX fusion protein (200 μg/ml) (20 μg/injection); 3: CpG-DNA+CMX consisted of CpG-DNA (200 μg/ml) (20 μg/injection) with the rCMX fusion protein (200 μg/ml). As controls, the adjuvant and the recombinant proteins were formulated alone at the same concentration used in the vaccine formulations. All vaccines were prepared immediately before animal inoculation followed by strong vortexing. In addition, as vaccine control, a group of animals was vaccinated with BCG (106 CFU/100 μL).
Experimental design
Six groups of 6–7 adults (8-10 weeks old) female BALB/c were used in this study. The animals from groups CpG-DNA+CMX, Advax4 (adjuvant control), Advax4+CMX, rCMX alone, Advax4+ECMX, and rECMX alone were injected three times intramuscularly with 100μL of each vaccine with a 30-day interval. One day before each vaccination, blood was collected to obtain serum for analysis. Thirty days after the last immunization, all animals were challenged with Mtb. The animals in the BCG group were subcutaneously vaccinated once and 90 days post immunizations were challenged with Mtb. Infection control groups were composed of animals intramuscularly injected with saline that were then challenged (n = 6) or not (n = 7) with M. tuberculosis. In order to evaluate the immune response elicited by the vaccine and the bacterial load, three animals from each group were euthanized by cervical dislocation at 45 days post M. tuberculosis infection (). Quadriceps muscles after vaccination or lungs lobes were collected, fixed with Paraphormaldehyde and H&E stained at different experimental points to evaluate the histology.
Blood collection and indirect ELISA for rCMX and rECMX
Blood from the caudal vein was obtained and the samples were incubated for 1 h at 37°C, centrifuged at 800 x g and the serum obtained were frozen at −20°C. The indirect ELISA was performed as previously published with fewer modifications to optimize the reaction to rECMX (de Sousa et al, 2012) as follows: 100 μL of each serum sample were diluted to 1:200 (rCMX) or 1:800 (rECMX) in 1% skim milk in saline.
Mycobacterium tuberculosis infection
To determine protective efficacy, previously quantified and stocked at −80°C, M. tuberculosis H37RV strain was thawed and the concentration adjusted to 106 CFU/mL in saline 0.05% Tween 80. The animals were injected intravenously with 100 µL of the M. tuberculosis suspension by the caudal vein. The inoculum was plated in 7H11 supplemented with OADC in order to check the challenge dose. Also, one day after the M. tuberculosis challenge, one animal from each group was euthanized by cervical dislocation and the lungs were collected, homogenized, and plated onto 7H11 supplemented with OADC in order to obtain the initial bacterial load.
Specific immune responses to rCMX and rECMX
Forty-five days post M. tuberculosis infection, three animals from each group were euthanized and the lungs and spleens were used to evaluate the specific immune response and the level of infection. Lung and spleen cells were collected from the animals and homogenized as described by Da Costa et al. (2014).Citation8 The cells were then stimulated with ConA (10 µg/mL), rCMX (10 µg/mL) or rECMX (10 µg/mL). Non stimulated cells from all animals were used as control. Monensin (3 µM; BD Pharmingen) was added to the cells that were incubated for 4 h at 37°C in a CO2 incubator. After this period, using buffer containing 5% paraformaldehyde, the cells were incubated with anti-CD4-FITC (clone: RM4-5 – eBioscience) for 20 minutes. The intracellular staining was performed after incubating the cells with Perm Wash buffer (BD Cytofix/Cytoperm Kit) using anti- IFN-γ-PE (clone: XMG1.2 eBioscience) and anti-IL-17-PERCP (clone: TC11-18H10 – BD Pharmigen). To evaluate lymph node cells, draining lymph nodes (popliteal and inguinal) from vaccinated BALB/c mice were collected two days post vaccination. Cells were fixed and treated with anti-gp38-FITC (clone: 8.1.1 – Novus Biologicals), anti-CD31-Pe-Cy7 (clone: 390 – BD Pharmigen), anti-F4/80-FITC (clone: BM8 – Novus Biologicals) and anti-CD11b-PE (clone: M1-70 – eBioscience). Cell phenotypes were acquired using a FACSVerse (BD Biosciences) leased to the Instituto de Patologia Tropical e Saúde Pública/Universidade Federal de Goiás. Lymph node endothelial cells evaluation was performed by lymphocyte gating and exclusion based upon size and granularity. The lymphocytes in the spleen or lungs were gated by size and granularity before fluorometric analysis using a FlowJo Software 8.7. At least 50,000 lymphocytes were acquired per sample.
Bacterial load evaluation
To evaluate bacillary load after vaccination, the apical lung lobe from each mouse was collected 45 days after infection, homogenized, diluted and cultured on 7H11 agar medium, supplemented with OADC. Plaques were incubated in a CO2 incubator at 37ºC. CFU counting was done manually 28 days later.
Statistical analysis
The data were digitalized using Microsoft Office Excel 2013 and GraphPad Prism version 6.0. The average and standard deviation were calculated for each experimental group. Variances among the groups were determined by One-Way ANOVA. Using Dunnet test as a post test, the differences between the groups were defined using a 95% confidence interval.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest in this study.
Authors’ contributions
BPOS developed the experiments and wrote the draft. MRNC carried out the histopathological analyses. NP provided the Advax4. APJK, AK and NP designed the experiments and wrote the manuscript. All authors read and critically revised the manuscript.
Acknowledgments
Vaxine Pty Ltd, Adelaide Australia provided the Advax4 adjuvant. Development of Advax adjuvants was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health through Contracts AI061142, HHSN272200800039C and HHSN272201400053C. Conselho Nacional de Ciência e Tecnologia (CNPq) of Ministry of Science and Technology of Brazil supported the CMX and ECMX development and the animal testing contract: 303675/2015-2. This publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or CNPq/Brazil. BPOS, MMT and RBM received fellowship from CNPq. This publication is part of BPOS dissertation of their Master degree.
References
- World Health Organisation. Global tuberculosis report 2016. ISBN 9789241565394. Available at: http://www.who.int/tb/publications/global_report/en/
- Manabe YC, Bishai WR. Latent Mycobacterium tuberculosis-persistence, patience, and winning by waiting. Nat Med. 2000;6:1327–9. https://doi.org/10.1038/82139 PMID:11100115
- Fletcher HA, Schrager L. TB vaccine development and the End TB Strategy: importance and current status. Trans R Soc Trop Med Hyg. 2016;110:212–8. https://doi.org/10.1093/trstmh/trw016 PMID:27076508
- Dockrell HM. Towards new TB vaccines: What are the challenges?. Pathog Dis. 2016;74:ftw016. https://doi.org/10.1093/femspd/ftw016 PMID:26960944
- Agger, EM. Novel adjuvant formulations for delivery of anti-tuberculosis vaccine candidates. Adv Drug Deliv Rev. 2016;1:73–82. https://doi.org/10.1016/j.addr.2015.11.012
- de Sousa EM, da Costa AC, Trentini MM, de Araújo Filho JA, Kipnis A, Junqueira- Kipnis AP. Immunogenicity of a fusion protein containing immunodominant epitopes of Ag85C, MPT51, and HspX from Mycobacterium tuberculosis in mice and active TB infection. PLoS One. 2012;7:e47781. https://doi.org/10.1371/journal.pone.0047781 PMID:23133523
- Junqueira-Kipnis AP, de Oliveira FM, Trentini MM, Tiwari S, Chen B, Resende DP, Silva BD, Chen M, Tesfa L, Jacobs WR Jr, et al. Prime-boost with Mycobacterium smegmatis recombinant vaccine improves protection in mice infected with Mycobacterium tuberculosis. PLoS One. 2013;8:e78639. https://doi.org/10.1371/journal.pone.0078639 PMID:24250805
- da Costa AC, Costa-Júnior Ade O, de Oliveira FM, Nogueira SV, Rosa JD, Resende DP, Kipnis A, Junqueira-Kipnis AP. A new recombinant BCG vaccine induces specific Th17 and Th1 effector cells with higher protective efficacy against tuberculosis. PLoS One 2014;9:e112848. https://doi.org/10.1371/journal.pone.0112848 PMID:25398087
- Chatterjee S, Dwivedi VP, Singh Y, Siddiqui I, Sharma P, Van Kaer L, Chattopadhyay D, Das G. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog. 2011;7:e1002378. https://doi.org/10.1371/journal.ppat.1002378 PMID:22102818
- Cooper PD, Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising β-D-[2 ->1] poly(fructo-furanosyl) α-D-glucose polymers. Glycobiology. 2011;21:595–606. https://doi.org/10.1093/glycob/cwq201 PMID:21147758
- Wong TM, Petrovsky N, Bissel SJ, Wiley CA, Ross TM. Delta inulin-derived adjuvants that elicit Th1 phenotype following vaccination reduces respiratory syncytial virus lung titers without a reduction in lung immunopathology. Hum Vaccin Immunother. 2016;12:2096–2105. https://doi.org/10.1080/21645515.2016.1162931 PMID:27215855
- Petrovsky N, Larena M, Siddharthan V, Prow NA, Hall RA, Lobigs M, Morrey J. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. J Virol. 2013;87:10324–33. https://doi.org/10.1128/JVI.00480-13 PMID:23864620
- Bielefeldt-Ohmann H, Prow NA, Wang W, Tan CS, Coyle M, Douma A, Hobson-Peters J, Kidd L, Hall RA, Petrovsky N. Safety and immunogenicity of a delta inulin adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals. Veterinary Research. 2014;45:130. https://doi.org/10.1186/s13567-014-0130-7 PMID:25516480
- Honda-Okubo Y, Ong CH, Petrovsky N. Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection. Vaccine. 2015;33:4892–900. https://doi.org/10.1016/j.vaccine.2015.07.051 PMID:26232344
- Li L, Honda-Okubo Y, Li C, Sajkov D, Petrovsky N. Delta inulin adjuvant enhances plasmablast generation, Expression of activation-induced cytidine deaminase and B-Cell affinity maturation in human subjects receiving seasonal influenza vaccine. PLoS One. 2015;10:e0132003. https://doi.org/10.1371/journal.pone.0132003 PMID:26177480
- Honda-Okubo Y, Kolpe A, Li L, Petrovsky N. A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax™) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine. 2014;32:4651–9. https://doi.org/10.1016/j.vaccine.2014.06.057 PMID:24958701
- McPherson C, Chubet R, Holtz K, Honda-Okubo Y, Barnard D, Cox M, Petrovsky N. Development of a SARS coronavirus vaccine from recombinant spike protein plus delta inulin adjuvant. Methods Mol Biol. 2016;1403:269–84. https://doi.org/10.1007/978-1-4939-3387-7_14 PMID:27076136
- Rodriguez-Del Rio E, Marradi M, Calderon-Gonzalez R, Frande-Cabanes E, Penadés S, Petrovsky N, Alvarez-Dominguez C. A gold glyco-nanoparticle carrying a Listeriolysin O peptide and formulated with Advax™ delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine. 2015;33:1465–73. https://doi.org/10.1016/j.vaccine.2015.01.062 PMID:25659269
- Hess JA, Zhan B, Torigian AR, Patton JB, Petrovsky N, Zhan T, Bottazzi ME, Hotez PJ, Klei TR, Lustigman S, et al. The immunomodulatory role of adjuvants in vaccines formulated with the recombinant Antigens Ov-103 and Ov-RAL-2 against Onchocerca volvulus in mice. PLoS Negl Trop Dis. 2016;10:e0004797. https://doi.org/10.1371/journal.pntd.0004797 PMID:27387453
- Feinen B, Petrovsky N, Verma A, Merkel TJ. Advax-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clin Vaccine Immunol. 2014;21:580–6. https://doi.org/10.1128/CVI.00019-14 PMID:24554695
- Gordon D, Kelley P, Heinzel S, Cooper P, Petrovsky N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled Phase 1 study. Vaccine. 2014;32:6469–77. https://doi.org/10.1016/j.vaccine.2014.09.034 PMID:25267153
- Gordon DL, Sajkov D, Honda-Okubo Y, Wilks SH, Aban M, Barr IG, Petrovsky N. Human Phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax™ delta inulin adjuvant. Vaccine. 2016;34:3780–6. https://doi.org/10.1016/j.vaccine.2016.05.071 PMID:27342914
- Heddle R, Russo P, Petrovsky N, Hanna R, Smith A. Immunotherapy – 2076. A controlled study of delta inulin-adjuvanted honey bee venom immunotherapy. World Allergy Organ J. 2013;6:P158.
- Petrovsky N. Freeing vaccine adjuvants from dangerous immunological dogma. Expert Rev Vaccines. 2008;7:7–10. https://doi.org/10.1586/14760584.7.1.7 PMID:18251687
- Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Safety. 2015;38:1059–74. https://doi.org/10.1007/s40264-015-0350-4 PMID:26446142
- Honda-Okubo Y, Saade F, Petrovsky N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012;30(36):5373-81.
- Da Costa AC, Resende DP, Santos BPO, Zoccal KF, Faccioli LH, Kipnis A, Junqueira-Kipnis AP. Modulation of macrophage responses by CMX, a fusion protein composed of Ag85c, MPT51, and HspX From Mycobacterium tuberculosis. Front Microbiol 2017;8:623.of adaptive immune responses. Vaccine. 2017;30:5373–81.
- Trentini MM, Oliveira FM, Kipnis A, Junqueira-Kipnis AP. The role of neutrophils in the induction of specific Th1 and Th17 during vaccination against tuberculosis. Front Microbiol. 2016;7:898. https://doi.org/10.3389/fmicb.2016.00898 PMID:27375607
- Tamburini BA, Burchill MA, Kedl RM. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat Commun. 2014;5:3989. https://doi.org/10.1038/ncomms4989 PMID:24905362
- Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. https://doi.org/10.1084/jem.178.6.2249 PMID:7504064
- Bourque SL, Davidge ST, Adams MA. The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1288–95. https://doi.org/10.1152/ajpregu.00397.2010 PMID:21368267
- Garcia-Pelayo MC, Bachy VS, Kaveh DA, Hogarth PJ. BALB/c mice display more enhanced BCG vaccine induced Th1 and Th17 response than C57BL/6 mice but have equivalent protection. Tuberculosis (Edinb). 2015;95:48–53. https://doi.org/10.1016/j.tube.2014.10.012 PMID:25467292
- Maroof A, Yorgensen YM, Li Y, Evans JT. Intranasal vaccination promotes detrimental Th17-Mediated immunity against influenza infection. PLoS Pathog. 2014;10:e1003875. https://doi.org/10.1371/journal.ppat.1003875 PMID:24465206
- Liu W, Xu Y, Yan J, Shen H, Yang E, Wang H. Ag85B synergizes with ESAT-6 to induce efficient and long-term immunity of C57BL/6 mice primed with recombinant Bacille Calmette-Guerin. Exp Ther Med. 2017;13:208–214. PMID:28123491
- Reither K, Katsoulis L, Beattie T, Gardiner N, Lenz N, Said K, Mfinanga E, Pohl C, Fielding KL, Jeffery H, et al. Safety and immunogenicity of H1/IC31®, an adjuvanted TB subunit vaccine, in HIV-infected adults with CD4+ lymphocyte counts greater than 350 cells/mm3: a phase II, multi-centre, double-blind, randomized, placebo-controlled trial. PLoS One. 2014;9:e114602. https://doi.org/10.1371/journal.pone.0114602 PMID:25490675
- Sreejit G, Ahmed A, Parveen N, Jha V, Valluri VL, Ghosh S, Mukhopadhyay S. The ESAT-6 protein of Mycobacterium tuberculosis interacts with beta-2-microglobulin (β2M) affecting antigen presentation function of macrophage. PLoS Pathog. 2014;10:e1004446. https://doi.org/10.1371/journal.ppat.1004446 PMID:25356553
- Singh V, Kaur C, Chaudhary VK, Rao KV, Chatterjee S. M. tuberculosis secretory protein ESAT-6 induces metabolic flux perturbations to drive foamy macrophage differentiation. Sci Rep. 2015;5:12906. https://doi.org/10.1038/srep12906 PMID:26250836