ABSTRACT
The poor immune response elicited by trivalent influenza vaccines (TIVs) in children can be enhanced by the addition of adjuvants. This observer-blind, randomized Phase III trial assessed the immunogenicity and safety of the MF59-adjuvanted trivalent influenza vaccine FLUAD® (aTIV) and a non-adjuvanted TIV, in healthy children (aged 6 to <72 months) from 3 centers in Mexico, during the 2014–2015 season. The primary objectives were to assess the non-inferiority of aTIV to TIV, measured by geometric mean titers (GMTs), and the safety of aTIV and TIV. Seroconversion was one of several secondary objectives. In total, 287 children were enrolled. The non-inferiority criteria for GMTs and seroconversion were met for aTIV for all 3 vaccine strains. Lower bounds of the 95% confidence intervals for all 3 aTIV:TIV vaccine ratios were >2, showing that the immunogenicity of aTIV was superior to that of TIV for all 3 strains. Solicited adverse events (AEs) were experienced more frequently with aTIV than TIV by younger children (aged 6 to <36 months), but were more frequent with TIV than aTIV in older children (aged 36 to <72 months) who had been vaccinated previously. More unsolicited AEs were associated with aTIV than the TIV. All AEs were of mild or moderate severity. No deaths, serious AEs, or AEs leading to premature withdrawal were reported. Overall, aTIV was highly immunogenic and was well tolerated in healthy children 6 to <72 months of age. These results indicate that aTIV may be a beneficial addition to national pediatric vaccination programs.
Introduction
Influenza is a serious disease as it can lead to severe morbidity and mortality in at-risk groups such as children.Citation1 Children experience the highest attack rates for influenzaCitation1 and while infections can be self-limiting in many cases, the risk of hospitalization is increased in this population compared with healthy adults.Citation2 Children can also disseminate influenza in households and the community as they shed virus for longer periods than adults, leading to influenza infections in others, including those at-risk (e.g. older adults [aged ≥65 years], those with co-morbidities, and younger children).Citation3,4
Influenza was one of the top 10 causes of death in Mexico in 2014 and therefore it is considered a public health priority.Citation5 In 2013, there were 4480 cases of confirmed influenza across all age groups, which led to 314 deaths, the majority of whom were vulnerable or at-risk.Citation5 An assessment of those hospitalized for influenza-like illness in Mexico City confirmed influenza A/H3N2 and B in 25 and 29% respectively, of those aged ≤18 years. Furthermore, of the different viruses assessed, the influenza virus was the most commonly identified.Citation6
Vaccination is the most effective method for preventing influenza and seasonal influenza vaccination is recommended by the World Health Organization (WHO) for children aged 6 months to 5 years.Citation7 For example, estimates from the Centers for Disease Control and Prevention indicate that influenza vaccination averted approximately 7.2 million illnesses, 3.1 million medically attended illnesses, and 90,000 hospitalizations associated with influenza, across all ages, in the 2013–2014 influenza season in the United States alone.Citation8 In Mexico, seasonal influenza vaccination has been shown to significantly (p = 0.00002) reduce influenza-related hospitalizations in children aged <5 years, from 7.5% before the vaccination program to 3.4% after vaccination was introduced.Citation9 However, children tend to have a weak and short-lived immune response to current non-adjuvanted trivalent influenza vaccines (TIVs) because their immune systems are immature.Citation2,4,10 The addition of adjuvants to trivalent influenza vaccines (aTIVs) can enhance the immune response, potentially improving protection in this population.Citation2,11-13
The influenza vaccine FLUAD® includes the oil-in-water adjuvant MF59, which has been shown to boost the immune response in children, adults, and elderly individuals.Citation10,12,14-16 Results from several clinical trials in adults and the elderly have demonstrated the immunogenicity of FLUAD in these patient populations and it has been licensed for influenza prophylaxis in older adults since 1997.Citation14,15,17 Results from a large Phase III trial in over 6000 children showed that higher and more durable hemagglutination inhibition (HI) titers against both homologous and heterologous influenza strains were induced following vaccination with FLUAD (n = 3125) than with the TIV.Citation11 In agreement with previous reports, reactogenicity rates were higher in FLUAD recipients than non-adjuvanted vaccine recipients, and the majority of events were mild or moderate in severity.Citation11 Results from another Phase III trial that included over 4500 young children (aged 6–72 months), showed that FLUAD (n = 1934) was associated with an increase in vaccine efficacy, antibody responses, and durability compared with a non-adjuvanted influenza vaccine.Citation12 Antibody titers of ≥40 against heterologous strains were also induced by 2 doses of FLUAD in ≥95% of recipients. The proportion of children aged 6–<36 months that experienced an adverse event (AE) was comparable between vaccination groups, with a relative risk of a solicited AE of 1.04 (95% confidence interval [CI] 0.98–1.09) for aTIV:TIV.Citation12 FLUAD was recently licensed in Canada for seasonal vaccination of children aged 6–<24 months.Citation18 FLUAD is currently approved in 38 countries, including Canada and many European countries, and it was recently approved by US Food and Drug Administration (FDA) for the prevention of seasonal influenza in individuals aged ≥65 years.Citation19
This Phase III trial aimed to assess the safety and immunogenicity of FLUAD (aTIV), compared with a non-adjuvanted influenza vaccine (Fluzone®; TIV), in healthy children at 3 centers in Mexico. The results will provide further evidence of the tolerability and immunogenicity of FLUAD in a pediatric population.
Results
Children
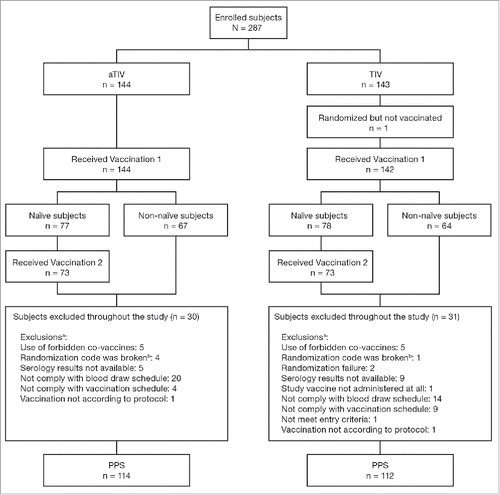
In total, 287 healthy children were enrolled, 144 in the aTIV group and 143 in the TIV group (). Similar numbers of children were excluded from the per protocol population of each vaccine group; 30 from the aTIV group and 31 from the TIV group (). The most common reason for exclusion for both groups was non-compliance with the blood draw schedule. Similar numbers of children were enrolled by the 3 centers (n = 95, 117, and 75).
Figure 1. Study flow. aTIV, adjuvanted trivalent influenza vaccine; PPS, per protocol set; TIV, trivalent influenza vaccine. a a child can have more than 1 exclusion; b accidentally unblinded.
The vaccine groups were well balanced at baseline (). The mean age was 29.5 months for the aTIV group and 30.1 months for TIV, the split of males to females was approximately even and 99% of children in either arm were Hispanic. Overall, more children in both vaccine groups were vaccine-naïve and there was an even distribution of children across the 3 age groups in both vaccine groups ().
Table 1. Subject demographic characteristics at baseline.
Immunogenicity
The non-inferiority criteria for geometric mean titers (GMTs) were met for all 3 virus strains in the overall population at 21 days after the last vaccination (, ). The GMTs were higher in the aTIV group than in the TIV group at 21 days after the last vaccination. The mean GMT ratios (95% CIs) for aTIV:TIV were: 4 (3–6) for A(H1N1) strain, 3 (2–4) for A(H3N2) strain, and 5 (3–6) for the B strain.
Table 2. Geometric mean HI titers and vaccine group ratios at 21 days after last vaccination in the overall population (children aged 6 to <72 months).
Figure 2. GMT ratios of aTIV to TIV for the 3 vaccine strains in children aged 6–<72 months, 21 days after the last vaccination. The mean GMT ratio is indicated by the diamond and the bars represent the 95% confidence intervals. aTIV, adjuvanted trivalent influenza vaccine; GMT, geometric mean titer; TIV, trivalent influenza vaccine.
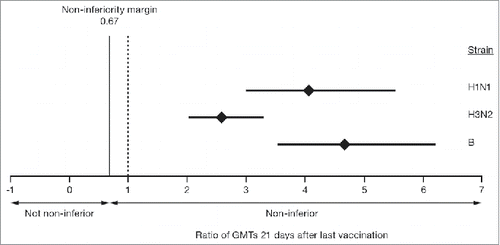
This protocol prospectively allowed for a superiority analysis in the event that the primary objective of GMT non-inferiority was met. The GMT ratio of aTIV to TIV was evaluated, with greater margins than the non-inferiority cutoff of 0.67. Lower bounds of the 95% CIs for all 3 aTIV:TIV vaccine ratios were >2, showing that the immunogenicity elicited by aTIV was superior to that of TIV for the 3 vaccine strains tested ().
Analysis of GMTs by age subgroups showed that they were higher with aTIV than with TIV in all 3 groups and for all 3 vaccine strains (). The GMT vaccine group ratios across all 3 strains were largest in the youngest (6–<18 months) age subgroup and smallest in the oldest (36–<72 months) age subgroup.
Table 3. Geometric mean titers and ratios at 21 days after last vaccination, by age group.
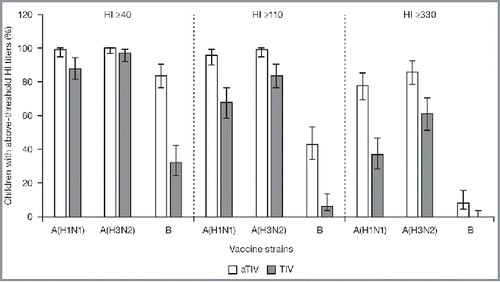
At 21 days after the last vaccination, the seroconversion rate following vaccination with aTIV met the non-inferiority criteria for all 3 virus strains in the overall population (). When analyzed by age, the seroconversion rate was greater for aTIV than for TIV in all 3 groups, for all 3 strains. The difference between the 2 vaccines was particularly pronounced for the B strain (). The proportion of children achieving HI titers ≥40, and the more stringent HI titer assessments, ≥110, and ≥330, 21 days after the last vaccination was higher for aTIV than for TIV, for all 3 virus strains and the 95% CIs did not overlap for the A(H1N1) and B strains (). When immunogenicity for the aTIV was analyzed separately in children who were vaccine-naïve (not received ≥2 doses of seasonal influenza vaccine since 1 July 2010) and non-naïve (received ≥2 doses of seasonal influenza vaccine since 1 July 2010), vaccine status appeared to have little effect on the response for all 3 strains. GMTs for the aTIV were of a similar magnitude for the 2 groups, with larger geometric mean ratios (GMRs) for the vaccine-naïve than for the non-naïve group. For the aTIV, the proportion of children achieving HI titers ≥40, ≥110, and ≥330 was also similar for vaccine-naïve and non-naïve, for all 3 strains and at all 3 thresholds. For the TIV, vaccine status did appear to influence GMTs as they were higher for the non-naïve than for the naïve group. GMRs varied by strain for the TIV; the GMR was larger for the non-naïve than for the naïve group for the A(H1N1) strain, while the reverse was true for the A(H3N2) strain. The GMRs were strain similar for the 2 groups for the B strain. For the TIV, the proportion of children achieving HI titers ≥40, ≥110, and ≥330 was higher in the non-naïve than in the naïve group for all 3 strains. These differences were particularly pronounced at the highest threshold for A(H1N1) and A(H3N2).
Figure 3. Differences between aTIV and TIV in seroconversion rate for the 3 vaccine strains in children aged 6–<72 months, 21 days after the last vaccination. The mean GMT ratio is indicated by the diamond and the bars represent the 95% confidence intervals. Non-inferiority criteria were met when the lower bound of the 2-sided 95% CI for the vaccine group difference in seroconversion rates was ≥−10% for each of the 3 strains, indicated by the dashed line.
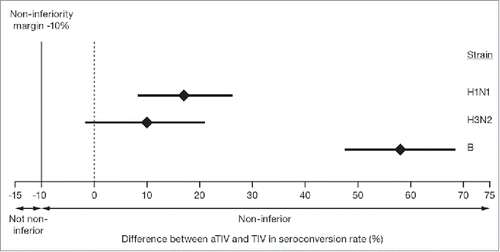
Table 4. Seroconversion rates at 21 days after last vaccination, by age group.
Safety
The number of children aged 6–<36 months in the non-naïve group (n = 44) was smaller than the number in the naïve group (n = 137). In vaccine-naïve children aged 6 to <36 months, the proportion experiencing any solicited AEs after any vaccination was higher in the group that received aTIV (74%) than in those who received TIV (59%). In addition, this difference between the proportions decreased from the first vaccination to the second vaccination (). The most common solicited local and systemic events in vaccine-naïve children aged 6 to <36 months were injection-site tenderness (aTIV 45%; TIV 32%) and irritability (aTIV 37%; TIV 25%), respectively. The proportion of non-naïve children aged 6–<36 months experiencing any solicited AE was higher with aTIV than with TIV (). The most common solicited local and systemic events in this group were injection-site tenderness (aTIV 41%; TIV 19%) and diarrhea (aTIV 14%; TIV 27%), respectively.
Table 5. Overview of solicited AEs by age group: A, children aged 6 to <36 months; B, children aged 36 to <72 months and by vaccination status.Footnote*
In the 36–<72 months age group, the number of vaccine-naïve children was too small for any meaningful analysis (). In the non-naïve group aged 36 to <72 months, slightly more children in the TIV group compared with the aTIV group experienced any event, which is likely to result from a difference in the rate of local events (). The most common solicited local and systemic events in the non-naïve group aged 36 to <72 months were injection-site pain (aTIV 32%; TIV 40%) and change in eating habits (aTIV 18%; TIV 8%), respectively. The majority of solicited local and systemic AEs in both age groups were of mild or moderate intensity. Severe injection-site pain was reported for 3 children only; 2 aged 6 to <36 months (1 vaccine-naïve and 1 non-naïve) and 1 aged 36 to <72 months (vaccine non-naïve), all had received aTIV. In vaccine-naïve children aged 6 to <36 months severe solicited systemic reactions were reported for 3 who received aTIV (irritability n = 1 and diarrhea n = 2) and 5 who received TIV (persistent crying, vomiting, diarrhea, all n = 1 and irritability n = 2). Severe solicited systemic reactions were reported for 2 non-naïve children aged 36 to <72 months only (chills and myalgia, both n = 1) and both had received aTIV. No severe systemic reactions were reported for non-naïve children aged 6 to <36 months or for vaccine-naïve children aged 36 to <72 months.
The rate of unsolicited AEs reported for children (aged 6 to <72 months) was of a similar magnitude for aTIV and TIV, in both those who were vaccination-naïve (45% and 38%, respectively) and those who were non-naïve (14% and 9%, respectively) (). The most commonly reported unsolicited AE was nasopharyngitis for aTIV (22%) and pharyngitis (14%) for TIV, in vaccine-naïve children. In non-naïve children nasopharyngitis was the most commonly reported unsolicited AE for both aTIV and TIV (both 3%). The proportion of children that experienced medically attended AEs (MAAEs) was comparable between the vaccine groups for both the naïve and non-naïve groups, with far fewer being reported by non-naïve children (). No deaths, no serious adverse events (SAEs), and no AEs leading to premature withdrawal were reported ().
Table 6. Overview of unsolicited events, by vaccination status.Footnote*
Discussion
The increased risk of influenza infection in children, compared with healthy adults, has prompted many countries, including those in North America, to incorporate annual influenza vaccinations into their healthcare programs, as recommended by the WHO.Citation7 The currently available TIVs elicit a sub-optimal immune response in children, spurring the development of vaccines with adjuvants such as AS03 or MF59, which stimulate more robust antibody production against the influenza antigens.
The results from this study show that the aTIV was non-inferior to the TIV for all 3 vaccine strains tested in healthy children aged 6–<72 months in Mexico, during the 2014–2015 season. The magnitude of the GMT ratios shows that the aTIV has a superior immune response than that of TIV against all 3 virus strains tested. These findings support those of previous studies of aTIV in children that have shown higher immune responses, compared with TIV.Citation11,12 These findings are unlikely to be due to differences between the 2 brands of vaccine as they contained the same antigens at the same concentrations and similar excipients, as well as being administered in the same way. Therefore, it is most likely to be the inclusion of the MF59 adjuvant in the vaccine that improved the immune response of the aTIV compared with the TIV.
While efficacy of the adjuvanted vaccine has been shown previously, with a relative efficacy versus TIV of 75% (95% CI: 55–87)Citation12 the superiority of the adjuvanted vaccine over non-adjuvanted vaccines has not been demonstrated before. Although 2 previous studies assessing aTIV in young children have shown high immune responses, neither have shown superiority of aTIV over TIV.Citation11,12 One studyCitation11 failed to meet the pre-specified criteria to show superiority, which required GMT and seroconversion rates to reach statistical significance: although the superiority criteria for GMT were met, those for seroconversion were not. The second studyCitation12 could not demonstrate superiority as this was not prospectively included in the study design. Nonetheless, the consistently higher GMTs reported with aTIV in both studies, against both homologous and heterologous influenza strains, and the superior immunogenicity of aTIV compared with TIV in the present study underscore the immunologic benefit derived from the MF59 adjuvant.
In this study, HI titers were higher in the aTIV group than the TIV group, for ≥40, ≥110, and ≥330 thresholds, and for all 3 strains. Assessment of HI titers ≥110 and ≥330 is important because the use of HI ≥40 as a correlate of protection is based on studies in adults and has been shown to be inappropriate for evaluating responses in children, based on antibody titers against H3N2.Citation20 Antibody titers of ≥110 for H3N2 have been shown to predict a 50% clinical protection rate, which is similar to the rate for titers of ≥40 in adults. However, the ≥330 cutoff was shown to predict an 80% clinical protection rate for H3N2, which may offer a public health benefit.Citation20
The robust immunogenicity demonstrated by the aTIV is important for the pediatric population as children tend to have a poor response to non-adjuvanted TIV while experiencing the highest attack rates for influenza and a high burden of morbidity.Citation2,4 In Mexico, the H1N1 and H3N2 strains are known to be endemic and were identified as the predominant circulating strains during the 2013–2014 winter.Citation5 Furthermore, a recent sero-analysis has shown that, despite high transmission rates of A(H1N1) during the 2009 pandemic in Mexico, a large proportion of the population, including children, have no or low levels of A(H1N1)pdm09 neutralizing antibodies, and so they remain susceptible to infection.Citation21 Together these findings illustrate the need for a vaccine that induces a strong immune response in children. In Mexico, the influenza vaccination program is based on the use of TIVs, therefore the aTIV assessed in this study may be a useful addition as it has shown superior immunogenicity to a TIV.
Cross-reactive immune responses can also be beneficial in children when vaccine strains and circulating viruses are mismatched. Heterologous responses were not assessed in the study reported here, but it is highly likely that the pattern of increased cross-reactive responses elicited by the MF59-adjuvanted vaccine, as reported in other prior pediatric studies,Citation11,12 could also have occurred in the children included in this study.
The results from this study show that the aTIV was well tolerated, supporting inclusion of this vaccine in pediatric seasonal influenza vaccination programs. The proportions of children experiencing solicited AEs were higher for aTIV than they were for TIV, but no AEs led to withdrawal from the study. The pattern of unsolicited AEs was similar for aTIV and TIV and no SAEs were reported. Overall, the safety results described here are aligned with those from previous reports,Citation11,12 with no new emergent concerns.
AS03 is another proprietary adjuvant used in influenza vaccines to enhance the immune response. MF59 and AS03 are both oil-based adjuvants that contain squalene, but α-tocopherol is also present in AS03,Citation22 but not MF59.Citation14 The AS03-adjuvanted monovalent pandemic (H1N1) vaccine in children has been shown to be effective in preventing influenza-related hospitalizationCitation23,24 and to be clinically superior to a non-adjuvanted pandemic monovalent vaccine.Citation25 An early study of an AS03-adjuvanted TIV has shown that this vaccine is also immunogenic.Citation26 Safety analyses have shown that while the AS03-adjuvanted monovalent vaccine is generally well tolerated, there may be an increased risk of febrile seizures, although the risk was less than that associated with contracting pandemic influenza,Citation27 and there may also be an increased risk of narcolepsy,Citation28 which has been tentatively associated with α-tocopherol.Citation29 These results support the use of non-aluminum based adjuvants to enhance the immune response to influenza vaccines and also illustrate how the safety profile of vaccines can vary.
This study has some limitations. The study is small and there were few older children (aged 36–<72 months) in the vaccine-naïve group, making sub-analyses of this population challenging. However, this is to be expected as most children will have received at least 1 vaccination by this age, and so it is reflective of the prevailing clinical practice. In addition, many children were excluded from immunogenicity study analyses owing to non-compliance with the blood draw schedule. This non-compliance was an unavoidable consequence of a concurrent national vaccination campaign, which was initiated by the government after more than half the subjects in this study had been enrolled. Assessment of the immune responses against heterologous strains in this population would also have provided useful additional data describing the breadth of immunogenicity of these vaccines. One strength of this study is that the inclusion and analysis of both vaccine-naïve and non-naïve children makes it more reflective of actual clinical practice. The randomized, controlled, observer-blind design of the study has allowed robust analysis of the data and hence clear conclusions to be drawn.
Conclusions
The aTIV was highly immunogenic and well tolerated in healthy children in Mexico. The HI titers elicited show that the aTIV is not only non-inferior to TIV with respect to GMT and seroconversion rates, but also that the GMT data for aTIV are superior to TIV. Overall, these findings suggest that the aTIV may be a beneficial addition to pediatric vaccination programs.
Methods
Study design
This was an observer-blind, multicenter, randomized Phase III trial that assessed the safety and immunogenicity of vaccination with FLUAD, an aTIV, compared with an unadjuvanted TIV (Fluzone®) in healthy children in Mexico. The trial was conducted at 3 centers in Mexico, between 23 October 2014 and 20 May 2015.
The primary objectives were: to demonstrate non-inferiority of aTIV to TIV, measured by GMTs and to assess the safety of the aTIV and TIV. Secondary objectives included: assessment of non-inferiority of aTIV to TIV by seroconversion; evaluation of immunogenicity of aTIV and TIV by GMRs and the proportion of children with HI titers ≥40, ≥110, and ≥330; and if non-inferiority was established, to evaluate the GMT ratio of aTIV to TIV using margins greater than the non-inferiority cutoff.
Children
Healthy male and female children aged 6–<72 months whose parent(s) or guardian(s) had voluntarily given written informed consent and who could comply with the trial procedures were included in the study. Children and their parent(s) or guardian(s) also had to agree to have serum samples stored for future testing (Table S1).
Major exclusion criteria included: any progressive, unstable, or uncontrolled clinical conditions or a fatal prognosis; hypersensitivity to any vaccine components; a history of seizures, severe neurological disorders or Guillain-Barré syndrome; abnormal functioning of the immune system; contraindication of intramuscular (IM) vaccination; or any clinical condition or planned procedure that might interfere with the trial schedule or results. Receipt of influenza (within 6 months) or other vaccines (within 14 days) also precluded enrollment (Table S1).
Children were stratified 1:1:1 by age group; 6–<18 months, 18–<36 months, and 36–<72 months and were assigned randomly 1:1 within the strata to receive vaccination with aTIV (FLUAD) or the TIV (Fluzone®), according to the prescribing information, on Day 1 (). Centers for conducting this study were selected based on their experience in clinical research and their access to a pediatric population. At each center, randomization was assigned based on the given subject number, using a validated web-based system, which automatically generated the group assignment in the specified ratio.
Previous influenza vaccination status for eligible children was defined as: “vaccine non-naïve” – children who had received 2 or more doses of seasonal influenza vaccine since 1 July 2010; “vaccine-naïve” – children who had not received 2 or more doses of seasonal influenza vaccine since 1 July 2010.
Vaccines
The aTIV (batch numbers: #IA142503 and IA142501) and the comparator TIV (batch number: IAU1189AC) contained ≥15µg HA of A/H1N1 (California/2009), A/H3N2 (Texas/2012), and B influenza (Massachusetts/2012), according to the WHO recommendations for 2014/2015. Both vaccines were administered IM; in the anterolateral aspect of the thigh to subjects aged <36 months, or in the deltoid muscle of the (preferably) non-dominant arm to subjects aged >36 months.
Children aged 6–<36 months received a 0.25 mL dose of vaccine and those aged ≥36 months received a 0.5 mL dose of vaccine, as recommended by medical guidelines. Children who were influenza non-naïve received 1 vaccination with either aTIV or TIV on Day 1. Children who were vaccine-naïve received 2 vaccinations with either aTIV or TIV on Days 1 and 29.
Study endpoints
GMTs were assessed by HI assay from blood samples (each approximately 5 mL) taken on Day 1 and on Day 22 for non-naïve children and on Day 1 and Day 50 for naïve children. HI titers were also used to calculate the proportion of children achieving seroconversion on Day 22 for non-naïve and Day 50 for naïve children for all 3 vaccine strains and GMRs from baseline. The proportion of children with a HI titer ≥40, ≥110, and ≥330 on Day 1 and on Day 22 for non-naïve children and on Day 1 and Day 50 for naïve children was also assessed.
The HI assay is a standard, widely used measure based on the binding of anti-hemagglutinin antibodies blocking the interaction between influenza viruses and red blood cells. HI assays were conducted by Novartis or a designated laboratory and, very briefly, involved: a standard number of red blood cells were incubated with serially diluted sera in the presence of viral isolates, and red blood cell agglutination recorded.
Solicited local and systemic AEs were collected using a diary and unsolicited AEs were collected through spontaneous reporting. The diary card was completed daily, reporting solicited local and systemic AEs from Day 1 to Day 7, following each vaccination. For non-naïve children, all unsolicited AEs, along with any treatments, were recorded from Day 1 to Day 22. In naïve children, all unsolicited AEs, along with any treatments, were recorded from Day 1 to Day 29. Following the second vaccination, the diary card was completed for a further 6 days and unsolicited AEs were recorded for a further 21 days. Solicited local reactions included: ecchymosis, erythema, induration, swelling; and tenderness in children aged 6–<36 months; or pain in children 36–<72 months. Local reactions were categorized as: none (0 mm), any (≥1 mm). Systemic reactions included: change in eating habits, vomiting, diarrhea, and fever ≥38°C. In children aged 6–<36 months, sleepiness, persistent crying, irritability were also included, while in children aged 36–<72 months, chills, myalgia, arthralgia, headache, and fatigue were evaluated. The severity of all AEs was classified as mild, moderate, or severe by the investigator, based on specific criteria for solicited AEs, and the relationship of an AE to the study treatment was defined by the investigator as not, possibly, or probably related. SAEs were recorded by the investigator and assessed for the relationship to the vaccine.
Statistical analyses
Children were recruited from central and southern areas of the country, as well as the metropolitan area of Mexico City, meaning the sample was representative of the general population. Sample size was determined based on previous trial data to demonstrate that at 21 days after the last vaccination, the GMT ratios of aTIV to TIV were non-inferior, with the lower limit of the 2-sided 95% CI above 0.67 (−0.176 on log10 scale) for each vaccine strain (1-sided α = 2.5%). With 126 subjects per group, a single test has a power of 92% for H1N1 and >99% for H3N2 and B. As there were 3 comparisons, the resulting power was 90%. Therefore, to account for subject withdrawals, 141 children were recruited to each group.
The per protocol set was used for all the immunogenicity analyses. The primary analyses were repeated using the full analysis set as a measure of sensitivity. Safety endpoints were reported by vaccine group and included: the proportion of children reporting solicited local and systemic AEs from Day 1 to Day 7 following each vaccination; the proportion of children reporting unsolicited AEs from Day 1 to Day 22 for non-naïve children and from Day 1 to Day 50 for naïve children; and the proportion of children with MAAEs, AEs leading to withdrawal, and SAEs from Day 1 to Day 22 for non-naïve children and from Day 1 to Day 50 for naïve children.
Immunogenicity endpoints, including GMTs, seroconversion, GMRs, and HI titers were reported by vaccine group on Days 1 and 22 for non-naïve children and on Days 1 and 50 for naïve children. Seroconversion was defined as HI ≥40 subject with a pre-vaccination HI titer <10; a minimum 4-fold increase HI titer for children with a pre-vaccination HI titer ≥10, on Day 22 for non-naïve children or Day 50 for naïve children. GMRs were calculated as Day 22/Day 1 for non-naïve children or Day 50/Day1 for naïve children. GMTs and associated CIs were determined using analysis of covariance with factors for vaccine group, age group, naïve/non-naïve, and center.
The primary objective was determined to have been met if the GMT ratios of aTIV to TIV 21 days after the last vaccination were demonstrated to be non-inferior, with the lower limit of the 2-sided 95% CI being above 0.67 for each vaccine strain. Non-inferiority of seroconversion for aTIV compared with TIV would be shown if the lower limit of the 95% CI around the difference in seroconversion rates between aTIV and TIV was higher than −10%. If non-inferiority of GMT ratios 21 days after the last vaccination was demonstrated, then “higher non-inferiority”/superiority was tested. The superiority margin would be increased by 0.01 unit (on the log scale) and superiority would be demonstrated if the lower limit of the 2-sided 95% CI was at least equal to the margin. Sample size was calculated based on data from previous studies. A single test with 126 children in each vaccine group has a power of 92% for H1N1 and >99% for H3N2 and B strains. Therefore, the overall power is 90%. To account for dropouts, 141 children were planned to be included in each group.
Ethics
This study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki, and was approved by local ethics committees.
Abbreviations
| AE | = | adverse event |
| aTIV | = | adjuvanted trivalent influenza vaccine |
| CI | = | confidence interval |
| GMR | = | geometric mean ratio |
| GMT | = | geometric mean titer |
| HI | = | hemagglutination inhibition |
| MAAE | = | medically attended adverse event |
| SAE | = | serious adverse event |
| TIV | = | trivalent influenza vaccine |
| WHO | = | World Health Organization. |
Disclosure of potential conflicts of interest
FC was employed by the Novartis group of companies at the time of the study conduct, analysis, and completion. ACV and GVZ declare no conflict of interest. MG was employed by the Novartis group of companies at the time of the study conduct, analysis, and completion. EH is employed by Seqirus (formerly part of Novartis Vaccines and Diagnostics Inc.). WJ was employed by the Novartis group of companies at the time of the study conduct analysis and completion. AKA was employed by the Novartis group of companies at the time of study conduct, analysis, and completion. SP was employed by the Novartis group of companies at the time of study conduct, data analysis, and clinical study report (CSR) completion. ACV and GVZ were the principal investigators in this study. None of the above have any financial interest in the study data.
Author contributions
FC was responsible for medical monitoring for the country. ACV and GVZ provided analysis and interpretation of data and developed, reviewed, and finalized the manuscript. MG was responsible for study management, country oversight, and data collection. EH provided input to data analysis and interpretation, review, and scientific input to each draft and finalization of the CSR. WJ provided statistical guidance for the study design, sample size calculation, data analysis, and interpretation, and finalizing the CSR. AKA contributed to the study design, data collection, manuscript review, and finalization. SP provided input to the data analysis and interpretation, review, and scientific input to each draft and finalization of CSR.
Trial registration
ClinicalTrials.gov: NCT02255279
Previous presentations
These data have not been presented previously.
Acknowledgments
The trial was funded by Novartis Vaccines and Diagnostics, Inc. Note that the influenza vaccine business of Novartis Vaccines and Diagnostics, Inc., was acquired by the CSL group on July 31, 2015, and is currently doing business as Seqirus Inc., a CSL company.
The authors thank the children and their parents/guardians for taking part in this trial. The authors also thank Dr. Carlos Medina Pech for contributions to the study administration, collating data, and assistance in developing the CSR, Srikanth Secunlapuram for assistance in developing the CSR and Valerio Romolini for assistance with statistical analysis of the study data.
The authors also thank Dr. Emma Fulkes (PAREXEL) and Melanie Meister-Broekema (PAREXEL) for manuscript writing assistance and its coordination.
References
- World Health Organization. Influenza (seasonal). Fact sheet no. 211. World Health Organization. Available at: http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed September 9, 2016.
- Banzhoff A, Stoddard JJ. Effective influenza vaccines for children: a critical unmet medical need and a public health priority. Hum Vaccin Immunother. 2012;8:398-402. doi:10.4161/hv.18561. PMID:22327501
- Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season: effect on school absenteeism, parental absenteeism from work, and secondary illness in families. Arch Pediatr Adolesc Med. 2002;156:986-91. doi:10.1001/archpedi.156.10.986. PMID:12361443
- Ruf BR, Knuf M. The burden of seasonal and pandemic influenza in infants and children. Eur J Pediatr. 2014;173:265-76. doi:10.1007/s00431-013-2023-6. PMID:23661234
- Mexican Department of Health. Influenza technical report. Mexican Department of Health. Available at: https://www.gob.mx/cms/uploads/attachment/file/20816/documento_tecnico_influenza.pdf. Accessed December 5, 2017.
- Galindo-Fraga A, Ortiz-Hernandez AA, Ramirez-Venegas A, Vazquez RV, Moreno-Espinosa S, Llamosas-Gallardo B, Perez-Patrigeon S, Salinger M, Freimanis L, Huang CY, et al. Clinical characteristics and outcomes of influenza and other influenza-like illnesses in Mexico City. Int J Infect Dis. 2013;17:e510-e517. doi:10.1016/j.ijid.2013.01.006. PMID:23416208
- World Health Organization. Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87:461-76. PMID:23210147
- Reed C, Kim IK, Singleton JA, Chaves SS, Flannery B, Finelli L, Fry A, Burns E, Gargiullo P, Jernigan D, et al. Estimated influenza illnesses and hospitalizations averted by vaccination–United States, 2013–14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:1151-4. PMID:25503917
- Aranda-Romo S, Comas-Garcia A, Garcia-Sepulveda CA, Hernandez-Salinas AE, Pina-Ramirez M, Noyola DE. Effect of an immunization program on seasonal influenza hospitalizations in Mexican children. Vaccine. 2010;28:2550-5. doi:10.1016/j.vaccine.2010.01.034. PMID:20117263
- Puig-Barbera J, Perez-Vilar S, Diez-Domingo J. MF59-adjuvanted seasonal influenza vaccine in young children. Expert Rev Vaccines. 2011;10:1519-28. doi:10.1586/erv.11.131. PMID:22043952
- Nolan T, Bravo L, Ceballos A, Mitha E, Gray G, Quiambao B, Patel SS, Bizjajeva S, Bock H, Nazaire-Bermal N, et al. Enhanced and persistent antibody response against homologous and heterologous strains elicited by a MF59-adjuvanted influenza vaccine in infants and young children. Vaccine. 2014;32:6146-56. doi:10.1016/j.vaccine.2014.08.068. PMID:25223266
- Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, Baehner F, Borkowski A, Tsai TF, Clemens R. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365:1406-16. doi:10.1056/NEJMoa1010331. PMID:21995388
- Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev. 2013;26:476-92. doi:10.1128/CMR.00097-12. PMID:23824369
- O'Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines. 2007;6:699-710. doi:10.1586/14760584.6.5.699. PMID:17931151
- Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine. 2001;19:2673-80. doi:10.1016/S0264-410X(00)00499-0. PMID:11257408
- Vesikari T, Forsten A, Herbinger KH, Cioppa GD, Beygo J, Borkowski A, Groth N, Bennati M, von SF. Safety and immunogenicity of an MF59((R))-adjuvanted A/H5N1 pre-pandemic influenza vaccine in adults and the elderly. Vaccine. 2012;30:1388-96. doi:10.1016/j.vaccine.2011.12.009. PMID:22192847
- Tsai TF. Fluad(R)-MF59(R)-adjuvanted influenza vaccine in older adults. Infect Chemother. 2013;45:159-74. doi:10.3947/ic.2013.45.2.159. PMID:24265964
- Moore DL. Vaccine recommendations for children and youth for the 2015/2016 influenza season. Paediatr Child Health. 2015;20:389-94. doi:10.1093/pch/20.7.389. PMID:26526862
- US Food and Drug Administration. FDA approves first seasonal influenza vaccine containing an adjuvant. US FDA. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm474295.htm. Accessed December 5, 2017.
- Black S, Nicolay U, Vesikari T, Knuf M, Del GG, Della CG, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011;30:1081-5. doi:10.1097/INF.0b013e3182367662. PMID:21983214
- Veguilla V, Lopez-Gatell H, Lopez-Martinez I, Aparicio-Antonio R, Barrera-Badillo G, Rojo-Medina J, Gross FL, Jefferson SN, Katz JM, Hernandez-Avila M, et al. A large proportion of the Mexican population remained susceptible to A(H1N1)pdm09 infection one year after the emergence of 2009 influenza pandemic. PLoS One. 2016;11:e0150428. doi:10.1371/journal.pone.0150428. PMID:27003409
- Garçon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines. 2012;11:349-66. doi:10.1586/erv.11.192. PMID:22380826
- Gilca R, Deceuninck G, De Serres G, Boulianne N, Sauvageau C, Quach C, Boucher FD, Skowronski DM. Effectiveness of pandemic H1N1 vaccine against influenza-related hospitalization in children. Pediatrics. 2011;128:e1084-91. doi:10.1542/peds.2010-3492. PMID:21987710
- Örtqvist Å, Bennet R, Hamrin J, Rinder MR, Lindlad H, Öhd JN, Eriksson M. Long term effectiveness of adjuvanted influenza A (H1N1)pdm09 vaccine in children. Vaccine. 2015;33:2558-61.
- Nolan T, Roy-Ghanta S, Montellano M, Weckx L, Ulloa-Gutierrez R, Lazcano-Ponce E, Kerdpanich A, Palazzi Safadi MA, Cruz-Valdez A, Litao S, et al. Relative Efficacy of AS03-Adjuvanted Pandemic Influenza A(H1N1) vaccine in children: results of a Controlled, Randomized Efficacy Trial. J Infect Dis. 2014;210:545-57. doi:10.1093/infdis/jiu173. PMID:24652494
- Carmona Martinez AC, Salamanca de la Cueva I, Boutet P, Vanden Abeele C, Smolenov I, Devaster J-M. A phase 1, open-label safety and immunogenicity study of an AS03-adjuvanted trivalent inactivated influenza vaccine in children aged 6 to 35 months. Hum Vaccin Immunother. 2014;10:1959-1968. doi:10.4161/hv.28743. PMID:25424805
- Bakken IJ, Kari Modalsli Aaberg KM, Ghaderi S, Gunnes N, Trogstad L, Magnus P, Håberg SE. Febrile seizures after 2009 influenza A(H1N1) vaccination and infection: a nationwide registry-based study. BMC Infect Dis. 2015;15:506. doi:10.1186/s12879-015-1263-7. PMID:26553258
- Miller E, Nick Andrews N, Stellitano L, Stowe J, Winstone AM, Shneerson J, Verity C. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ. 2013;346:f794. doi:10.1136/bmj.f794. PMID:23444425
- Ahmed SS, Steinman L. Narcolepsy and influenza vaccination-induced autoimmunity. J Autoimmun. 2014;50:1-11. doi:10.1016/j.jaut.2014.01.033. PMID:24559657