ABSTRACT
The RepliVax vaccine platform(RV) is based on flavivirus genomes that are rationally attenuated by deletion. The self-limiting infection provided by RV has been demonstrated to be safe, highly immunogenic and efficacious for several vaccine candidates against flaviviruses. Here respiratory syncytial virus (RSV) F, influenza virus HA, and simian immunodeficiency virus (SIV) Env proteins were expressed in place of either prM-E or C-prM-E gene deletions of the West Nile (WN) virus genome. The resulting RV-RSV, -influenza and -SIV vaccine prototypes replicated efficiently in complementing helper cells expressing the WN structural proteins in trans. Expressed antigens exhibited correct post-translational processing and the RV recombinants were shown to be highly attenuated and immunogenic in mice, eliciting strong antigen-specific antibodies as well as detectable T-cell responses. These data support the utility of RV vectors for development of vaccines against non-flavivirus targets including rabies and HIV.
Introduction
The use of viruses as delivery vehicles of foreign antigens offers the opportunity to develop new effective vaccines with the potential of eliciting robust and long-lasting immune responses (both B- and T-cell) without adjuvant. The RepliVax (RV) approach was initially developed as a novel replication-defective vaccine platform against flavivirus pathogens.Citation1,2 Flaviviruses include several major pathogens such as yellow fever (YF), dengue (DEN) types 1–4, Japanese encephalitis (JE), West Nile (WN), and tick-borne encephalitis (TBE) viruses that are transmitted by arthropods. They are small enveloped plus-strand RNA viruses. The genomic RNA of ∼11,000 nt in length contains a single open reading frame encoding the C-prM-E structural proteins (prM is a precursor for mature M) and nonstructural proteins NS1 – NS5. RV vaccine candidates against flavivirus targets are generated by deleting most of the capsid C gene in a desired flavivirus genome (); such constructs are also referred to as single component pseudo infectious viruses (sPIV).Citation1 These RV recombinants are propagated in helper cells supplying the C protein in trans. In normal cells, sPIVs undergo only a single round of replication, which leads to high level of attenuation in vivo. RV sPIV mimics infection of an intact flavivirus efficiently stimulating protective immunity. We have previously shown in a head-to-head comparison that RV prototypes are as immunogenic and efficacious in mice and hamsters as available live attenuated vaccines (LAVs) against flaviviruses.Citation3 Recently we developed a RV-TBE vaccine candidate that elicited a significant and more durable TBE virus-specific neutralizing antibody response after a single dose in NHP than three human doses of a licensed inactivated TBE vaccine.Citation4
Figure 1. RepliVax vaccine platform principle. A. RV vaccines against flavivirus targets are constructed by deleting most of the capsid protein gene in the flavivirus genome; the main flavivirus immunogen is represented by empty virus-like particles (VLPs) encoded by the prM-E genes eliciting neutralizing antibodies. The prM-E genes can be either homologous (e.g., as in RV-WN) or heterologous to the backbone (as in RV-TBE containing TBE virus specific prM-E genes in the WN backbone). RV vaccine candidates against non-flavivirus targets, e.g. RSV, influenza, etc., are engineered to express a desired transgene in place of the deleted structural proteins of the vector. The configuration of the expression product (presented on the cell surface, soluble protein, or VLP) depends on transgene design. B. RV candidates against non-flavivirus targets are propagated in helper cells expressing the deleted structural protein genes (e.g., C-prM-E cassette) in trans. In vivo, they undergo a single round of replication eliciting transgene-specific antibody and T-cell responses, without spread to surrounding cells.
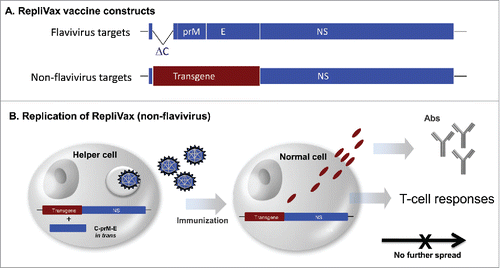
The prototype flavivirus LAV, YF 17D vaccine, is considered to be one of the strongest human immunogens. It infects and activates antigen presenting cells and induces robust and durable, possibly life-long immunity after a single dose.Citation5 New vaccine candidates against non-flavivirus targets capable of similarly eliciting durable immunity are desirable, which warrants the development of flavivirus vaccine vectors as delivery vehicles. The principle of developing flavivirus vectors has been previously demonstrated with the Kunjin virus replicon.Citation6 Expression of the heterologous immunogen can be manipulated by the insert design to target proteins for secretion, cytoplasmic or cell surface presentation, or release as virus like particles (VLPs). Here we expressed several non-flavivirus immunogens, specifically, respiratory syncytial virus (RSV) F, influenza HA, and SIV Env proteins in place of structural protein gene deletions in the WN (NY99 strain) RV vector, and evaluated the vaccine potential of resulting recombinants.
Results and discussion
In our previous studies on RV-TBE vaccine candidates, the WN virus strain NY99 backbone (encoding the WN NS proteins) outperformed other flavivirus backbones in terms of the yields in helper cells and enabled high immunogenicity and efficacy in mice and nonhuman primates (NHP).Citation4 Therefore we further explored delivery of non-flavivirus transgenes for proof-of-concept (POC) using this backbone (the principle is illustrated in and ) and evaluated immunogenicity of the RV-vectored constructs against established historical controls, such as formalin-inactivated or live viruses.
RV-RSV F
Antibodies against the RSV fusion glycoprotein F neutralize RSV infectivity and the protein is a target antigen for RSV vaccine development.Citation7 We constructed a RV candidate containing a truncated form of the F protein from a clinical isolate (MSA-1) of RSV (trF) expressed in place of the prM-E gene deletion of the WN genome (; amino acid residues at the vector-insert junctions are provided). The trF cassette lacks the C-terminal anchor of the F protein to enable efficient secretion from cells and potentiate immunogenicity.Citation8 The resulting RV-RSV F sPIV was recovered and propagated in BHK-WN C-prM-E helper cells. It grew to titers in excess of 6 log10 FFU/ml, and similar numbers of foci were detected by both anti-RSV F and anti-WN antibodies indicating that all infectious viral particles contained the trF gene (). Efficient trF expression was confirmed by intracellular staining of infected cells with anti-RSV F Mab () and Western blot. To confirm attenuation, highly sensitive 2–3 day old ICR suckling mice were inoculated by the IC route with 5 log10 FFU dose of RV-RSV F and no deaths or adverse clinical signs were observed during the 21-day observation period. This was similar to the RV-WN vaccine control previously shown to be avirulent in suckling mouse neurovirulence tests at doses up to 6 log10 FFU and in contrast to the YF 17D benchmark LAV control which caused mortality with an LD50 of 0.5 PFU (in 2–3 day old mice)Citation3 (). Thus the RV-RSV F recombinant sPIV is highly attenuated and efficiently expresses the trF product.
Figure 2. RV-RSV F candidate. A. The truncated F gene was codon optimized and contains a chimeric WN prM/RSV F signal sequence and FMDV 2A cleavage element at the C terminus. It was expressed in place of the WN virus prM-E genes. Amino acid sequences at WN vector – trF insert gene junctions are shown. Growth curve of the recombinant was determined in BHK helper cells (expressing the WN C-prM-E proteins) at MOI 0.001. Titration of samples was performed in Vero cells using immunostaining of foci with a mouse anti-RSV F Mab (5353C75) or a WN NS1 Mab 8152 (Chemicon). Fluorescence of infected Vero cells following immunostaining using the RSV F Mab is shown. B. Neurovirulence of RV-RSF F variant and benchmark control YF 17D was tested in 2–3 day-old ICR suckling mice. LD50 values are shown. C. RSV specific neutralizing antibody titers were determined in sera of BALB/c mice immunized with RV-RSV F (trF) or empty RV vector (v) administered twice by IP or IM routes at indicated doses, or two IM doses of formalin-inactivated RSV (FI-RSV) at week 8, or one IN dose (6 log10 PFU) of live RSV at week 4 (RSV). PRNT50 assay was performed in Vero cells using staining of plaques with RSV F Mab 5353C75.
Immunogenicity of RV-RSV F in mice was compared to that of live or formalin inactivated RSV. BALB/c mice (6–8 weeks old) were immunized twice at weeks 0 and 4 by the IM or IP routes with RV-RSV F or empty RV vector (negative control) at doses 6 or 7 log10 FFU (). Mice in control groups were immunized intranasally (IN) once with 6 log10 PFU of live RSV (Long strain), or IM twice with a formalin-inactivated RSV formulation (FI-RSV) prepared using 4.3 log10 PFU of virus per dose as previously described.Citation9 RSV-neutralizing antibody responses measured in sera at week 8 (4 weeks after second dose) were significantly higher for RV-RSV F (all dose and immunizing route groups) compared to the FI-RSV control. Immunogenicity of RV-RSV F at 6 log10 FFU dose (IM and IP) was comparable to live RSV immunization (week 4), and much higher at 7 log10 FFU dose than live RSV (). The PRNT50 titers correlated with RSV F-specific binding IgG titers determined by ELISA (not shown). This result demonstrates that RV-RSV F is highly immunogenic in the mouse model.
RV-Flu HA
Additional POC studies on the WN RV vector were performed where expression and immunogenicity was assessed using the hemagglutinin (HA) gene of influenza virus. The advantage of generating a virus-vectored influenza vaccine is the induction of both neutralizing/hemagglutination inhibiting antibodies and robust T-cell responses, without adjuvants, that may potentiate protection and durability of immunity.Citation10 The full-length codon optimized HA gene of H1N1 influenza virus strain A/New Caledonia/20/1999 (Accession # AAP34324) was cloned in place of the prM-E gene deletion in the same fashion as shown for RSV F in . This antigen was chosen because the A/New Caledonia/20/1999 vaccine strain is capable of eliciting a broad humoral response against influenza virus isolates from several different time periods.Citation11 The RV-Flu HA variant was recovered in BHK helper cells, with equivalent peak titers (∼ 7 log10 FFU/ml) detected by staining with both anti-HA and anti-WN MAbs. The protein was properly folded and efficiently delivered to the cell surface of infected cells as evidenced by immunostaining of formalin fixed cells with HA stem- (C179) and head-specific (ITM-003–001M2) MAbs (not shown).
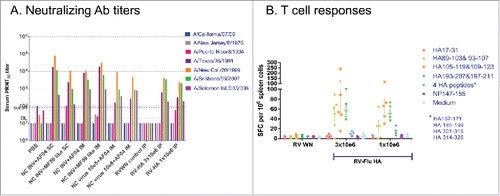
Immunogenicity of RV-Flu HA recombinant was assessed in the mouse model. BALB/c mice were immunized twice (days 0 and 21) with RV-Flu HA (1 × 106 or 3 × 106 FFU) by the IP route, or A/New Caledonia/20/1999 virus which was either formalin-inactivated (1.5 μm HA/dose) or live (5 or 6 log10 PFU/dose) and formulated with adjuvants AF04 or MF59 in-house preparationCitation12 administered by IM or SC routes. Pronounced neutralizing (PRNT50) antibody titers were detected on day 35 in mouse sera from all vaccine immunized groups (but not PBS diluent and RV-WN negative controls) against several H1N1 strains, specifically, A/Texas/36/1991, A/New Caledonia/20/1999, A/Brisbane/59/2007 and A/Solomon Islands/03/2006 strains, but not A/California/07/09, A/New Jersey/8/1976 and A/Puerto Rico/8/1934 (). The magnitude of antibody response elicited by RV-Flu HA (without adjuvant) was comparable to adjuvanted influenza virus immunogen groups, both inactivated and live virus preparations. HAI titers were also determined and they generally correlated with PRNT50 titers (not shown). HA specific T cell responses were detected in RV-Flu HA groups by ELISPOT using several peptides derived from HA (A/New Caledonia/20/1999), but not NP as expected, on day 7 (after the first dose) and days 28, 35 and 49 (after two doses), as illustrated for day 35 in . These results indicate the applicability of the RV platform to the influenza vaccine target. Importantly, the breadth of HA antibody response induced by RV-Flu HA was the same as induced by influenza virus with adjuvants; this however requires further investigation using the same routes and against appropriate standard-of-care controls (split inactivated virus antigen without adjuvant). RV-Flu HA immunization also elicited detectable HA specific T cell responses in the absence of adjuvant.
Figure 3. RV-Flu HA immunogenicity in mice compared to A/New Caledonia/20/1999 inactivated (INV) or live virus formulated with AF04 or MF59-like adjuvants. BALB/c mice were immunized twice on days 0 and 21 by the indicated routes. A. Neutralizing Ab titers determined in pooled sera at day 35 by PRNT50 assay against a panel of indicated H1N1 strains. B. Influenza specific T cell responses in RV-Flu HA and RV-WN negative control groups determined by ELISPOT in splenocytes on day 35 using a panel of indicated influenza virus peptides from A/New Caledonia/20/1999 HA or NP (not expressed by RV-Flu HA) proteins; peptide designations include amino acid numbers. ELISPOT counts in adjuvanted influenza virus groups were similar to RV-Flu HA (not shown).
RV-SIV Env
The field of HIV vaccine development has been reinvigorated by the promising results of the RV-144 Phase III trial in Thailand in which vaccination with ALVAC vectored and subunit protein immunogens resulted in 31.2% efficacy,Citation13 stimulating further exploration of new antigen designs and delivery systems. Therefore as a POC, we evaluated delivery of SIV Env (gp120) by WN RV vector. Synthetic Env (gp120) cassette of SIV (GenBank accession number ADM52218.1) modified by silent nt changes to eliminate direct sequence repeats of ≥ 8 nt to increase insert stability, with NHP codon preference (DNA2.0 algorithm) was expressed in RV WN vector in place of the C-prM-E deletion (in the same fashion as shown for RSV F in but together with the upstream delta-C deletion of RV-WNCitation1). In the Env cassette, the native signal sequence and the transmembrane and cytoplasmic domains were replaced with those from rabies virus G protein to facilitate Env transport to the cell surface and potential release of Env from cells due to the rabies G transmembrane domain.Citation14,15 The resulting RV-SIV Env construct grew to titers in excess of 6 log10 FFU/ml.
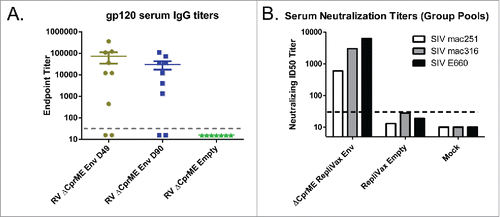
To evaluate immunogenicity of RV-SIV Env prototype, BALB/c mice were immunized with the sPIV at 6 log10 FFU/dose twice (days 0 and 29) by the IP route. High titers of gp120 specific binding IgG (ELISA) and SIV neutralizing antibodies were observed in sera of RV-SIV Env immunized mice collected on days 49 and 90 ( and ). Importantly, distinct SIV strains (mac251, mac316 and E660) were efficiently neutralized (), indicating that a broad immune response was elicited. Magnitude, durability, and breadth of antibody responses against different circulating HIV viruses are necessary features of an effective HIV vaccine.
Figure 4. Immunogenicity of RV-SIV Env. BALB/c mice (6–8 weeks old) were immunized twice on days 0 and 29. A. Binding IgG titers in individual sera determined on days 49 and 90 against subunit SIV gp120. B. Neutralizing Ab titers were determined in pooled sera of RV-SIV Env immunized mice on days 49 and 90 against SIV pseudoviruses with envelopes from indicated SIV strains (by Monogram Biosciences, San Francisco, CA); day 49 titers are shown.
Conclusion
In conclusion, we have expressed several immunogens from RSV, influenza virus, and SIV using the single-cycle RV (WN virus strain NY99) technology, demonstrating that the WN virus backbone can be used to efficiently express heterologous inserts. The recombinant sPIVs were found to be highly attenuated and immunogenic in mice. Immunogenicity in mice was similar or higher than that of historical controls, i.e. formalin-inactivated or live virus-based formulations. Collectively the data indicate applicability of the RV platform to developing new effective vaccines against non-flavivirus pathogens capitalizing on the property of flaviviruses to elicit strong and highly durable protective immunity. Further studies are required using relevant animal models. In this regard, the RV approach was applied to rabies and HIV targets yielding promising vaccine candidates evaluated in dogs and NHP, respectively (to be published elsewhere).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
RepliVax is currently a registered trademark of the Board of Regents of the University of Texas System.
References
- Mason PW, Shustov AV, Frolov I. Production and characterization of vaccines based on flaviviruses defective in replication. Virology. 2006;351:432-43.[https://doi.org/10.1016/j.virol.2006.04.003]. PMID:16712897
- Suzuki R, Fayzulin R, Frolov I, Mason PW. Identification of mutated cyclization sequences that permit efficient replication of West Nile virus genomes: Use in safer propagation of a novel vaccine candidate. J Virol. 2008;82:6942-51.[https://doi.org/10.1128/JVI.00662-08]. PMID:18480453
- Rumyantsev AA, Giel-Moloney M, Liu Y, Gao QS, Zhang ZX, Catalan J, Frolov I, Almond J, Kleanthous H, Pugachev KV. Characterization of the RepliVax platform for replication-defective flavivirus vaccines. Vaccine. 2011;29:5184-94.[https://doi.org/10.1016/j.vaccine.2011.05.032]. PMID:21620917
- Rumyantsev AA, Goncalvez AP, Giel-Moloney M, Catalan J, Liu Y, Gao QS, Almond J, Kleanthous H, Pugachev KV. Single-dose vaccine against tick-borne encephalitis. Proc Natl Acad Sci USA. 2013;110:13103-8.[https://doi.org/10.1073/pnas.1306245110]. PMID:23858441
- Monath TP, Cetron MS, Teuwen D. Yellow fever vaccine. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. 5th ed. Philadelphia (PA): Saunders/Elsevier; 2008. p. 959-1055.
- Pijlman GP, Suhrbier A, Khromykh AA. Kunjin virus replicons: An RNA-based, non-cytopathic viral vector system for protein production, vaccine and gene therapy applications. Expert Opin Biol Ther. 2006;6:135-45.[https://doi.org/10.1517/14712598.6.2.135] PMID:16436039
- Kahn JS, Schnell MJ, Buonocore L, Rose JK. Recombinant vesicular stomatitis virus expressing respiratory syncytial virus (RSV) glycoproteins: RSV fusion protein can mediate infection and cell fusion. Virology. 1999;254:81-91.[https://doi.org/10.1006/viro.1998.9535]. PMID:9927576
- Li X, Sambhara S, Li CX, Ewasyshyn M, Parrington M, Caterini J, James O, Cates G, Du RP, Klein M. Protection against respiratory syncytial virus infection by DNA immunization. J Exp Med. 1998;188:681-8.[https://doi.org/10.1084/jem.188.4.681]. PMID:9705950
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422-34.[https://doi.org/10.1093/oxfordjournals.aje.a120955]. PMID:4305198
- Tripp RA, Tompkins SM. Virus-vectored influenza virus vaccines. Viruses. 2014;6:3055-79.[https://doi.org/10.3390/v6083055]. PMID:25105278
- Carter DM, Darby CA, Lefoley BC, Crevar CJ, Alefantis T, Oomen R, Anderson SF, Strugnell T, Cortes-Garcia G, Vogel TU, et al. Design and characterization of a computationally optimized broadly reactive hemagglutinin vaccine for H1N1 influenza viruses. J Virol. 2016;90:4720-34.[https://doi.org/10.1128/JVI.03152-15]. PMID:26912624
- Haensler J, Probeck P, Su J, Piras F, Dalencon F, Cotte JF, Chambon V, Iqbal SM, Hawkins L, Burdin N. Design and preclinical characterization of a novel vaccine adjuvant formulation consisting of a synthetic TLR4 agonist in a thermoreversible squalene emulsion. Int J Pharm. 2015;486:99-111.[https://doi.org/10.1016/j.ijpharm.2015.03.028]. PMID:25794609
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209-20.[https://doi.org/10.1056/NEJMoa0908492]. PMID:19843557
- Mebatsion T, Conzelmann KK. Specific infection of CD4+ target cells by recombinant rabies virus pseudotypes carrying the HIV-1 envelope spike protein. Proc Natl Acad Sci USA. 1996;93:11366-70.[https://doi.org/10.1073/pnas.93.21.11366]. PMID:8876141
- Dietzschold B, Wiktor TJ, Macfarlan R, Varrichio A. Antigenic structure of rabies virus glycoprotein: Ordering and immunological characterization of the large CNBr cleavage fragments. J Virol. 1982;44:595-602. PMID:6183450
