ABSTRACT
Tuberculosis (TB) remains among the world's leading cause of mortality. For its control, studies of TB vaccines are needed. Since live-attenuated Mycobacterium bovis bacillus Calmette-Guérin (BCG) is the only TB vaccine currently in use, studies on the protective role of BCG are required. In this study, we analyzed host cells purified directly from whole blood of human immunodeficiency virus (HIV)-negative volunteers, comprising adult healthy donors (HD) and neonates (umbilical cord bloods, UCB), with the aim to directly compare in vitro immune responses with distinct BCG strains in human mononuclear cells. The Moreau, Pasteur, and Danish BCG strains were used to infect mononuclear cells in vitro for 48 h; bacilli viability and cell-death were subsequently detected by flow cytometry. In addition, cell culture supernatants were used in cytokine detection assays. Overall, the Moreau BCG strain induced higher levels of apoptosis than the Pasteur and Danish BCG strains in both the HD and UCB groups (p-value < 0.05), and a human monocytic cell-line mirrored those cell-death patterns after BCG infection. The Moreau BCG strain, exclusively, induced Th1 cytokines at the highest levels in cells from adults (p-value < 0.05) when compared with both Pasteur and Danish BCG strains, whereas TGF-β1 levels were reduced significantly (p-value < 0.01) in the HD group when cells were infected with the Moreau BCG vaccine. As expected, eight out of 22 pro-inflammatory cytokines were secreted at significant levels (p-value < 0.05) above the baseline rates in all BCG-infected cell cultures, in the HD group only. When analyzing these results, we excluded confounding factors related to storage and viability of the BCG strains used. These findings suggest that Moreau BCG is a more potent immunostimulating agent than the Pasteur and Danish BCG strains. Clinical trials will be needed to confirm these findings.
Introduction
Tuberculosis (TB) is the leading cause of death from infectious disease, and is an enormous public health problem.Citation1 The global TB burden is concentrated in the developing world. Although the incidence of this disease has declined over the last decade, high case numbers persist in endemic countries like Brazil.Citation1 Recent strategies to reduce TB have relied on additional preventive efforts, including improvements to the current TB vaccine. The TB burden cannot be controlled without further improvements in preventive methods.Citation1
The live-attenuated Mycobacterium bovis bacillus Calmette-Guérin (BCG) is the only vaccine approved for preventing TB in humans. The BCG vaccine is not a single organism, but comprises genotypically and phenotypically differing strains (also known as substrains) (reviewed byCitation2). On the basis of comparative studies that have uncovered modifications comprising both deletions and insertions of genetic material and two independent tandem duplications, the BCG vaccines were divided into evolutionarily “early-shared strains” (Group I) and more attenuated evolutionarily “late-shared strains” (Groups II to IV).Citation3 Notably, Group I strains are more efficacious than Groups II, III and IV strains.Citation4 To examine this hypothesis in an in vitro human model, we tested here three widely used BCG vaccines: the Moreau BCG (Group I), the Danish BCG (Group III) used in Czech Republic, Denmark, Estonia, Finland, Greece, France, Hungary, Italy, Ireland, India, Latvia, Lithuania, Malta, the Netherlands, Norway, Slovakia, Slovenia, Sweden, England, South Africa and Switzerland, and the Pasteur BCG strains (Group IV) used in both Poland and Serbia.Citation5 The BCG vaccine currently used in Brazil is the Moreau strain only; however, little is known about its protective properties, or the immune response that it induces in comparison with the effects of other BCG strains. Although Moreau BCG elicits a strong delayed-type hypersensitivity reaction in skin tests, relatively few in vitro studies have examined the origin of this protective response (reviewed byCitation2).
BCG vaccination results in a robust cellular immune response against M. tuberculosis, although the protective efficacy of this response varies.Citation6 Although the BCG vaccine protects reasonably well against both infantile meningitis and miliary TB disease, it remains ineffective in protecting against pulmonary TB in several settings; this shortcoming has led to an increase in the global TB burden.Citation7 Despite progress in understanding transcriptional responses, signaling mechanisms, and sub-cellular processes related to the immune response to TB infection, cell-cell interactions following mycobacterial infections, mostly between cells in the monocyte lineage, remain unclear.Citation8
To maintain homeostasis, dead cells are often cleared from tissues by host phagocytes. Monocytes and macrophages are also responsible for neutralizing potential threats posed by internalized debris. The role of apoptosis in TB has been extensively debated (reviewed byCitation9). On the one hand, studies support that apoptosis is an effective innate-immunity defense mechanism that M. tuberculosis is able to partially inhibit. On the other hand, apoptosis can also boost the induction of M. tuberculosis-specific immune response, so that excessive apoptosis would be detrimental for the bacterium. Mycobacteria have been shown to induce macrophage apoptosis; inhibition of apoptosis by virulent tubercle bacilli might be an evasive strategy that creates a safe environment within the host suitable for intracellular replication and colonization of neighboring cells (reviewed byCitation10). In fact, the appropriate cell-death pattern is important for a protective immune response; macrophage apoptosis represents a critical innate host response to control M. tuberculosis infection and limit disease.Citation11 For instance, immune-activating danger signals released during either necrotic or necroptotic cell-death initiate an inflammatory response. Alternatively, apoptosis triggered by attenuated, non-virulent mycobacteria plays a significant role in shaping immune responses to these infections.Citation11 The specific cell-death pattern therefore plays a critical role in the ensuing immune response. Dysfunction in this pattern-specific response might cause immunopathology via an influx of inappropriate immune cells, breakdown of granulomas, and progress to cavitation, which can increase disease transmission.Citation12 Conversely, the secretion of the anti-inflammatory cytokines IL-10 and TGF-β1 modulates the immune response to ameliorate tissue damage.Citation13
Owing to the uncertainty about the importance of strain diversity, and failures in protection against TB since the original Pasteur BCG vaccine was disseminated between 1924 and 1927,Citation14 the present study aimed to directly compare in vitro immune responses in Moreau, Pasteur (actual), and Danish BCG-infected human mononuclear cells. Previous studies have shown that appropriate cell-death pattern is an important defense against mycobacteria; host cell apoptosis has been associated with a decrease in pathogen viability.Citation10,11,15,16 Thus, we categorize here the monocyte cell-death as the readout that might differentiate those strains after infection. We also compare several soluble factors released during BCG-mononuclear cell interactions.
Results
The Moreau BCG vaccine induced higher levels of apoptosis than the Pasteur and Danish BCG strains in both groups studied
Mononuclear cells participate in the primary inflammatory response to mycobacteria, but little is known about the interactions of distinct M. bovis BCG strains with human monocytes (reviewed byCitation17,18). Thus, the present study infected cells with the Moreau, Pasteur, or Danish BCG vaccine strain from (1) adults vaccinated with BCG during childhood and (2) neonates who had never been exposed to mycobacteria. summarizes these findings. After infection with the Moreau BCG strain, we observed a significant increase (p ≤ 0.05) in monocyte apoptosis in healthy donor adults (HD) and newborns' umbilical cord bloods (UCB) groups. Moreover, only cells from UCB group showed an increase in necrosis (p ≤ 0.05) under the Moreau, but neither Pasteur nor Danish BCG infections (3.4% & 0.6; 33.5% & 4.2; 5.2% & 1.3 and 4.7% & 1.1, at baseline and 48 h of Moreau, Pasteur and Danish BCG-infections, respectively). Thus, the necrosis data mirrored the apoptosis patterns after BCG infection in the UCB group. In addition, the heating cycle performed in baseline samples caused an enhancement in necrosis (p ≤ 0.05) in both groups, as expected (51.3% & 15.0 and 55.3% & 29.1, at HD and UCB groups, respectively). We minimized potential variability related to storage and viability of the BCG strains used, since all three BCG vaccine strains used throughout the study showed virtually the same pattern ().
Figure 1. (A) The apoptosis levels (%) in healthy donor (closed symbols) and umbilical cord blood (opened symbols) groups representing the baseline (circle) and Moreau (square), Pasteur (diamond) and Danish (triangle) BCG strains in 48 h in vitro infection of human mononuclear cells. Data points denote individual donors and horizontal bars represent median apoptosis values in each condition. *p-value ≤ 0.05; **p-value ≤ 0.01; ***p-value ≤ 0.001, according to the Dunn's paired test. The representative histograms show bacilli viability (%) of the (B) Moreau, (C) Pasteur and (D) Danish BCG strains, evaluated by a specific nucleic acid dye (green-fluorescent SYTO-9).
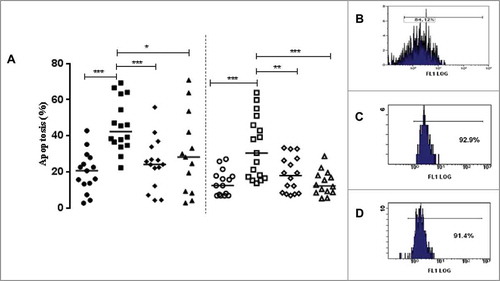
With the aim to provide proof of concept that primary monocytes increase in apoptosis levels after the Moreau BCG infection only, we used a smaller set of experiments (n = 9) to test the THP-1 cell-line in our in vitro model. This step allowed us (1) to circumvent any potential, intrinsic lymphocyte interference and (2) to confirm the previous results in primary mononuclear cell cultures for apoptosis (46.6% & 1.0 vs. 6.9% & 1.2; 10.4% & 6.0 and 10.9% & 2.7, at 48 h of Moreau BCG- vs. baseline, Pasteur and Danish BCG-infected THP-1 cell-line conditions, respectively: p-values ≤ 0.02). Thus, the THP-1 cell-line mirrored the cell-death patterns after M. bovis BCG infections found in both groups studied.
The Moreau BCG vaccine induced the highest Th1 cytokines, but the lowest TGF-β1 levels, in cells from adults when compared with both Pasteur and Danish BCG strains
Pro-inflammatory cytokines have been shown to play a key role in the induction of apoptosis in vitro,Citation16,19 particularly when monocytes are infected with mycobacteria.Citation20 Therefore, we assayed the levels of 22 cytokines in supernatants of mononuclear cells from both groups infected with the Moreau, Pasteur, or Danish BCG strains. Overall, only three cytokines comprising the type-1 pattern were found to be modulated in both groups (). Since the BCG vaccine is given at birth and induces a Th1-polarized immune response in newborns,Citation21 we focused on a type-1 skewed response. Corroborating previous findings,Citation22 we found a significant increase (p-value ≤ 0.05) in the levels of pro-inflammatory cytokines IL-2 (at least 1.8 fold), GM-CSF (at least 1.7 fold), IFN-γ (at least 1.9 fold), and TNF-α (at least 1.9 fold) in the Moreau BCG-infected cell cultures from the HD group (). Remarkably, this result reflected that observed with apoptosis (). All three BCG strains failed to trigger consistent TNF-α production in the UCB group (); such a failure was reported previously for the Moreau BCG vaccine.Citation8 No other differences were found for the UCB group. In the HD group, eight out of 22 cytokines assessed in the BCG-infected cell cultures were significantly increased in abundance (p-value ≤ 0.05), regardless of the infecting strain (, and ). Conversely, we detected a very significant reduction (p-value ≤ 0.0005) in TGF-β1 levels with Moreau BCG infection (). Lastly, the IL-18 levels decreased regardless of the infecting strain (). Although differences were not statistically significant for Danish BCG vaccine, we observed the highest values under baseline conditions. It is not possible to determine whether this result was spurious owing to the samples analyzed, or representative of outliers in this particular population. In light of the dramatic discrepancy observed, further analyses with larger sample sizes should clarify the role of this cytokine in infections by all BCG strains. We found no group or vaccine strain-dependent differences in IL-1α, IL-4, IL-5, IL-7, IL-10, IL-12p70, IL-13, IL-23, IL-27, or MCP-1 levels.
Figure 2. (A) IL-2, (B) GM-CSF, (C) IFN-γ and (D) TNF-α levels (pg/mL) in healthy donor (Y1, closed symbols) and umbilical cord blood (Y2, opened symbols) groups representing the baseline, uninfected cells (circle) and Moreau (square), Pasteur (diamond) and Danish (triangle) BCG strains in 48 h in vitro infection of human mononuclear cells. Data points denote individual donors and horizontal bars represent median cytokine values in each condition. *p-value ≤ 0.05; **p-value ≤ 0.005; ***p-value ≤ 0.0005, according to the Dunn's paired test.
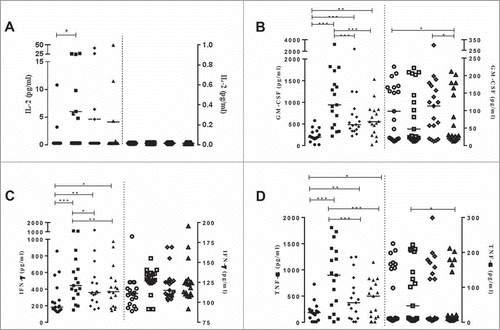
Table 1. The cytokine levels induced by Moreau, Pasteur and Danish BCG strains after 48 h in vitro infection of human mononuclear cells in the healthy donor group (n = 18) except the IL-18 levels assessed in the umbilical cord blood group (n = 23).
Additional analysis shows a strong modulation induced by BCG vaccines for all cytokines in adult healthy donors
A previous study of circulating biomarkers found that pro-inflammatory IL-18 plasma levels positively correlated with inflammatory IL-23 in cord blood, but not in adult blood.Citation23 This pattern may imply an ex vivo induction of IL-17 by IL-23 and IL-18.Citation24 Motivated by this prior finding, and to further elucidate potential associations between apoptosis and cytokines, the correlation analysis among the three BCG strains were found to be strong and positive (r-value > 0.7) for all cytokines with high levels in the HD group, directly showing modulation induced by the BCG vaccine, regardless of the strain employed (data not shown). Conversely, we failed to identify association between apoptosis levels in Moreau BCG-infected cell cultures from the HD group vs. those same pro-inflammatory cytokines, namely IL-2 (r-value = 0.3; p-value = n.s.), GM-CSF (r-value = −0.0; p-value = n.s.), IFN-γ (r-value = 0.2; p-value = n.s.) and TNF-α (r-value = 0.3; p-value = n.s.). No other correlations were found for the UCB group.
Discussion
In the present study, the immunological activities of mononuclear cells following infection with three different BCG strains were assessed and compared in order to examine the in vitro immune responses to the attenuated M. bovis BCG vaccines employed worldwide. The main findings were that the BCG Moreau vaccine is the most immunostimulating of the three strains in vitro tested. Hence, BCG Moreau induced the highest levels of apoptosis and Th1-type cytokines, while TGF-β1 levels were reduced. Pro-inflammatory cytokines were evoked after infection with all three BCG strains in mononuclear cells from adult donors only.
In prior studies, we revealed an increase in cell-death events in BCG Moreau-infected mononuclear cells from both vaccinated and unvaccinated study participants, followed by the induction of different endogenous compounds that might promote specific cell-death.Citation8,25 Reflecting the distinct virulence properties of different mycobacterial species, the Moreau BCG vaccine appears to induce an adequate Th1 skewed response in immune cells. Prior findings have found that the Japan BCG, another Group I strain, similarly induced higher levels of the Th1 cytokines, even stronger immunogenicity in infants, and a lower level of the Th2 cytokines than the Danish BCG strain, which suggest that early BCG vaccines may even be superior to the later ones that are more widely used.Citation3 In support of this hypothesis, we demonstrated that Moreau BCG strain differentially induced the highest in vitro apoptosis levels and pro-inflammatory cytokines secretion from human mononuclear cells, when compared with the distinct Pasteur and Danish BCG strains. Importantly, the cell-death results were similar in cells from unlike origins, i.e., cells from individuals with sensitization to mycobacteria (healthy adults vaccinated with M. bovis BCG when they were young) and those unexposed to mycobacteria (immunologically naïve newborns).
Conversely, a prior study found no substantial differences in efficacy between “early-shared strains” vs. “late-shared strains” of BCG, and only small variances in efficacy among the latter.Citation4 The authors concluded that the “late-shared strains” are not less potent than an “early-shared strains” and argues against strain differences as a major factor in the variability of outcomes in BCG vaccine trials.
A seminal study demonstrated that 48 h of infection of human macrophages with M. tuberculosis at a low multiplicity of infection (MOI) leads to specific cell-death.Citation15 Using different infectivity rates, Welin and colleagues (2011)Citation26 also found that macrophages infected with M. tuberculosis at relatively high – but not low – MOIs for 48 h exhibited suggestive characteristics that the principal cell-death pathway was not apoptosis. However, Chen and colleagues (2006)Citation15 found that higher MOIs also led to the release of copious amounts of the pro-inflammatory IL-1β, whereas a lower MOI resulted in nearly absence of IL-1β secretion. In light of the four different MOIs previously tested, in the present study we used an intermediate 2:1 M. bovis BCG-host cell rate, which produced a cell-death frequency that was similar to the rate with a ratio of 5:1 (unpublished data).
In a study conducted to identify alterations in the expression of 165 genes in human monocyte-derived dendritic cells (DCs) infected with M. tuberculosis vs. Pasteur and Japan BCG, EBI3 expression was found to be upregulated by M. tuberculosis, relative to that with Japan BCG strain.Citation27 EBI3 was found to be an IL-12p40-related polypeptide of IL-27 that may play a role in regulating cell-mediated immune responses.Citation28 In addition, Japan BCG was the only strain that failed to induce the release of a significant amount of IL-10 in host cell cultures, in contrast with the marked increase of TNF-α expression in these cultures.Citation27 In another gene expression study, only five genes were upregulated in bovine macrophages infected with a virulent strain of M. bovis, in contrast with 172 genes upregulated in cells infected with an attenuated counterpart.Citation29 These genes included an apoptosis regulator that, in a separate study of unrelated model, was also upregulated in bovine monocytes infected in vitro with live attenuated respiratory syncytial virus vaccine.Citation30 In this study, apoptosis increased, with higher levels of TNF-α and IL-1β than in cells infected with the parental and virulent wild-type strains. Higher rates of apoptosis in BCG-infected macrophages have been associated with stronger DC-mediated cross-priming of T cells, leading to protection against M. tuberculosis (reviewed byCitation31). Moreover, in agreement with our findings, the vaccine had the ability to induce pro-inflammatory cytokines. Notably, we found significantly heightened levels of necrosis in the UCB group only; this observation highlighted the importance of accurately determining the type of macrophage cell-death.Citation8,25
We observed that the highest levels of prototypic Th1 cytokines, which are key inducers of cell-death,Citation32 were released in response to the Moreau BCG vaccine in cells from the HD group. Here, we confirmed previous results, and expanded the list of Th1 cytokines induced in vitro by the Moreau BCG vaccine to include GM-CSF.Citation22 GM-CSF is essential to the generation of a protective immune response against M. tuberculosis, because mice lacking GM-CSF are unable to control bacterial loads, and succumb to disease.Citation33 GM-CSF has been shown to inhibit granulocyte spontaneous apoptosis.Citation34 More recently, several inflammatory cytokines, including GM-CSF, were found to delay specific cell-death; a higher MOI of BCG and increased GM-CSF levels inhibited spontaneous apoptosis of stimulated, mature neutrophils in vitro.Citation35 Importantly, protection from apoptosis coincided with a reduction in the number of necrotic cells.
In our study, infection with the Moreau BCG strain of mononuclear cells from immunized individuals efficiently induced the pro-inflammatory IL-2, accompanied by a reduction in the levels of TGF-β, a prototypic regulatory Th3 cytokine.Citation36 However, all three strains exhibited reduced IL-18 production, in contrast with an increase in IL-6, IL-8, IL-17, G-CSF, and MIP-1β levels. In a recent study, interactions between the BCG vaccine and host cells appeared to be dispensable, as IL-18, TGF-β, and GM-CSF were involved in Connaught BCG-induced iNK cell receptor modulation.Citation37 Remarkably, exogenous IL-18 reduced in vitro NKp46 levels, and TGF-β reduced NKp30 and NKG2D expression. Whether these reductions impacted polarization of the immune response after BCG vaccine administration remains a matter of debate.
Finally, a given experimental model was challenged with M. bovis after vaccination with 11 different BCG strains.Citation38 The survival of animals immunized with Danish BCG was shorter than those with Moreau BCG strain. Another study investigating the recall response one year after immunization at gene expression profiling level of children given either Moreau, Danish or Japan BCG strains at birth found that both Moreau and Danish BCG vaccines induced significantly higher levels of IFN-γ, IL-12p40 and IL-27 mRNA. Rather, Japan BCG-immunized newborns expressed higher levels of IL-1, IL-6 and IL-24, suggesting a higher pro-inflammatory response.Citation39
A limitation of the present study was the lack of inclusion of BCG vaccine strains from the most immunogenic cluster (Group I), other than the Moreau strain. It has been hypothesized that underrepresentation of Group I BCG strains (Moreau, Russia, and Japan) might reduce the efficacy of the recombinant BCG vaccines (reviewed byCitation2). Although neither the Danish (Group III) nor Pasteur (Group IV) BCG strains have been shown to induce greater immunogenicity than that of induced by Group I strains, there are essentially no surrogate indicators to precisely predict BCG vaccine efficacy.Citation40 Finally, another limitation of this study is its lack of demographical data. Healthy donor adult samples were obtained from whom there is no available information on tuberculin skin test positivity. As stated earlier, anonymous donations policy was enforced by the institutional review boards (IRBs) approvals precluding records of clinical characteristics, but the only secure information is clearly that those Brazilian individuals have been vaccinated with Moreau BCG strain only (BCG vaccination in Brazil is mandatory after birth). Additional studies with increased sample sizes are warranted to confirm the present results.
In summary, the Moreau BCG strain induced apoptosis more efficaciously than the Pasteur and Danish BCG strains. Hence, “early-shared strains” of BCG vaccine (including Moreau) have greater potential to induce specific cell-death in already sensitized human mononuclear cells than do “late-shared strains” of BCG (Pasteur and Danish). Taken together with our findings, Brosch and colleagues (2007)Citation3 formerly suggested that early BCG vaccines may confer better protection against TB, a possibility that would benefit from formal evaluation in clinical trials. On the other hand, the Moreau BCG strain induced different in vitro regulatory and pro-inflammatory cytokines than those two others. Overall, these results suggest that Moreau BCG is a more potent immunostimulating agent than the Pasteur and Danish BCG strains.
Currently, while the protection afforded against TB and the concomitant induced immune response may vary between BCG vaccines, there is scarce information to recommend one particular strain. Our study intended to fulfill this dramatic gap. Thus, distinguishing BCG strains with superior protection would have a critical outcome on TB control, preventing massive numbers of cases of severe TB and deaths, particularly in children. To generate an immune response by BCG vaccination that eradicates M. tuberculosis in a host, we must continue to determine both protective and pathogenic immune responses to M. tuberculosis infection. Greater knowledge of the natural- and vaccine-induced responses to this infection will drive improvements in immune-based interventions. Further studies are underway to confirm these findings.
Patients and methods
Study population
Specimens tested in this study were collected between November 2010 and July 2016 from two groups of donors at different sites in Rio de Janeiro. The first group of specimens was from healthy donor adults (HD; n = 18); these specimens were collected by a transfusion medicine and hemotherapy unit at a private blood bank (anonymous donations from individuals aged ≥ 18 years). The second group of specimens was from newborns’ umbilical cord bloods (UCB; n = 17), collected using umbilical cord puncture procedures on full-term placentas, performed on disease-free mothers with uncomplicated deliveries at the Gaffree Guinle State University Hospital (HUGG). The UCB cells were collected promptly after delivery. These samples were also used in a previous study to test BCG in vitro T-cell immune responses.Citation22 The BCG vaccination in Brazil is universal after birth since 1967. The HUGG/UNIRIO (protocol # 59117015.0.0000.5258) and IOC-Fiocruz (protocol # 35775014.0.0000.5248) IRBs approved the study procedures. All study participants (healthy adults, mothers of neonates, authorities, or the children's parents or guardians) provided written informed consent.
Peripheral blood mononuclear cells (PBMC) and cord blood mononuclear cells (CBMC) purification, BCG vaccine strains, and co-culture
Both PBMCs and CBMCs were separated within no more than 24 h (average 5 h) of obtaining blood specimens from all study participants, and cultured as described elsewhere.Citation22 In vitro infections of freshly isolated PBMCs and CBMCs with the Moreau (at single dose, individual batches of sealed glass vials containing liquid suspension with approximately 1 × 107 viable bacilli), Pasteur, and Danish BCG strains (both at single dose, individual batches of vials containing frozen suspensions with approximately 1 × 107 viable bacilli each) were performed at a MOI of 2:1 (bacilli:host cell). Prior assessment has established that the infectivity rates remained comparable for MOI 1:1, 2:1 and 10:1, as did the cell-death rates for the two latter (data not shown). In addition, no difference has been found between the aforementioned frozen and liquid lots (Fig. S1). Accordingly, a previous study has shown that MOI 1:1 was not detrimental, but MOI 10:1 induced apoptosis in macrophages.Citation41 Furthermore, at MOI lesser then 10:1, BCG and M. tuberculosis H37Ra are much stronger apoptosis inducers than all virulent strains, including wild-type M. bovis, M. tuberculosis H37Rv, and Erdman as well as clinical isolates (reviewed byCitation42). Thus, the MOI used in this study is congruent with others using BCG as well. Since considerable variability among BCG vaccine lots may occur, and in order to linearize the MOI for all 3 strains, bacteria were prompt quantified by reaching the optical density (OD 600) = 1.0 (mid-log phase at 30 × 107 CFU/mL). The respective batches were used once for each infection, and subsequently discarded. Pasteur and Danish BCG ampoules were thawed shortly before the infection of cells. The viabilities of bacilli were assessed by an immunofluorescence kit (LIVE/DEAD BacLight, Invitrogen Co., USA), which uses a green-fluorescent SYTO-9 dye (FL-1) for nucleic acid stain and allows one to reliably distinguish live and dead bacteria with the aid of a flow cytometer. Cultured PBMCs or CBMCs (1 × 106 cells each) in RPMI medium (Sigma Immunochemicals, USA) supplemented with 10% human AB serum were kept at 37°C in a humidified 5% CO2 atmosphere in individual 12 × 75 mm sterile polystyrene tubes (Falcon, Corning Inc., USA). Previous experiments with these tubes showed a better viability of cells when compared to conventional culture plates.Citation43 PBMCs and CBMCs were used for subsequent cell-death analysis, and supernatants stored at −70°C in order to be used further in cytokine detection assays.
A parallel process was used to establish the human acute monocytic leukemia cell line THP-1 in a monocyte-like state. Cells were grown, expanded, cultured in tubes (1 × 106 cells), and then infected with the Moreau, Pasteur, and Danish BCG strains (MOI of 2:1). Next, cells were labeled and analyzed following the same apoptosis detection protocol as below described.
Cell-death assay
PBMCs and CBMCs from each donor, as well as THP-1 cell-line, were cultured for 48 h, a period earlier established following optimization assays.Citation8 Negative control cultures (baseline) were left uninfected for the same period. Positive control cells were subjected to the following protocol just before staining to induce cell necrosis: An ordinary, controlled temperature water bath chamber was utilized to heat cells directly in those culture tubes for subsequent analysis by flow cytometry. The chamber maintained the solution within the culture tubes in about 90°C for 10 min. The apparatus was preheated for 10 min prior to use to minimize the thermal transients. Immediately following heat shock, culture tubes were placed in cold ice (roughly 4°C) until 10 min prior to analysis.Citation44 After incubation, cells were labeled using a cell-death detection kit, as specified by the manufacturer (TACS, R&D Systems, USA), and immediately analyzed by flow cytometry (FACSCalibur, Becton Dickinson, USA). At baseline, monocytes in PBMC and CBMC were present in similar frequencies ranging from 10 to 35% of mononuclear cells.Citation45 Importantly, monocyte apoptosis is preceded by down-regulation of the CD14 surface receptor expression, precluding that phenotyping strategy.Citation46 Therefore, after lymphocyte population exclusion based on light scattering properties, cell-death events were analyzed (Fig. S2). Hence, the combination of annexin V-FITC (FL-1) and propidium iodide (FL-3) labeling for the differentiation between apoptotic (A), necrotic (N) and viable (L) cells in a gating strategy for determining monocytes cell-death was applied. Thresholds and statistical markers were set for positivity by means of negative, baseline matched controls as a reference. All data were expressed as the percentage of stained-positive bright cells.
Cytokine detection assays
Cell-free supernatants were thawed once and subsequently assayed to determine the concentrations of human IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-17, GM-CSF, G-CSF, MCP-1, MIP-1β, IFN-γ, and TNF-α, using the Bio-Plex protein multiarray system kits, which uses Luminex-based technology (Bio-Rad, USA). In parallel, we performed enzyme-linked immunosorbent assay (ELISA) on human IL-1α, IL-18, IL-23, IL-27, and TGF-β1, using commercial kits (R&D Systems, USA). We performed these assays according to the manufacturers’ instructions.
Statistical evaluation
Data were compared between groups by the Friedman test (a nonparametric test for matched data), and for cytokines only p-values were calculated for multiple testing using Dunn's test. P-values were adjusted to correct for multiple testing considering the level of 0.05 as the threshold of significance. We used the GraphPad InStat software to perform these tests. Cytokine production values and the THP-1 cell-death levels are reported as medians & interquartile range (IQR). Spearman's rank correlation coefficient was calculated to analyze the relationship of each cytokines level in response to each BCG strain.
Disclosure of potential conflicts of interest
None of the authors have a commercial association that poses a conflict of interest.
KHVI_A_1382788_Supplemental.zip
Download Zip (378.1 KB)Acknowledgments
The authors are grateful to Tiago Toledo-Pinto (Leprosy Laboratory, IOC/FIOCRUZ), Victor Fonseca, Thaíze Pedro, Vitor Lima, Jessica Lima, Leticia Moreira, Julie Santana and Rayanne Souza (Clinical Immunology Laboratory, IOC/FIOCRUZ), and Drs. Alvaro Bertho (Immunoparasitology Laboratory, IOC/FIOCRUZ) and Paulo Totino (Laboratory of Malaria Research, IOC/FIOCRUZ) for their help during technical procedures. The authors are in debt with HUGG staff for their service in the clinical practices. The Moreau BCG vaccine was a gift of the Ataulpho de Paiva Foundation, whereas Pasteur and Danish BCG strains were provided by Dr. Milton Moraes (Leprosy Laboratory, IOC/FIOCRUZ).
This work was presented at the 11th ALAI Congress (Immunocolombia), Oct 13–16, 2015, Medellin, Colombia.
Additional information
Funding
References
- Dirlikov E, Raviglione M, Scano F. Global tuberculosis control: Toward the 2015 targets and beyond. Ann Intern Med. 2015;163(1):52-8. doi:10.7326/M14-2210. PMID:25915859
- Antas PR. Crucial requirement for standardization during the development of novel recombinant BCG vaccines: Does the corresponding substrain background matter? Hum Vaccin Immunother. 2016;12(12):3099-102. doi:10.1080/21645515.2016.1212145. PMID:27454883
- Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, Dos Santos S, Duthoy S, Lacroix C, Garcia-Pelayo C, et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A. 2007;104(13):5596-601. doi:10.1073/pnas.0700869104. PMID:17372194
- Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Commonly administered BCG strains including an evolutionarily early strain and evolutionarily late strains of disparate genealogy induce comparable protective immunity against tuberculosis. Vaccine. 2009;27(3):441-5. doi:10.1016/j.vaccine.2008.10.058. PMID:19007841
- Ritz N, Hanekom WA, Robins-Browne R, Britton WJ, Curtis N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev. 2008;32(5):821-41. doi:10.1111/j.1574-6976.2008.00118.x. PMID:18616602
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(9):698-702. doi:10.1001/jama.1994.03510330076038. PMID:8309034
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282(7):677-86. doi:10.1001/jama.282.7.677. PMID:10517722
- Simas CJ, Silva DP, Ponte CG, Castello-Branco LR, Antas PR. Patterns of in vitro cell-death, metaloproteinase-9 and pro-inflammatory cytokines in human monocytes induced by the BCG vaccine, Moreau strain. Vaccine. 2011;29(38):6446-50. doi:10.1016/j.vaccine.2011.06.093. PMID:21745518
- Delogu G, Provvedi R, Sali M, Manganelli R. Mycobacterium tuberculosis virulence: Insights and impact on vaccine development. Future Microbiol. 2015;10(7):1177-94. doi:10.2217/fmb.15.26. PMID:26119086
- Fratazzi C, Arbeit RD, Carini C, Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. Macrophage apoptosis in mycobacterial infections. J Leukoc Biol. 1999;66(5):763-4. PMID:10577507
- Divangahi M, Behar SM, Remold H. Dying to live: How the death modality of the infected macrophage affects immunity to tuberculosis. Adv Exp Med Biol. 2013;783:103-20. doi:10.1007/978-1-4614-6111-1_6. PMID:23468106
- Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, Pereira DR, Randall TD, Pedrosa J, Cooper AM, et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207(8):1609-16. doi:10.1084/jem.20100265. PMID:20624887
- Campisi L, Cummings RJ, Blander JM. Death-defining immune responses after apoptosis. Am J Transplant. 2014;14(7):1488-98. doi:10.1111/ajt.12736. PMID:24903539
- Benevolo-de-Andrade TC, Monteiro-Maia R, Cosgrove C, Castello-Branco LR. BCG Moreau Rio de Janeiro: An oral vaccine against tuberculosis-review. Mem Inst Oswaldo Cruz. 2005;100(5):459-65. doi:10.1590/S0074-02762005000500002. PMID:16184220
- Chen M, Gan H, Remold HG. A mechanism of virulence: Virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J Immunol. 2006;176(6):3707-16. doi:10.4049/jimmunol.176.6.3707. PMID:16517739
- Keane J, Balcewicz-Sablinska MK, Remold HG, Chupp GL, Meek BB, Fenton MJ, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65(1):298-304. PMID:8975927
- van CR, Ottenhoff TH, Van der Meer JW. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev. 2002;15(2):294-309. doi:10.1128/CMR.15.2.294-309.2002. PMID:11932234
- Kleinnijenhuis J, van CR, Netea MG. Trained immunity: Consequences for the heterologous effects of BCG vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):29-35. doi:10.1093/trstmh/tru168. PMID:25573107
- Friedlander RM, Gagliardini V, Rotello RJ, Yuan J. Functional role of interleukin 1 beta (IL-1 beta) in IL-1 beta-converting enzyme-mediated apoptosis. J Exp Med. 1996;184(2):717-24. doi:10.1084/jem.184.2.717. PMID:8760825
- Hernandez MO, Neves I, Sales JS, Carvalho DS, Sarno EN, Sampaio EP. Induction of apoptosis in monocytes by Mycobacterium leprae in vitro: A possible role for tumour necrosis factor-alpha. Immunology. 2003;109(1):156-64. doi:10.1046/j.1365-2567.2003.01630.x. PMID:12709029
- Kativhu CL, Libraty DH. A model to explain how the Bacille Calmette Guerin (BCG) vaccine drives interleukin-12 production in neonates. PLoS One. 2016;11(8):e0162148. doi:10.1371/journal.pone.0162148. PMID:27571272
- Ponte C, Peres L, Marinho S, Lima J, Siqueira M, Pedro T, De Luca P, Cascabulho C, Castello-Branco LR, Antas PR. In vitro T-cell profile induced by BCG Moreau in healthy Brazilian volunteers. Hum Vaccin Immunother. 2015;11(2):450-7. doi:10.4161/21645515.2014.970954. PMID:25483636
- Antas PR, Pedro TQ, Santiago EA, Lima JR, Silva FC, Melca LA, Ponte CG. Human neonates display altered ex vivo monokine production related to healthy adults. Immunol Lett. 2016;170:64-7. doi:10.1016/j.imlet.2015.12.004. PMID:26687810
- Chen JM, Jiang GX, Li QW, Zhou ZM, Cheng Q. Increased serum levels of interleukin-18, -23 and -17 in Chinese patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2014;38(5–6):321-9. doi:10.1159/000360606. PMID:25138786
- Lima J, Siqueira M, Pedro T, Ponte C, Peres L, Marinho S, Castello-Branco LR, Antas PRZ. The role of host soluble inflammatory mediators induced by the BCG vaccine for the initiation of in vitro monocyte apoptosis in healthy Brazilian volunteers. J Inflamm (Lond). 2015;12:60. doi:10.1186/s12950-015-0105-0. PMID:26516315
- Welin A, Eklund D, Stendahl O, Lerm M. Human macrophages infected with a high burden of ESAT-6-expressing M. tuberculosis undergo caspase-1- and cathepsin B-independent necrosis. PLoS One. 2011;6(5):e20302. doi:10.1371/journal.pone.0020302. PMID:21637850
- Sanarico N, Colone A, Grassi M, Speranza V, Giovannini D, Ciaramella A, Colizzi V, Mariani F. Different transcriptional profiles of human monocyte-derived dendritic cells infected with distinct strains of Mycobacterium tuberculosis and Mycobacterium bovis bacillus Calmette-Guerin. Clin Dev Immunol. 2011;2011:741051. doi:10.1155/2011/741051. PMID:21436989
- Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70(2):1143-53. PMID:8551575
- Caimi K, Blanco F, Soria M, Bigi F. Transcriptional response of bovine monocyte-derived macrophages after the infection with different Argentinean Mycobacterium bovis isolates. Biomed Res Int. 2013;2013:458278. doi:10.1155/2013/458278. PMID:23484118
- Taylor G, Wyld S, Valarcher JF, Guzman E, Thom M, Widdison S, Buchholz UJ. Recombinant bovine respiratory syncytial virus with deletion of the SH gene induces increased apoptosis and pro-inflammatory cytokines in vitro, and is attenuated and induces protective immunity in calves. J Gen Virol. 2014;95(Pt 6):1244-54. doi:10.1099/vir.0.064931-0. PMID:24700100
- Behar SM, Woodworth JS, Wu Y. Next generation: Tuberculosis vaccines that elicit protective CD8+ T cells. Expert Rev Vaccines. 2007;6(3):441-56. doi:10.1586/14760584.6.3.441. PMID:17542758
- Vandenbroeck K, Goris A. Cytokine gene polymorphisms in multifactorial diseases: Gateways to novel targets for immunotherapy? Trends Pharmacol Sci. 2003;24(6):284-9. doi:10.1016/S0165-6147(03)00131-7. PMID:12823954
- Gonzalez-Juarrero M, Hattle JM, Izzo A, Junqueira-Kipnis AP, Shim TS, Trapnell BC, Cooper AM, Orme IM. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol. 2005;77(6):914-22. doi:10.1189/jlb.1204723. PMID:15767289
- Cox G, Gauldie J, Jordana M. Bronchial epithelial cell-derived cytokines (G-CSF and GM-CSF) promote the survival of peripheral blood neutrophils in vitro. Am J Respir Cell Mol Biol. 1992;7(5):507-13. doi:10.1165/ajrcmb/7.5.507. PMID:1384583
- Suttmann H, Lehan N, Bohle A, Brandau S. Stimulation of neutrophil granulocytes with Mycobacterium bovis bacillus Calmette-Guerin induces changes in phenotype and gene expression and inhibits spontaneous apoptosis. Infect Immun. 2003;71(8):4647-56. doi:10.1128/IAI.71.8.4647-4656.2003. PMID:12874345
- Toussirot EA. Oral tolerance in the treatment of rheumatoid arthritis. Curr Drug Targets Inflamm Allergy. 2002;1(1):45-52. doi:10.2174/1568010023344850. PMID:14561205
- Marras F, Bozzano F, Bentivoglio G, Ugolotti E, Biassoni R, Moretta L, De Maria A. Receptor modulation and functional activation of human CD34+ Lin- -derived immature NK cells in vitro by Mycobacterium bovis Bacillus Calmette-Guerin (BCG). Eur J Immunol. 2012;42(9):2459-70. doi:10.1002/eji.201242375. PMID:22736333
- Ladefoged A, Bunch-Christensen K, Guld J. The protective effect in bank voles of some strains of BCG. Bull World Health Organ. 1970;43(1):71-90. PMID:4921094
- Wu B, Huang C, Garcia L, Ponce de Leon A, Osornio JS, Bobadilla-del-Valle M, Ferreira L, Canizales S, Small P, Kato-Maeda M, et al. Unique gene expression profiles in infants vaccinated with different strains of Mycobacterium bovis bacille Calmette-Guerin. Infect Immun. 2007;75(7):3658-64. doi:10.1128/IAI.00244-07. PMID:17502394
- Saikolappan S, Estrella J, Sasindran SJ, Khan A, Armitige LY, Jagannath C, Dhandayuthapani S. The fbpA/sapM double knock out strain of Mycobacterium tuberculosis is highly attenuated and immunogenic in macrophages. PLoS One. 2012;7(5):e36198. doi:10.1371/journal.pone.0036198. PMID:22574140
- Periasamy S, Tripathi BN, Singh N. Mechanisms of Mycobacterium avium subsp. paratuberculosis induced apoptosis and necrosis in bovine macrophages. Vet Microbiol. 2013;165(3–4):392-401. doi:10.1016/j.vetmic.2013.03.030. PMID:23639474
- Lee J, Remold HG, Ieong MH, Kornfeld H. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J Immunol. 2006;176(7):4267-74. doi:10.4049/jimmunol.176.7.4267. PMID:16547264
- Santos DO, Coelho JG, Neri ECL, Campos-Souza IC, Ponte CGG, Antas PRZ. The differential in vitro presentation of Mycobacterium leprae antigens by human dendritic cells is determined by the mechanism of host cell adhesion. J Clin Cell Immunol. 2016;7:443.
- Song AS, Najjar AM, Diller KR. Thermally induced apoptosis, necrosis, and heat shock protein expression in 3D culture. J Biomech Eng. 2014;136(7):0710061-10. doi:10.1115/1.4027272.
- Sohlberg E, Saghafian-Hedengren S, Bremme K, Sverremark-Ekstrom E. Cord blood monocyte subsets are similar to adult and show potent peptidoglycan-stimulated cytokine responses. Immunology. 2011;133(1):41-50. doi:10.1111/j.1365-2567.2011.03407.x. PMID:21323661
- Heidenreich S. Monocyte CD14: A multifunctional receptor engaged in apoptosis from both sides. J Leukoc Biol. 1999;65(6):737-43. PMID:10380893
