ABSTRACT
The 2014–15 Ebola outbreak in West Africa highlighted the potential for large disease outbreaks caused by emerging pathogens and has generated considerable focus on preparedness for future epidemics. Here we discuss drivers, strategies and practical considerations for developing vaccines against outbreak pathogens. Chimpanzee adenoviral (ChAd) vectors have been developed as vaccine candidates for multiple infectious diseases and prostate cancer. ChAd vectors are safe and induce antigen-specific cellular and humoral immunity in all age groups, as well as circumventing the problem of pre-existing immunity encountered with human Ad vectors. For these reasons, such viral vectors provide an attractive platform for stockpiling vaccines for emergency deployment in response to a threatened outbreak of an emerging pathogen. Work is already underway to develop vaccines against a number of other outbreak pathogens and we will also review progress on these approaches here, particularly for Lassa fever, Nipah and MERS.
Progression of the vectored vaccine approach: Success of rapid clinical production and testing of Ebola and malaria vaccine vectors
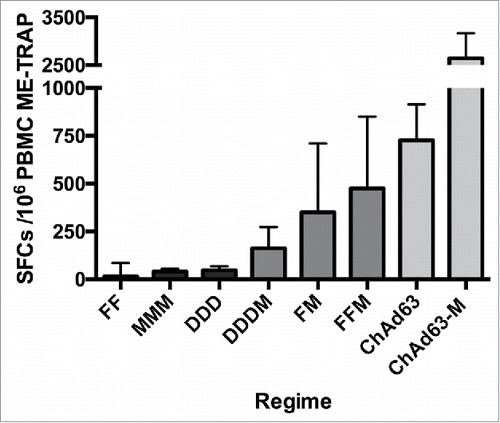
Since the first documented report of the use of an engineered virus to induce a protective immune response,Citation1 clinical testing of numerous potential vaccine vectors has been undertaken against a broad range of diseases. Over many years of preclinical development, a series of new vector or vaccination regimens have demonstrated improved immunogenicity: in particular, antigen-specific antibody and/or T cell responses have been increased through iterative rounds of vector vaccine development. This is well illustrated by the development of malaria vaccines against P. falciparum encoding the ME-TRAP antigen, where vaccine-induced T cell responses have increased from 44 IFN-γ spot-forming cells per million peripheral blood mononuclear cells (SFC) after DNA vaccination, to 850 SFC after a single vaccination with a simian adenovirus-vectored vaccine (). Importantly, viral vectors have not shown age-limitations in their use, with comparable T cell responses observed following vaccination with a modified vaccinia Ankara (MVA) vector expressing the influenza A antigens NP+M1 in healthy older adults (aged 50–60, 60–70, 80+ years) compared to a younger adult population (aged 18–55 years).Citation2 In addition, age de-escalation studies of chimpanzee adenovirus 63 (ChAd63) ME-TRAP in West-African children have demonstrated potent T cell and antibody responses in immunised children as young as 1 week of age.Citation3,4
Table 1. Comparison of cellular immune responses with different delivery methods for the same malaria antigen (ME-TRAP) at seven days after the final vaccination. Immunogenicity as measured by ex vivo interferon-gamma ELISPOT using the same ELISPOT method and peptide pools in the same lab.
The urgent need for a treatment or vaccine intervention during the West-African Ebola outbreak saw five vectored vaccines tested concurrently in Phase I trials; three non-replicating adenoviruses of different serotypes, MVA and Vesicular stomatitis virus (VSV), all encoding the ebolavirus glycoprotein (GP). All vaccines were primarily tested for their ability to induce high levels of antibodies against GP, as this correlated with protection observed in non-human primates, although cell-mediated immunity has also been shown to play a protective role with some vectors.Citation5,6 While it is not straightforward to directly compare antibody levels induced by the different vectors due to the range of assays employed by different groups, responses following a single vaccination with ChAd3, Ad26 and rVSV were detectable within 28 days, with a very significant enhancement in antibody responses observed when adenoviral prime vaccinations were followed by an MVA boost.Citation7,8 Humoral immunogenicity induced by various viral vectors encoding Ebolavirus (EBOV) glycoprotein is summarised in . Although initially developed as a platform for inducing T cell responses, single vaccinations with ChAd63 have demonstrated good antibody induction against malaria antigens, which could be enhanced by boosting with an MVA.Citation9-12 Ad-MVA regimens induced IgG responses that were maintained for at least 180 days after immunisation.Citation7,13
Table 2. Comparative humoral immunogenicity of viral vectors encoding Ebolavirus glycoprotein.
Prime-boost vaccination with adenoviral and MVA vectored vaccines is now well-stablished as a safe and robust strategy for inducing both cellular and humoral immunity against malaria and ebolavirus, with the addition of an MVA boost increasing both the magnitude and the breadth of the T cell response ( and ).Citation13,14 Either vaccine can act as prime or boost, as demonstrated in a novel Phase I Ebola vaccine trial with AdHu26 and the multivalent MVA BN-Filo vaccines.Citation7 Although the highest T cell and antibody responses to ChAd3 MVA were observed with a four to eight-week interval between prime and boost, reducing the interval to one week still induced comparable T cell responses to the eight-week interval. However, the shorter prime-boost interval did lead to a reduction in antibody responses including neutralising antibodies.Citation13
Figure 1. Comparative T cell immunogenicity of different viral vector regimens encoding the same pre-erythrocytic malaria antigen, ME-TRAP, as measured by ex-vivo interferon-γ ELISpot assays. F, Fowlpox (FP9); M, MVA; D, DNA; SFC, SFC, spot-forming cells; PBMC, peripheral blood mononuclear cells.
MVA vectors have been successfully used to boost responses in adults induced by vaccination with BCG in infancy, demonstrating the potential of the MVA vector to boost any pre-existing T cell memory responseCitation15 The development of multivalent MVA vectors, such as MVA BN-Filo which encodes four proteins from three ebolavirus species and Marburg virus, is also a potentially important tool for reducing the number of vaccine products that might need to be manufactured, by encoding protective antigens from several strains of the same pathogen or from multiple pathogens into the same vaccine construct (reviewed inCitation16) The large genome of MVA allows insertion of a larger amount of foreign DNA compared with other viral vectors including adenoviruses.
Consistent with previous studies, strong T cell responses to the EBOV glycoprotein were observed after a single ChAd3 administration and significantly enhanced after an MVA boost, but were undetectable after rVSV vaccination.Citation17 Only the rVSV vaccine was assessed for efficacy during the outbreak in a ring-vaccination trial where volunteers were stratified into immediate or delayed vaccination groups following exposure.Citation18 The significant reduction in Ebola cases from 10 days after vaccination in this Guinea trial highlights the need to induce a rapid immune response in an outbreak scenario, with a single-dose vaccine remaining the most manageable option. For a rapid response in an outbreak setting, an early induction of protective immunity would be prioritised over durability. However, for immunisation of healthcare workers and other first responders in anticipation of a potential outbreak in the future, durability would be more important than rapid induction of immunity. For the former application, single dose vaccines will be most desirable, whereas for durable immunity a multi-dose regimen would likely be acceptable and could be required.
Viral vector biology influences the choice of vaccine platform
Several viral vectors currently have the potential to serve as single dose vaccine platforms for the purpose of outbreak preparedness, having shown robust immunogenicity in clinical trials (reviewed inCitation19). However, in order to achieve high vaccine effectiveness, it is equally important to consider parameters affected by vector biology, such as manufacturability, stability and safety of the vaccine.
A key factor is the manner in which the vaccine antigen is encoded and expressed. In adenoviral vectored vaccines, the antigen is typically placed under the control of a heterologous, strong promoter, and encoded in an independent expression cassette which is inserted into a well-characterised location in the adenoviral genome. This is most commonly the E1 locus. Concurrent deletion of the adenoviral E1 genes at this locus renders the virus replication incompetent. Vector production can therefore only take place in complementing cell lines expressing the E1 genes, such as HEK-293 or PER.C6®.Citation20 Typical genetic engineering methods for antigen insertion into adenoviral vectors include plasmid-based homologous recombination in E. coli,Citation21 bacterial artificial chromosome (BAC)-based recombineering,Citation22 or in vitro Gateway® recombination.Citation23 The placement of such antigen expression cassettes within the viral genome leads to de-novo expression of the antigen in the vaccine target cells, which in turn results in a strong humoral as well as cellular immune response against the antigen. More recently, the capsid-incorporation approach has shown promise for the induction of antigen-specific antibodies using modified adenoviral vectors.Citation24 Here, antigenic epitopes or entire antigens are engineered to be part of adenoviral capsid proteins and are thus displayed on the surface of the viral vector, for recognition by the immune system. However, as the capsid-display strategy has not yet been evaluated in clinical trials, this review will focus on traditionally engineered adenoviral vectors with antigen cassettes at the E1 locus.
The first adenoviral vaccine vectors to be developed were based on human adenovirus serotype 5 (HAd5), a species C adenovirus which commonly infects humans. However, it was found that pre-existing anti-HAd5 antibodies which are present in a large proportion of the human population could significantly dampen the humoral and cellular immune response to the vaccine antigen.Citation25 Various strategies have since been explored to circumvent this problem: the use of alternative human serotypes, such as HAd26 or HAd35,Citation26 re-engineering the capsid of HAd5 to prevent antibody recognition,Citation27 and the use of simian adenoviral vectors against which there is no pre-existing immunity.Citation28 As discussed above, chimpanzee adenoviral vectors (ChAds) have successfully been used in clinical trials against a variety of diseases.
ChAds are non-enveloped viruses, meaning that the antigen (e.g. a membrane glycoprotein) is not present on the surface of the vector, but is expressed at high levels once the vector enters the target cells of the vaccinated individual. This is in contrast to VSV-based vaccine vectors, which, as enveloped viruses, are designed to incorporate glycoprotein antigens into their viral lipid membrane and thus display the antigen on the virus surface, in addition to expressing it upon entry into the target cell.Citation29 Crucially, VSV-based vectors carrying heterologous glycoprotein antigens are generally deleted for their endogenous glycoprotein (VSV-G), which implies that it falls to the vaccine antigen to fulfil the role of functional viral fusion protein as an essential component for vector propagation during manufacture as well as for target cell entry. This important requirement for functionality inherently affects the choice of antigen for VSV-based vectors, as some viral glycoprotein antigens are either not functional by themselves (e.g. Nipah virus glycoprotein G needs glycoprotein FCitation30 or are not incorporated into the VSV membrane without modification (e.g. HIV envCitation31). In addition, while adenoviral vectors can equally well encode antigens which are not membrane-bound glycoproteins (e.g. Ebolavirus nucleoprotein, HIV gag), VSV vectors carrying such antigens rely on the endogenous glycoprotein (VSV-G) for viral entry. Since the full-length VSV-G protein is implicated in neurotropism,Citation32 a genetically attenuated vector carrying a truncated VSV-G has been developed,Citation33 which has an acceptable safety profile in healthy adults.Citation34
Having thus weighed up some of the characteristics of the two most clinically advanced vectors for emergency preparedness platforms, it becomes apparent why vector biology can have significant implications for vaccine safety. Specifically, tissue tropism and replication competency of the viral vector have to be taken into consideration. Intuitively, a replication-deficient vector (such as ChAd) carries less safety risks than a replication competent, albeit attenuated, vector (such as VSV), since the inability to replicate prevents dissemination of the vector throughout the body. Accordingly, transgene expression of replication-deficient adenoviral vectors was shown to be confined to the injection site and the draining lymph nodes,Citation35 whereas recent Phase I/II trials of rVSV-ZEBOV found evidence of viral vector replication in synovial fluid and skin lesions, presumed to be a result of Ebolavirus glycoprotein-specific tissue tropism of the vaccine.Citation8 These findings underline the difficulty in predicting the safety profile of VSV-based vaccines, since tissue tropism will be highly dependent on the chosen glycoprotein antigen. In contrast, adenoviral vectors have a well-characterised safety profile, across a range of age groups, which is largely independent of the nature of the antigen.Citation36
Lastly, vector biology may also significantly impact vaccine manufacture and delivery. For emergency preparedness stockpiling, each vaccine might need to be produced at a scale of 500,000 – 2 million doses, with the option to quickly increasing manufacture to perhaps 4–6 million doses or more in case of an outbreak, depending on the specific pathogen. Of the two most clinically advanced platforms, VSV and adenoviruses, the latter can likely meet this requirement more easily: GMP-compliant large-scale adenoviral vector production facilities exist in many countries, related to the regular use of adenoviral vectors not only in prophylactic vaccines but also in some cancer and gene-therapy trials. One potential drawback of ChAd-based viral vectors compared to human Ad vectors is the need for vector optimisation or cell line engineering to ensure high viral yields during virus production. For example, ChAd vectors may need to contain certain E4 genes from HAd5 in order to grow to high titers in current HAd5-E1-transcomplementing cell lines, as was demonstrated with the ChAdOx1 vector.Citation23 Alternatively, producer cell lines can be engineered to increase viral yield.Citation37 However, this need for optimisation has not been a hurdle to large-scale manufacturing so far. GMP-compliant VSV-vector production has also been developed in recent years, and scalable manufacture of rVSV is now possible.Citation38 Once a stockpile has been produced, vector stability during storage and deployment is critical. Most viral vectored vaccines are stable for >5 years at −70°C, and a 2–8°C cold chain is required for distribution and storage of adenoviral vectors. One study assessing the recently deployed rVSV-ZEBOV vaccine observed a significant loss of viral titres at a temperature of 4°C after 2 weeks,Citation39 whereas adenoviral vectors were shown to be stable for 20 days at room temperature in a sucrose buffer.Citation40 In addition, sensitivity of any VSV-based vaccine to pH changes is presumably dependent on the specific envelope glycoprotein (i.e. the vaccine antigen). Overall, vaccine stability in terms of temperature and pH range would therefore likely be variable across a panel of putative VSV-based outbreak vaccines, since the glycoprotein will differ from vaccine to vaccine. In the case of an adenoviral vectored vaccine, on the other hand, variation in stability is expected to be minimal, since the vaccine antigen is not present in the viral capsid, and the composition of the virus particles would be very similar across different vaccines. Of note, new approaches for thermostabilisation have recently been developed for adenoviral vectors, such as immobilization of viral particles in a sugar glass on a filterCitation41 or the use of biocompatible additives to slow down the degradation of virus particles.Citation40 These improvements are expected to have a significant impact on the deployment of vectored vaccines in challenging climates such as sub-Saharan Africa. In human populations, pre-existing immunity to simian-derived adenoviral vectors is, unsurprisingly, less prevalent than immunity to human adenoviruses, and antibodies to some simian vectors, such as ChAdOx1, appear to be particularly rare.Citation23 Anti-vector immunity to the backbone of simian viruses increased after vaccination but is relatively short-lived. As a result, reuse of the same vectors has been successful for boosting after 6 or more months in clinical trials.Citation42
Limitations of the traditional approach
The traditional approach to vectored vaccine design has been to identify an immunogenic antigen from the pathogen, construct the vector in the chosen platform and then assess immunogenicity and efficacy in murine models, prior to further testing in higher species and progression to the next stage of vaccine development. A significant obstacle in this approach is that the pathogen must be infectious in rodents if the efficacy of the vaccine is to be assessed preclinically and therefore the data may rely on mouse-adapted or chimeric pathogens ( summarises common mouse models for evaluating candidate vaccines for outbreak pathogens).
In the case of MERS CoV or SARS CoV, preclinical vaccine candidates could be tested in murine models with a mouse adapted strain of virus,Citation43,44 but for newly emerging pathogens, establishing a mouse model could take significant time, in particular for evaluation of numerous viral isolates or serial passaging of a virus in mice. Alternatively, use of neonatal mice or knockout mice (e.g. of IFN-α/βR, STAT-1) have been required to mimic human disease for Ebola, Marburg, Lassa, Nipah, or Zika viruses in mice, and only through expression of human DPP4 (receptor for MERS CoV) in mouse lungs could infection of mice with MERs CoV be achieved.Citation45 While these mouse models may prove useful in drug discovery, if a significant component of the immune response is compromised, it is unlikely that protection observed in pre-clinical studies will be consistent with the protective immune response required in humans.
A new strategy for developing vaccines against outbreak pathogens
A more economical and achievable strategy than traditional approaches to vaccine development and deployment would be to focus on manufacturing small stockpiles of vaccine using a common platform technology. ChAd vectored vaccines provide a good example of a suitable vaccine platform, which has been identified as one of significant interest by the WHO R&D Blueprint process. The overall strategy would be to generate suitable stockpiles for emergency response use having previously demonstrated safety and immunogenicity of each vaccine up to Phase II trials in the target geographical regions. These products could be stored in relevant locations for each disease and, in the event of an outbreak emerging, could be deployed in a ring vaccination program similar to that employed in a Phase III trial in Guinea of the rVSV ZEBOV vaccine during the West African Ebola outbreak.Citation18 Such a deployment would need to be made under the provisions of policies for use of unapproved medicinal products, such as the FDA Expanded Access program, also known as “compassionate use”, or other emergency use legislation. This would require fulfilment of certain conditions including that no comparable or satisfactory therapy is available, that the risk of harm from the vaccine is not greater than the risk of disease and that there is sufficient evidence of the safety and effectiveness of the product to support its use in the given circumstances.Citation46 In this context, a vaccine for an outbreak pathogen, based on a well-developed platform, such as ChAd vectors, with evidence of efficacy from a relevant animal model would be likely to gain approval for use in a limited setting. Based on research, manufacturing and clinical trial costs for the ChAd3 vectored vaccine developed for Ebola, vaccines might be stockpiled for just $50 million per disease, representing a fraction of the cost of bringing a vaccine through to licensure. Deployment would provide the efficacy data in humans required for approval by a national regulator, increasing the likelihood of the vaccine progressing through the later stages of development.
Tackling future outbreak threats
To improve responsiveness to epidemics, in 2015 the WHO published a list of nine diseases requiring urgent vaccine R&D to prevent public health emergencies in the future. This list was revised in 2017, and key characteristics of the diseases prioritised by the WHO are summarised in . The process of prioritising diseases took into account properties of the causative pathogen e.g. transmissibility, host-based factors such as immunopathology, clinical aspects including ease of accurate diagnosis, availability of countermeasures and mortality, public health capacity and epidemiological factors.Citation47 Research and development priorities for these diseases include development of suitable diagnostic tests, assessment of potential treatments, identification of key knowledge gaps, production platforms, behavioural interventions and acceleration of vaccine development. Preparation of sufficient quantities of safe and efficacious vaccines against potential outbreak pathogens is an extremely effective strategy. However, a lack of access to dedicated long-term funding has hampered vaccine development for outbreak pathogens in recent decades.Citation48 As well as limiting the number of new vaccines being developed, the number of facilities with the capacity to biomanufacture vaccines is also limited, which is a significant issue for outbreak preparedness.Citation49 In addition, WHO recognised that generally applicable platform technologies for rapid vaccine development are required and have set out to identify and prioritise the leading platforms.
Table 3. Mouse models for evaluating candidate vaccines for outbreak pathogens.
Table 4. Characteristics of the priority diseases identified in the WHO R&D blueprint (revised 2017).
To address these issues, the Coalition for Epidemic Preparedness Innovations (CEPI) was launched in January 2017, bringing together funders including the Wellcome Trust, the Bill and Melinda Gates Foundation, and the governments of Norway, Germany, Japan and others.Citation50 The initial fund is $460 million, with the European Commission also pledging co-funding of €250 million and further funding due to be confirmed from the Government of India by the end of 2017. The fund will initially focus on the Nipah, Lassa and MERS viruses, aiming to bring two candidate vaccines through development against each disease. CEPI also aims to promote technical and institutional platforms to improve responsiveness to future epidemics. The approach undertaken by CEPI will advance vaccine development for diseases where research to date has been limited. This is in large part due to the lack of market potential for such vaccines in conjunction with the huge costs involved over a long period of time to provide a vaccine, from pre-clinical development through to licensure, estimated at upwards of $200 million to $500 million per vaccine.Citation51 Therefore, the funding required to license a vaccine for each of the priority diseases highlighted by the WHO blueprint would run into many billions of dollars, and opportunities to assess the efficacy of these vaccines in humans would be rare.
Prioritising vaccine development for the greatest threats
Although Ebola virus disease (EVD) has been described since 1976, the outbreak that began in 2014 was larger than all the previous episodes combined, potentially due to a mutation in the glycoprotein that occurred immediately prior to the rapid increase in the number of EVD cases.Citation52,53 Although not sufficiently advanced to be deployed immediately during the outbreak itself, several vaccines against ebolaviruses had already been manufactured to Good Manufacturing Practice (GMP) standards providing a rare opportunity to undertake phase I trials very rapidly and then assess efficacy against disease.
The 2014 outbreak provided a much-needed impetus to improve pandemic preparedness for emerging pathogens. To this end, the three identified viruses as targets for vaccine development, by CEPI have known potential to cause outbreaks with high mortality: MERS-CoV, Nipah virus and Lassa virus.
Nipah virus
Nipah Virus (NiV) is a recently-recognised and highly pathogenic zoonotic paramyxovirus that can cause severe disease in man with high associated fatality rates (up to 100%).Citation54 Outbreaks have occurred in Malaysia, Singapore and India with almost annual occurrence in Bangladesh. Human-to-human transmission is common in Bangladesh and has also been documented in India.Citation55 Several species of pteropid fruit bats are known to be host reservoirs of NiV, with accumulating evidence that both NiV and other paramyxoviruses can circulate worldwide in bats.Citation54-56 The high fatality rate, direct infection from natural reservoirs, infection following amplification in susceptible domestic livestock such as pigs, documented human-to-human transmission, and the potential ability to transverse the globe, all emphasise the pandemic potential of NiV.Citation56
There are no clinically approved vaccines against NiV, however, one therapeutic approach (monoclonal antibody therapy) has recently completed a phase I clinical trial with results still to be reported.Citation57 While monoclonal antibody treatment may be efficacious in a short window post-exposure, this treatment option is not suitable for large-scale use, and as such, vaccine development is a key research focus for the prevention of NiV-mediated disease. Advantageously, there are a number of animal models of NiV infection which are used in vaccine development programs and are considered to sufficiently mirror NiV-induced pathogenesis observed in humans, e.g. the hamster, ferret and African Green Monkey (AGM) models.Citation58-60
While vaccine-mediated cellular immunity has been demonstrated to play a role in protection in preclinical models of NiV infection,Citation61 the most advanced vaccine modalities demonstrating clear efficacy across multiple animal models have primarily induced humoral immunity. A soluble glycoprotein (sG) subunit vaccine from the related henipavirus Hendra virus (HeV) is an extensively studied vaccine that can protect ferrets and AGM from experimental challenge with NiV or HeV. Prime-boost regimens with adjuvanted HeV-sG subunit proteins are efficacious in stringent NiV challenge models, across a range of doses (4–100ug), and with pre-challenge neutralising antibody titres as low as 1:28.Citation62,63 The HeV sG vaccine (Equivac® HeV) has been licensed to vaccinate horses in Australia against HeV.Citation64 A number of viral vectored vaccines have also been tested and show promising immunogenicity and/or efficacy against NiV-mediated disease. These include poxvirus (canarypoxvirus ALVAC strain), vesicular stomatitis virus (VSV), rabies virus (RABV), adeno-associated virus (AAV), Newcastle disease virus (NDV) and Venezuelan equine encephalitis virus (VEEV); this topic has recently been comprehensively reviewed.Citation56,65
Lassa virus
Lassa virus (LASV) is a medically relevant arenavirus which produces conditions ranging from asymptomatic infection to a lethal haemorrhagic fever, Lassa fever (LF). Annually, LASV appears to infect between 300,000 to 500,000 individuals with mortality rates ranging from 2% to in excess of 50% in outbreaks.Citation66,67 LF is an endemic zoonosis in parts of West Africa including Nigeria, Liberia, Sierra Leone and Guinea, with more recent studies highlighting the spread of LASV into surrounding areas e.g. Mali, Benin and Ghana. This epidemiology suggests that efficacy trials of Lassa fever vaccines could be conducted successfully in countries such as Nigeria and Sierra Leone.
The common African rat (Mastomys natalensis) is the zoonotic reservoir for LASV and is thought to facilitate the ease of LASV spread to humans. Despite the recurrent and high disease incidence with associated significant morbidity and mortality, there are no approved vaccines. Currently, LF treatment relies on supportive care and, where available, the administration of the antiviral drug ribavirin.Citation68 There continues to be an unmet need for medical interventions that can curb the spread of LASV and avert the morbidity and mortality associated with potential viral dissemination into a large geographical area due to the zoonotic reservoir.Citation69,70
The first clinically available vaccine for the prevention of an arenavirus haemorrhagic fever was Candid #1, a live-attenuated vaccine against Junin virus infection, available through the Argentine National Immunization Plan.Citation71 Unfortunately, the development of a LASV vaccine has not progressed as rapidly. Cellular immunity is thought to be critical for survival of LF infection, with early T cell activation associated with a better clinical outcome.Citation72,73 Recent studies focusing on the early stages of LF in non-human primates (NHP) have confirmed previous observations that early and strong T-cell responses are associated with effective control of virus replication and recovery, while fatal LASV infection of NHP has been associated with a lack of peripheral T-cell activation.Citation73,74 It has also been demonstrated that some vaccination strategies primarily aimed to elicit LASV-specific humoral immunity are not effective, e.g. gamma-irradiated LASV.Citation75
The development of LASV vaccines has involved a number of different platform technologies including non-replicating vaccine approaches, such as inactivated LASV virus, virus-like particles (VLPs), and DNA vaccines, as well as replication-competent vaccine strategies (both recombinant and re-assortment viral vectored vaccines). The four replication-competent LASV vaccine candidates that have been extensively studied are based on vaccinia virus,Citation76,77 vesicular stomatitis virus,Citation78 Mopeia virus (MOPV)Citation79 and yellow fever virus (YFV) 17D vectorsCitation80 with all of these vaccine candidates tested in different animal models, including NHPs.
Efficacy testing in animal models that mimic the major pathophysiological and immunological features of human LF are a prerequisite before licensure. Rodents are an obvious first species to establish immunogenicity, but as LASV has a rodent host reservoir and the response to LASV varies depending on mouse strain, age and inoculation route, rodents are not suitable as a valid LF disease model. Guinea pigs are the most sensitive model to study lung pathology,Citation81,82 while common marmosets (CM) are surrogates to study liver involvement.Citation83 However, LASV-infected rhesus and cynomolgus monkeys are considered the gold-standard models and are the only available and relevant challenge models for human LF.
The YFV vaccine strain 17D has been genetically manipulated to express the LASV glycoprotein and was designed to control both diseases, YF and LF, in areas of overlapping incidence in West Africa.Citation84 While it can protect guinea pigs,Citation80 it has failed to protect marmosets and is genetically unstable.Citation86,87 In addition, while recombinant vesicular stomatitis virus (rVSV) expressing LASV glycoprotein was protective in nonhuman primate challenge, the protection was not sterile and LASV viremia could be measured post-infection.Citation85
LASV and MOPV are closely related Old World arenaviruses that can exchange genomic segments (reassort) during coinfection. Clone ML29, encodes the major antigens of LASV and also MOPV antigens. Preclinically, both marmosets and guinea pigs have survived an otherwise fatal LASV infection.Citation86,87 Recent studies have demonstrated that SIV-infected rhesus macaques respond well to ML29 vaccination, and survive when challenged with a heterologous lethal arenavirus strain (LCMV-WE) indicating that ML29 is both safe and immunogenic in immuno-compromised animals.Citation88
Another vaccine vector that proved effective in guinea pigs against LASV challenge is a Venezuelan equine encephalitis virus (rVEE) replicon particle expressing GP or NP.Citation89 Animals were fully protected against LASV challenge after prime/boost/boost immunization with this vector. One of the most promising vaccines is vaccinia virus encoding LASV glycoprotein; nonhuman primates vaccinated with this vaccine candidate were protected against challenge.Citation90,91 However, despite several promising vaccine candidates in pre-clinical evaluation, none has yet advanced to a clinical trial in humans.
Novel coronaviruses: MERS CoV and SARS CoV
Several novel coronaviruses have emerged over the last decade, causing outbreaks mainly in the Middle East region and Asia, in Saudi Arabia, Jordan, Qatar and China in particular. An epidemic of Severe Acute Respiratory Syndrome (SARS) was reported in 2003, which started in China and caused over 8000 cases with between 10 and 50% mortality depending on age.Citation92 The causative agent was identified as a novel coronavirus, SARS CoV, not previously identified as infectious to humans,Citation93 with bats and civets as natural reservoirs.Citation94,95 Middle Eastern Respiratory Syndrome (MERS) was first reported in 2012 in a man who became ill in Saudi Arabia.Citation96 The isolation of another novel coronavirus followed, known as MERS CoV, which has subsequently caused nearly 1900 cases and 670 deaths.Citation97 Dromedary camels are a reservoir, although transmission also occurs from human to human.Citation98
Strategies for producing effective coronavirus vaccines have focussed on expression of either the spike protein or nucleocapsid proteins or, in some cases a combination of both, in a range of vectors including rabies viruses, VSV and VEE (reviewed inCitation99,100). A report from a recent workshop in Riyadh on countermeasures for MERS CoV bringing together funders, public health experts and researchers concluded that progress with vaccine development is still hindered by the lack of animal models for evaluating efficacy.Citation100 Small animals do not naturally express a functional form of the dipeptidyl peptidase 4 (DPP4) receptor; however, transgenic mice expressing human DPP4 are susceptible to infection.Citation101,102 Despite this advance, mouse models are likely to be less useful for the assessment of immune correlates than larger animal models such as rhesus macaques and common marmosets, which exhibit the severe clinical syndromes observed in humans.Citation103,104 MVA and ChAd viral vectors for MERS have reached GMP manufacture, while a DNA vaccine is now being tested in clinical trials.Citation105,106
Progress with development of chimpanzee adenovirus vectors for outbreak pathogens
In May 2017, the first cases in an outbreak of EVD were reported in the Bas Uele Province in the Democratic Republic of the Congo (DRC).Citation107 This area shares a border with the Central African Republic and is particularly remote and difficult to access. As the causative species has been identified as Zaire ebolavirus, the rVSV-ZEBOV vaccine is being considered at the time of writing, for deployment in a ring vaccination design to protect contacts and frontline healthcare workers (HCWs).Citation108 This fresh outbreak is the 8th to occur in the DRC and highlights the potential utility of vaccination to protect HCWs, particularly where remote locations present significant logistical challenges for responding to and containing outbreaks. Maintaining the current momentum for developing vaccines against outbreak pathogens is crucial, and as such, simian adenoviruses are uniquely fit for purpose as an effective vaccine platform, not in small part due to their predictable safety profile, stability, manufacturability, but most importantly owing to their immunogenicity. Therefore, a single-antigen pathogen-specific ChAd vector vaccine could be suitable as a single dose approach for rapid induction of protective immunity in an outbreak, but for durable protection for potential first responders a ChAd prime, MVA boost approach could be more effective.
Novel vaccines against outbreak pathogens are under development in a range of simian adenovirus serotypes including ChAd3, ChAd63 and ChAdOx1 (reviewed inCitation109) and for the human vectors AdHu26 and AdHu5. Application of a pipeline approach to developing vaccines for outbreak pathogens can greatly accelerate the output of candidate vaccines as the key processes, such as generation of constructs, production of virus stocks, defining preclinical immunogenicity, and GMP manufacture can be substantially standardized. An approach that is currently being adopted for at least twelve potential outbreak pathogens using standardized preclinical processes (), with several advancing to GMP manufacture and clinical testing. The latter include vaccines against MERS-CoV, Rift Valley fever virus, Zika virus and Chikungunya virus.
Table 5. Status of chimpanzee adenovirus vector (ChAd) vaccine development for a range of outbreak pathogens at the Jenner Institute, University of Oxford (as May 2017). The genetic background for all vectors is ChAdOx1 (a species E modified chimpanzee adenovirus based on isolate Y25).Citation23 Antigens are inserted at the E1 locus via Gateway® recombination. For preclinical immunogenicity testing, mice typically receive a single-dose of 108 infectious units (intramuscular).
The key bottlenecks for this approach are the identification of vaccine antigens and the availability of appropriate animal models of disease. For preparations to be made to counter future threats, some knowledge of emerging pathogens is required, and yet detailed epidemiological surveillance for many infectious diseases remains limited in regions where incidence is greatest.Citation110 Recent data suggests that around 60% of emerging infectious diseases are zoonotic with the majority originating in wildlife, requiring surveillance among livestock animals and wildlife species, as well as in humans.Citation111 Although Ebola outbreaks have occurred sporadically since 1976, the pace of vaccine development for Ebola has been slow with most vaccines undergoing preclinical evaluation for more than 5 years before the start of Phase I clinical trials. The 2014–15 outbreak provided much needed momentum for public health experts and the research community to improve preparedness for future epidemics.Citation112 In order to continue to improve our preparedness for future outbreaks, epidemiological surveillance and vaccine development will need to accelerate substantially.
References
- Moss B, Smith GL, Gerin JL, Purcell RH. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984;311:67-9. doi:10.1038/311067a0. PMID:6472464
- Antrobus RD, Lillie PJ, Berthoud TK, Spencer AJ, McLaren JE, Ladell K, Lambe T, Milicic A, Price DA, Hill AV, et al. A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PLoS One. 2012;7:e48322. doi:10.1371/journal.pone.0048322. PMID:23118984
- Bliss CM, Drammeh A, Bowyer G, Sanou GS, Jagne YJ, Ouedraogo O, Edwards NJ, Tarama C, Ouedraogo N, Ouedraogo M, et al. Viral Vector Malaria Vaccines Induce High-Level T Cell and Antibody Responses in West African Children and Infants. Mol Ther. 2017;25:547-59. doi:10.1016/j.ymthe.2016.11.003. PMID:28153101
- Sophie Roetynck, Victorine Mensah, Ebrima Kanteh, Georgina Bowyer, Amy Ndaw, Francis Oko, Carly Bliss, Riccardo Cortese, Alfredo Nicosia, Rachel Roberts, et al. Immunogenicity of ChAd63/MVA ME-TRAP malaria vectored vaccines when given with routine childhood vaccines in Gambian infants and neonates: a randomized controlled trial.. Atlanta (GA) USA: American Society of Tropical Medicine and Hygiene; 2016.
- Sullivan NJ, Hensley L, Asiedu C, Geisbert TW, Stanley D, Johnson J, Honko A, Olinger G, Bailey M, Geisbert JB, et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med. 2011;17:1128-31. doi:10.1038/nm.2447. PMID:21857654
- Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol. 2009;7:393-400. doi:10.1038/nrmicro2129. PMID:19369954
- Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E, Nuthall E, Voysey M, Silva-Reyes L, McElrath MJ, et al. Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial. JAMA. 2016;315:1610-23. doi:10.1001/jama.2016.4218. PMID:27092831
- Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J, Eickmann M, Finckh A, Goncalves AR, Hooper JW, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2015;15:1156-66. doi:10.1016/S1473-3099(15)00154-1. PMID:26248510
- Sheehy SH, Duncan CJ, Elias SC, Collins KA, Ewer KJ, Spencer AJ, Williams AR, Halstead FD, Moretz SE, Miura K, et al. Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Mol Ther. 2011;19:2269-76. doi:10.1038/mt.2011.176. PMID:21862998
- Sheehy SH, Duncan CJ, Elias SC, Biswas S, Collins KA, O'Hara GA, Halstead FD, Ewer KJ, Mahungu T, Spencer AJ, et al. Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PLoS One. 2012;7:e31208. doi:10.1371/journal.pone.0031208. PMID:22363582
- de Barra E, Hodgson SH, Ewer KJ, Bliss CM, Hennigan K, Collins A, Berrie E, Lawrie AM, Gilbert SC, Nicosia A, et al. A phase Ia study to assess the safety and immunogenicity of new malaria vaccine candidates ChAd63 CS administered alone and with MVA CS. PLoS One. 2014;9:e115161. doi:10.1371/journal.pone.0115161. PMID:25522180
- Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, de Barra E, Havelock T, Bowyer G, Poulton ID, et al. Evaluation of the Efficacy of ChAd63-MVA Vectored Vaccines Expressing Circumsporozoite Protein and ME-TRAP Against Controlled Human Malaria Infection in Malaria-Naive Individuals. J Infect Dis. 2015;211:1076-86. doi:10.1093/infdis/jiu579. PMID:25336730
- Ewer K, Rampling T, Venkatraman N, Bowyer G, Wright D, Lambe T, Imoukhuede EB, Payne R, Fehling SK, Strecker T, et al. A Monovalent Chimpanzee Adenovirus Ebola Vaccine Boosted with MVA. N Engl J Med. 2016;374:1635-46. doi:10.1056/NEJMoa1411627. PMID:25629663
- Ewer KJ, O'Hara GA, Duncan CJ, Collins KA, Sheehy SH, Reyes-Sandoval A, Goodman AL, Edwards NJ, Elias SC, Halstead FD, et al. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun. 2013;4:2836. doi:10.1038/ncomms3836. PMID:24284865
- McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240-4. doi:10.1038/nm1128. PMID:15502839
- Lauer KB, Borrow R, Blanchard TJ. Multivalent and Multipathogen Viral Vector Vaccines. Clin Vaccine Immunol. 2017;24. doi:10.1128/CVI.00298-16. PMID:27535837.
- Dahlke C, Kasonta R, Lunemann S, Krähling V, Zinser ME, Biedenkopf N, Fehling SK, Ly ML, Rechtien A, Stubbe HC, et al. Dose-dependent T-cell Dynamics and Cytokine Cascade Following rVSV-ZEBOV Immunization. EBioMedicine. 2017;19:107-118. Epub 2017 Apr 5; doi:10.1016/j.ebiom.2017.03.045. PMID:28434944.
- Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet. 2017;389:505-18. doi:10.1016/S0140-6736(16)32621-6. PMID:28017403
- Mire CE, Geisbert TW, Feldmann H, Marzi A. Ebola virus vaccines – reality or fiction? Expert Rev Vaccines. 2016;15:1421-30. doi:10.1080/14760584.2016.1178068. PMID:27078187
- Fallaux FJ, Bout A, van der Velde I, van den Wollenberg DJ, Hehir KM, Keegan J, Auger C, Cramer SJ, van Ormondt H, van der Eb AJ, et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther. 1998;9:1909-17. doi:10.1089/hum.1998.9.13-1909. PMID:9741429
- Reddy PS, Ganesh S, Hawkins L, Idamakanti N. Generation of recombinant adenovirus using the Escherichia coli BJ5183 recombination system. Methods Mol Med. 2007;130:61-8. PMID:17401164
- Ruzsics Z, Lemnitzer F, Thirion C. Engineering adenovirus genome by bacterial artificial chromosome (BAC) technology. Methods Mol Biol. 2014;1089:143-58. doi:10.1007/978-1-62703-679-5_11. PMID:24132484
- Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC, Hill AV, Cottingham MG. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 2012;7:e40385. doi:10.1371/journal.pone.0040385. PMID:22808149
- Matthews QL. Capsid-incorporation of antigens into adenovirus capsid proteins for a vaccine approach. Mol Pharm. 2011;8:3-11. doi:10.1021/mp100214b. PMID:21047139
- Wu L, Zhang Z, Gao H, Li Y, Hou L, Yao H, Wu S, Liu J, Wang L, Zhai Y, et al. Open-label phase I clinical trial of Ad5-EBOV in Africans in China. Hum Vaccin Immunother. 2017;13:1-8. doi:10.1080/21645515.2017.1342021.
- Geisbert TW, Bailey M, Hensley L, Asiedu C, Geisbert J, Stanley D, Honko A, Johnson J, Mulangu S, Pau MG, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol. 2011;85:4222-33. doi:10.1128/JVI.02407-10. PMID:21325402
- Gu L, Icyuz M, Krendelchtchikova V, Krendelchtchikov A, Johnston AE, Matthews QL. Development of an Ad5H3 Chimera Using the “Antigen Capsid-Incorporation” Strategy for an Alternative Vaccination Approach. Open Virol J. 2016;10:10-20. doi:10.2174/1874357901610010010. PMID:27335626
- Morris SJ, Sebastian S, Spencer AJ, Gilbert SC. Simian adenoviruses as vaccine vectors. Future Virol. 2016;11:649-59. doi:10.2217/fvl-2016-0070
- Whitt MA, Geisbert TW, Mire CE. Single-Vector, Single-Injection Recombinant Vesicular Stomatitis Virus Vaccines Against High-Containment Viruses. Methods Mol Biol. 2016;1403:295-311. doi:10.1007/978-1-4939-3387-7_16. PMID:27076138
- Aguilar HC, Iorio RM. Henipavirus membrane fusion and viral entry. Curr Top Microbiol Immunol. 2012;359:79-94. PMID:22427111
- Owens RJ, Rose JK. Cytoplasmic domain requirement for incorporation of a foreign envelope protein into vesicular stomatitis virus. J Virol. 1993;67:360-5. PMID:8093220
- van den Pol AN, Dalton KP, Rose JK. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J Virol. 2002;76:1309-27. doi:10.1128/JVI.76.3.1309-1327.2002. PMID:11773406
- Clarke DK, Nasar F, Chong S, Johnson JE, Coleman JW, Lee M, Witko SE, Kotash CS, Abdullah R, Megati S, et al. Neurovirulence and immunogenicity of attenuated recombinant vesicular stomatitis viruses in nonhuman primates. J Virol. 2014;88:6690-701. doi:10.1128/JVI.03441-13. PMID:24696472
- Fuchs JD, Frank I, Elizaga ML, Allen M, Frahm N, Kochar N, Li S, Edupuganti S, Kalams SA, Tomaras GD, et al. First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Vesicular Stomatitis Virus Human Immunodeficiency Virus-1 gag Vaccine (HVTN 090). Open Forum Infect Dis. 2015;2:ofv082. PMID:26199949
- Yang TC, Dayball K, Wan YH, Bramson J. Detailed analysis of the CD8+ T-cell response following adenovirus vaccination. J Virol. 2003;77:13407-11. doi:10.1128/JVI.77.24.13407-13411.2003. PMID:14645597
- Wold WS, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. 2013;13:421-33. doi:10.2174/1566523213666131125095046. PMID:24279313
- Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Béthune MP, Kostense S, Penders G, Helmus N, Koudstaal W, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77:8263-71. doi:10.1128/JVI.77.15.8263-8271.2003. PMID:12857895
- Ausubel LJ, Meseck M, Derecho I, Lopez P, Knoblauch C, McMahon R, Anderson J, Dunphy N, Quezada V, Khan R, et al. Current good manufacturing practice production of an oncolytic recombinant vesicular stomatitis viral vector for cancer treatment. Hum Gene Ther. 2011;22:489-97. doi:10.1089/hum.2010.159. PMID:21083425
- Arnemo M, Viksmoen Watle SS, Schoultz KM, Vainio K, Norheim G, Moorthy V, Fast P, Røttingen JA, Gjøen T. Stability of a Vesicular Stomatitis Virus-Vectored Ebola Vaccine. J Infect Dis. 2016;213:930-3. doi:10.1093/infdis/jiv532. PMID:26563239
- Pelliccia M, Andreozzi P, Paulose J, D'Alicarnasso M, Cagno V, Donalisio M, Civra A, Broeckel RM, Haese N, Jacob Silva P, et al. Additives for vaccine storage to improve thermal stability of adenoviruses from hours to months. Nat Commun. 2016;7:13520. doi:10.1038/ncomms13520. PMID:27901019
- Dulal P, Wright D, Ashfield R, Hill AV, Charleston B, Warimwe GM. Potency of a thermostabilised chimpanzee adenovirus Rift Valley Fever vaccine in cattle. Vaccine. 2016;34:2296-8. doi:10.1016/j.vaccine.2016.03.061. PMID:27020712
- O'Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, Goodman AL, Edwards NJ, Reyes-Sandoval A, Bird P, et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. The Journal of infectious diseases. 2012;205:772-81. doi:10.1093/infdis/jir850. PMID:22275401
- van Doremalen N, Munster VJ. Animal models of Middle East respiratory syndrome coronavirus infection. Antiviral Res. 2015;122:28-38. doi:10.1016/j.antiviral.2015.07.005. PMID:26192750
- Day CW, Baric R, Cai SX, Frieman M, Kumaki Y, Morrey JD, Smee DF, Barnard DL. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology. 2009;395:210-22. doi:10.1016/j.virol.2009.09.023. PMID:19853271
- Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ Jr, Baric RS, Enjuanes L, Gallagher T, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111:4970-5. doi:10.1073/pnas.1323279111. PMID:24599590
- Holbein ME, Berglund JP, Weatherwax K, Gerber DE, Adamo JE. Access to Investigational Drugs: FDA Expanded Access Programs or “Right-to-Try” Legislation? Clin Transl Sci. 2015;8:526-32. doi:10.1111/cts.12255. PMID:25588691
- WHO. Blueprint for R&D preparedness and response to public health emergencies due to highly infectious pathogens.. Geneva: WHO,. 2015:7.
- Serdobova I, Kieny MP. Assembling a global vaccine development pipeline for infectious diseases in the developing world. Am J Public Health. 2006;96:1554-9. doi:10.2105/AJPH.2005.074583. PMID:16873743
- Plotkin SA, Mahmoud AA, Farrar J. Establishing a Global Vaccine-Development Fund. N Engl J Med. 2015;373:297-300. doi:10.1056/NEJMp1506820. PMID:26200974
- Butler D. Billion-dollar project aims to prep vaccines before epidemics hit. Nature.. Vol. 541: Springer Nature, 2017:444-5. doi:10.1038/nature.2017.21329
- Andre FE. How the research-based industry approaches vaccine development and establishes priorities. Dev Biol (Basel). 2002;110:25-9. PMID:12477303
- Diehl WE, Lin AE, Grubaugh ND, Carvalho LM, Kim K, Kyawe PP, McCauley SM, Donnard E, Kucukural A, McDonel P, et al. Ebola Virus Glycoprotein with Increased Infectivity Dominated the. 2013–2016 Epidemic. Cell. 2016;167:1088-98 e6. doi:10.1016/j.cell.2016.10.014. PMID:27814506
- Urbanowicz RA, McClure CP, Sakuntabhai A, Sall AA, Kobinger G, Müller MA, Holmes EC, Rey FA, Simon-Loriere E, Ball JK. Human Adaptation of Ebola Virus during the West African Outbreak. Cell. 2016;167:1079-87 e5. doi:10.1016/j.cell.2016.10.013. PMID:27814505
- WHO. Nipah virus outbreaks in the WHO South-East Asia Region. Available at: http://www.searo.who.int/entity/emerging_diseases/links/nipah_virus_outbreaks_sear/en/. Accessed February 10. 2017.
- Clayton BA. Nipah virus: transmission of a zoonotic paramyxovirus. Curr Opin Virol. 2017;22:97-104. doi:10.1016/j.coviro.2016.12.003. PMID:28088124
- Broder CC, Weir DL, Reid PA. Hendra virus and Nipah virus animal vaccines. Vaccine. 2016;34:3525-34. doi:10.1016/j.vaccine.2016.03.075. PMID:27154393
- ANZCTR. A Randomized, Double-blind, Placebo-controlled Study of the Safety, Tolerability, Pharmacokinetics and Immunogenicity of Various Dosing Regimens of Intravenous anti-Hendra Virus Antibody (mAb m102.4) in Healthy Subjects. Available at: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368110&isReview=true. Accessed 10th February. 2017.
- Wong KT, Grosjean I, Brisson C, Blanquier B, Fevre-Montange M, Bernard A, Loth P, Georges-Courbot MC, Chevallier M, Akaoka H, et al. A golden hamster model for human acute Nipah virus infection. Am J Pathol. 2003;163:2127-37. doi:10.1016/S0002-9440(10)63569-9. PMID:14578210
- Bossart KN, Zhu Z, Middleton D, Klippel J, Crameri G, Bingham J, McEachern JA, Green D, Hancock TJ, Chan YP, et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009;5:e1000642. doi:10.1371/journal.ppat.1000642. PMID:19888339
- Geisbert TW, Daddario-DiCaprio KM, Hickey AC, Smith MA, Chan YP, Wang LF, Mattapallil JJ, Geisbert JB, Bossart KN, Broder CC. Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS One. 2010;5:e10690. doi:10.1371/journal.pone.0010690. PMID:20502528
- DeBuysscher BL, Scott D, Marzi A, Prescott J, Feldmann H. Single-dose live-attenuated Nipah virus vaccines confer complete protection by eliciting antibodies directed against surface glycoproteins. Vaccine. 2014;32:2637-44. doi:10.1016/j.vaccine.2014.02.087. PMID:24631094
- Bossart KN, Rockx B, Feldmann F, Brining D, Scott D, LaCasse R, Geisbert JB, Feng YR, Chan YP, Hickey AC, et al. A Hendra virus G glycoprotein subunit vaccine protects African green monkeys from Nipah virus challenge. Sci Transl Med. 2012;4:146ra07. doi:10.1126/scitranslmed.3004241
- Pallister J, Middleton D, Wang LF, Klein R, Haining J, Robinson R, Yamada M, White J, Payne J, Feng YR, et al. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 2011;29:5623-30. doi:10.1016/j.vaccine.2011.06.015. PMID:21689706
- Broder CC, Xu K, Nikolov DB, Zhu Z, Dimitrov DS, Middleton D, Pallister J, Geisbert TW, Bossart KN, Wang LF. A treatment for and vaccine against the deadly Hendra and Nipah viruses. Antiviral Res. 2013;100:8-13. doi:10.1016/j.antiviral.2013.06.012. PMID:23838047
- Satterfield BA, Dawes BE, Milligan GN. Status of vaccine research and development of vaccines for Nipah virus. Vaccine. 2016;34:2971-5. doi:10.1016/j.vaccine.2015.12.075. PMID:26973068
- Fisher-Hoch SP, Tomori O, Nasidi A, Perez-Oronoz GI, Fakile Y, Hutwagner L, McCormick JB. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. BMJ. 1995;311:857-9. doi:10.1136/bmj.311.7009.857. PMID:7580496
- McCormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155:437-44. doi:10.1093/infdis/155.3.437. PMID:3805771
- McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, Elliott LH, Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986;314:20-6.
- Mylne AQ, Pigott DM, Longbottom J, Shearer F, Duda KA, Messina JP, Weiss DJ, Moyes CL, Golding N, Hay SI. Mapping the zoonotic niche of Lassa fever in Africa. Trans R Soc Trop Med Hyg. 2015;109:483-92. doi:10.1093/trstmh/trv047. PMID:26085474
- Sogoba N, Feldmann H, Safronetz D. Lassa fever in West Africa: evidence for an expanded region of endemicity. Zoonoses Public Health. 2012;59 Suppl 2:43-7. doi:10.1111/j.1863-2378.2012.01469.x. PMID:22958249
- Ambrosio A, Saavedra M, Mariani M, Gamboa G, Maiza A. Argentine hemorrhagic fever vaccines. Hum Vaccin. 2011;7:694-700. doi:10.4161/hv.7.6.15198. PMID:21451263
- Mahanty S, Bausch DG, Thomas RL, Goba A, Bah A, Peters CJ, Rollin PE. Low levels of interleukin-8 and interferon-inducible protein-10 in serum are associated with fatal infections in acute Lassa fever. J Infect Dis. 2001;183:1713-21. doi:10.1086/320722. PMID:11372023
- ?A3B2 tlsb=-0.15pt?>Russier M, Pannetier D, Baize S. Immune responses and Lassa virus infection. Viruses. 2012;4:2766-85. doi:10.3390/v4112766. PMID:23202504
- Prescott JB, Marzi A, Safronetz D, Robertson SJ, Feldmann H, Best SM. Immunobiology of Ebola and Lassa virus infections. Nat Rev Immunol. 2017. doi:10.1038/nri.2016.138. PMID:28111475
- McCormick JB, Mitchell SW, Kiley MP, Ruo S, Fisher-Hoch SP. Inactivated Lassa virus elicits a non protective immune response in rhesus monkeys. J Med Virol. 1992;37:1-7. doi:10.1002/jmv.1890370102. PMID:1619397
- Auperin DD, Esposito JJ, Lange JV, Bauer SP, Knight J, Sasso DR, McCormick JB. Construction of a recombinant vaccinia virus expressing the Lassa virus glycoprotein gene and protection of guinea pigs from a lethal Lassa virus infection. Virus Res. 1988;9:233-48. doi:10.1016/0168-1702(88)90033-0. PMID:3354260
- Clegg JC, Lloyd G. Vaccinia recombinant expressing Lassa-virus internal nucleocapsid protein protects guineapigs against Lassa fever. Lancet. 1987;2:186-8. doi:10.1016/S0140-6736(87)90767-7. PMID:2885642
- Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Möller P, Wagner R, Volchkov V, Klenk HD, Feldmann H, Ströher U. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78:5458-65. doi:10.1128/JVI.78.10.5458-5465.2004. PMID:15113924
- Lukashevich IS, Patterson J, Carrion R, Moshkoff D, Ticer A, Zapata J, Brasky K, Geiger R, Hubbard GB, Bryant J, et al. A live attenuated vaccine for Lassa fever made by reassortment of Lassa and Mopeia viruses. J Virol. 2005;79:13934-42. doi:10.1128/JVI.79.22.13934-13942.2005. PMID:16254329
- Jiang X, Dalebout TJ, Bredenbeek PJ, Carrion R Jr, Brasky K, Patterson J, Goicochea M, Bryant J, Salvato MS, Lukashevich IS. Yellow fever 17D-vectored vaccines expressing Lassa virus GP1 and GP2 glycoproteins provide protection against fatal disease in guinea pigs. Vaccine. 2011;29:1248-57. doi:10.1016/j.vaccine.2010.11.079. PMID:21145373
- Jahrling PB, Smith S, Hesse RA, Rhoderick JB. Pathogenesis of Lassa virus infection in guinea pigs. Infect Immun. 1982;37:771-8. PMID:6749685
- Walker DH, Wulff H, Lange JV, Murphy FA. Comparative pathology of Lassa virus infection in monkeys, guinea-pigs, and Mastomys natalensis. Bull World Health Organ. 1975;52:523-34. PMID:821625
- Carrion R, Jr., Brasky K, Mansfield K, Johnson C, Gonzales M, Ticer A, Lukashevich I, Tardif S, Patterson J. Lassa virus infection in experimentally infected marmosets: liver pathology and immunophenotypic alterations in target tissues. J Virol. 2007;81:6482-90. doi:10.1128/JVI.02876-06. PMID:17409137
- Bredenbeek PJ, Molenkamp R, Spaan WJ, Deubel V, Marianneau P, Salvato MS, Moshkoff D, Zapata J, Tikhonov I, Patterson J, et al. A recombinant Yellow Fever 17D vaccine expressing Lassa virus glycoproteins. Virology. 2006;345:299-304. doi:10.1016/j.virol.2005.12.001. PMID:16412488
- Geisbert TW, Jones S, Fritz EA, Shurtleff AC, Geisbert JB, Liebscher R, Grolla A, Ströher U, Fernando L, Daddario KM, et al. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2005;2:e183. doi:10.1371/journal.pmed.0020183. PMID:15971954
- Falzarano D, Feldmann H. Vaccines for viral hemorrhagic fevers–progress and shortcomings. Curr Opin Virol. 2013;3:343-51. doi:10.1016/j.coviro.2013.04.007. PMID:23773330
- Lukashevich IS. Advanced vaccine candidates for Lassa fever. Viruses. 2012;4:2514-57. doi:10.3390/v4112514. PMID:23202493
- Zapata JC, Poonia B, Bryant J, Davis H, Ateh E, George L, Crasta O, Zhang Y, Slezak T, Jaing C, et al. An attenuated Lassa vaccine in SIV-infected rhesus macaques does not persist or cause arenavirus disease but does elicit Lassa virus-specific immunity. Virol J. 2013;10:52. doi:10.1186/1743-422X-10-52. PMID:23402317
- Pushko P, Geisbert J, Parker M, Jahrling P, Smith J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J Virol. 2001;75:11677-85. doi:10.1128/JVI.75.23.11677-11685.2001. PMID:11689649
- Fisher-Hoch SP, Hutwagner L, Brown B, McCormick JB. Effective vaccine for lassa fever. J Virol. 2000;74:6777-83. doi:10.1128/JVI.74.15.6777-6783.2000. PMID:10888616
- Olschlager S, Flatz L. Vaccination strategies against highly pathogenic arenaviruses: the next steps toward clinical trials. PLoS Pathog. 2013;9:e1003212. doi:10.1371/journal.ppat.1003212. PMID:23592977
- Cherry JD. The chronology of the. 2002–2003 SARS mini pandemic. Paediatr Respir Rev. 2004;5:262-9. doi:10.1016/j.prrv.2004.07.009. PMID:15531249
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-66. doi:10.1056/NEJMoa030781. PMID:12690092
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276-8. doi:10.1126/science.1087139. PMID:12958366
- Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676-9. doi:10.1126/science.1118391. PMID:16195424
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814-20. doi:10.1056/NEJMoa1211721. PMID:23075143
- WHO. Middle East respiratory syndrome coronavirus (MERS-CoV) – Saudi Arabia. Available at: http://www.who.int/csr/don/26-january-2017-mers-saudi-arabia/en/. Accessed February 10. 2017.
- Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nature reviews Microbiology. 2013;11:836-48. doi:10.1038/nrmicro3143. PMID:24217413
- Choi J, Kim MG, Oh YK, Kim YB. Progress of Middle East respiratory syndrome coronavirus vaccines: a patent review. Expert Opin Ther Pat. 2017;27:1-11.
- Excler JL, Delvecchio CJ, Wiley RE, Williams M, Yoon I-K, Modjarrad K, Boujelal M, Moorthy VS, Hersi AS, Kim JH. Toward Developing a Preventive MERS-CoV Vaccine-Report from a Workshop Organized by the Saudi Arabia Ministry of Health and the International Vaccine Institute, Riyadh, Saudi Arabia, November 14–15, 2015. Emerg Infect Dis. 2016;22(8). doi:10.3201/eid2208.160229.
- Agrawal AS, Garron T, Tao X, Peng BH, Wakamiya M, Chan TS, Couch RB, Tseng CT. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol. 2015;89:3659-70. doi:10.1128/JVI.03427-14. PMID:25589660
- Cockrell AS, Peck KM, Yount BL, Agnihothram SS, Scobey T, Curnes NR, Baric RS, Heise MT. Mouse dipeptidyl peptidase 4 is not a functional receptor for Middle East respiratory syndrome coronavirus infection. J Virol. 2014;88:5195-9. doi:10.1128/JVI.03764-13. PMID:24574399
- Falzarano D, de Wit E, Feldmann F, Rasmussen AL, Okumura A, Peng X, Thomas MJ, van Doremalen N, Haddock E, Nagy L, et al. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014;10:e1004250. doi:10.1371/journal.ppat.1004250. PMID:25144235
- Munster VJ, de Wit E, Feldmann H. Pneumonia from human coronavirus in a macaque model. N Engl J Med. 2013;368:1560-2. doi:10.1056/NEJMc1215691. PMID:23550601
- Wang L, Shi W, Joyce MG, Modjarrad K, Zhang Y, Leung K, Lees CR, Zhou T, Yassine HM, Kanekiyo M, et al. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun. 2015;6:7712. doi:10.1038/ncomms8712. PMID:26218507
- Clinicaltrials.gov. NCT02670187. Phase I, Open Label Dose Ranging Safety Study of GLS-5300 in Healthy Volunteers. Available at: https://clinicaltrials.gov/ct2/show/NCT02670187?term=NCT02670187&rank=1. Accessed 10th May. 2017.
- WHO. Ebola Situation Report- 5th June. 2017. Ebola Situation Report- 5th June. 2017, 2017:1-6 .
- Cohen J. As Ebola outbreak grows, question of using vaccine becomes more urgent. Science. 2017.
- Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AV, Dorrell L. Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol. 2016;41:47-54. doi:10.1016/j.coi.2016.05.014. PMID:27286566
- King DA, Peckham C, Waage JK, Brownlie J, Woolhouse ME. Epidemiology. Infectious diseases: preparing for the future. Science. 2006;313:1392-3.
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990-3. doi:10.1038/nature06536. PMID:18288193
- Butler D. How to beat the next Ebola. Nature. 2015;524:22-5. doi:10.1038/524022a. PMID:26245563
- Walther M, Thompson FM, Dunachie S, Keating S, Todryk S, Berthoud T, Andrews L, Andersen RF, Moore A, Gilbert SC, et al. Safety, immunogenicity, and efficacy of prime-boost immunization with recombinant poxvirus FP9 and modified vaccinia virus Ankara encoding the full-length Plasmodium falciparum circumsporozoite protein. Infect Immun. 2006;74:2706-16. doi:10.1128/IAI.74.5.2706-2716.2006. PMID:16622207
- McConkey SJ, Reece WH, Moorthy VS, Webster D, Dunachie S, Butcher G, Vuola JM, Blanchard TJ, Gothard P, Watkins K, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9:729-35. doi:10.1038/nm881. PMID:12766765
- Webster DP, Dunachie S, Vuola JM, Berthoud T, Keating S, Laidlaw SM, McConkey SJ, Poulton I, Andrews L, Andersen RF, et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci U S A. 2005;102:4836-41. doi:10.1073/pnas.0406381102. PMID:15781866
- Bejon P, Mwacharo J, Kai O, Mwangi T, Milligan P, Todryk S, Keating S, Lang T, Lowe B, Gikonyo C, et al. A phase 2b randomised trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS Clin Trials. 2006;1:e29. PMID:17053830
- Ogwang C, Kimani D, Edwards NJ, Roberts R, Mwacharo J, Bowyer G, Bliss C, Hodgson SH, Njuguna P, Viebig NK, et al. Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci Transl Med. 2015;7:286re5.
- Rampling T, Ewer K, Bowyer G, Venkatraman N, Wright D, Lambe T, Imoukhuede EB, Payne R, Fehling SK, Strecker T, et al. A Monovalent Chimpanzee Adenovirus Ebola Vaccine – Preliminary Report. N Engl J Med. 2015;372(5):1-10. PMID:25629663.
- Zhu FC, Hou LH, Li JX, Wu SP, Liu P, Zhang GR, Hu YM, Meng FY, Xu JJ, Tang R, et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet. 2015;385:2272-9. PMID:25817373
- Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, Yerly S, Dayer JA, Kraehling V, Kasonta R, et al. Phase 1 Trials of rVSV Ebola Vaccine in Africa and Europe – Preliminary Report. N Engl J Med. 2016;374(17):1647-60. Epub 2015 Apr 1. doi:10.1056/NEJMoa1502924.
- Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, Doumbia M, Traore A, Coulibaly F, Kodio M, Onwuchekwa U, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2016;16:31-42. PMID:26546548
- Nakayama E, Saijo M. Animal models for Ebola and Marburg virus infections. Frontiers in microbiology. 2013;4:267. PMID:24046765
- Yun NE, Seregin AV, Walker DH, Popov VL, Walker AG, Smith JN, Miller M, de la Torre JC, Smith JK, Borisevich V, et al. Mice lacking functional STAT1 are highly susceptible to lethal infection with Lassa virus. J Virol. 2013;87:10908-11. PMID:23903830
- Dhondt KP, Mathieu C, Chalons M, Reynaud JM, Vallve A, Raoul H, Horvat B. Type I interferon signaling protects mice from lethal henipavirus infection. J Infect Dis. 2013;207:142-51. PMID:23089589
- Bente DA, Alimonti JB, Shieh WJ, Camus G, Ströher U, Zaki S, Jones SM. Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J Virol. 2010;84:11089-100. doi:10.1128/JVI.01383-10. PMID:20739514
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19:720-30. doi:10.1016/j.chom.2016.03.010. PMID:27066744
- Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, et al. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4:e29. doi:10.1371/journal.ppat.0040029. PMID:18282093
- Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14:763-72. doi:10.1016/S1473-3099(14)70718-2. PMID:24837566
