ABSTRACT
Objective: The aims of this study were to estimate the cost-effectiveness of the Haemophilus influenzae type b (Hib) vaccine for the prevention of childhood pneumonia, meningitis and other vaccine-preventable diseases in mainland China from a societal perspective and to provide information about the addition of the Hib vaccine to Chinese immunization programs.
Methods: A decision tree and the Markov model were used to estimate the costs and effectiveness of the Hib vaccine versus no Hib vaccine for a birth cohort of 100,000 children in 2016. The disease burden was estimated from the literature, statistical yearbooks and field surveys. Vaccine costs were calculated from government reports and the United Nations International Children's Emergency Fund (UNICEF) website. The WHO cost-effectiveness thresholds were used to evaluate the Hib vaccine intervention. A one-way sensitivity analysis and probabilistic sensitivity analysis were performed to evaluate the parameter uncertainties.
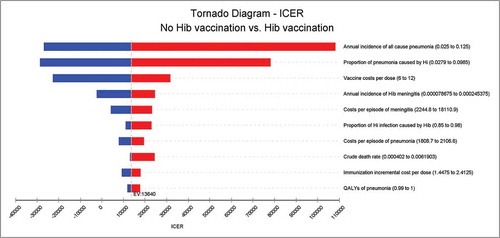
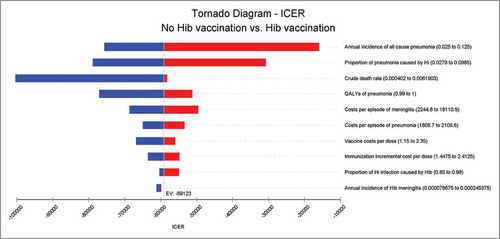
Results: Within the hypothetical cohort, under a vaccination coverage of 90%, the Hib vaccine could reduce 91.4% of Hib pneumonia and 88.3% of Hib meningitis; the Hib vaccine could also prevent 25 deaths, 24 meningitis sequelae cases and 9 hearing loss cases caused by Hib infection. From a societal perspective, the incremental cost-effectiveness ratio (ICER) of the Hib vaccine compared with no vaccination was US$ 13,640.1 at the market price, which was less than 3 times the GDP per capita of China in 2016. The ICER of the Hib vaccine was US$ −59,122.9 at the UNICEF price, indicating a cost savings. The largest portion of the uncertainty in the result was caused by the annual incidence of all-cause pneumonia, proportion of pneumonia caused by Hi, vaccine costs per dose, annual incidence of Hib meningitis and costs per episode of meningitis. The models were robust considering parameter uncertainties.
Conclusion: The Hib vaccine is a cost-effective intervention among children in mainland China. The cost of Hib vaccine should be reduced, and it should be introduced into Chinese immunization programs.
Background
Haemophilus influenzae type b (Hib) is a bacterium that causes meningitis, epiglottitis, pneumonia, arthritis and cellulitis in children under 5 years of age.Citation1 Watt, JPCitation2 estimates that 1.11 million cases of serious illness and 19,000 deaths due to Hib in children under 5 years of age occurred in China in 2000, with approximately 14% and 5% of the total cases and deaths worldwide occurring in China, respectively. The case fatality rate (CFR) of Hib meningitis is 3%-6% despite appropriate antimicrobial treatment, and sequelae occur in 15%-30% of survivors, including hearing impairment and other neurologic problems.Citation1,Citation3
Hib vaccines are safe and effective in preventing the morbidity and mortality of diseases caused by Hib. The reported Hib diseases in countries in which Hib vaccines have been introduced have been dramatically reduced, regardless of their development level and economic status.Citation4,Citation5 Vaccination is the only effective means of preventing Hib disease and has become increasingly important as Hib antibiotic resistance grows. The World Health Organization (WHO) recommends that all countries introduce the Hib vaccine into their national immunization programs. By 2015, 192 of the 194 WHO member countries (except China and Thailand) had introduced the Hib vaccine into their immunization programs.
The Hib vaccine was firstly introduced in China in 1997. By 2015, there were 6 Hib single-component vaccines and 3 Hib-containing combined vaccines approved by the China Food and Drug Administration. All the Hib vaccines are non-Expanded Program Immunization (EPI) vaccines, which rely on out-of-pocket payment by parents. The 4 doses of Hib single-component vaccine can be as expensive as US$ 40, which is not affordable for most parents in China's western region and in other economically undeveloped areas. Consequently, the coverage of the 2nd dose of the Hib vaccine remained at approximately 46.7% among target children in 2014.Citation6
In 2017, the State Council of China asked the health and financial departments to gradually introduce safe, effective and affordable vaccines into the Chinese EPI. The aim of this study was to analyze the cost-effectiveness of the Hib vaccine for the prevention of childhood pneumonia, meningitis and other vaccine-preventable diseases in mainland China from a societal perspective and to provide decision-makers with crucial information about adding the Hib vaccine into the Chinese EPI.
Results
Impact of the Hib vaccine
The reduction in disease cases following Hib vaccination strategies was compared with the results of non-vaccination (). Within the hypothetical cohort, under a vaccination coverage of 90%, the Hib vaccine could reduce 91.4% of Hib pneumonia cases and 88.3% of Hib meningitis cases; the Hib vaccine could also prevent 25 deaths, 24 meningitis sequelae cases and 9 hearing loss cases.
Table 1. Markov cohort stages of Hib vaccination compared with the non-vaccination strategy.
Cost-effectiveness
The projected health outcomes and costs for each vaccination strategy compared with those of no vaccination are shown in . The incremental cost-effectiveness ratio (ICER) of Hib vaccination compared with no vaccination was US$ 13,640.1 at the market price, which was less than the cost-effectiveness threshold recommended by the WHO, i.e., 3 times the gross domestic product (GDP) per capita of China in 2016.
Table 2. Estimated outcomes and costs of vaccination strategies per child.
The ICER of Hib vaccination compared with no vaccination was US$ −59,122.9 at the United Nations International Children's Emergency Fund (UNICEF) price, which indicated a cost savings.
Sensitivity analyses
and illustrated the results of the one-way sensitivity analysis of the Hib vaccine strategy versus non-vaccination. The projected ICER of Hib vaccination versus no vaccination at the market price was most sensitive to the annual incidence of all- cause pneumonia, proportion of pneumonia caused by Hi, vaccine costs per dose, annual incidence of Hib meningitis and costs per episode of meningitis; none of the single parameter could alter the cost savings at the UNICEF price. Taking all parameter uncertainties into account, the model remained robust and reliable.
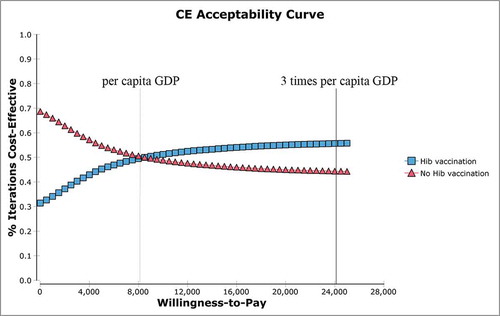
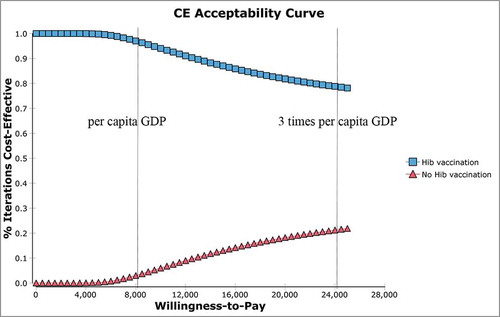
The results of the probabilistic sensitivity analysis indicated that in Hib simulations, 55.7% showed cost-effectiveness and 44.3% did not at the market price (); 78.2% showed cost-effectiveness, and 21.8% did not at the UNICEF price ().
Discussion
To the best of our knowledge, this is the first study to comprehensively evaluate the cost-effectiveness and health benefits of the Hib vaccine in mainland China. Compared with the non-vaccination strategy, these projected vaccination programs substantially reduced the disease burden and mortality of Hib diseases and reduced medical costs for the treatment of Hib invasive diseases. The Hib vaccine is particularly important for Hib diseases because antibiotic resistance makes treatment more difficult and expensiveCitation7 and because the overuse of antibiotics is very common in China.Citation8 The ICER per quality-adjusted life year (QALY) averted by the Hib vaccine was cost-effective at the market price and cost-saving at the UNICEF price based on the WHO thresholds.
The ICER per QALY was higher at the market price than in other studies conducted in both lower-middle- and upper-middle-income countries eligible for financial support for immunization programs from the GAVI Alliance,Citation9 such as Vietnam,Citation10 Indonesia,Citation11 and India.Citation12 The ICER per QALY was similar to Taiwan's ICER in 1994 (US$ 33,258.9, per capita GDP: 17,007)Citation13, but lower than Taiwan's ICER in 2007 (US$ 108,616.4, per capita GDP: 18,256).Citation14
In the past, disability-adjusted life year (DALY) was the only summary measure for which consistent estimates were available across all parts of the world, and the DALY is recommended by WHO for economic evaluations of immunization programs,Citation15 such as economic evaluations of Hib vaccines in Vietnam,Citation10 India Citation12 and low- and middle-income countries.Citation9 Recently, suitable QALY weights have been readily available, and QALY has been widely used in the assessment of health intervention of pneumococcal vaccines.Citation16-21 QALY and DALY are complementary concepts and are derived from the product of the number of years lived and by the quality of those years. QALY and DALY use utility weights of health states and disability weights, respectively, to reflect the burden of the same states.Citation22 Hib diseases start in the very early years of life and are of short duration; therefore, the QALY gained may exceed the DALY saved.Citation23 Therefore, the intervention may be more cost-effective in the ICER per QALY gained than in the ICER per DALY saved.
In the published articleCitation2,Citation9-12, Hib disease was divided into three different groups, such as meningitis, pneumonia and less common non-pneumonia-non-meningitis; the time horizons were for the lifetime of the patients. In our study, Hib disease was divided into Hib pneumonia, Hib epiglottitis, Hib meningitis, meningitis sequelae and hearing loss; more detailed results were provided in this study than previously published articles. The reasons of 5 – years – time horizon in this study were as follows: 1) Almost all Hib cases occurred among children under 5 years of age. Most children acquired immunity by asymptomatic infection in the 5–6 years of age. 2) The duration of protection by the Hib vaccine did not continue throughout the lifetime. 3) There is no Hib disease surveillance in China. Thus, obtaining most of the disease burden and epidemiological data among children and adults older than 5 years is difficult.
Although the Hib vaccine has been recommended for inclusion in national immunization schedules in all countries, Hib immunization was not included as a national routine immunization program in China because there was no routine surveillance of Hib disease or cost-effectiveness analysis, and the cost of the Hib vaccine itself was very high.
Our study highlighted that vaccination costs had a significant impact on the cost-effectiveness of the projected Hib vaccines. In most developing countries or GAVI-eligible countries where Hib vaccines have been routinely implemented, the vaccine cost is estimated to range from US$ 1 to US$ 3 per dose.Citation9,Citation24 In China, a variety of Hib vaccines that are not included in the free routine immunization must be paid for by the consumer, which negatively affects vaccination coverage. The full cost of the EPI could be dramatically increased after the addition of Hib into the EPI. The cost of the EPI was nearly US$ 30 per child for the all EPI vaccines, disease surveillance and special investigation in 2016.Citation25 A total Hib vaccine cost of US$ 40 is more expensive than other EPI vaccines in China. Therefore, unless the vaccine price falls, it will be difficult for the government to add the Hib vaccine to the Chinese EPI.
The Monte Carlo simulation incorporated parameter uncertainty into the analysis, and it was found that variations in cost-effectiveness were explained by only a few variables. As might have been expected, the incidences of Hib pneumonia and meningitis caused the largest part of the uncertainty which is consistent with other studies.Citation9 Hib pneumonia is important both because it is the most frequent type of Hib disease and because of the relatively wide uncertainty range. The burden of clinical and etiologic-specific pneumonia is intrinsically difficult to determine and remains the most important ambiguity when making conclusions about the value of the Hib vaccine. Because the reliability of the data for Hib meningitis is considerably better than for Hib pneumonia, the uncertainty range is reduced. However, because of the high cost of lost productivity attributable to meningitis sequelae, the incidence of Hib meningitis was the more important determinant of cost-effectiveness from a societal perspective.
The limitations of this review include the following. 1) There was a lack of local data for some parameters, including the incidence and costs of epiglottitis, the costs of meningitis sequelae and the case fatality rates (CFRs) of sequelae and hearing loss. However, the conclusion was not altered by these parameters according to the sensitivity analyses. 2) The cost-effectiveness of the Hib vaccine might be underestimated due to the incidences and costs of Hib diseases other than meningitis, pneumonia and epiglottitis. 3) A wide margin of sensitivity surrounding the incidence of Hib pneumonia and meningitis reflects different scenarios in mainland China.
Conclusion
The Hib vaccine is a cost-effective intervention among children in China. The cost of the Hib vaccine should be reduced, and it should be introduced into Chinese immunization programs.
Methods
Model overview and analytic framework
A decision tree and Markov model were designed to simulate the health state of a theoretical birth cohort of 100,000 infants and to estimate the costs, benefits and health outcomes of the Hib vaccine using TreeAge Pro 2017 (TreeAge Software, Inc., Williamstown, MA). The decision-tree and Markov model diagram is presented in . In the model, every infant was assumed to enter the model in the presence of each option (Hib vaccination or no Hib vaccination) and to be healthy but exposed to the risk of contracting Hib pneumonia, Hib meningitis, or Hib epiglottitis, depending on the vaccination status and efficacy. Meningitis sequelae and hearing loss were also considered in the model. The Markov model cycled in yearly increments for 5 years.
Figure 5. Decision tree and Markov model showing different vaccination strategies and possible subsequent events for each child. The “Protected by vaccine” and “Not protected by vaccine” arms are clones of the “No vaccination” arm.
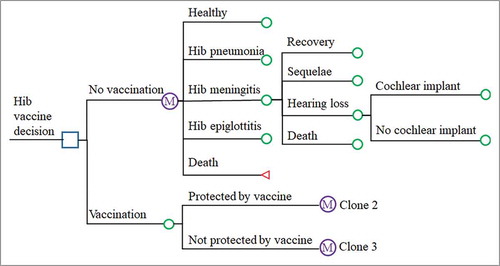
The parameters in the model were derived from the literature, statistical yearbooks and field surveys. Model outputs included the numbers of Hib pneumonia cases, Hib epiglottitis cases, Hib meningitis cases, meningitis sequelae, hearing loss cases, deaths, and QALYs. Disease burden and costs of cellulitis, arthritis, osteomyelitis and bacteremia caused by Hib were omitted due to a lack of local data.
Several assumptions were included in the model: (1) the theoretical population would be protected from Hib infection after administration of the Hib vaccine or Hib infection; (2) there were no systematic studies of health utility in China and no regional differences in health utilities (the QALY values in this model were obtained from the literature); and (3) the costs and loss of QALY due to adverse events were omitted from the analysis because the Hib vaccine was known to be safe.
Epidemiological data
presents the point and interval estimation of the parameters in the Markov model for the base-case and sensitivity analyses. Demographic information, including the crude death rate, was derived from the Chinese Health Statistical Yearbook.Citation26 The incidence density of pneumonia was based on a retrospective study of the disease burden of community-acquired pneumonia among children under 5 years of age in Gansu Province during 2015–2016.Citation27 The incidence of Hib meningitis was obtained based on the Chinese disease burden of bacterial meningitis.Citation28 The rates of Hib meningitis, sequelae, hearing loss and case fatality were derived from a retrospective study of bacterial meningitis in China in 2016.Citation29 From this study, 17.6% of cases of Hib meningitis involved a physical / intellectual / mental disability, of which required special education. Furthermore, 6.9% of children with meningitis had hearing problems, of which 40% required a cochlear hearing device.Citation30
Table 3. Point and interval estimation of parameters in the Markov model.
The CFRs of sequelae, hearing loss and epiglottitis were the same as the crude death rateCitation26 due to the lack of hospital-based CFR data. Due to a lack of research in China on epiglottitis, data from a Japanese studyCitation31 were applied because of regional homogeneity. The plausible range of the base-case value was subjected to a sensitivity analysis.
The proportion of the pneumonia caused by Hi was obtained from a Chinese systemic review of the etiology of children with pneumonia. Serotype b is responsible for approximately 95% of all cases of invasive disease due to Hi.Citation4 In the pre-vaccine era, Hib was responsible for 75%-90% of epiglottitis cases in children.Citation22,Citation32
Disease burden
The analysis was conducted from societal perspectives. The cost data for the direct medical costs per case of Hib-related disease included fees for hospitalization, physician consultations, diagnostic tests, operations and nursing as well as medication expenses.
We conducted a retrospective study of the disease burden of pneumonia among children under 5 years of age from 2015–2016,Citation27 in which the average cost per case was US$ 936.1, including US$ 672.1 of direct costs and US$ 264.0 of indirect costs. The total cost per Hib meningitis case was US$ 10,215.2 based on the Chinese disease burden of bacterial meningitis.Citation29 There were no available data on the costs of meningitis sequelae. The cost of meningitis sequelae was based on the disease burden of Japanese encephalitis.Citation33 The costs associated with the long-term sequelae of meningitis, including hearing loss and special education for learning disabilities, were also included in the analysis. The annual cost (US$ 3469.1) and proportion (65%) of special education were derived from the Statistical Yearbook of Chinese Education 2015.Citation34 The cost of epiglottitis was assumed to have homogeneity with the direct medical cost of adenoid disease from the Chinese Health Statistical Yearbook.Citation26
If the cost data used in this analysis were derived from previous years or some provinces, the GDP per capitaCitation35 and a discounted rate were used to adjust the cost data into 2016 Chinese RMB at the national level. All costs were presented in 2016 US dollars (1 US$ = 6.64 RMBCitation36). Both costs and effectiveness were discounted at 5% (range: 3%-8%).Citation37
Vaccine coverage
The Hib immunization schedule follows the recommendation by the manufacturers. Infants should receive a 3-dose primary series of Hib vaccine (starting at 2 or 3 months, with at least a 4-week interval between doses and completion within 6 months of age) and a booster dose at the age of 18–24 months.
Based on the national immunization coverage survey, the coverage of new vaccines integrated into the EPI after 2008 was above 90% overall.Citation38 We assumed that the Hib vaccine would be funded by the government through the EPI and would attain coverage near 90%, similar to the EPI vaccines.
Immunization cost and vaccine cost
According to the country's application of the Hib vaccine into the existing immunization services, no EPI staff salary or office expenses were needed. Hence, only the incremental cost of disease surveillance, training, transportation, media and publicity, injection supplies and subsidies were included. We conducted a retrospective study of the incremental EPI costs in 2016; the incremental EPI costs were US$ 1.9 per dose.
The average price of the Hib vaccine in China was US$ 10.0 per dose on the current tariff. The average price of the DTP-HepB-Hib vaccine in a single-dose liquid presentation was US$ 2.0 from the UNICEF website.Citation24
Vaccine efficacy
The model considered both the direct vaccine efficacy and a herd protection effect on unvaccinated children. For 3 doses of the Hib vaccine, we used 95% (95% CI: 82%-99%) and 91% (95% CI: 73%-97%) as the level of vaccine effectiveness against invasive Hib disease and meningitis, respectively, based on a systematic review of the effectiveness of the Hib vaccine.Citation39
Major declines in the levels of nasopharyngeal Hib colonization have also been observed following introduction of Hib conjugate vaccines,Citation40,Citation41 suggesting that widespread use of the vaccine has resulted in herd protection. The United States experience with herd immunity of pneumococcal conjugate vaccine was approximately 30% of the direct effect.Citation42 The herd protection effect in the unvaccinated population was set at 20% of the efficacy of the vaccine due to the serotype shift impact on herd effects.Citation22,Citation43; the rates of Hib vaccine protection against invasive diseases and meningitis were 19.0% and 18.2%, respectively.
Effectiveness parameters
In this study, health outcomes were measured by QALYs gained. Health utility values were measured on an interval scale, on which 0 denoted a state of health equivalent to death and 1 denoted perfect health. QALYs for health states were derived from published studies.Citation44-47
Cost-effectiveness analysis
ICERs were used to compare the 2 alternative strategies. The Chinese Government has not determined an official policy for using cost-effectiveness evidence for health care decision-making. According to WHO-recommended thresholds,Citation48 the Hib vaccine can be considered very cost-effective or cost-effective when the ICER is lower than the annual per capita GDP or between 1 and 3 times the annual per capita GDP, respectively. These values were US$ 8,129.5 and US$ 24,388.5,Citation35 respectively.
Sensitivity analyses
To measure the uncertainty in our analysis, we conducted one-way sensitivity analysis and probabilistic sensitivity analysis. Based on the literature, we determined a range of plausible values for each parameter entered into the model. In cases in which ranges could not be unfound in the literature, we used ±25% as a conservative estimate. A tornado diagram was used to present parameters in which variations changed the ICER. In addition, parameters were generated using Monte Carlo simulations (number of samples = 100,000) for the probabilistic sensitivity analysis to evaluate the impact of parameter uncertainty on the ICER.
Abbreviations
| CFR | = | Case fatality rate |
| DALY | = | Disability-adjusted life year |
| EPI | = | Expanded Program Immunization |
| GAVI | = | Global Alliance for Vaccines and Immunization |
| GDP | = | Gross domestic product |
| Hib | = | Haemophilus influenzae type b |
| ICER | = | Incremental cost-effectiveness ratio |
| QALY | = | Quality-adjusted life year |
| UNICEF | = | United Nations International Children's Emergency Fund |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Hamborsky J, Kroger A, Wolfe C. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Washington D.C: Public Health Foundation; 2015.
- Watt JP, Wolfson LJ, O'Brien KL, Henkle E, Deloria-Knoll M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: Gglobal estimates. Lancet. 2009;374(9693):903–11. doi:10.1016/S0140-6736(09)61203-4. PMID:19748399.
- Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: A meta-analysis. Pediatr Infect Dis J. 1993;12(5):389–94. doi:10.1097/00006454-199305000-00008. PMID:8327300.
- WHO. Haemophilus influenzae type b (Hib) Vaccination WHO position paper: July 2013-Recommendations. Vaccine. 2013;31(52):6168–9. doi:10.1016/j.vaccine.2013.10.045. PMID:24156921.
- Watt JP, Chen S, Santosham M. Haemophilus influenzae type b conjugate vaccine: Review of observational data on long term vaccine impact to inform recommendations for vaccine schedule. 2012 [ accessed March 2, 2017]. http://cdrwww.who.int/immunization/sage/meetings/2013/april/4_Hib_Review_observational_data_Watt.pdf.
- Ping Y, Yaling J, Jingshan Z, Lei C, Lingsheng C, Jian C, Qiyou X, Huaqing W. Surveillance of Category II Vaccines in China, 2014. Chinese Journal of Vaccines and Immunization. 2016;22(2):143-148+158.
- Whitney CG, Klugman KP. Vaccines as tools against resistance: the example of pneumococcal conjugate vaccine. Seminars in pediatric infectious diseases. 2004;15(2):86–93. doi:10.1053/j.spid.2004.01.011. PMID:15185191.
- Yang YH, Fu SG, Peng H, Shen AD, Yue SJ, Go YF, Yuan L, Jiang ZF. Abuse of antibiotics in China and its potential interference in determining the etiology of pediatric bacterial diseases. Pediatr Infect Dis J. 1993;12(12):986–8. doi:10.1097/00006454-199312000-00004. PMID:8108225.
- Griffiths UK, Clark A, Hajjeh R. Cost-effectiveness of Haemophilus influenzae type b conjugate vaccine in low- and middle-income countries: Regional analysis and assessment of major determinants. J Pediatr. 2013;163(1):S50-S9 e9. doi:10.1016/j.jpeds.2013.03.031.
- Le P, Griffiths UK, Anh DD, Franzini L, Chan W, Swint JM. Cost-effectiveness of Haemophilus influenzae type b vaccine in Vietnam. Vaccine. 2015;33(36):4639–46. doi:10.1016/j.vaccine.2015.05.050. PMID:26044493.
- Broughton EI. Economic evaluation of Haemophilus influenzae type B vaccination in Indonesia: A cost-effectiveness analysis. J Public Health (Oxf). 2007;29(4):441–8. doi:10.1093/pubmed/fdm055. PMID:17875589.
- Gupta M, Prinja S, Kumar R, Kaur M. Cost-effectiveness of Haemophilus influenzae type b (Hib) vaccine introduction in the universal immunization schedule in Haryana State, India. Health Policy Plan. 2013;28(1):51–61. doi:10.1093/heapol/czs025. PMID:22407018.
- Xiaoping H. Cost-Effectiveness Analysis of Hib Vaccination Program in Taiwan. 1994 [ accessed May 3, 2017]. http://www.cdc.gov.tw/uploads/files/4e94658b-c9f2-4661-ae45-3bbd48d85fd3.pdf.
- Ziying S. Cost-Effectiveness Analysis of Hemophilus influenzae type b (Hib) Vaccination under 5 years of age in Taiwan[dissertation]. Tainan: Chang Jung Christian University; 2007.
- Department of Immunization, Vaccines and Biologicals. WHO guide for standardization of economic evaluations of immunization programmes. Geneva: WHO Press; 2008.
- Zhao D, Gai Tobe R, Cui M, He J, Wu B. Cost-effectiveness of a 23-valent pneumococcal polysaccharide vaccine immunization programme for the elderly in Shanghai, China. Vaccine. 2016;34(50):6158–65. doi:10.1016/j.vaccine.2016.11.003. PMID:27838068.
- Wu DB, Roberts C, Lee VW, Hong LW, Tan KK, Mak V, Lee, K K. Cost-effectiveness analysis of infant universal routine pneumococcal vaccination in Malaysia and Hong Kong. Hum Vaccin Immunother. 2016;12(2):403–16. doi:10.1080/21645515.2015.1067351. PMID:26451658.
- van Hoek AJ, Miller E. Cost-Effectiveness of Vaccinating Immunocompetent >/ = 65 Year Olds with the 13-Valent Pneumococcal Conjugate Vaccine in England. PLoS One. 2016;11(2):e0149540. doi:10.1371/journal.pone.0149540. PMID:26914907.
- Newall AT, Reyes JF, McIntyre P, Menzies R, Beutels P, Wood JG. Retrospective economic evaluation of childhood 7-valent pneumococcal conjugate vaccination in Australia: Uncertain herd impact on pneumonia critical. Vaccine. 2016;34(3):320–7. doi:10.1016/j.vaccine.2015.11.053. PMID:26657187.
- Maurer KA, Chen HF, Wagner AL, Hegde ST, Patel T, Boulton ML, Hutton, D W. Cost-effectiveness analysis of pneumococcal vaccination for infants in China. Vaccine. 2016;34(50):6343–49. doi:10.1016/j.vaccine.2016.10.051. PMID:27810315.
- Delgleize E, Leeuwenkamp O, Theodorou E, Van de Velde N. Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: A comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model. BMJ Open. 2016;6(11):e010776. doi:10.1136/bmjopen-2015-010776. PMID:27903558.
- Plotkin SA, Orenstein WA, Offit PA. Vaccine. Philadelphia, PA: Elsevier; 2013.
- Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan. 2006;21(5):402–8. doi:10.1093/heapol/czl018. PMID:16877455.
- UNICEF. Vaccine Price Data. 2017 [ accessed March 2 2017]. https://www.unicef.org/supply/files/DTP-HepB-Hib.pdf.
- Ministry of Finance of the People's Republic of China. Subsidies of public health service, 2016. Beijing: 2016 [accessed March 2 2017]. http://sbs.mof.gov.cn/zxzyzf/ggwsfwbzzj/201607/t20160721_2367374.html.
- National Health and Family Planning Commission of the PRC. China Health and Family Planning Statistical Yearbook. Beijing: Peking Union Medical College Press; 2016.
- Guijun N, Xuxia W, Shiwen L, Yuying Z, Bingling Z, Xiaoshu Z, Yixing L, Zundong Y, Weizhong Y. Retrospective Investigation on Diseases Burden of Children with Community Acquired Pneumonia under 5 Years in Baiyin City of Gansu Province, 2015–2016. Chinese Journal of Vaccines and Immunization. 2017;23(1):18-21+12.
- Jinfang S, Hongyan Y, Shicheng Y, Yuehua H, Qiqi W, Wandi P, Maigeng Z, Shiwei L, Yichong L. Disease Burden of three kinds of bacterial meningitis in China, 1990 and 2010. Disease Surveillance. 2015;30(12):1008–13.
- Wenting L. Study on economic burden of bacterial meningitis in China[dissertation]. Beijing: Chinese Center for Disease Control and Prevention; 2016.
- Bingkui S. Investigation on neonatal hearing screening status in Zhengzhou, 2009–2013. Maternal and Child Health Care of China. 2015;30(4):571–3.
- Takeuchi M, Yasunaga H, Horiguchi H, Fushimi K. The burden of epiglottitis among Japanese children before the Haemophilus influenzae type b vaccination era: An analysis using a nationwide administrative database. Journal of infection and chemotherapy: Official journal of the Japan Society of Chemotherapy. 2013;19(5):876–9. doi:10.1007/s10156-013-0585-x. PMID:23512615.
- Kessler A, Wetmore RF, Marsh RR. Childhood epiglottitis in recent years. Int J Pediatr Otorhinolaryngol. 1993;25(1-3):155–62. doi:10.1016/0165-5876(93)90049-9. PMID:8436460.
- Yin Z, Beeler Asay GR, Zhang L, Li Y, Zuo S, Hutin YJ, Ning G, Sandhu HS, Cairns L, Luo H, et al. An economic evaluation of the use of Japanese encephalitis vaccine in the expanded program of immunization of Guizhou province, China. Vaccine. 2012;30(37):5569–77. doi:10.1016/j.vaccine.2012.05.068. PMID:22698453.
- Huanzhong X, Changwei Q. Educational statistics yearbook of China. 2015. Beijing: China Statistics Press; 2016.
- National Bureau of Statistics of China. China Statistical Yearbooks. Beijing: 2016 [accessed Feb 20, 2017].http://www.stats.gov.cn/english/Statisticaldata/AnnualData/.
- The People's Bank of China. Exchange Rate. 2017 [ accessed Apirl 20, 2017]. http://www.pbc.gov.cn/diaochatongjisi/resource/cms/2017/01/2017011816584588958.pdf.
- Guoen L, Shanlian H, Jiuhon W. China Guidelines for Pharmacoeconomic Evaluations. China Journal of Pharmaceutical Economics. 2011; 30(S3):3-50.
- Lei C, Huaqing W, Jinghan Z, Ping Y, Lingsheng C, Guomin Z, Keyu J. National Immunization Coverage Survey in China after Integrated more Vaccines into EPI Since 2008. Chinese Journal of Vaccines and Immunization. 2012;18(5):419-424+478.
- O'Loughlin RE, Edmond K, Mangtani P, Cohen AL, Shetty S, Hajjeh R, Mulholland K. Methodology and measurement of the effectiveness of Haemophilus influenzae type b vaccine: Ssystematic review. Vaccine. 2010;28(38):6128–36. doi:10.1016/j.vaccine.2010.06.107.
- Adegbola RA, Secka O, Lahai G, Lloyd-Evans N, Njie A, Usen S, Oluwalana C, Obaro S, Weber M, Corrah T, et al. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: A prospective study. Lancet. 2005;366(9480):144–50. doi:10.1016/S0140-6736(05)66788-8. PMID:16005337.
- Dagan R, Fraser D, Roitman M, Slater P, Anis E, Ashkenazi S, Kassis I, Miron D, Leventhal A. Effectiveness of a nationwide infant immunization program against Haemophilus influenzae b. The Israeli Pediatric Bacteremia and Meningitis Group. Vaccine. 1999;17(2):134–41. doi:10.1016/S0264-410X(98)00165-0. PMID:9987147.
- Ray GT, Whitney CG, Fireman BH, Ciuryla V, Black SB. Cost-effectiveness of pneumococcal conjugate vaccine: Evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J. 2006;25(6):494–501. doi:10.1097/01.inf.0000222403.42974.8b. PMID:16732146.
- Tyo KR, Rosen MM, Zeng W, Yap M, Pwee KH, Ang LW, Shepard DS. Cost-effectiveness of conjugate pneumococcal vaccination in Singapore: Comparing estimates for 7-valent, 10-valent, and 13-valent vaccines. Vaccine. 2011;29(38):6686–94. doi:10.1016/j.vaccine.2011.06.091. PMID:21745516.
- Bennett JE, Sumner W, 2nd, Downs SM, Jaffe DM. Parents' utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154(1):43–48. PMID:10632249.
- Oostenbrink R, HA AM, Essink-Bot ML. The EQ-5D and the Health Utilities Index for permanent sequelae after meningitis: A head-to-head comparison. J Clin Epidemiol. 2002;55(8):791–99. doi:10.1016/S0895-4356(02)00448-1. PMID:12384194.
- Maurer KA, Chen HF, Wagner AL, Hegde ST, Patel T, Boulton ML, Hutton DW. Cost-effectiveness analysis of pneumococcal vaccination for infants in China. Vaccine. 2016;34(50):6343–49. doi:10.1016/j.vaccine.2016.10.051. PMID:27810315.
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31-32):4203–14. doi:10.1016/j.vaccine.2004.05.003. PMID:15474710.
- WHO-CHOICE. Threshold values for intervention cost-effectiveness by Region. 2005 [ accessed Feb 7, 2017]. http://www.who.int/choice/costs/CER_levels/en/.
- Qiu J, Yu C, Ariyaratne TV, Foteff C, Ke Z, Sun Y, Zhang L, Qin F, Sanderson G. Cost-Effectiveness of Pediatric Cochlear Implantation in Rural China. Otol Neurotol. 2017;38(6):e75–e84. doi:10.1097/MAO.0000000000001389. PMID:28379918
- Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: A systematic review and meta-analysis. Lancet Infectious diseases. 2010;10(5):317–328. doi:10.1016/S1473-3099(10)70048-7. PMID:20417414
- Yang Y, Leng Z, Lu D. Pediatric Haemophilus influenzae type b meninngitis in Hefei city: an epidemiologic study. National Medical Journal of China. 1998;78(4):251–253. PMID:10923476