ABSTRACT
Prior to availability of an effective vaccine, an estimated 4 million cases of varicella occurred annually in the United States, resulting in 10,000 hospitalizations and over 100 deaths. With the increased usage of a two-dose varicella vaccine (as recommended by the ACIP), approval of other VZV-containing products and the adoption of varicella vaccination in additional countries, the demand for VZV-containing vaccines has increased. This study (NCT02062502) evaluated the safety, tolerability, and immunogenicity of VARIVAX™ (VAR, varicella vaccine live) manufactured using a new seed manufacturing process (VARNSP) compared to the currently licensed VAR.
Healthy children 12–23 months were randomized (1:1) into Group 1 (2 doses of VARNSP given concomitantly with M-M-R™ II, ∼3 months apart) versus Group 2 (2 doses of VAR given concomitantly with M-M-R™ II, ∼3 months apart). Serum samples collected prior to vaccination on Day 1 and 6 weeks Postdose 1 were tested for antibody to VZV using a glycoprotein enzyme-linked immunosorbent assay (gpELISA). Safety was assessed Days 1 to 42 following each vaccination.
Six weeks Postdose 1, the response rate (percent of subjects with VZV antibody titer ≥5 gpELISA units/mL) of VARNSP was non-inferior compared to VAR. Vaccine-related adverse events (AEs) were comparable with the exception of measles-like rash, where a greater number of rashes were observed with VAR than VARNSP. The 2 vaccination groups were comparable with incidence rates of AEs, injection-site AEs, vaccine-related AEs, systemic AEs, and serious AEs.
This new process is an important innovation for the extreme demand of sustaining sufficient supplies of varicella vaccine to protect our communities against diseases caused by VZV.
KEYWORDS:
Introduction
Varicella is a generalized illness that has an incubation period of about 12 to 16 days, is highly contagious, and is characterized by a papulovesicular rash that typically resolves in 5 to 6 days.Citation1 Complications of varicella include secondary bacterial infection, encephalitis, and pneumonia. Less common complications include nephritis, arthritis, orchitis, uveitis, thrombocytopenia, purpura fulminans, Reye's syndrome, and death. Although immunity following varicella infection is generally long-lasting, the virus may persist in latent form in the peripheral nerve tissue (ganglia). As a result of immune senescence due to advanced age, stress, and/or immunocompromising conditions, herpes zoster (or shingles) may develop as a result of the reactivation of latent varicella-zoster virus (VZV).Citation2,3
Before the introduction of a varicella vaccine (VARIVAX™; Merck & Co., Inc., Kenilworth, NJ, USA), an estimated 4 million cases of varicella occurred annually in the US,Citation4 resulting in 10,000 yearly hospitalizations and over 100 deaths.Citation4-6 VARIVAX™ was licensed in 1995 on the basis of studies that demonstrated single-dose efficacy of 70% to 95% against clinical disease and 95% against severe disease over a 7- to 10-year follow-up period.Citation7 Following licensure, vaccine coverage has increased to an estimated 90% of the pediatric population in the US,Citation8,9 reducing varicella incidence by up to 91%,Citation8 and varicella-related hospitalizations by 75% to 88%.Citation10,11 During the first 12 years following licensure of VARIVAX™ in the US, varicella-related mortality was reduced by 88%, including a 97% reduction in individuals under 20 years of age and a 96% reduction in individuals under 50 years of age.Citation12 Recent epidemiologic studies have demonstrated that VARIVAX™ affords long-term protection against varicella, and also imparts herd immunity to unvaccinated individuals in settings where vaccine adoption is widespread.Citation13-15 Following the recommendation of a two-dose varicella vaccination schedule by the ACIP,Citation11 the approval of other VZV-containing products (ZOSTAVAX™ and ProQuad™; Merck & Co., Inc., Kenilworth, NJ, USA),Citation16,17 and the adoption of varicella vaccination in additional countries worldwide, the demand for vaccines containing VZV has increased. The VARIVAX™ NSP will extend the availability of VZV (Oka/Merck)-containing vaccines. This study (NCT02062502) evaluated the safety, tolerability, and immunogenicity of a formulation of VAR that contains Oka/Merck-VZV manufactured using the NSP (VARNSP) compared to the currently licensed VAR. The study was designed to demonstrate that a single dose of VARNSP induces acceptable VZV antibody responses 6 weeks Postdose 1 that are non-inferior to those induced by VAR.
Results
Subjects
Overall, 611/611 (100%) randomized subjects received Dose-1 of study vaccine; and among subjects who received Dose-1, 90.5% (553/611) received Dose-2 (). Approximately 87.2% (533/611) of subjects completed the study (received both doses, had all blood samples collected, and completed the 42-day safety data after each vaccination). The most commonly cited reason for exclusion was a missing blood sample after Dose-1. In general, the number of subjects who were excluded from the per-protocol analyses at each deviation category was comparable between groups (Supplementary Table 1). All subjects who received a vaccine dose were included in the safety analysis.
Figure 1. Subject Disposition * One patient experienced serious adverse events of acute respiratory distress syndrome, drowning, and respiratory failure (considered severe in intensity and not related to the study vaccine). These events were fatal.
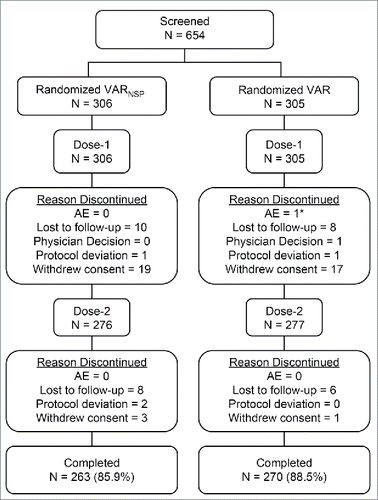
The number of subjects who discontinued was evenly distributed across the two groups. The most common reasons for discontinuation from the study were withdrew consent (6.5%) and lost to follow-up (5.2%). One subject in the VAR group died due to an SAE (acute respiratory distress syndrome and respiratory failure due to drowning) following Dose-1: deemed not related to the study vaccine by study investigator.
The majority of subjects across both groups were white (79.7%); however, they were generally similar with respect to sex and age (). The distribution of baseline VZV serostatus (>93% in either group) were generally comparable between the 2 groups. The most common prior medications taken by both vaccination groups within 14 days of Dose-1 were analgesics (31, 5.1%; acetaminophen) and antibacterials for systemic use (27, 4.4%). The most common prior medications taken by both vaccination groups within 14 days of Dose-2 were antibacterials for systemic use (27, 4.9%). A greater number of subjects received antibacterials for systemic use in the VARNSP group (6.9%) than in the VAR group (2.9%) within 14 days of Dose 2, a majority of which were amoxicillin for treatment of ear infection.
Table 1. Demographics.
Immunogenicity
The results demonstrate acceptability and non-inferiority of the response rate 6 weeks Postdose 1 in VARNSP as compared to VAR (p<0.001) and non-inferiority of the VZV antibody GMCs in VARNSP as compared to VAR (p<0.001) ().
Table 2. Summary of VZV Antibody Response Rates and Geometric Mean Concentrations (GMCs) After Dose-1.
A secondary supportive analysis on the Postdose 1 VZV antibody seroconversion rate was performed. The VZV antibody seroconversion rates after the first dose were comparable between vaccination groups, with 100.0% of the VARNSP group and 99.6% of the VAR group (99.8% of subjects overall). The VARNSP group had baseline (2.9 versus 1.9) and Postdose 1 GMCs (18.1 versus 15.5), and a percentage of subjects with antibody titers ≥5 gpELISA units/mL (92.3% versus 94.7%), a geometric mean fold rise (GMFR) (6.2 versus 8.0), and a percentage of subjects with ≥4-fold rise in antibody response (69.2% versus 89.5%).
Safety
In subjects with safety follow-up (), the 2 vaccination groups were comparable in terms of the incidence rates of AEs overall, injection-site AEs, vaccine-related AEs, systemic (non-injection-site) AEs, and serious AEs (SAEs); all injection-site AEs were considered to be vaccine-related. Overall, 72.1% of the subjects reported at least one AE after Dose-1 and 58.8% reported at least one AE after Dose-2. The number of subjects with vaccine-related AEs Postdose 1 were generally similar in the VARNSP group and the VAR group (risk difference [95% CI] of -2.4 [-10.5, 5.7]).
Table 3. Adverse Event (AE) Summary (Days 1 to 42 Following Each Vaccination).
The proportion of subjects with at least one injection-site AEs Day 1 to Day 42 post either Dose-1 or Dose-2 ranged from 30.7% (low Postdose 2) to 40.6% (high Postdose 1), with the majority being mild to moderate in intensity across both doses (); all injection-site AEs were considered vaccine-related. From Days 1 to 5 after Dose-1 (), the proportion of subjects with at least one Vaccine Report Card (VRC)-prompted injection-site AE in VARNSP group (112 [38.5%]) was comparable to that in the VAR group (113 [38.6%]) with a risk difference (VARNSP versus VAR) of -0.1% (95% CI [-8.0%, 7.8%]).
Table 4. Analysis of VRC-Solicited Injection-Site AE and Unsolicited Injection-Site AE (Incidence >1% in One or More Vaccination Groups) - Days 1 to 5 (All Subjects as Treated Population).
Postdose 1, 132 subjects (45.4%) from the VARNSP group and 140 subjects (47.8%) from the VAR group experienced vaccine-related AEs; 33 subjects (11.3%) in the VARNSP group and 40 subjects in the VAR group reported systemic vaccine-related AEs. Postdose 2, 92 subjects (33.2%) from the VARNSP group and 101 subjects (36.6%) from the VAR group experienced vaccine-related AEs; 12 subjects (4.3%) in the VARNSP group and 11 subjects (4.0%) in the VAR group reported systemic vaccine-related AEs.
The majority of subjects in both vaccination groups reported at least 1 systemic AE from Day 1 to Day 42 Postdose 1 (175 [60.1%] subjects in the VARNSP group and 164 [56.0%] subjects in the VAR group). Most systemic AEs by system organ class were infections and (34.0% of subjects from the VARNSP group and 28.3% of subjects from the VAR group) and skin and subcutaneous tissue disorders (17.5% of subjects from the VARNSP group and 21.2% of subjects from the VAR group). The most frequently reported systemic clinical AE by specific term (≥10% in any vaccination group) was pyrexia (14.8% of subjects from the VARNSP group and 15.0% of subjects from the VAR group).
Postdose 2 many subjects in both vaccination groups reported at least 1 systemic AE (127 [45.8%] subjects in the VARNSP group and 122 [44.2%] subjects in the VAR group). Most systemic AEs were infections (25.6% of subjects from the VARNSP group and 27.9% of subjects from the VAR group). The most frequently reported systemic clinical AE (≥10% in any vaccination group) was upper respiratory tract infection (10.5% of subjects from the VARNSP group and 9.1% of subjects from the VAR group).
For both time periods (Days 1 to 42 Postdose 1 and Postdose 2), the proportions of subjects with VRC-prompted rash () were comparable between the 2 vaccination groups, with the exception of measles-like rash, where a greater number of measles-like rashes were observed in the VAR group than in the VARNSP group during Days 1 to 42 Postdose 1 only (2.4% versus 0.3%, p=0.034). All these rashes were considered to be vaccine-related, and none were reported to be SAEs. No subjects in either vaccination group during either time period experienced mumps-like symptoms, rubella-like rash, or zoster-like rash. The 2 vaccination groups were comparable with respect to elevated body temperature. Postdose 1, 27 subjects (9.5%) in the VARNSP group and 30 subjects (10.5%) in the VAR group reported a maximum temperature ≥102.2°F (≥39.0°C); risk difference -1.0 (95% CI: -6.0, 4.0; p=0.696). Postdose 2, 21 subjects (8.1%) in the VARNSP group and 23 subjects (8.6%) in the VAR group reported a maximum temperature ≥102.2°F (≥39.0°C); risk difference -0.5 (95% CI: -5.3, 4.4; p=0.845).
Table 5. Rashes and Mumps like Symptoms Prompted for on Vaccine Report Card (VRC) (All Subjects as Treated Population).
Five (5) subjects experienced SAEs Days 1 to 42, 2 subjects in the VARNSP group and 3 subjects in the VAR group, following either dose of study vaccine (). No subjects experienced a vaccine-related SAE. A 13-month-old female, in the VAR group died due to SAEs of acute respiratory distress syndrome and respiratory failure caused by drowning (severe in intensity and not related to the study vaccine) 5 days Postdose 1.
Table 6. Serious Adverse Event (SAE) Listing.
Discussion
Following the recommendation by the ACIP of a two-dose varicella vaccination schedule,Citation11 the approval of other VZV-containing products, and the adoption of varicella vaccination in additional countries, the demand for vaccines containing VZV (Oka/Merck) has increased.Citation18,19 There is little doubt about the successes of the US immunization programs for measles, mumps, rubella, and varicella. Cases of rubellaCitation20 and measlesCitation21 in the US have essentially been eliminated, although some recent outbreaks have occurred.Citation22 Additionally, mumps and varicella are well controlled.Citation23
Continuing the successes of these programs depends on maintaining an adequate and available supply of vaccines, and VARNSP represents an important addition to that effort. All vaccine products in the VZV (Oka/Merck) family are derived from a common bulk. New manufacturing methods were implemented to increase the efficiency of the seed manufacturing process. The VARNSP results in less master seed consumption due to the increased efficiency of the stock seed process without changes to the final product for administration and will extend the availability of VZV (Oka/Merck)-containing vaccines.
This study evaluated the immunogenicity, safety, and tolerability of VARNSP (vaccine manufactured by the new process) in comparison with the currently licensed VAR process. A single dose of VARNSP in healthy children 12 to 23 months of age induces VZV antibody response rates and GMCs 6 weeks Postdose 1 which are both acceptable and non-inferior to those induced by VAR. VARNSP was well-tolerated with AE, elevated body temperature, and rash profiles that were comparable to that of VAR.
The strength of this study was that it was a well-controlled, blinded study with over 87% of all subjects completing the study. However, a limitation of the study was that we only assessed immunogenicity data after the first vaccination. This study was a standard non-inferiority study design in vaccine clinical research, as such a larger number of enrolled subjects would have increased the overall power of the study.
Methods and materials
Design
This was a randomized, double-blind, multicenter, comparator-controlled clinical trial conducted in 34 sites within the US from March 2014 to October 2015. The protocol was conducted in accordance with principles of Good Clinical Practice (GCP), including obtaining written informed consent from each participant's parent(s) or legal guardian(s) prior to study entry, and was approved by the human studies committees applicable to each study site. VARNSP and VAR were packaged identically, so that treatment blind/masking was maintained. The subject, the investigator, and Sponsor personnel or delegate(s) who were involved in the treatment or clinical evaluation of the subjects, were unaware of the treatment group assignments. Randomization occurred centrally using an automated phone system. Subjects were assigned randomized treatment in a 1:1 ratio to VARNSP and VAR, respectively. No stratification based on age, sex, or other characteristics was used in this trial. The investigator/co-investigators from each site were fully responsible and accountable for the study conduct and reporting data from each respective study site.
Subjects
Healthy children 12 to 23 months of age with a negative history for varicella, herpes zoster, measles, mumps, and rubella and without prior immunization against these diseases were eligible for the study. Exclusion criteria included: receipt of any measles, mumps, rubella, or varicella vaccine, either alone or in any combination during the study; receipt of systemic immunomodulatory steroids 3 months prior or systemic steroids within 7 days prior to entering study; had a history of allergy reaction to any vaccine component; had received salicylates within 14 days prior to vaccination; history of seizure disorder; febrile illness (≥102.2°F [39.0°C] oral equivalent) within 72 hours prior to study entry; any congenital or acquired immune deficiency, neoplastic disease, or immunosuppression; exposure to varicella, herpes zoster, measles, mumps, or rubella in the last 4 weeks prior to the study vaccination; history of thrombocytopenia; currently participating in (within 30 days to enrollment); or scheduled to participate in any other clinical trial other than a surveillance study during the planned study period for this trial.
Subjects were allocated to a vaccination group using a randomized schedule generated by the study statistician. Subjects were randomized into 1 of 2 vaccination groups (ratio 1:1). Group 1 (∼300 subjects) received 2 doses of VARNSP, given concomitantly with Measles, Mumps, and Rubella Virus Vaccine Live (MMR; M-M-R™ II, Merck & Co., Inc., Kenilworth, NJ, USA), approximately 3 months apart. Group 2 (∼300 subjects) received 2 doses of VAR, given concomitantly with MMR, approximately 3 months apart according to US ACIP recommendations. Based on approximately 300 subjects per group, and with an expected evaluability rate of 90%, the study provided 90% power across the primary immunogenicity hypotheses.
Vaccines
VAR and VARNSP are live, attenuated, lyophilized vaccines for the prevention of varicella in children 12 months and older. MMR is a live, attenuated, lyophilized vaccine for the prevention of measles, mumps, and rubella in children 12 months and older. VAR and VARNSP are indistinguishable in appearance. All vaccines (VAR, VARNSP, and MMR) were packaged in single-dose glass vials with a multilingual booklet label and stored according to the product insert.Citation24 VARNSP implemented 2 changes to the seed manufacturing process: 1) use of a new cell culture platform for the viral propagation step, and 2) new method to add VZV-infected MRC-5 cells to the cell culture platform. These changes increase the efficiency of the seed manufacturing process by producing greater quantities of the viral components already used in the current vaccine formulation process. These changes did not alter the vaccine formulation process itself, but moving forward will meet the increase demand for varicella-containing products.
Immunogenicity
The primary immunogenicity analyses were based on the Per-Protocol population. The Per-Protocol population was defined as subjects who received 1 dose of VARNSP or VAR according to their vaccination group assignment, adhered to study instructions, and provided serum samples within the appropriate day ranges. The Per-Protocol population excluded subjects due to important deviations from the protocol that could substantially affect the results of the primary immunogenicity endpoints.
Serum samples were collected from all subjects at two time points, prior to vaccination on Day 1 (baseline), and 6 weeks Postdose 1. Serum samples were tested for antibody to VZV using a glycoprotein enzyme-linked immunosorbent assay (gpELISA).Citation25 The antibody response rate for VZV was defined as the percent of subjects with a post vaccination VZV antibody titer ≥5 gpELISA units/mL for subjects whose baseline VZV antibody titer was <1.25 gpELISA units/mL. Immunogenicity to measles, mumps, and rubella was not evaluated since no changes were made to the manufacturing process of measles, mumps, or rubella. All serum specimens were sent and analyzed at PPD Vaccines and Biologics, LLC, Wayne, Pennsylvania, USA, on dry ice.
The primary immunogenicity objectives were: (1) to demonstrate that a single dose of VARNSP induces VZV antibody responses non-inferior to those induced by VAR; (2) the VZV antibody GMCs; and (3) to demonstrate that a single dose of VARNSP induces an acceptable antibody response rate to VZV 6 weeks Postdose 1.
The immunogenicity endpoints were: (1) VZV antibody response rate defined as percent of subjects with VZV antibody titer ≥5 gpELISA units/mL 6 weeks Postdose 1 among subjects who were seronegative to VZV antibody (titer <1.25 gpELISA units/mL) at baseline; (2) VZV antibody GMC defined as post vaccination antibody GMCs 6 weeks Postdose 1; (3) VZV antibody seroconversion rate defined as proportion of subjects with baseline VZV antibody titer <1.25 gpELISA units/mL and with post vaccination VZV antibody titer ≥1.25 gpELISA units/mL at Postdose 1; (4) GMFR for subjects who were initially seropositive (baseline titer ≥1.25 gpELISA units/mL) at Postdose 1; and (5) percent of subjects achieving ≥4-fold rise in antibody titer from baseline at Postdose 1.
Safety
The safety objective (secondary study objective) was to assess the overall safety and tolerability of VARNSP when administered to children 12 to 23 months of age; however, no formal hypothesis was tested. The primary safety endpoints were: fever (temperature ≥102.2°F [≥39.0°C] oral equivalent) from Days 1 to 42 after each vaccination; varicella-like, zoster-like, measles-like or rubella-like rashes, or mumps-like symptoms occurring from Days 1 to 42 following each vaccination; and solicited injection-site reactions (redness, swelling, pain/tenderness) of any intensity or size from Days 1 to 5 following each vaccination. Parents/guardians recorded daily axillary temperatures and any injection-site or systemic adverse events (AEs) using a (VRC).Citation26
Subjects were followed for 42 days following each vaccination visit for unsolicited injection-site and systemic AEs. All subjects were followed for serious AEs (SAEs) from the time of enrollment into the study through 180 days Postdose 2. During the phone call on Day 271 (Day 180 Postdose 2), a scripted questionnaire was used to determine if any SAEs had occurred since Day 43, Postdose 2. Subject's parent/legal guardian was also instructed to contact study personnel immediately if a varicella-or herpes zoster-like rash developed. Subjects were required to be seen at the clinic within 72 hours of the rash presentation.
Statistical analyses
The first primary endpoint evaluating the non-inferiority of the VZV antibody response rate was based on a one-sided non-inferiority test. This comparison was conducted at the one-sided α=0.025 level with a non-inferiority margin of 10 percentage points and was conducted based on methods developed by Miettinen and Nurminen for testing the non-inferiority of 2 proportions.Citation27 The statistical criterion for non-inferiority required the lower bound of the 95% CI on the difference in VZV antibody response rates [VARNSP minus VAR] to exclude a decrease of 10 percentage points or more.
The second primary endpoint evaluating the non-inferiority of the VZV antibody GMT was based on a one-sided non-inferiority test, conducted at the α=0.025 (one-sided) level. The statistical criterion for non-inferiority required that the lower bound of the two-sided 95% CI for the GMT ratio [VARNSP/VAR] be >0.67. A ratio of 0.67 [VARNSP/VAR] corresponded to a 1.5-fold decrease of GMT in the VARNSP group compared with the VAR group.
The third primary endpoint evaluating the acceptability of the VZV antibody response rate for VARNSP was based on a one-sided, one-sample exact binomial test (conducted at the α=0.025 one-sided level). This comparison tested H0: p≤0.76 versus Ha: p>0.76 (where p was the VZV antibody response rate in subjects who received VARNSP). Rejecting H0 was equivalent to requiring the lower bound of the one-sample 95% CI for the response rate to be above 76%.
Abbreviations
| AAP | = | American Academy of Pediatrics |
| ACIP | = | Advisory Committee on Immunization Practices |
| AE | = | Adverse event |
| CI | = | Confidence interval |
| ELISA | = | Enzyme-linked immunosorbent assay |
| GMC | = | Geometric mean concentration |
| GMFR | = | Geometric mean fold rise |
| gpELISA | = | Glycoprotein enzyme-linked immunosorbent assay |
| MMR | = | MMR™ II: Measles, mumps, and rubella virus vaccine live |
| NSP | = | New seed process |
| Oka/Merck-VZV | = | Oka/Merck strain varicella-zoster virus |
| SAE | = | Serious adverse event |
| VAR | = | Varicella vaccine |
| VARNSP | = | Varicella vaccine New Seed Process |
| VRC | = | Vaccination report card |
| VZV | = | Varicella-zoster virus |
Disclosure of potential conflicts of interest
Dr. Shelly Senders received a clinical study grant from the sponsor.
Nickoya Bundick, Jianing Li, Carol Zecca, and Frans Helmond are employees of the sponsor and may hold stock and/or stock options from the sponsor.
Author contributions
Senders, Bundick, Li, Zecca, and Helmond: analysis and interpretation of data, and preparation of manuscript.
All co-authors approved the final version of the manuscript.
V210-063 study investigators
| • | California: K. Coverston; A. Ituriaga; S. Khamis; V. Sanchez-Bal | ||||
| • | Colorado: A. Garscadden; K. Lesh | ||||
| • | Florida: R. Lopez | ||||
| • | Georgia: E Anderson | ||||
| • | Kentucky: W. Daly; J. Hedrick | ||||
| • | Louisiana: T. Latiolais | ||||
| • | Massachusetts: C. Marchant | ||||
| • | Michigan: M. Levinson | ||||
| • | New York: K Bromberg; R. Dracker | ||||
| • | North Carolina: E.R. Franklin | ||||
| • | Ohio: E. Malacaman | ||||
| • | Pennsylvania: C. Duffy | ||||
| • | South Carolina: M. Leonardi | ||||
| • | South Dakota: J. McAreavey | ||||
| • | Tennessee: C. Jordan | ||||
| • | Texas: R. Rupp | ||||
| • | Utah: B. Eberhard; A. Gabrielsen | ||||
Sponsor's role
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (sponsor). Although the sponsor formally reviewed a penultimate draft, the opinions expressed are those of the authorship and may not necessarily reflect those of the sponsor.
KHVI_A_1388479_Supplemental.doc
Download MS Word (58 KB)Acknowledgments
The authors would like to thank:
| – | All the subjects who participated in this study and their parents or legal guardian. | ||||
| – | Julie Gardner for her guidance and review of this manuscript. | ||||
| – | Jon Stek for his guidance and review of this manuscript. | ||||
Funding
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
References
- Hope-Simpson R. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc R Soc Med. 1965;58:9–20. PMID:14267505.
- Brisson M1 EW, Law B, Gay NJ, Walld R, Brownell M, Roos LL, De Serres G. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127:305–14. doi:10.1017/S0950268801005921. PMID:11693508.
- Guess HA BD, Melton LJ 3rd, Kurland LT. Epidemiology of herpes zoster in children and adolescents: a population-based study. Pediatrics 1985;76:512–7. PMID:3863086.
- Wharton M. The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am 1996;10:571–81. doi:10.1016/S0891-5520(05)70313-5. PMID:8856352.
- Galil K, Brown C, Lin F, Seward J. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr Infect Dis J. 2002;21:931–5. doi:10.1097/00006454-200210000-00009.
- Meyer PA, Seward JF, Jumaan AO, Wharton M. Varicella mortality: trends before vaccine licensure in the United States, 1970–1994. J Infect Dis. 2000;182:383–90. doi:10.1086/315714. PMID:10915066.
- Kuter BJ, Weibel RE, Guess HA, Matthews H, Morton DH, Neff BJ, Provost PJ, Watson BA, Starr SE, Plotkin SA. Oka/Merck varicella vaccine in healthy children: final report of a 2-year efficacy study and 7-year follow-up studies. Vaccine 1991;9:643–7. doi:10.1016/0264-410X(91)90189-D. PMID:1659052.
- Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS, Gargiullo P, Perella D, Civen R, Seward JF. Changing varicella epidemiology in active surveillance sites–United States, 1995–2005. J Infect Dis. 2008;197 Suppl 2:S71–5. doi:10.1086/522156. PMID:18419413.
- Luman ET, Ching PL, Jumaan AO, Seward JF. Uptake of varicella vaccination among young children in the United States: a success story in eliminating racial and ethnic disparities. Pediatrics. 2006;117:999–1008. doi:10.1542/peds.2005-1201. PMID:16585293.
- Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. JAMA. 2005;294:797–802. doi:10.1001/jama.294.7.797. PMID:16106004.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF, Advisory Committee on Immunization Practices, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1–40. PMID:17585291.
- Marin M, Zhang JX, Seward JF. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics. 2011;128:214–20. doi:10.1542/peds.2010-3385. PMID:21788222.
- Duncan JR, Witkop CT, Webber BJ, Costello AA. Varicella seroepidemiology in United States air force recruits: A retrospective cohort study comparing immunogenicity of varicella vaccination and natural infection. Vaccine. 2017;35:2351–7. doi:10.1016/j.vaccine.2017.03.054. PMID:28359621.
- Lopez AS, Zhang J, Marin M. Epidemiology of Varicella During the 2-Dose Varicella Vaccination Program – United States, 2005–2014. Mmwr-Morbid Mortal W. 2016;65:902–5. doi:10.15585/mmwr.mm6534a4.
- Baxter R, Ray P, Tran TN, Black S, Shinefield HR, Coplan PM, Lewis E, Fireman B, Saddier P. Long-term effectiveness of varicella vaccine: a 14-Year, prospective cohort study. Pediatrics. 2013;131:e1389–96. doi:10.1542/peds.2012-3303. PMID:23545380.
- Robinson CL, Romero JR, Kempe A, Pellegrini C, Advisory Committee on Immunization Practices, Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger – United States, 2017. Mmwr-Morbid Mortal W. 2017;66:134–5. doi:10.15585/mmwr.mm6605e1.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF, Advisory Committee on Immunization Practices CfDC, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1–40. PMID:17585291.
- Marin M, Broder KR, Temte JL, Snider DE, Seward JF, Centers for Disease C, et al. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1–12. PMID:20448530.
- Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR, Centers for Disease C, Prevention. Update on recommendations for use of herpes zoster vaccine. Mmwr-Morbid Mortal W. 2014;63:729–31.
- Reef SE, Cochi SL. The evidence for the elimination of rubella and congenital rubella syndrome in the United States: a public health achievement. Clin Infect Dis. 2006;43 Suppl 3:S123–5. doi:10.1086/505943. PMID:16998770.
- Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16–17 March 2000. J Infect Dis. 2004;189 Suppl 1:S43–7. doi:10.1086/377696. PMID:15106088.
- Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS, Centers for Disease Control and Prevention. Measles – United States, January 4-April 2, 2015. Mmwr-Morbid Mortal W. 2015;64:373–6.
- Adams DA, Jajosky RA, Ajani U, Kriseman J, Sharp P, Onwen DH, et al. Summary of notifiable diseases–United States, 2012. Mmwr-Morbid Mortal W. 2014;61:1–121.
- FDA-Center for Drug Evaluation and Research. VARIVAX® – Varicella Virus Vaccine Live [Package Insert]. Kenilworth, NJ: Merck & Co., Inc.; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142812.pdf. [accessed September 11, 2017]
- Hammond O, Wang Y, Green T, Antonello J, Kuhn R, Motley C, Stump P, Rich B, Chirmule N, Marchese RD. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J Med Virol. 2006;78:1679–87. doi:10.1002/jmv.20754. PMID:17063506.
- Norquist JM, Khawaja SS, Kurian C, Mast TC, Liaw KL, Robertson MN, Evans B, Gutsch D, Saddier P. Adaptation of a previously validated vaccination report card for use in adult vaccine clinical trials to align with the 2007 FDA Toxicity Grading Scale Guidance. Hum Vaccin Immunother. 2012;8:1208–12. doi:10.4161/hv.21408. PMID:22906942.
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–26. doi:10.1002/sim.4780040211. PMID:4023479.
