ABSTRACT
Influenza is one of the major infectious causes of excess mortality, hospitalization, and an increase in healthcare expenditure in all countries. In an increasingly ageing population, many members are exposed to flu-related complications. Vaccination coverage rates for the elderly in most European countries, such as Italy, are not satisfactory, and have been decreasing with time due to a sense of skepticism toward vaccination. Nowadays, many types of vaccines are available on the Italian market to prevent influenza illness. Many studies have proven their effectiveness in preventing influenza-related complications in specific risk groups. Any vaccine can be crucial to avoid complications, hospitalizations and death, but use of the most appropriate vaccine could optimize the result at a very modest cost. General practitioners (GPs) should encourage their patients to take the influenza vaccination to prevent complications or deaths. Health authorities should give GPs the opportunity to choose the appropriate vaccines tailored to specific patients.
Introduction
Influenza is one of the major infectious causes of excess mortality, hospitalization and an increase in healthcare expenditure in all countries.Citation1–3 An increasingly ageing population, and a longer life expectancy have brought about an increase in the quota of chronically ill patients and fragile subsets of the population, exposing many members to influenza-related complications. These complications latter include not only respiratory distress – resulting from both influenza and frequent cases of secondary pneumonia – but also conditions related to ischemic cardiopathy, cerebrovascular accidents or diseases, and diabetes.Citation4 The primary objective of influenza vaccination is to reduce the number of flu-related cases of complications and deaths. Influenza immunization can obtain these results to a significant degree, though the results may vary from year to year. The spread of the epidemic, the match between the circulating strains and the vaccine strains, and the age and immunity status of the population can influence the impact of vaccination. The aim of the current work is to describe the types of influenza vaccine available on the Italian market and their most appropriate uses and make recommendations, starting from the epidemiology of influenza in Italy.
Epidemiological surveillance in Italy
In Italy, an integrated surveillance system – Influnet – is in place. It involves a seasonal survey of Influenza like Illness (ILI), which is conducted through a network of general practitioners (GPs) and pediatricians. The aim is to estimate the weekly incidence of influenza cases in winter season, to determine the duration and intensity of the epidemic. According to Influnet, ILI affects between 4% and 12% of the Italian population every year. Moreover, since 2009/10, an enhanced surveillance system has been put in place to deal with severe and complicated clinical cases of influenza.Citation5 Every region in Italy must report to the Ministry of Health and the National Health Institute (ISS) all influenza cases – complicated or severe – that require hospitalization in the Intensive Care Unit (ICU) and/or Extra Corporeal Membrane Oxygenation (ECMO).Citation6
In the 2015/16 season in Italy, the influenza epidemic reached its peak in the eighth week of 2016, with 6.1 cases per 1,000 patients. The epidemic (incidence >2.36 cases/1000 patients) lasted 12 weeks.Citation7 The cumulative incidence was 82 cases/1000 patients, which was, however, low compared with previous seasonal surveys. Cumulative incidence in the paediatric population (0-4 years) was 227/1000, while it was 165/1000 in the 5 – 14-year-old age group. Predictably, age and incidence are inversely related, as demonstrated by the minimal incidence registered among the geriatric population (29/1000 for those aged 65 or older).
With regard to severe disease outcomes, 89 cases were registered in a population with a mean age of 57 years, and 32 confirmed influenza deaths in a population with a mean age of 59 years. Seventy-six percent of those severe cases, and 63% of the deaths surveyed showed the presence of at least one pre-existing chronic disease, for which the influenza vaccine is recommended. Only 9.7% of these patients with pre-existing chronic conditions (severe cases together with subjects who died) were immunized in the 2015/16 season.Citation8
The 2016/2017 season in Italy has been characterized by an early start and a rapid increase in the circulation of the influenza virus since December 2016. The seasonal peak of the circulation was recorded starting the first week of January, followed by a gradual decrease in the second half of January. The number of estimated cases from the beginning of the surveillance was 5,441,000. According to the calculation of the epidemic thresholds by the Moving Epidemic Method (MEM) developed by the ECDC (European Centre for Disease Prevention and Control), the last season for Italy was classified as a mean intensity season. The highest weekly incidence rates were registered in the age groups of 0–4 years and 5–14 years. Between the end of 2016 and the end of January 2017 two weekly incidence peaks were registered in these two age groups. The values reached 24.9/1000 and 18.5/1000 in the paediatric population (0-4 years) and 13.3/1000 and 11.2/1000 in the 5–14 age group. The cumulative incidence rates were not shown.Citation9
Virological surveillance
The presence of the well-known phenomena of antigenic drift and antigenic shift make the accurate surveillance of the influenza viruses an absolute imperative at the international level. This can be achieved through the identification of pathogens in accredited laboratories. Every year, the World Health Organization (WHO) releases the composition of the vaccine for the forthcoming season, based on the information on the viral strains in circulation and on the ILI-related data collected and presented on the Global Influenza Surveillance Network (WHO).Citation10 In winter 2014/15 in Italy, viruses belonging to the subtype H1N1pdm09 were predominantly isolated (52%), especially compared with H3N2 viral strains (41%). The remaining 7% of the type A strains were not been subtyped. Type-B influenza viruses belonging to the B/Yamagata/16/88 and B/Victoria/2/87 lineages were simultaneously in circulation. However, the viruses belonging to the B/Yamagata lineage were largely predominant (97%).Citation11
Over the 2015/16 season, 8,971 clinical samples were tested and analyzed in Italian laboratories: 2,456 (27%) resulted positive to the influenza virus. Strains A and B were in circulation simultaneously, but the type-B viruses were predominant (57%) when compared with type A (43%). As for type A, the subtype H3N2 viruses were mainly isolated and identified (56%) as compared with the H1N1pdm09 strains (35%). The remaining 9% of the type-A strains was not subcategorized. The type-B influenza viruses belonging to the lineages B/Yamagata/16/88 and B/Victoria/2/87 were in circulation at the same time, with a large prevalence of the B/Victoria lineage (95%).Citation11
The results of the 2016/17 season showed a prevalence of influenza type-A strains (95%) belonging to the subtype H3N2 (99% of type-A strains). The phylogenetic analyses of type-B viruses, which represented 4.9% of the positive influenza samples, showed a predominant circulation of viruses belonging to the B/Yamagata lineage (B/Phuket/3073/2013-like virus).Citation12 Data on age distribution of influenza viruses are not available.
For the 2017/18 influenza season in the northern hemisphere, the WHO recommends the following trivalent influenza vaccine (TIV) composition: A/Michigan/45/2015 (H1N1)pdm09-like virus, A/Hong Kong/4801/2014 (H3N2)-like virus and B/Brisbane/60/2008-like virus. Quadrivalent influenza vaccines (QIV) should contain the above mentioned three viruses and a B/Phuket/3073/2013-like virus.Citation13
Immunization coverage in Italy
Over the last few years, in Italy, like in many other industrialized countries, a certain degree of skepticism toward vaccinations has increased in the population. This has had clear repercussions in terms of a lower extent of coverage, both in the case of mandatory or recommended vaccines for early childhood, and, predominantly, that of influenza vaccines. Indeed, between 2000/01 and 2016/17 in Italy, the lowest immunization coverage (48.6%) in the elderly population was registered in 2014/15 (). In the last two influenza seasons (2015/16 and 2016/17), vaccination coverage among the elderly increased slightly to 52.0%.Citation14
Figure 1. Influenza vaccination coverage among the elderly (subjects over 65 years) in Italy between 2000/01 and 2016/17.
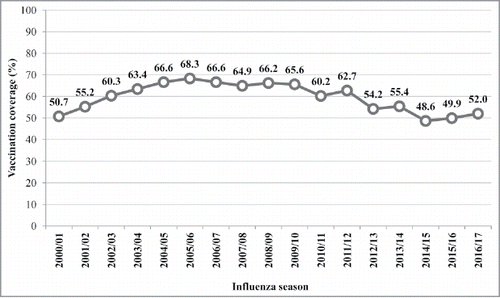
However, a lot still needs to be done to close the gap that has grown over the last 10 years, and reach the targets established by the National Vaccination Plan 2017/19 (75% as the minimum achievable coverage threshold).Citation15
Vaccines on the Italian market
Split vaccines
Split vaccines are influenza virus particles disrupted by diethyl ether or detergent treatment. While split vaccine does contain all viral proteins, the original viral particulate organization and viral ssRNA are mostly lost. This lead to the loss of some of the inherent immunogenicity of the particle.Citation16 However, due to their adequate immunogenicity, tolerability, and relative ease of production, split vaccines are wide in use. Apart from a TIV formulation, a QIV one has existed since 2014 as well.
Since 1983, with the evolution and co-circulation of two distinct lineages of influenza type-B virus,Citation17 the conventional trivalent influenza vaccines have seemed to show a limited ability to induce effective protection when major or minor mismatches between the influenza B vaccine component and the circulating strains occur.
QIV contains one virus from each of the two influenza B lineages (one B/Victoria virus and one B/Yamagata virus), whereas TIV contains one influenza B virus from one lineage. The results of the clinical trials in all the studied populations – children, adolescents, adults and the elderly – have shown that the Flu-QIV induces an immune response not inferior to that of TIV to the viral strains in common. This indicates the absence of immunological interference after the addition of a fourth viral strain. It is also superior from the immunological point of view with respect to the virus B strain, which is absent in TIVs.Citation18 The safety profile of the Flu-QIV overlaps with that of the TIVs.Citation19
Some impact assessments Citation20–21 indicate that compared to the TIV, the use of QIV allows the prevention of additional flu-related cases, hospitalization and death. In particular, the analysis of the cost-effectiveness of the QIV and TIV influenza vaccination have shown that Flu-QIV is more cost-effective compared with the TIVs. In particular, given the risk of a “mismatch” of the TIV with the type B viruses in some influenza seasons, the use of the QIV would lead to a significant reduction in the number of influenza cases and resource-saving associated with such cases. This can partially compensate for the higher cost of the vaccine. In the base case of a cost-effectiveness model applied to the Italian population, it is estimated that the use of the QIV, as compared with the TIV, would prevent 1,413,887 cases of influenza, 169,638 complications, 41,862 hospitalizations, 127,776 outpatient services, and 20,905 deaths, considering the entire Italian population and a time horizon of a lifetime.Citation22
QIVs are, thus, designed to provide a broader protection against the influenza B viruses in circulation. These are indicated for young subjects, who are at risk with no immunocompromising conditions, and for healthcare professionals.
Subunit vaccines
Subunit vaccines contain hemagglutinin (HA) and neuraminidase (NA) purified proteins. They lack inner antigens and lipopolysaccharides. These are frequently used in TIV formulations. They are available for QIV vaccines as well, though those are not available on the Italian market. These have moderate immunogenicity and are well tolerated by humans, especially children. However, two doses are needed to guarantee the vaccine's effectiveness during epidemics.Citation23
Adjuvanted subunit vaccine
Adjuvanted inactivated vaccine contains HA and NA purified antigens, adjuvanted with MF59. MF59 is an oil-in-water emulsion, which consists of 150 nm-sized biodegradable squalene oil droplets stabilized by non-ionic surfactants.Citation24 MF59 adjuvanted trivalent vaccine was licensed in EU, and around 47 million doses have been distributed since 1997. Many studies Citation25–29 on its safety and efficacy were carried out between 1997 and 2006. These studies found substantial differences between adjuvanted and non-adjuvanted vaccines in terms of adverse reactions. Unlike aluminum salts, MF59 does not create a deposit of adjuvant and antigen in the injection site. This constitutes a release system that promotes an antigen's captation in the immune system cells. MF59 also activates immune local cells (rapid activation of linfocitary precursors CD4+) with an immune-strengthening effect. Thanks to its characteristics, it can be recommended for all kinds of population – with respect to age, or chronic conditions determining immunodeficiency, or for those who need any stimulation or enhanced immunogenicity.
The immunogenicity of the MF59 adjuvanted vaccine has been studied in various clinical trials Citation25–30 and its advantages in some population groups show consistent results. One of the studies was designed to prove the consistency between the effects of various lots and the profile of antibody response.Citation30 The methodology compared the efficacy of adjuvanted and non-adjuvanted vaccines on the healthy elderly or adults with chronic diseases. The study's results showed a better response using adjuvanted vaccine instead of a non-adjuvanted (split) one. The response was better not only for seroconversion but also for antibody geometric mean titer (GMT) increase. The same study also showed a better response not only against homologous but also heterovariant virus strains. So, we can speculate that adjuvanted vaccine gives a broader protection and protects against virus antigenic drift. Adjuvanted influenza vaccines are less likely to be ineffective even when the vaccine strains do not perfectly match the circulating virus strains.Citation30
Another study aimed at testing the effectiveness of adjuvanted vaccine included 170,000 vaccinated subjects aged over 65 years in Lombardia, a region in northern Italy. This study analyzed two groups of subjects. In the first one, the subjects were given adjuvanted vaccine (n = 88,449), while in the second group, the subjects were given non-adjuvanted vaccine (n = 82,539), following the local vaccine recommendations. The main outcome was to estimate the risk of hospitalization due to pneumonia related to influenza or influenza-like syndromes. During the three considered seasons (2006/07, 2007/08 and 2008/09) the risk of hospitalization, before each influenza season, was higher for the subjects who were given adjuvanted vaccine (+17%), because they generally were more frail than subjects who were given TIV. Nevertheless, in the peak influenza weeks, 25% less hospitalization was recorded for the adjuvanted group compared with the non-adjuvanted one. In fact, Italian health authorities recommended the adjuvanted trivalent influenza (aTIV) vaccine for high-risk patients. Hence, the patients who received the aTIV vaccine were generally older, with more functional limitations and a higher co-morbidity rate. Such patients may, therefore, have a higher baseline hospitalization rate.Citation31
In another study, the adjuvanted vaccine was compared with one subunit and one split vaccine among infants and young children. The adjuvanted influenza vaccine had a higher antibody responses to matched and mismatched strains, a faster onset of immunogenicity, a longer duration, and enhanced protection against influenza in young children. There were higher levels of reactogenicity with the adjuvanted vaccine, but most of the events were mild and transient. Rates of unsolicited adverse events, and severe adverse events were similar in all the groups.Citation32
Virosome vaccine
Virosomes represent a vaccine presentation form that closely mimics the native virus.Citation33 Virosomes are reconstituted influenza virus envelopes consisting of HA, NA, and viral phospholipids which lack the viral genetic material.Citation34 Virosomes are produced from the influenza virus through a detergent solubilization and removal procedure. Functionally reconstituted influenza virosomes preserve the receptor-binding and membrane-fusion activity of the viral HA. These functional characteristics of virosomes form the basis for their enhanced immunogenicity. Since the virosomes lack the viral RNA, the binding and fusion of virosomes to cells do not imply any infection of the cells. The virosome vaccine has high immunogenicity for all age groups, and thanks to the good tolerability, it can be gven to children under 12 years (liposomes are the only adjuvants that can be given to children). The dosage of the virosome vaccine is 0.25 ml between six and 35 months, and 0.5 ml for all other age groups.
Intradermal vaccine
Beyond the standard intramuscular split vaccines, an intradermal influenza split vaccine has been currently licensed. It has been proven to induce non-inferior immune responses at a 9 μg HA dose compared with the standard 15 μg HA in adults.Citation35 This dose-sparing effect is likely to be mediated by the high density of antigen presenting cells (APCs) in the skin, which can capture the antigen and transport it to the lymph nodes, or capture the viral antigen after its migration to the lymph nodes.Citation16 However, the elderly still need a normal dose of 15μg when they receive an intradermal influenza vaccine. There is no indication for children. It is administered with a micro needle (1.5 mm) 10 times thinner than conventional needles.
Intranasal vaccine
Intranasal vaccine consists of the live-attenuated influenza virus. In contrast to inactivated vaccines, the live-attenuated influenza vaccine (LAIV) induces strong mucosal IgA responses and cell-mediated immune responses, which are effective for the prevention of influenza.Citation36 It is administered intranasally using the supplied prefilled, single-use sprayer containing 0.2 ml of vaccine. About 0.1 ml is sprayed into each nostril. LAIV is approved for use in the 2-18-year-old age group. But the ACIP recommended that the LAIV was not to be used in 2016/17 in light of its low effectiveness against influenza A(H1N1)pdm09 in the United States in 2013/14 and 2015/16. In Italy, the LAIV is authorized by the AIFA (Italian Agency of Medicines), but is not commercialized.
Influenza vaccination recommendations
For the influenza season 2016/2017, the CDC continues to recommend a routine annual influenza vaccination for those aged ≥6 months without contraindications.Citation37 The correct campaign should consider the timing and indications of vaccination for different populations groups.
Optimally, vaccination should be done before the onset of influenza activity in the community. Children aged between six months and eight years, who require two doses for their first influenza vaccination, should receive their first dose as soon as possible after the vaccine becomes available and the second dose at least four weeks later. The majority of adults have a protective antibody response within two weeks of vaccination.Citation38–39 Vaccination should continue to be offered as long as the influenza viruses are circulating and the unexpired vaccine is available. Questions related to the ideal time for vaccination have been raised with respect to the early availability of the vaccine compared with the typical onset and the peak of influenza activity. Efforts should be made to ensure that as many people as possible are vaccinated before influenza strikes the community. On the other hand, protective antibody levels decrease over time after the vaccination. Some recent studies have shown that early vaccination of adults, particularly the elderly, might contribute to reduced protection later in the season.Citation40-41 However, it is noteworthy that revaccination is not recommended in the same season. Vaccination programs should be balanced between maximizing the duration of vaccine-induced protection through the season and avoiding missing an opportunity to vaccinate or vaccinating after the onset of influenza. summarizes the recommendations of the CDC and the Italian Ministry of Health to prevent influenza.
Table 1. Vaccination recommendations of the CDC and the Italian Ministry of Health to prevent influenza.
Vaccination is particularly important for groups of people who have an increased risk of influenza or related complications.Citation42
It is important to improve vaccination among healthcare workers through the knowledge of influenza transmission and vaccination and other multi-component interventions so that they can protect themselves and their patients.Citation43–44
The influenza vaccine is not contraindicated for people who are allergic to eggs but only for those who have shown a severe allergic reaction to influenza vaccines. Studies on the experience of the use of inactivated influenza vaccines, and more recently, LAIV, indicate that severe allergic reactions to the currently available egg-based influenza vaccines are unlikely for those who are allergic to eggs.Citation45
The “Vaccine Calendar for life, 2016”,Citation46 is a document that arose from a consolidated partnership between two Italian Scientific Societies – the Italian Society of Hygiene, Preventive Medicine and Public Health (SItI) and the Italian Society of Pediatrics (SIP) – and the main federations representing primary care for children, the Italian Federation of Paediatricians (FIMP), and for adults, the Italian Federation of General Medicine (FIMMG). According to the calendar, the vaccine choice is based on the patient's age and should be treated as follows:
| · | Six months through three years: Subunit/TIV. | ||||
| · | 3–70 years: Split QIV for risk groups according to national recommendations, except for the patients who need enhanced immunogenicity based on the physician's advice (intradermal vaccine from 60-year-old subjects or adjuvanted vaccine for people aged over 65 years). | ||||
| · | >70 years: Adjuvanted vaccine or high-dose intradermal vaccine for enhanced immune response. | ||||
Since 2012, WHO has recommended the development of QIV, which reflects the current epidemiology of influenza more accurately and which would improve the effectiveness of the vaccine, optimizing the control of this public health threat.Citation47
Data on the effectiveness of adjuvanted vaccines are still under discussion in scientific literature, especially in terms of reduction of mortality, complications, and the number of hospitalization days.Citation48 However, against all three homologous strains and the heterologous A strains, the MF59-adjuvanted vaccine gives higher rate of seroprotection to subjects aged over 65 years at three or four weeks after vaccination than non-adjuvanted vaccines do. The MF59-adjuvanted vaccine enhances the immunogenicity of influenza vaccination among the elderly and promises to be a strong tool for influenza prevention in populations with known suboptimal response. Immunogenicity can be defined as the ability of a vaccine to induce an immune response, and in the case of the influenza virus, it is measured by the hemagglutination-inhibition (HI) test. For the influenza vaccination, anti-HI antibodies can inhibit the penetration of the virus into the target cells by binding the haemagglutinin. Actually, it is reasonable to use the level of HI antibodies to infer the efficacy of the vaccine when their function is exactly known. Although there is no fixed cut-off for predictive level, some studies conducted on healthy volunteers have shown a clear reverse correlation between the anti-HI antibody level and the probability of being infected. Accordingly, higher anti-HI titers reduce the likelihood of illness, and an inverse relationship exists between anti-HI titers and the attack rate during an epidemic. The protective capacity of the influenza vaccine increases with increasing antibody titers.Citation49–50 The data from clinical trials and observation studies on safety and effectiveness attest to the safety of MF-59 and its ability to enhance the effectiveness of influenza vaccines in the elderly and children.Citation31,Citation51–61
Skills of communication and adequate management of adverse events following vaccinations are of utmost importance for the achievement of immunization coverage. In fact, in Italy, incorrect assessment and the inability to handle information on deaths not related to a vaccine have paradoxically led to an increase in the number of deaths and complications predominantly due to the non-use of influenza vaccines. In the winter 2014/15, which was characterized by high influenza activity, the collapse of the vaccine coverage following the so-called “Fluad case” Citation62 has heavily influenced the occurrence of a large number of complications, hospital admissions, and deaths in Italy.Citation63–64
Conclusions
The availability of different types of vaccines to prevent influenza illness and the results of their effectiveness and immunogenicity in different age groups or populations at risk should prompt GPs to encourage their patients to undergo influenza vaccination to prevent complications and deaths. Health authorities should give GPs the opportunity to choose the most appropriate vaccines tailored to specific patients.
In conclusion, the use of any influenza vaccine is crucial to avoid complications, hospitalization and death. But the use of the most appropriate vaccine for each patient optimizes the result at a very modest cost. Increasing the value of public health offer through a value-based approach can improve the quality of health services and preserve its sustainability.
Disclosure of potential conflicts of interest
No potential conflicts of interest to declare.
Financial disclosure statement
Authors have not received any funding for this work.
References
- Vestergaard LS, Nielsen J, Krause TG, Espenhain L, Tersago K, Bustos Sierra N, Denissov G, Innos K, Virtanen MJ, Fouillet A, et al. Excess all-cause and influenza-attributable mortality in Europe, December 2016 to February 2017. Euro Surveill. 2017;22(14):pii=30506.
- Rolfes MA, Foppa IM, Garg S, Flannery B, Brammer L, Singleton JA, et al. Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths Averted by Vaccination in the United States. 2016 Dec 9 [Date Cited]; https://www.cdc.gov/flu/about/disease/2015-16.htm
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086-96. doi:10.1016/j.vaccine.2007.03.046.
- Siriwardena AN. Increasing evidence that influenza is a trigger for cardiovascular disease. J Infect Dis. 2012;206(11):1636–8. doi:10.1093/infdis/jis598.
- Italian Network for Surveillance of Influenza - InfluNet. [Italian] Available at: http://www.iss.it/iflu/
- Italian Ministry of Health. Circular 19 November 2009. [Italian] Available at: http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=0&codLeg=50960&parte=1%20&serie=. Last accessed 18.07.2017]
- Italian Network for Surveillance of Influenza – InfluNet. Epidemiologic surveillance of Flu season 2015/2016. [Italian] Available at: http://www.iss.it/binary/iflu/cont/Influnet_stagione_2015_2016.pdf
- Italian Ministry of Health. Prevention and control of influenza: recommendations for the influenza season 2016–2017. [Italian] Available at: http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2016&codLeg=55586&parte=1%20&serie=null
- Italian National Health Institute (ISS). Epidemiologic surveillance of influenza - INFLUNET. Report n. 27. 3 May 2017. [Italian] Available at: (http://www.iss.it/binary/iflu/cont/Influnet_2017_17.pdf)
- Global Influenza Surveillance and Response System (GISRS). Available at: http://www.who.int/influenza/gisrs_laboratory/en/
- Italian Ministry of Health. Prevention and control of influenza: recommendations for the influenza season 2015–2016. [Italian] Available at: http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=0&codLeg=52703&parte=1%20&serie=
- Italian National Health Institute (ISS). Virologic surveillance of influenza - INFLUNET. Report n. 24. 3 May 2017. [Italian] Available at: (http://www.iss.it/binary/fluv/cont/Agg.Vir_03_05_17.pdf)
- WHO. Recommended composition of influenza virus vaccines for use in the 2017–2018 northern hemisphere influenza season. March 2017. Available at: http://www.who.int/influenza/vaccines/virus/recommendations/201703_recommendation.pdf?ua=1
- Italian Ministry of Health. Influenza vaccination coverage in Italy. Seasons 2000-2001/2016-2017. Available at: http://www.salute.gov.it/imgs/C_17_tavole_19_allegati_iitemAllegati_0_fileAllegati_itemFile_3_file.pdf.
- Italian Ministry of Health. National Plan for Vaccination Prevention 2017-2019. [Italian] Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf
- Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007;25(4):659–63. doi:10.1016/j.vaccine.2006.08.026.
- Trucchi C, Alicino C, Orsi A, Paganino C, Barberis I, Grammatico F, Canepa P, Rappazzo E, Bruzzone B, Sticchi L, et al. Fifteen years of epidemiologic, virologic and syndromic influenza surveillance: A focus on type B virus and the effects of vaccine mismatch in Liguria region, Italy. Hum Vaccin Immunother. 2017;13(2):456–463. doi:10.1080/21645515.2017.1264779.
- Kieninger D, Sheldon E, Lin WY, Yu CJ, Bayas JM, Gabor JJ, Esen M, Fernandez Roure JL, Narejos Perez S, Alvarez Sanchez C, Feng Y, Claeys C, Peeters M, Innis BL, Jain V. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years. BMC Infect Dis. 2013;13:343. doi:10.1186/1471–2334-13-343.
- Fluarix-Tetra® Summary of Product Characteristics. [Italian] Available at: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000231_043132_RCP.pdf&retry=0&sys=m0b1l3.
- Crépey P, de Boer PT, Postma MJ, Pitman R. Retrospective public health impact of a quadrivalent influenza vaccine in the United States. Influenza Other Respir Viruses. 2015;9 Suppl 1:39–46. doi:10.1111/irv.12318.
- Kittikraisak W, Chittaganpitch M, Gregory CJ, Laosiritaworn Y, Thantithaveewat T, Dawood FS, Lindblade KA. Assessment of potential public health impact of a quadrivalent inactivated influenza vaccine in Thailand. Influenza Other Respir Viruses. 2016;10(3):211–9. doi:10.1111/irv.12361.
- Barbieri M, Silvestri R, Boccalini s, De Waure C. Analisi di costo-efficacia della vaccinazione anti-influenzale in Italia. QIJPH. 2015, Volume 4, Number 5:70–84. [Italian]
- Gianchecchi E, Trombetta C, Piccirella S, Montomoli E. Evaluating influenza vaccines: progress and perspectives. Future Virology. 2016, 11(5):379–393. doi:10.2217/fvl-2016-0012.
- Soema PC, Kompier R, Amorij JP, Kersten GF. Current and next generation influenza vaccines: Formulation and production strategies. Eur J Pharm Biopharm. 2015;94:251–63. doi:10.1016/j.ejpb.2015.05.023.
- Banzhoff A, Pellegrini M, Del Giudice G, Fragapane E, Groth N, Podda A. MF59®‐adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influenza and Other Respiratory Viruses. 2008;2(6):243–249. doi:10.1111/j.1750-2659.2008.00059.x. PMID:19453401
- Ruf BR, Colberg K, Frick M, Preusche A. Open, randomized study to compare the immunogenicity and reactogenicity of an influenza split vaccine with an MF59- adjuvanted subunit vaccine and a virosome-based subunit vaccine in elderly. Infection. 2004;32(4):191–8. doi:10.1007/s15010-004-3204-z.
- Frey S, Poland G, Percell S, Podda A. Comparison of the safety, tolerability, and immunogenicity of a MF59-adjuvanted influenza vaccine and a non-adjuvanted influenza vaccine in non-elderly adults. Vaccine. 2003;21(27-30):4234–7. doi:10.1016/S0264-410X(03)00456-0.
- Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology. 2003;49(3):177–84. doi:10.1159/000069172.
- Podda A, Del Giudice G. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev Vaccines. 2003;2(2):197–203. doi:10.1586/14760584.2.2.197.
- Del Giudice G, Hilbert AK, Bugarini R, Minutello A, Popova O, Toneatto D, Schoendorf I, Borkowski A, Rappuoli R, Podda A. An MF59-adjuvanted inactivated influenza vaccine containing A/Panama/1999 (H3N2) induced broader serological protection against heterovariant influenza virus strain A/Fujian/2002 than a subunit and a split influenza vaccine. Vaccine. 2006;24(16):3063–5. doi:10.1016/j.vaccine.2006.01.015.
- Mannino S, Villa M, Apolone G, Weiss NS, Groth N, Aquino I, Boldori L, Caramaschi F, Gattinoni A, Malchiodi G, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol. 2012;176(6):527–33. doi:10.1093/aje/kws313.
- Nolan T, Bravo L, Ceballos A, Mitha E, Gray G, Quiambao B, Patel S, Bizjajeva S, Bock H, Nazaire-Bermal N, et al. Enhanced and persistent immune response against homologous and heterologous strains elicited by an MF59®-adjuvanted influenza vaccine in infants and young children. 2013. V70_29 poster. 7th Vaccine and ISV Congress 2013. 27–29 October 2013 - Sitges Barcelona, Spain. Available at: https://www.researchgate.net/publication/273945702_Enhanced_and_persistent_immune_response_against_homologous_and_heterologous_strains_elicited_by_an_MF59R-adjuvanted_influenza_vaccine_in_infants_and_young_children.
- Herzog C, Hartmann K, Künzi V, Kürsteiner O, Mischler R, Lazar H, Glück R. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009 16;27(33):4381–7. doi:10.1016/j.vaccine.2009.05.029.
- Wilschut J. Influenza vaccines: the virosome concept. Immunol Lett. 2009;122(2):118–21. doi:10.1016/j.imlet.2008.11.006.
- Ansaldi F, de Florentiis D, Durando P, Icardi G. Fluzone(®) Intradermal vaccine: a promising new chance to increase the acceptability of influenza vaccination in adults. Expert Rev Vaccines. 2012;11(1):17–25. doi:10.1586/erv.11.154.
- Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults. Drugs. 2011;71(12):1591–622. doi:10.2165/11206860-000000000-00000.
- CDC. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2016-17 Influenza Season, MMWR 2016;65(5);1–54.
- Gross PA, Russo C, Dran S, Cataruozolo P, Munk G, Lancey SC. Time to earliest peak serum antibody response to influenza vaccine in the elderly. Clin Diagn Lab Immunol 1997;4:491–2. PMID:9220171
- Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis 1995;171:198–203. doi:10.1093/infdis/171.1.198. PMID:7798664
- Ochiai H, Shibata M, Kamimura K, Niwayama S. Evaluation of the efficacy of split-product trivalent A(H1N1), A(H3N2), and B influenza vaccines: reactogenicity, immunogenicity and persistence of antibodies following two doses of vaccines. Microbiol Immunol 1986;30:1141–9. doi:10.1111/j.1348-0421.1986.tb03043.x. PMID:3807793
- Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, Cho GJ, Hwang TG, Kim WJ. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28(23):3929–35. doi:10.1016/j.vaccine.2010.03.067.
- European Centre for Disease Prevention and Control. Seasonal influenza vaccination and antiviral use in Europe – Overview of vaccination recommendations and coverage rates in the EU Member States for the 2013–14 and 2014–15 influenza seasons. Stockholm: ECDC. 2016
- Bonaccorsi G, Santomauro F, Porchia BR, Niccolai G, Pellegrino E, Bonanni P, Lorini C. Beliefs and Opinions of Health Care Workers and Students Regarding Influenza and Influenza Vaccination in Tuscany, Central Italy. Vaccines 2015, 3(1), 137–147. doi:10.3390/vaccines3010137. PMID:26344950
- Bonaccorsi G, Lorini C, Porchia BR, Niccolai G, Martino G, Giannarelli L, Santomauro F. [Influenza vaccination: coverage and risk perception among students of the health professions at Florence University, Italy]. Ann Ig. 2013;25(3):181–9.
- Des Roches A, Paradis L, Gagnon R, Lemire C, Bégin P, Carr S, Chan ES, Paradis J, Frenette L, Ouakki M, et al; PCIRN (Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network). Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol. 2012;130(5):1213–1216.e1. doi:10.1016/j.jaci.2012.07.046.
- SItI, SIP, FIMP, FIMG. Vaccine Calendar for life, 2016. III Edition. [Italian] Calendario Vaccinale per la vita. Available at: https://www.sip.it/il-calendario-per-la-vita-iii-edizione-2016.
- WHO. Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012;87(47):461–76.
- Trippoli S. Comparison between MF59-adjuvanted vaccine and non -adjuvanted vaccines: data are still lacking about clinical outcomes (Comment). PubMed Commons, available at http://www.ncbi.nlm.nih.gov/pubmed/26022564#cm26022564_11401 accessed 17 August 2015.
- Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35(1):69–75. doi:10.1093/oxfordjournals.bmb.a071545.
- Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011;30(12):1081–5. doi:10.1097/INF.0b013e3182367662.
- Black S. Safety and effectiveness of MF-59 adjuvanted influenza vaccines in children and adults. Vaccine. 2015;33 Suppl 2:B3–5. doi:10.1016/j.vaccine.2014.11.062.
- Del Giudice G, Rappuoli R. Inactivated and adjuvanted influenza vaccines. Curr Top Microbiol Immunol. 2015;386:151–80. PMID:25038938
- Frey SE, Reyes MR, Reynales H, Bermal NN, Nicolay U, Narasimhan V, Forleo-Neto E, Arora AK. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine. 2014;32(39):5027–34. doi:10.1016/j.vaccine.2014.07.013.
- Baldo V, Baldovin T, Floreani A, Fragapane E, Trivello R; Family Medicine Group. Response of influenza vaccines against heterovariant influenza virus strains in adults with chronic diseases. J Clin Immunol. 2007;27(5):542–7. doi:10.1007/s10875-007-9100-4.
- Squarcione S, Sgricia S, Biasio LR, Perinetti E. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine. 2003;21(11-12):1268–74. doi:10.1016/S0264-410X(02)00401-2.
- Gasparini R, Pozzi T, Montomoli E, Fragapane E, Senatore F, Minutello M, Podda A. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur J Epidemiol. 2001;17(2):135–40. doi:10.1023/A:1017919305501. PMID:11599686
- Baldo V, Menegon T, Bonello C, Floreani A, Trivello R; Collaborative Group. Comparison of three different influenza vaccines in institutionalised elderly. Vaccine. 2001;19(25-26):3472–5. doi:10.1016/S0264-410X(01)00060-3.
- Menegon T, Baldo V, Bonello C, Dalla Costa D, Di Tommaso A, Trivello R. Influenza vaccines: antibody responses to split virus and MF59-adjuvanted subunit virus in an adult population. Eur J Epidemiol. 1999;15(6):573–6. doi:10.1023/A:1007594911541.
- De Donato S, Granoff D, Minutello M, Lecchi G, Faccini M, Agnello M, Senatore F, Verweij P, Fritzell B, Podda A. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine. 1999;17(23-24):3094–101. doi:10.1016/S0264-410X(99)00138-3.
- Minutello M, Senatore F, Cecchinelli G, Bianchi M, Andreani T, Podda A, Crovari P. Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine. 1999 ;17(2):99–104. doi:10.1016/S0264-410X(98)00185-6.
- Villa M, Black S, Groth N, Rothman KJ, Apolone G, Weiss NS, Aquino I, Boldori L, Caramaschi F, Gattinoni A, et al. Safety of MF59-adjuvanted influenza vaccination in the elderly: results of a comparative study of MF59-adjuvanted vaccine versus nonadjuvanted influenza vaccine in northern Italy. Am J Epidemiol. 2013;178(7):1139–45. doi:10.1093/aje/kwt078.
- EMA Press Office. No evidence that Fluad vaccine caused deaths in Italy. EMA Committee review reassures Member States over safety of flu vaccine. EMA/749142/2014. 3 December 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/12/WC500177992.pdf.
- Signorelli C, Odone A, Conversano M, Bonanni P. Deaths after Fluad flu vaccine and the epidemic of panic in Italy. BMJ. 2015;350:h116. doi:10.1136/bmj.h116.
- Levi M, Sinisgalli E, Lorini C, Santomauro F, Chellini M, Bonanni P. The "Fluad Case" in Italy: Could it have been dealt differently? Hum Vaccin Immunother. 2017;13(2):379–384. doi:10.1080/21645515.2017.1264738.
