?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: We actively followed a cohort of nursery school children in Suzhou, China to assess the impact of vaccination with trivalent influenza vaccine on the prevention of influenza like illness (ILI).
Methods: We enrolled children aged 36 to 72 months from 13 nursery schools in Suzhou starting two weeks after vaccination during October 2015-February 2016. Every school-day, teachers reported the names of students with ILI to study clinicians, who collected the student's nasopharyngeal swab or throat swab, either at a study clinic or the child's home. Swabs were sent to the Suzhou Center for Disease Control and Prevention's laboratory for influenza testing by RT-PCR.
Results: In total, 3278 children were enrolled; 83 (3%) were lost to follow-up, while 3195 (vaccinated: 1492, unvaccinated: 1703) were followed for 24 weeks. During the study, 40 samples tested positive; 17 in the vaccinated (B Victoria: 12; A(H1N1)pdm09: 5) and 23 in the unvaccinated group (B Victoria: 10; B Yamagata: 2; A(H1N1)pdm09: 11). The VE estimates were: 16% overall (95%CI:-58%,56%), 48% (−47%,84%) for influenza A(H1N1)pdm09, 43% (−650%,98%) for influenza B Yamagata, and −37% (−227%,42%) for influenza B Victoria. Data were analyzed by vaccinated and unvaccinated groups based on enrollees' vaccination records.
Conclusions: The VE for A(H1N1)pdm09 was moderate but not significant. Mismatching of B lineage may have compromised trivalent influenza vaccine effectiveness during the 2015–2016 influenza season among nursery school children in Suzhou, China. Additional larger studies are warranted to inform policy related to quadrivalent influenza vaccine licensure in China in the future.
Introduction
Seasonal influenza epidemics pose a heavy economic burden on modern society.Citation1–3 The incidence of influenza disease is high among children, and some studies suggest incidence among children is higher than among older adults.Citation4,5 Seasonal influenza vaccination is the most effective method of preventing influenza virus infection and its potentially severe complications. Both the World Health Organization (WHO)Citation6 and the Chinese Center for Disease Control and PreventionCitation7 recommend annual seasonal influenza vaccination for groups at highest risk for developing severe illness from influenza infection, including young children. However, seasonal influenza vaccination coverage among young children in China is still low, with estimates ranging from 8% to 26% in 2009–2012 in five provinces and municipalities (Beijing, Shandong, Hunan, Henan and Sichuan).Citation8 Vaccine efficacy from randomized control trials and vaccine effectiveness from observational studies among healthy children in developed countries has ranged from 40%−70%.Citation9–11 Similar findings were reported in several seasons in China when circulating influenza viruses were well-matched to vaccine strains.Citation12–15
Improving our understanding of how to effectively prevent influenza infection among nursery school children is particularly important given that children in this age group have a longer duration of pre- and post-symptomatic influenza virus shedding than adultsCitation16 and, therefore, may infect family members and classmates prior to symptom onset. Well-timed administration of pediatric vaccination would likely decrease influenza illness among adults.Citation17 Additional data on seasonal influenza vaccine effectiveness for nursery school children in the local setting may inform local vaccine policy to promote increased vaccination coverage among young children in China.
We conducted a community-based prospective cohort study among nursery school children in Suzhou, China to evaluate seasonal influenza vaccine effectiveness during the 2015–2016 influenza season.
Results
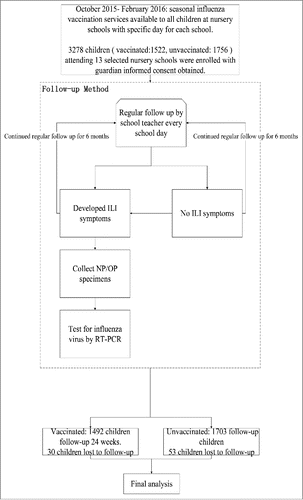
A total of 3278 children were recruited from 13 selected nursery schools among 24 nursery schools in Suzhou. Among them, 41% (1329/3278) were aged 60–72 months, 33% (1079/3278) were aged 48–59 months, and 27% (870/3278) were aged 36–47 months. Among the 3278 enrollees, 83 (3% of all enrolled children; 2% vaccinated and 3% unvaccinated, p = 0.057) were lost to follow-up because their family left Suzhou.
Among which, 1522 children were enrolled as vaccinated group and 1492 children were enrolled as unvaccinated group.The median ages were 56.8 months and 56.6 months for the vaccinated and unvaccinated children (p = 0.679), respectively. The influenza vaccine coverage in private nursery schools was higher than the coverage in public schools (52.4% vs. 43.9%, p = 0.002). Compared with the unvaccinated children, the proportion of vaccinated children with medical insurance was higher (68% vs. 65%, p<0.05). For the parents' educational background, compared with the vaccinated children, unvaccinated children's mother had higher proportion of higher educational background (52% vs. 48%, p<0.05), however, that difference was not found in the father part. Other characteristics, including sex, comorbidity (defined as any of the following: congenital heart disease, asthma and other chronic lung disease, neuromuscular disease, kidney disease, blood dyscrasia and HIV), family income and respiratory illness within the past 2 weeks were similar between vaccinated and unvaccinated groups ().
Table 1. Characteristics of enrolled children aged 3 to 6 years in Suzhou, 2015–2016.
The influenza vaccine coverage among recruited children was 46% (1522/3278). By age group, coverage was 43% (378/870) among children aged 36–47 months; 49% (534/1079) among children 48–59 months, and 46% (610/1329) among children 60–72 months.
There were no significant differences in the incidence of ILI symptom development, clinical visits due to ILI, hospitalization due to ILI and purchase of medicines due to ILI between the vaccinated group and unvaccinated group during follow-up. ().
Table 2. Incidence of influenza-like illness and relative health care seeking behavior during 6 months follow-up period, Suzhou 2015–16.
Table 3. Number of laboratory-confirmed influenza like illnesses and the unadjusted vaccine effectiveness (VE) by sub-type and lineage in the 2015/2016 influenza season in Suzhou.
During the follow-up period, 237 of 1522 (16%) vaccinated children developed ILI symptoms and respiratory swabs were collected for 70% (165/236). In the unvaccinated group, 296 of 1756 (17%) children developed ILI symptoms and respiratory swabs were collected for 69% (203/294). Of the 368 respiratory swabs collected, 40 (11%) tested positive for influenza virus. Within the vaccinated group, 17 children had laboratory-confirmed influenza; 12 tested positive for influenza B Victoria (BV) and 5 tested positive for influenza A(H1N1)pdm09 virus. In the unvaccinated group, 23 children had laboratory-confirmed influenza; 10 tested positive for influenza BV, 2 tested positive for influenza B Yamagata (BY) and 11 tested positive for influenza A(H1N1)pdm09 virus (). The unadjusted VE against laboratory-confirmed influenza illness was not statistically significant, with a point estimate of 16% (95%CI:-58%,56%). The VE by type/subtype were also not significant; the point estimates were 43% (95% CI:−650%,98%) for BY, −37% (95% CI:-227%,42%) for BV and 48% (95% CI:-47%,84%) for A(H1N1)pdm09 ().
Discussion
In our community cohort surveillance among children aged 36 to 72 months old in Suzhou during November 2015 to July 2016, seasonal influenza vaccination coverage was 46% and the influenza positive rate among children with ILI was 11%. The predominating influenza viruses were influenza B Victoria and influenza A(H1N1)pdm09. The overall VE against laboratory confirmed influenza illness during the study period was not significant. While the overall VE point estimate was 16% (95% CI, −58%,56%), the point estimates for VE against influenza A(H1N1)pdm09 virus was 48% (95% CI,-47%,84%), against B Yamagata was 43% (95% CI,-650%,98%), and against B Victoria was −37% (95% CI,-227%,42%).
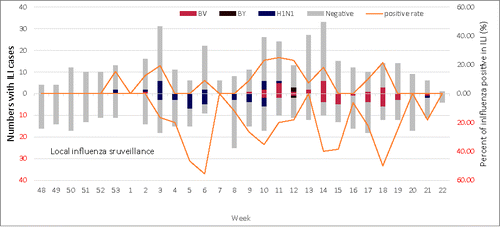
The point estimates for VE against influenza A(H1N1)pdm09 and B Yamagata in our study were quite similar to those described in the United States during the same season. However, the point estimate for the VE against laboratory-confirmed B Victoria ILI in our study was much lower than the VE against acute respiratory illness with laboratory-confirmed B Victoria among outpatients aged 2–17 years observed in the United States (−37% vs. 56%).Citation18 The influenza virus circulating pattern seen in this study was similar to local surveillance data (unpublished). The influenza virus circulating pattern seen in this study was similar to local surveillance data and that observed in other studies in the region (unpublished data). In Eastern Asia, the most predominant influenza virus type circulating during the 2015–2016 influenza season was influenza B, followed by the subtype influenza A(H1N1)pdm09 virus.Citation19 Chinese National Influenza Surveillance data further demonstrated that within the influenza B virus type, influenza B Yamagata lineage only predominated in the first few weeks of the season, replaced by influenza B Victoria lineage, followed by influenza A(H1N1)pdm09 virus.Citation20 An influenza B Yamagata virus rather than a Victoria virus was included in the 2015/2016 trivalent inactivated influenza vaccine (TIV). In the United States, the VE for inactivated vaccine described during the same season was 47% (adjusted VE for children 6m-8y; 95% CI: 31%−61%) where quadrivalent vaccine, which contains both influenza B lineages, represented more than half of the influenza vaccine supply in 2015–16.Citation21 In China, only TIV containing northern hemisphere vaccine strains recommended by WHO are licensed for use domestically.Citation7 TIV includes strains of influenza A(H1N1)pdm09, A(H3N2) and either B Victoria or B Yamagata viruses. Some previous reports have shown that there is no substantial cross-protection between the B lineages,Citation22–24 and therefore correctly predicting the predominant circulating B lineage can impact the VE of the trivalent vaccine. In the 2015–2016 influenza season, B Yamagata was the WHO-recommended B lineage included in the TIV. In our study, few influenza B Yamagata-associated ILI were detected in either the vaccinated or unvaccinated groups. Further, Suzhou's 2015–2016 local ILI surveillance data showed that the influenza B Victoria lineage predominated for the majority of the season (). Therefore, it is possible that the non-inclusion of an influenza B Victoria lineage strain in the 2015–16 northern hemisphere TIV contributed to the lower VE observed among young children in Suzhou in 2015–2016 compared to countries where QIV use was common.
In this study, we did not adjusted VE because, the related underlying medical conditions were balance between vaccinated and unvaccinated children. For the enrolled children, the proportion vaccinated was similar among migrant and resident children. Although the educational background of their parents was different, putting this variable into model tuning had little effect on the VE. There may be other unknown variables that we had not collected, which affect vaccine and vaccine outcomes, but we could not adjust the variables here. So in this article only, crude VE is reported.
This study has several limitations. First, the sample collection rate among children who reported ILI symptoms in the surveillance cohort was only 70% in the vaccinated group and 69% in the unvaccinated group. Further, teachers only conducted symptom screening during school days. Illnesses that occurred during weekends, short school holidays, and the longer Spring Festival were missed. Although symptom screening was the same for both vaccinated and unvaccinated children, the missed illness episodes combined with the low sample collection rate in both groups decreased our power to measure VE overall and especially by virus type, sub-type and lineage. While the VE point estimates in our study vary by influenza virus sub-type and lineage, and while they are similar to those described in the United States during the same season for A(H1N1)pdm0 and B Yamagata, the wide confidence intervals limit our ability to make definitive comparisons. Second, we selected nursery schools based on their interest in collaborating and their relatively high influenza vaccine coverage among young children in the previous two influenza seasons. The children in these nursery schools may not be representative of other nursery-school aged children in Suzhou, China. Finally, half of the children living in Suzhou were children of migrant workers. Although children of migrant workers have access to routine immunization services, their prior influenza vaccination records were not available, which limited our ability to assess the impact of prior vaccination on the VE observed during this study season.
Although the overall VE against laboratory-confirmed ILI among children aged 36–72 months in Suzhou, China during the 2015–2016 influenza season was not significant, the VE point estimates within this study varied by virus type/subtype. The lowest point estimate was for B Victoria lineage, which predominated for most of Suzhou's 2015–16 season and which was not included in the 2015–16 northern hemisphere TIV, the only vaccine currently licensed in China. Research designed to evaluate the impact of B lineage mismatch in the TIV may inform policy related to QIV licensure in China in the future.
Methods
Study site selection
This study was conducted in Suzhou municipality city, Jiangsu Province, located in eastern China. In 2014, Suzhou had 10 million residents; approximately half were migrants, and 10% were children less than 14 years of age.Citation25 Children of migrant workers have the same access to routine immunization services as children of Suzhou residents. Suzhou is an economically well-developed city with a Gross Domestic Product per capita ranked 7th in China in 2015.Citation26
Based on their interest in collaborating and their relatively high influenza vaccine coverage among young children in the previous two influenza seasons, we selected 2 districts of Suzhou as the study sites, Wuzhong and Taicang Districts. In each district, we selected both public and private nursery schools which typically enroll children aged 3–6 years residing in the surrounding neighborhoods. Inclusion criteria for nursery schools were: 1) the influenza vaccine coverage among students was at least 30% in the previous two seasons; 2) the number of enrolled students was no less than 200 (to facilitate the inclusion of a reasonable number of nursery schools for effective coordination); 3) the nursery school's administration was willing to participate.
Sample size
Based on previous health utilization surveys (unpublished), the incidence of influenza like illness (ILI) is approximately 6.5%, and the ILI attack rate is 39.0% in both vaccinated and unvaccinated children. The influenza positive rate among ILI cases is 10%. We assume that the influenza vaccine effectiveness is 45% and that 10% of the study subjects will withdraw are be lost to follow up. According to the formula:
Seasonal influenza vaccine program in Suzhou
The seasonal influenza vaccine is not included in the national immunization program in China. Despite this, local hospitals in Suzhou provide influenza vaccination services to each school for one week, usually during the months of November and December, with the specific timing of the services varying by district. Parents may elect to have their children vaccinated for seasonal influenza during this week, at an out-of-pocket cost of 10–15 US dollars. Students who are absent during the seasonal influenza vaccine services week have the opportunity to be vaccinated in the following week.
Enrollment
All children in selected nursery schools were eligible for enrollment. From October 2015 to February 2016, after local seasonal influenza vaccination services had ended, we enrolled all children whose parents provided written informed consent into two groups: the vaccinated group, children who had received seasonal influenza vaccination in the study season, and the unvaccinated group, children who had not received influenza vaccination in the study season. In China, per technical guidelines, any child 36 months of age or older receives only one dose of seasonal influenza vaccine, while two doses are recommended for children aged 6–35 months. The influenza vaccination histories of all enrolled children were verified by the study doctors through vaccination record review. Vaccine records were obtained from the local vaccine clinic, which hosts an electronic record system maintained by Suzhou CDC and includes complete vaccination records for >99% of children living in the district. The guardians of enrolled children completed a self-administrated questionnaire at the time of enrollment that collected demographic information, child's medical history, family health seeking behavior, and family health behaviors ().
Follow-up
Enrolled children were followed for six months starting two weeks after the scheduled vaccination day for each nursery school. The teachers in charge of each class in the selected nursery schools were trained to screen all enrolled children for illness on every school day. If a child was absent on any school day, the teacher called the child's home to ask about illness. Teachers identified children with influenza-like illness (ILI), which was defined as measured axillary temperature 38°C or higher, accompanied by cough or sore throat/inflamed pharynx. A repeat illness report within two weeks was counted as one episode. Guardians of children who met the criteria for ILI were encouraged to take them to the designated community health care centers within 3 days of symptom onset, and designated physicians in the health care centers collected nasopharyngeal swab (NP) or throat swab (OP) specimens from the children. If guardians stated that they would not go to one of the designated health care centers, study physicians collected samples in the home within 24 hours of the illness report ().
Laboratory test
Specimens were stored at −20°C in the community health care centers. Every Tuesday and Thursday, the specimens were transported to Suzhou CDC laboratory, one of the national ILI network laboratories. Viral RNA was extracted using high pure viral RNA kits (Roche, Shanghai, China) according to the manufacturer's instructions. Real-time reverse transcription polymerase chain reaction (rRT-PCR) was employed to test for influenza A or B viruses using influenza virus A/B dual fluorescent quantitative RT-PCR kits (Bio Perfectus Technology Co., Jiangsu, China). The influenza virus A subtype identification was performed using influenza A(H1N1)/A(H3N2)/A(H1N1)pdm09 real time RT-PCR kits (ZJ Bio-Tec Co., Shanghai, China). The influenza virus B lineage identification was performed using influenza B Yamagata /B Victoria real time RT-PCR kits (ZJ Bio-Tec Co., Shanghai, China).
Statistical analysis
Unadjusted vaccine effectiveness (VE) was estimated as: (1−RR) × 100%. The risk ratio (RR) was defined as the incidence rate of laboratory confirmed influenza within the vaccinated group divided by the incidence rate of laboratory confirmed influenza within the unvaccinated group. We added 0.5 to any cell of the 2 × 2 table that was zero.Citation27 Mid-P exact testCitation28 was used to compare two incidence rates by openepi.Citation29 Other statistical analyses were performed using R, version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria). Statistical tests were all 2-sided. The level of significance was defined as p<0.05 for all statistical tests and confidence intervals.
Ethics statement
This study was approved by the Institutional Review Board of the School of Public Health, Fudan University, and received a non-engaged determination from the U.S. Centers for Disease Control and Prevention. Written informed consent was obtained from parents or guardians on behalf of children participants enrolled in the study prior to enrollment.
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to disclose. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Acknowledgment
The authors thank Mark Thompson for his review of this paper.
References
- Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, Bridges CB, Grijalva CG, Zhu Y, Bernstein DI, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006; 355(1):31–40. doi:10.1056/NEJMoa054869. PMID:16822994
- Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig RL, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016; 16(1):481. doi:10.1186/s12889-016-3128-4. PMID:27278794
- Carrat F, Sahler C, Rogez S, Leruez-Ville M, Freymuth F, Le Gales C, Bungener M, Housset B, Nicolas M, Rouzioux C. Influenza burden of illness: estimates from a national prospective survey of household contacts in France. Arch Intern Med. 2002; 162(16):1842–8. doi:10.1001/archinte.162.16.1842. PMID:12196082
- Fowlkes A, Steffens A, Temte J, Di Lonardo S, McHugh L, Martin K, Rubino H, Feist M, Davis C, Selzer C, et al. Incidence of medically attended influenza during pandemic and post-pandemic seasons through the Influenza Incidence Surveillance Project, 2009–13. Lancet Respir Med. 2015; 3(9):709. doi:10.1016/S2213-2600(15)00278-7. PMID:26300111
- Cowling BJ, Chan KH, Fang VJ, Lau LL, So HC, Fung RO, Ma ES, Kwong AS, Chan CW, Tsui WW, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010; 362(23):2175–84. doi:10.1056/NEJMoa0911530. PMID:20558368
- WHO. Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012; 87(47):461–76. PMID:23210147.
- Feng L, Yang P, Zhang T, Yang J, Fu C, Qin Y, Zhang Y, Ma C, Liu Z, Wang Q, et al. Technical guidelines for the application of seasonal influenza vaccine in China (2014-2015). Hum Vaccin Immunother. 2015; 11(8):2077–101. doi:10.1080/21645515.2015.1027470. PMID:26042462
- Zhou L, Su Q, Xu Z, Feng A, Jin H, Wang S, Feng Z. Seasonal influenza vaccination coverage rate of target groups in selected cities and provinces in China by season (2009/10 to 2011/12). Plos One. 2013; 8(9):e73724. doi:10.1371/journal.pone.0073724. PMID:24040041
- Sugaya N, Nerome K, Ishida M, Matsumoto M, Mitamura K, Nirasawa M. Efficacy of inactivated vaccine in preventing antigenically drifted influenza type A and well-matched type B. JAMA. 1994; 272(14):1122–6. doi:10.1001/jama.1994.03520140052037. PMID:7933325
- Ritzwoller DP, Bridges CB, Shetterly S, Yamasaki K, Kolczak M, France EK. Effectiveness of the 2003–2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics. 2005; 116(1):153–9. doi:10.1542/peds.2005-0049. PMID:15995046
- Zhou L, Su Q, Xu Z, Feng A, Jin H, Wang S, Feng Z. Seasonal influenza vaccination coverage rate of target groups in selected cities and provinces in China by season (2009/10 to 2011/12). Plos One. 2013; 8(9):e73724. doi:10.1371/journal.pone.0073724. PMID:24040041
- Wang Y, Zhang T, Chen L, Greene C, Ding Y, Cheng Y, Yang C, Zeng S, Hua J, Zhou S, et al. Seasonal influenza vaccine effectiveness against medically attended influenza illness among children aged 6–59 months, October 2011–September 2012: A matched test-negative case–control study in Suzhou, China. Vaccine. 2016; 34(21):2460–5. doi:10.1016/j.vaccine.2016.03.056. PMID:27016650
- Yang Z, Dong Z, Fu C. Seasonal influenza vaccine effectiveness among children aged 6 to 59 months in southern China. Plos One. 2012; 7(1):e30424. doi:10.1371/journal.pone.0030424. PMID:22291953
- Fu C, He Q, Li Z, Xu J, Li Y, Lu J, Li K, Yang Q, Dong Z, Liu X, et al. Seasonal influenza vaccine effectiveness among children, 2010–2012. Influenza Other Resp. 2013; 7(6):1168–74. doi:10.1111/irv.12157
- Fu C, Xu J, Lin J, Wang M, Li K, Ge J, Thompson MG. Concurrent and cross-season protection of inactivated influenza vaccine against A(H1N1)pdm09 illness among young children: 2012–2013 case-control evaluation of influenza vaccine effectiveness. Vaccine. 2015; 33(25):2917–21. doi:10.1016/j.vaccine.2015.04.063. PMID:25921713
- Ng S, Lopez R, Kuan G, Gresh L, Balmaseda A, Harris E, Gordon A. The Timeline of Influenza Virus Shedding in Children and Adults in a Household Transmission Study of Influenza in Managua, Nicaragua. Pediatr Infect Dis J. 2016; 35(5):583–6. doi:10.1097/INF.0000000000001083. PMID:26910589
- Hodgson D, Baguelin M, van Leeuwen E, Panovska-Griffiths J, Ramsay M, Pebody R, Atkins KE. Effect of mass paediatric influenza vaccination on existing influenza vaccination programmes in England and Wales: a modelling and cost-effectiveness analysis. Lancet Public Health. 2017; 2(2):e74–81. doi:10.1016/S2468-2667(16)30044-5. PMID:28299371
- CDC, US.Seasonal Influenza Vaccine Effectiveness. 2005–2017. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm
- Mondiale de la Santé, Organisation, World Health Organization. Review of global influenza activity, October 2015-October 2016. Wkly Epidemiol Rec. 2016; 91(51–52):604–22. PMID:27995783.
- Chinese National influenza Center.Chinese Influenza Weekly Report. 2017 4/18. http://www.chinaivdc.cn/cnic/en/Surveillance/WeeklyReport/201609/t20160925_134340.htm
- CDC, US.Seasonal Influenza Vaccine Effectiveness, 2005–2016. 2017 /4/18. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm
- Chiu SS, Feng S, Chan KH, Lo JY, Chan EL, So LY, Cowling BJ, Peiris JS. Hospital-based vaccine effectiveness against influenza B lineages, Hong Kong, 2009–14. Vaccine. 2016; 34(19):2164–9. doi:10.1016/j.vaccine.2016.03.032. PMID:27013437
- Skowronski DM, Janjua NZ, Sabaiduc S, De Serres G, Winter AL, Gubbay JB, Dickinson JA, Fonseca K, Charest H, Bastien N, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis. 2014; 210(1):126–37. doi:10.1093/infdis/jiu048. PMID:24446529
- Camilloni B, Neri M, Lepri E, Iorio AM. Cross-reactive antibodies in middle-aged and elderly volunteers after MF59-adjuvanted subunit trivalent influenza vaccine against B viruses of the B/Victoria or B/Yamagata lineages. Vaccine. 2009; 27(31):4099–103. doi:10.1016/j.vaccine.2009.04.078. PMID:19410623
- Zou H. Monitoring report of Suzhou 12th Five-Year “women and children development plan” in 2014. 2017 /4/18. http://www.sztjj.gov.cn/info_detail.asp?id=22562
- Xinhuanet. The city GDP rankings of China in 2015. 2017 /4/18. http://www.tj.xinhuanet.com/news/2016-01/21/c_1117847129.htm
- Green JPHA. Measures of relative effect: the risk ratio and odds ratio. 2017 4/18. http://handbook.cochrane.org/chapter_9/9_2_2_2_measures_of_relative_effect_the_risk_ratio_and_odds.htm
- Fagerland MW, Lydersen S, Laake P. The McNemar test for binary matched-pairs data: mid-p and asymptotic are better than exact conditional. BMC Med Res Methodol. 2013; 13:91. doi:10.1186/1471-2288-13-91. PMID:23848987
- Kevin M. Sullivan AGDA.Comparing Two Person-Time Rates. 2017 4/18. http://www.openepi.com/PersonTime2/PersonTime2.htm