ABSTRACT
INTRODUCTION: The vaccination against Humanpapilloma Virus (HPV) is an effective strategy to prevent high-risk HPV infection and subsequent cervical carcinogenesis. Although the safety profile has been ascertained, the relation with the development of central nervous system (CNS) autoimmune disorders (AD) appears still controversial. Multiple Sclerosis (MS) is the most common cause of chronic neurological impairment in young people, typically striking females. The main purpose of this review was to assess the association between HPV vaccination and MS.
METHODS: The systematic review of the literature was carried out using 5 search engines: MEDLINE, SCOPUS, ISI WEB OF KNOWLEDGE, GOOGLE SCHOLAR and ClinicalTrial.gov. The web search was updated on January 2017. PRISMA checklist was adopted to address the content of the systematic review. The measures of outcome were reported as relative risk (RR) in cohort studies and odds ratio (OR) in case-control studies.
RESULTS: The systematic review identified 5 observational studies, 9 reviews, and 1 randomized clinical trials (RCT) pooled analysis. The RR of MS onset detected by cohort studies ranged from 1.54 (95%CI, 0.04–8.59) to 1.37 (95%CI, 0.74–3.20). Concerning case-control studies, the OR spanned from 0.3, (95%CI 0.1–0.9) to 1.60 (95%CI = 0.79–3.25) for the group exposed to HPV vaccination. No result was significant.
CONCLUSION: This review showed no significant association between HPV vaccination and MS. The low statistical power of the studies agreed with the low incidence of MS disease among general population. In order to overcome the shortcoming the research may be extended to the entire pattern of CNS ADs.
Introduction
Persistent infection with Human Papillomavirus (HPV) is considered the main agent involved in development of cervical cancer. According to the estimation, among 200 genotypes of HPV, 18 are strictly related to cervical cancerCitation1,Citation2 and nearly the 70% of cervical cancer cases are caused by HPV types 16 and 18.Citation3,Citation4 Moreover, HPV is considered the major etiological agent of anus squamous cell carcinoma and a significant contributor to a considerable proportion of squamous cell carcinoma of the vulva, vagina, penis, mouth, and oropharynx.Citation5 Vaccination is one of the current strategies to prevent high-risk HPV infection and subsequent cervical carcinogenesis.Citation6 The prophylactic vaccines currently available – a bivalent HPV-vaccine (2vHPV, Cervarix® GlaxoSmithKline), a quadrivalent vaccine (4vHPV, Gardasil®, Merck & Co.) and a nonavalent HPV vaccine (9vHPV, Gardasil® 9, Merck & Co.) – are noninfectious subunit vaccines composed by virus-like particles (VLPs).Citation7 The 2vHPV is indicated for prevention of cervical pre-cancers and cervical cancer associated with oncogenic HPV types 16 and 18. The 4vHPV protects against HPV types 6 and 11, which are the most common causes of genital warts, in addition to types 16 and 18. The Food and Drug Administration (FDA) approved the 4vHPV for use in females aged 9–26 years, in 2006Citation8 and the 2vHPV in 2009.Citation9
In 2015 the US FDA approved the 9vHPV, which has the potential to prevent roughly 90% of cervical, vulvar, vaginal, and anal cancers caused by HPV types 16, 18, 31, 33, 45, 52, and 58, and for the prevention of genital warts caused by HPV types 6 or 11.Citation10,Citation11 In October 2016, the Advisory Committee on Immunization Practices (ACIP) updated their recommendation, indicating 2 doses of HPV vaccine over a 12-months period for females and males before the 15th birthday and 3 doses over a 6-months period after that date.Citation12 The safety profiles of all HPV vaccines have been confirmed either by clinical trials or by clinical practice worldwide and 28 countries have included them in their immunization schedules. There has not been any absolute contraindication for their use so far.Citation9,Citation13-15 To date over than 270 million doses of HPV vaccines have been distributed worldwide.Citation16
Clinical trials data reported for all vaccines systemic adverse events after vaccination, such as fever, nausea, vomiting, dizziness, myalgia, and diarrhea, but severe adverse events (SAE), such as persistent headache, hypertension, gastroenteritis, and bronchospasm, were described in no more than 0.5% of cases.Citation13,Citation17 Currently, the most AE for the three vaccines is the ‘injection site reaction’, particularly described as pain, swelling, and erythema of light–moderate intensity.Citation13,Citation17,Citation18
Although their safety profile has been already established, the associations between HPV mass vaccinations and ADs appear to be still controversial,Citation19–21 specially given the high rate of autoimmune disease in adolescent girls and young women, the main target of HPV vaccination programs, that may lead to temporal association between vaccination and ADs and to their following report as AE.Citation22 Actually, Sutton et al reported five cases of multiple sclerosis (MS) occurred in young women between days 1 and 21 after 4vHPV administration and three of them had experienced prior episodes of demyelination. Moreover, the cases were noteworthy not only because of the temporal association with immunization date, but also for the atypical and multifocal aspect of their clinical presentation, as MS would require.Citation23,Citation24
In June 2013, the GACVS (Global Advisory Committee on Vaccine Safety) reviewed the updated data from USA, Australia, Japan, and the manufacturers of Cervarix (GlaxoSmithKline) and Gardasil (Merck). Serious AEs, such as Guillain-Barré syndrome, seizures, stroke, venous thromboembolism, anaphylaxis and other allergic reactions, have been investigated in more detail and they were not confirmed. The Committee reassured about the safety profile of the HPV vaccines concerning the potential association with autoimmune diseases, including MS.Citation25
In consideration of the current knowledge, a further assessment of the scientific literature was deemed appropriate, in order to explicitly determine a potential association between the HPV vaccine and the risk of developing MS.
Results
Study selection
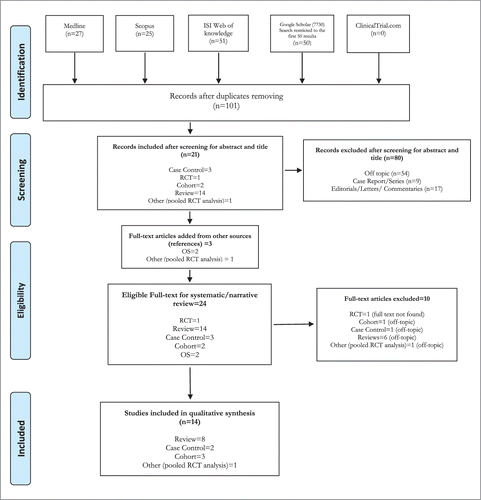
A total of 153 studies were identified by the search strategy conducted on five search engines: Medline, Scopus, ISI web of knowledge, GOOGLE Scholar and ClinicalTrials.gov. After adjusting for duplicates 101 articles remained. The screening for title and abstract identified 21 articles suitable for full-text reading, while 80 papers were excluded. Of these, 54 were discarded because after reviewing the abstracts it appeared that they clearly did not meet the topic criteria as described in methods section, while 9 case report/series and 17 editorials/letters/ commentaries were excluded because they did not match the design requirement. The bibliography was further screened as a source of additional evidence and three studies were drawn up from the provided references. The full text of the resulting 24 studies was examined in more detail. It followed that 9 studies did not meet the inclusion criteria as described and the full text of a randomized clinical trial (RCT) was not available.Citation26 Fifteen publications were finally selected for the qualitative synthesis: two case-control studies, three cohort studies, nine reviews, and one pooled RCT analysis. No unpublished relevant studies were found. (See )
Study characteristics
All 14 publication finally selected for the review were published in English. Three observational studies were European,Citation27-29 while two were carried out in US.Citation30,Citation31 Langer-Gould et al. was a nested case-control study from the retrospective cohort study carried out by Chao et al.Citation31 ()
Table 1. Studies included in Systematic Review.
The quality of the five observational studies was 6.2 on average, according the Newcastle-Ottawa score.Citation32 The highest score (7) was obtained by the two case-control study (Langer-Gould et al. and Grimaldi-Bensouda et al.)Citation27,Citation31 and by one cohort study (Scheller et al.),Citation28 while the lowest score (5) was obtained by two cohort studies (Willame et al. and Chao et al.).Citation29,Citation30 The incident rate (IR) of developing MS symptoms after HPV vaccination was computed mostly by the cohort studies, while the case-control studies calculated the odds ratio (OR) as a measure of risk to develop MS. The relative risks (RR) were computed from the IR. The aforementioned measures of outcome were available only for the female population and the mean age resulted 17 years. Aside from Langer-Gould et al. nested case-control study, the four original studies involved a total of 324,261 female participants. Either young women undergoing HPV-vaccination for the first time or catching up cohorts were included. Three studies out of four focused on 4vHPV effect,Citation27,Citation28,Citation30 while the association between MS and 2vHPV pertained only to the cohort study performed in United Kingdom.Citation29 Results of data extraction are reported in .
Table 2. Main Outcomes from Observational Studies.
Overall, the RR and the OR of developing MS symptoms was computed for different buffering risk windows from the exposure to vaccine, for a period of time spanning from a minimum of 14 days to a maximum of 3 years from the first dose administration.Citation31 The mean length of follow-up was 30 months. HPV vaccine was mainly administrated in three doses (See ).
The cohort study performed by Scheller et al. reported a lower risk of developing MS symptoms after the exposure to HPV vaccination (crude incidence rates: 6.12 events/100 000 person-years (95%CI, 4.86–7.69) and 21.54 events/100 000 person-years (95%CI, 20.90–22.20) for the vaccinated and unvaccinated periods). Analysis of risk was performed for different risk windows, starting within 179 days after the exposure and up until 2 years. Overall the adjusted OR was 0.90 for the vaccinated cohort and it was not significant (95%CI, 0.79–1.38). Results were stratified for age, nationality and time of each dose administration.Citation28 The other two cohort studies had a retrospective design and reported a higher IR (incidence x 100,000 person years) of MS onset among the vaccinated female population examined; with RR ranging from 1.54 (95%CI, 0.04–8.59) in Willame et al.,Citation29 to 1.37 (95%CI, 0.74–3.20) in Chao et al.Citation30 The outcome measures were adjusted for age, dose sequence, time from exposure. Results were not significant.
Regarding the case-control studies, the risk of MS onset after 3 years from the HPV vaccination was found 70% lower in the vaccinated group with respect to unexposed (OR = 0.3, 95%CI 0.1–0.9) by Grimaldi-Bensouda et al.Citation27 Conversely, Langer-Gould et al. found out a higher risk for the vaccinated group after three year from the administration (OR = 1.60, 95%CI = 0.79–3.25). By stratifying for age the OR turned out to be protective for over 50 years (OR = 0.88, 95%CI = 0.54–1.44), but the result was still not significant.Citation31
Langer-Gould et al. controlled the OR results for a total of four risk windows since HPV-vaccine exposure, the first of which was set after 14 days, and the final date tallied with the end of the follow up period, namely 3 years after the exposure. Grimaldi-Bensouda et al. evaluated the results after 2 years since vaccine administration ().
In both the case-control studies, the OR values were adjusted for age and geographical origin. Confounding factors such as ethnicity, concurrent infectious/parasitic diseases, comorbidities, hospitalization and number of Physician/Emergency Department visits resulted significant at 1% in Langer-Gould.Citation31 Grimaldi-Bensuda et al. accounted for: alcohol consumption, use of oral contraceptive, vaccination other than Gardasil over the 24 months before the index date and personal history of nervous autoimmune diseases.Citation27
Nine reviews were found through the systematic search, published between 2009 and 2017. Seven were narrative reviewsCitation33-39 while two were systematic reviews.Citation23,Citation40 No study performed a meta-analysis. As stated in methods, quality was evaluated through Amstar for systematic reviews and through Insa for narrative reviews. The median value of the quality score was 4.5 for the narrative reviews and 3.5 for the systematic reviews. Seven reviewsCitation23,Citation33-36,Citation38,Citation39 did not declared any sponsor, one reviewCitation40 was funded by public health institutions and oneCitation37 by a private foundation. Four reviewsCitation36,Citation38-40 did not declare the conflict of interest, three reviewCitation33,Citation35,Citation37 declared that no conflict of interest existed, two reviewsCitation23,Citation34 declares grants and other funds from the license holder of the HPV vaccines. In one caseCitation34 the first author was an employee of the license holder. All reviews investigated both bivalent and quadrivalent vaccine, with the exception of Gee et al., Vichnin et al. and Karussis et al, which reviewed the quadrivalent vaccine only.Citation33,Citation34,Citation36 All the authors, with the exception of Tomljenovic et al, claimed that there is no relation between the HPV vaccination and the development of MS or other demyelinating diseases.
One pooled analysis of RCT (Angelo et al)Citation41 was found through the systematic search, published in 2014. All the authors of the paper were employees of the license holder (GlaxoSmithKline). The paper is based on ongoing and completed RCT carried out by GlaxoSmithKline itself on the efficacy and safety of the bivalent HPV vaccine. 41862 women were included in the pooled analysis for the autoimmune diseases (including MS), 21358 in the intervention group (women with at least one administered dose of bivalent HPV vaccine) and 20504 in the control group, formed by women treated with placebo (Al(OH)3) or with at least one dose of other vaccines (i.e. HPA vaccines, HBV vaccines, quadrivalent HPV vaccine, meningococcal ACYW-135 vaccine, combined diphtheria, tetanus, acellular pertussis (adsorbed) vaccine with or without inactivated poliomyelitis vaccines). The authors did not found any association between cases and controls for Multiple Sclerosis within 1 year of any doses.
Risk of bias across studies
Authors did not combined data in a meta-analysis mainly for methodological reasons. Indeed the different effect measures applied, the variability of the confounding factors and the baseline risk may remarkably contribute to the undetectable amount of heterogeneity. For the aforementioned reasons the authors agreed to not perform further quantitative analysis.
Discussion
This study provides a substantial contribute on the safety profile of HPV vaccine as related to the risk of develop multiple sclerosis, including data from the available literature.
Vaccines could theoretically increase the risk of CNS ADS through mechanisms similar to those induced by infection. Hitherto, the mechanisms underlying immune mediated disorders are complex, and not fully understood. Infections are known to induce autoimmunity through expansion of autoreactive T-cell clones by molecular mimicry, later stimulation of autoreactive T-cell clones, or enhancement of antigen presentation by bystander activation, epitope spreading, adjuvant effect, or enhanced antigen presentation itself.Citation42 However, for some immune mediated diseases, an autoimmune mechanism has not been clearly defined. Thus, a viable approach would be to collect scientific evidence about all possible diseases for which an autoimmune-dependent mechanism, namely MS, has been postulated, even if not yet unequivocally established.Citation43
Since the introduction of HPV vaccines, neurological autoimmune diseases, including MS, have been under particularly careful investigation given their correspondingly high age-specific background incidence and further potential confounding factors.Citation22,Citation43-45
A retrospective register-based cohort study in Sweden and Denmark included almost 1 million girls aged 10–17 years, among whom almost 300 000 were vaccinated against HPV. The study results did not show evidence of any association between exposure to HPV vaccine and autoimmune, neurological, and venous thromboembolic adverse events.Citation46 Despite the potential bias represented by the limits of a self-reported symptoms recording, no increased risk of autoimmune diseases following 4vHPV vaccination was found in this study.Citation46 In accordance, no evidence of an increased risk of MS onset was confirmed for the bivalent vaccine 2vHPV-16/18 by Willame et al. in their retrospective cohort study, reporting a RR = 0.Citation29 The slightly increased risk of MS within the first 3 months after HPV vaccination (but not potential precursors such as Clinically Isolated Syndrome (CIS) or Acute Disseminated Encephalomyelitis (ADEM)) reported by Langer-Gould et al., was statistically non-significant.Citation31 Moreover, all new MS cases experienced a full recovery from their first attack. However, the power of the study was not sufficient to detect a statistically meaningful effect (92 vaccinated MS cases recorded before the index date versus 459 controls).Citation31
Concerning the buffering risk windows since the HPV-vaccine exposure, a substantial heterogeneity and a lack of knowledge about the appropriate length of follow-up should be remarked. By ACIP recommendations, the 4vHPV is administered in a 2 or 3-dose series.Citation47 The number of new MS cases was reported for each dose of vaccine administered in a retrospective cohort study performed by Chao et al. in US during 2012. For the four MS cases recorded, the average time from the onset of the symptoms was 73 days afterward the 4vHPV exposure. However, no higher risk of develop MS was identified.Citation30 In Langer-Gould the exposure (vaccination) was restricted to the following different time frames before the index date: 14 days, 30 days, 42 days, 90 days, 180 days, 1 year, and 3 years.Citation31 The highest adjusted OR value (3.25) was reported after 42 days from vaccination, but the result was not significant (95%CI = 0.80-13.29). After 3 years of follow up, Langer-Gould et al. found no significant results.Citation31 Overall, the mean length of follow up was 30 months; therefore, the long-term effects of the vaccine on MS development might have been underestimated. In fact, the mean age of women affected by full-blown MS disease could be ranged between 34.3748 and 46.9.Citation49 The one-year follow-up period was chosen in agreement with the FDA in Willame et al.Citation29
No significant association between the HPV vaccination and the development of MS or other demyelinating diseases was confirmed by the reviews. Agorastos et al. pointed out the need of accurately establish the baseline rates of autoimmune disorders, including acute demyelination, in the general population.Citation23 Pellegrino et al noted that, given the low risk of death from cervix cancer and the efficacy of the screening program, the vaccination should not be a public health measure, but rather a personal decision.Citation34 In contrast with the other authors, Tomljenovic et al. were very skeptical about the efficacy of the HPV vaccines in countries with effective screening program for the cervix cancer. About the safety, they contest the use in pre-clinical trials of inappropriate placebo for the controls, i.e. using aluminum as adjuvant in the control vaccine. Moreover, they found that the post-licensure cohort study of Chao et al. about the adverse events correlated with anti-HPV vaccination is prone to bias toward false negatives. Overall, Tomljenovic et al. were very doubtful about the safety of the HPV vaccination.Citation37
The French Regional Commission for Conciliation and Compensation had adjudicated on one case of MS. Another 14 cases of MS were reported through regional pharmacovigilance centers and/or the manufacturers to the European Medicines Agency. All 15 cases were declared as being of “doubtful” causality, according to the French grading system. In addition, a cohort study involving 2 million girls aged 12–16 did not show any increase in hospitalization rates for autoimmune diseases in the vaccinated cohort (2.1/10 000 patients/year) compared to not exposed (2.09/10 000 patients/year).Citation50
Epidemiological studies included in this systematic review showed no increased risk of MS. Nevertheless, the robustness of the results and the amount of the heterogeneity between studies should take into account several confounding factors. In particular of outmost importance are: the hetereogenity of risk windows, as discussed above; the possible action of HPV vaccine as a proinflammatory cofactor that may accelerate the transition to clear autoimmunity symptoms in patients with existing subclinical disease,Citation31 similarly to various comorbidities, upper respiratory tract and other infection, that are well known risk factors for MS relapsesCitation51; bias introduced by self-controlled symptoms and electronic health records; lack of medical record review; the additive effect of more dosesCitation30 and the effect of vaccine adjuvants, which formulation have not yet been clearly determined; risk adjustment for potential confounders, such as the health care system organization, equality and equity of access to public preventive health policy, opportunity or ability of taking part to the public vaccine programsCitation27,Citation39; others concurrent vaccinations.
A mainstay point that can undermine the generalizability of results is the low statistical power of the studies reviewed, due to the rarity of MS cases. Therefore, in order to obtain a significant result, the systematic review might be extended to the entire range of Neurological Autoimmune Diseases risk. Furthermore, considering that MS is more likely to occur among female rather than male, in a 2:1 ratio,Citation52 the male cohort should be included in the analysis. Finally, the temporal relationship and the plausibility between the exposure to the HPV vaccine and the MS onset is worth of further investigation, in order to quantitatively define the degree of association.
Methods
Eligibility criteria
The original systematic review of the literature included RCTs, Cohort and Case Control studies, afterwards the research was extended to review or meta-analysis studies. Letters, editorials, and overviews were not regarded for inclusion. Articles had to be English written and published within January 2017.
Participants of male and female gender who had undertaken both bivalent and quadrivalent schedule of HPV vaccine were considered. No limits concerning age, sample size and the geographic setting were set up. The occurrence of MS following the HPV vaccine was established as measure of outcome, no restriction was applied to the time risk window. Either the risk of developing MS symptoms in long run or following each vaccine dose administration were taken into account.
Information sources and search strategy
The following databases were employed for pertinent articles: MEDLINE, SCOPUS, ISI WEB OF KNOWLEDGE, GOOGLE SCHOLAR and ClinicalTrial.gov.
The keywords used for the research were: “((multiple sclerosis or demyelinating disease*) AND (Papillomavirus vaccin* OR HPV vaccin*))” for MEDLINE, “(multiple sclerosis OR demyelinating diseases) AND TITLE-ABS-KEY (hpv vaccine OR papillomavirus vaccine))” for SCOPUS, “(multiple sclerosis OR demyelinating disease*) AND TOPIC: (HPV vaccin* OR papillomavirus vaccin*)” for ISI WEB OF KNOWLEDGE, “HPV vaccine (all the words) “multiple sclerosis”(the whole sentence)” for GOOGLE SCHOLAR, “HPV vaccine” AND “multiple sclerosis” for ClinicalTrials.gov. The research was performed at January 2017.
Study selection, data collection process and data items
Two researchers independently screened all the papers for eligibility and a third reviewer performed the assessment in case of disagreement. The literature screening was carried out by title and abstract, the full text suitability was assessed afterwards. Two reviewers performed the data extraction process, collecting the following information: title, authors, year of publication, journal, study design, and if available, sample size, mean age of the sample, duration of follow up, risk measures.
Risk of bias in individual studies
In order to ascertain the validity of the studies selected for the data extraction process, the quality assessment of cohort and case-control studies was determined by applying Newcastle-Ottawa ScaleCitation32 tool. The systematic and the narrative reviews were judged using the AMSTARCitation53 and INSACitation54 tools, respectively. The presentation of the results of the present systematic review is in accordance with the PRISMA statement.Citation55
Summary measures and additional analysis
Either the incidence relative risk (IRR), the RR or ODDs ratios of developing MS after HPV vaccination were considered as primary measure of risk for data extraction. The search strategy was relative to any available formulation of HPV test: Cervarix, Gardasil and Gardasil-9. No time restrictions was applied to the adverse effect occurrence after HPV vaccine exposure. Both male and female gender were considered. Any additional quantitative analyses was deferred to the results of the systematic review and the data availability.
Disclosure of potential conflicts of interest
Authors have nothing to disclose.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Garland SM. Can cervical cancer be eradicated by prophylactic HPV vaccination? Challenges to vaccine implementation. Indian J Med Res. 2009;130:311–21. PMID:19901440.
- Woodman CBJ, Collins SI, Young LS. The natural history of cervical HPV infection: Unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi:10.1038/nrc2050. PMID:17186016.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin H-R, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi:10.1016/S1470-2045(10)70230-8. PMID:20952254.
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Muñoz N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26 Suppl 10:K1–16. doi:10.1016/j.vaccine.2008.05.064.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24 Suppl 3:S3/11–25.
- McGraw SL, Ferrante JM. Update on prevention and screening of cervical cancer. World J Clin Oncol. 2014;5:744–52. doi:10.5306/wjco.v5.i4.744. PMID:25302174.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89:12180–4. doi:10.1073/pnas.89.24.12180. PMID:1334560.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi:10.1056/NEJMoa061741. PMID:17494925.
- Approved Products >Cervarix [Internet]. [cited 2017 Sep 20]; Available from: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186957.htm
- Kirby T. FDA approves new upgraded Gardasil 9. Lancet Oncol. 2015;16:e56. doi:10.1016/S1470-2045(14)71191-X. PMID:25532625.
- Petrosky E, Bocchini JA, Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE, Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–4. PMID:25811679
- Meites E. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination — Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep [Internet] 2016 [cited 2017 Sep 20]; 65. Available from: https://www.cdc.gov/mmwr/volumes/65/wr/mm6549a5.htm
- Gonçalves AK, Cobucci RN, Rodrigues HM, de Melo AG, Giraldo PC. Safety, tolerability and side effects of human papillomavirus vaccines: a systematic quantitative review. Braz J Infect Dis Off Publ Braz Soc Infect Dis. 2014;18:651–9.
- Research C for BE and. Approved Products – Gardasil [Internet]. [cited 2017 Dec 1]; Available from: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm094042.htm
- Research C for BE and. Approved Products – Gardasil 9 [Internet]. [cited 2017 Dec 1]; Available from: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm426445.htm
- WHO | Global Advisory Committee on Vaccine Safety,<br>7–8 June 2017 [Internet]. WHO [cited 2017 Dec 1]; Available from: http://www.who.int/vaccine_safety/committee/reports/June_2017/en/
- Costa APF, Cobucci RNO, da Silva JM, da Costa Lima PH, Giraldo PC, Gonçalves AK. Safety of Human Papillomavirus 9-Valent Vaccine: A Meta-Analysis of Randomized Trials. J Immunol Res. 2017;2017:3736201. doi:10.1155/2017/3736201. PMID:28812030.
- Kang S, Kim KH, Kim YT, Kim YT, Kim JH, Song YS, Shin SH, Ryu HS, Han JW, Kang JH, et al. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: A randomized, placebo-controlled trial in 176 Korean subjects. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2008;18:1013–9.
- Klein NP, Hansen J, Chao C, Velicer C, Emery M, Slezak J, Lewis N, Deosaransingh K, Sy L, Ackerson B, et al. Safety of Quadrivalent Human Papillomavirus Vaccine Administered Routinely to Females. Arch Pediatr Adolesc Med 2012; 166:1140. doi:10.1001/archpediatrics.2012.1451. PMID:23027469.
- Geier DA, Geier MR. Quadrivalent human papillomavirus vaccine and autoimmune adverse events: A case–control assessment of the vaccine adverse event reporting system (VAERS) database. Immunol Res. 2017;65:46–54. doi:10.1007/s12026-016-8815-9. PMID:27406735.
- Guimarães LE, Baker B, Perricone C, Shoenfeld Y. Vaccines, adjuvants and autoimmunity. Pharmacol Res. 2015;100:190–209. doi:10.1016/j.phrs.2015.08.003. PMID:26275795.
- Siegrist C-A. Autoimmune diseases after adolescent or adult immunization: What should we expect?. CMAJ Can Med Assoc J J Assoc Medicale Can. 2007;177:1352–4. doi:10.1503/cmaj.071134.
- Agorastos T, Chatzigeorgiou K, Brotherton JML, Garland SM. Safety of human papillomavirus (HPV) vaccines: A review of the international experience so far. Vaccine. 2009;27:7270–81. doi:10.1016/j.vaccine.2009.09.097. PMID:19799849.
- Sutton I, Lahoria R, Tan I, Clouston P, Barnett M. CNS demyelination and quadrivalent HPV vaccination. Mult Scler Houndmills Basingstoke Engl. 2009;15:116–9. doi:10.1177/1352458508096868.
- WHO | Global Advisory Committee on Vaccine Safety, report of meeting held 12–13 June 2013 [Internet]. WHO [cited 2017 Nov 14]; Available from: http://www.who.int/vaccine_safety/committee/reports/Jun_2013/en/
- Moreira ED, Block SL, Ferris D, Giuliano AR, Iversen O-E, Joura EA, Kosalaraksa P, Schilling A, Van Damme P, Bornstein J, et al. Safety Profile of the 9-Valent HPV Vaccine: A Combined Analysis of 7 Phase III Clinical Trials. Pediatrics. 2016;138(2). pii: e20154387. doi:10.1542/peds.2015-4387. PMID:27422279
- Grimaldi-Bensouda L, Guillemot D, Godeau B, Bénichou J, Lebrun-Frenay C, Papeix C, Labauge P, Berquin P, Penfornis A, Benhamou P-Y, et al. Autoimmune disorders and quadrivalent human papillomavirus vaccination of young female subjects. J Intern Med. 2014;275:398–408. doi:10.1111/joim.12155. PMID:24206418.
- Scheller NM, Svanström H, Pasternak B, Arnheim-Dahlström L, Sundström K, Fink K, Hviid A. Quadrivalent HPV vaccination and risk of multiple sclerosis and other demyelinating diseases of the central nervous system. JAMA. 2015;313:54–61. doi:10.1001/jama.2014.16946. PMID:25562266.
- Willame C, Rosillon D, Zima J, Angelo M-G, Stuurman AL, Vroling H, Boggon R, Bunge EM, Pladevall-Vila M, Baril L. Risk of new onset autoimmune disease in 9- to 25-year-old women exposed to human papillomavirus-16/18 AS04-adjuvanted vaccine in the United Kingdom. Hum Vaccines Immunother. 2016;12:2862–71. doi:10.1080/21645515.2016.1199308.
- Chao C, Klein NP, Velicer CM, Sy LS, Slezak JM, Takhar H, Ackerson B, Cheetham TC, Hansen J, Deosaransingh K, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193–203. doi:10.1111/j.1365-2796.2011.02467.x. PMID:21973261.
- Langer-Gould A, Qian L, Tartof SY, Brara SM, Jacobsen SJ, Beaber BE, Sy LS, Chao C, Hechter R, Tseng HF. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol. 2014;71:1506–13. doi:10.1001/jamaneurol.2014.2633. PMID:25329096.
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 2017 Mar 27]; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Gee J, Weinbaum C, Sukumaran L, Markowitz LE. Quadrivalent HPV vaccine safety review and safety monitoring plans for nine-valent HPV vaccine in the United States. Hum Vaccines Immunother. 2016;12:1406–17. doi:10.1080/21645515.2016.1168952.
- Vichnin M, Bonanni P, Klein NP, Garland SM, Block SL, Kjaer SK, Sings HL, Perez G, Haupt RM, Saah AJ, et al. An Overview of Quadrivalent Human Papillomavirus Vaccine Safety: 2006 to 2015. Pediatr Infect Dis J. 2015;34:983–91. doi:10.1097/INF.0000000000000793. PMID:26107345.
- Handler NS, Handler MZ, Majewski S, Schwartz RA. Human papillomavirus vaccine trials and tribulations: Vaccine efficacy. J Am Acad Dermatol. 2015;73:759-767; quiz 767–768. doi:10.1016/j.jaad.2015.05.041.
- Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13:215–24. doi:10.1016/j.autrev.2013.10.003. PMID:24514081.
- Tomljenovic L, Spinosa JP, Shaw CA. Human papillomavirus (HPV) vaccines as an option for preventing cervical malignancies: (how) effective and safe? Curr Pharm Des. 2013;19:1466–87. PMID:23016780
- Orbach H, Agmon-Levin N, Zandman-Goddard G. Vaccines and autoimmune diseases of the adult. Discov Med. 2010;9:90–7. PMID:20193633
- Macartney KK, Chiu C, Georgousakis M, Brotherton JML. Safety of human papillomavirus vaccines: A review. Drug Saf. 2013;36:393–412. doi:10.1007/s40264-013-0039-5. PMID:23637071.
- Pellegrino P, Carnovale C, Pozzi M, Antoniazzi S, Perrone V, Salvati D, Gentili M, Brusadelli T, Clementi E, Radice S. On the relationship between human papilloma virus vaccine and autoimmune diseases. Autoimmun Rev. 2014;13:736–41. doi:10.1016/j.autrev.2014.01.054. PMID:24468416.
- Angelo M-G, David M-P, Zima J, Baril L, Dubin G, Arellano F, Struyf F. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf. 2014;23:466–79. doi:10.1002/pds.3554. PMID:24644063.
- Münz C, Lünemann JD, Getts MT, Miller SD. Antiviral immune responses: Triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9:246–58. doi:10.1038/nri2527. PMID:19319143.
- World Health Organization. Global Advisory Committee on Vaccine Safety Statement on the continued safety of HPV vaccination [Internet]. [cited 2017 Mar 12]; Available from: http://www.who.int/vaccine_safety/committee/topics/hpv/GACVS_Statement_HPV_12_Mar_2014.pdf?ua=1
- Siegrist C-A, Lewis EM, Eskola J, Evans SJW, Black SB. Human papilloma virus immunization in adolescent and young adults: A cohort study to illustrate what events might be mistaken for adverse reactions. Pediatr Infect Dis J. 2007;26:979–84. doi:10.1097/INF.0b013e318149dfea. PMID:17984802.
- Callréus T, Svanström H, Nielsen NM, Poulsen S, Valentiner-Branth P, Hviid A. Human papillomavirus immunisation of adolescent girls and anticipated reporting of immune-mediated adverse events. Vaccine. 2009;27:2954–8. doi:10.1016/j.vaccine.2009.02.106. PMID:19428906.
- Arnheim-Dahlström L, Pasternak B, Svanström H, Sparén P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ. 2013;347:f5906. doi:10.1136/bmj.f5906. PMID:24108159.
- HPV Vaccine Administration | Human Papillomavirus Vaccination | CDC [Internet]. [cited 2017 Mar 27]; Available from: https://www.cdc.gov/vaccines/vpd/hpv/hcp/administration.html
- Shaygannejad V, Rezaie N, Paknahad Z, Ashtari F, Maghzi H. The environmental risk factors in multiple sclerosis susceptibility: A case-control study. Adv Biomed Res. 2016;5:98. doi:10.4103/2277-9175.183665. PMID:27376037.
- Manouchehrinia A, Westerlind H, Kingwell E, Zhu F, Carruthers R, Ramanujam R, Ban M, Glaser A, Sawcer S, Tremlett H, et al. Age Related Multiple Sclerosis Severity Score: Disability ranked by age. Mult Scler. 2017;23(14):1938–1946. doi:10.1177/1352458517690618.
- WHO | Human papillomavirus vaccines safety (HPV) [Internet]. WHO [cited 2017 Apr 12]; Available from: http://www.who.int/vaccine_safety/committee/topics/hpv/dec_2013/en/
- Buljevac D, Flach HZ, Hop WCJ, Hijdra D, Laman JD, Savelkoul HFJ, van Der Meché FGA, van Doorn PA, Hintzen RQ. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain J Neurol. 2002;125:952–60. doi:10.1093/brain/awf098.
- Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention. Semin Neurol. 2008;28:17–28. doi:10.1055/s-2007-1019126. PMID:18256984.
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, Porter AC, Tugwell P, Moher D, Bouter LM. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol [Internet] 2007 [cited 2017 Mar 27]; 7. Available from: http://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-7-10 doi:10.1186/1471-2288-7-10.
- La Torre G, Backhaus I, Mannocci A. Rating for narrative reviews: concept and development of the International Narrative Systematic Assessment tool. Senses Sci. 2015;2:31–5.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi:10.1016/j.jclinepi.2009.06.006. PMID:19631507.